The Influence of Precursor pH on the Synthesis and Morphology of AuNPs Synthesized Using Green Tea Leaf Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
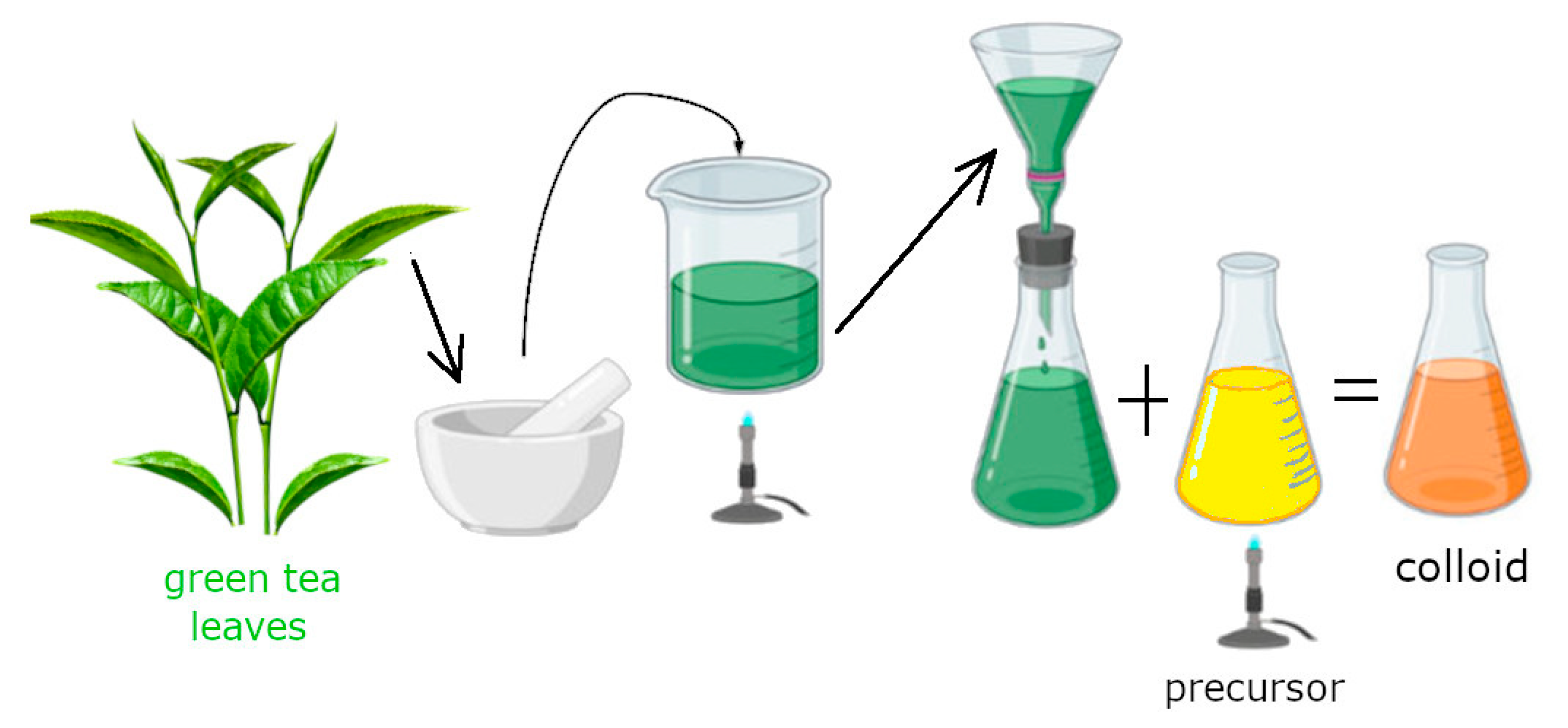
2.2. Solution Preparation and Synthesis of AuNPs
2.3. Methods
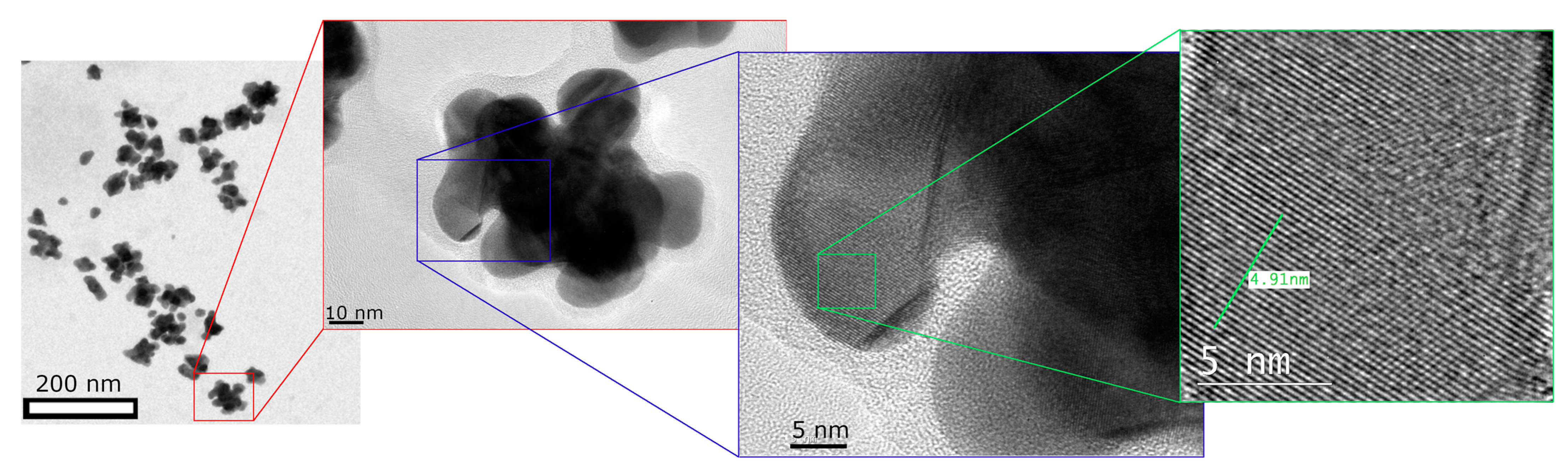
3. Results and Discussion
3.1. Green Tea Leaf
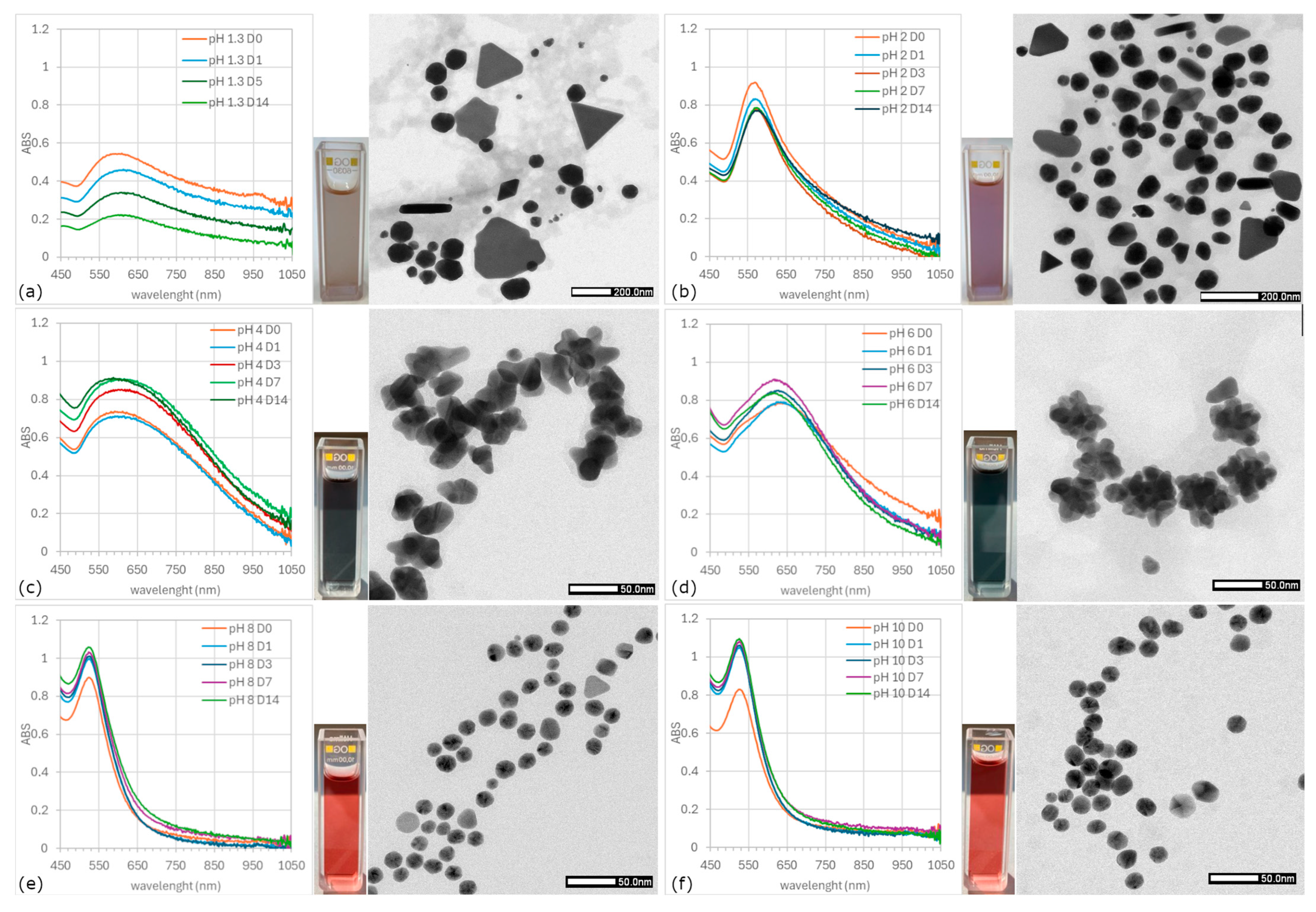
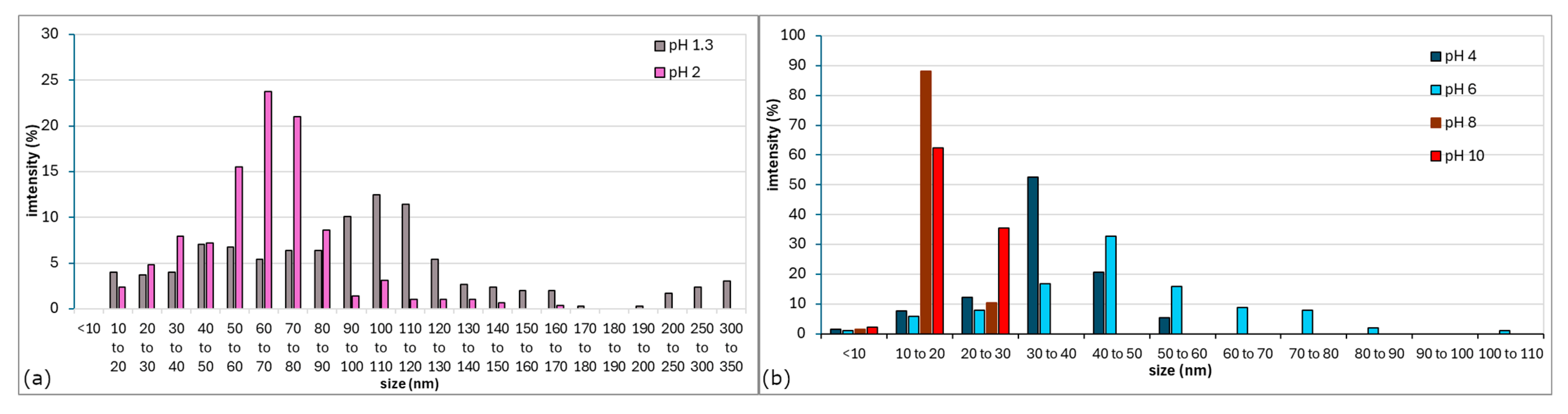
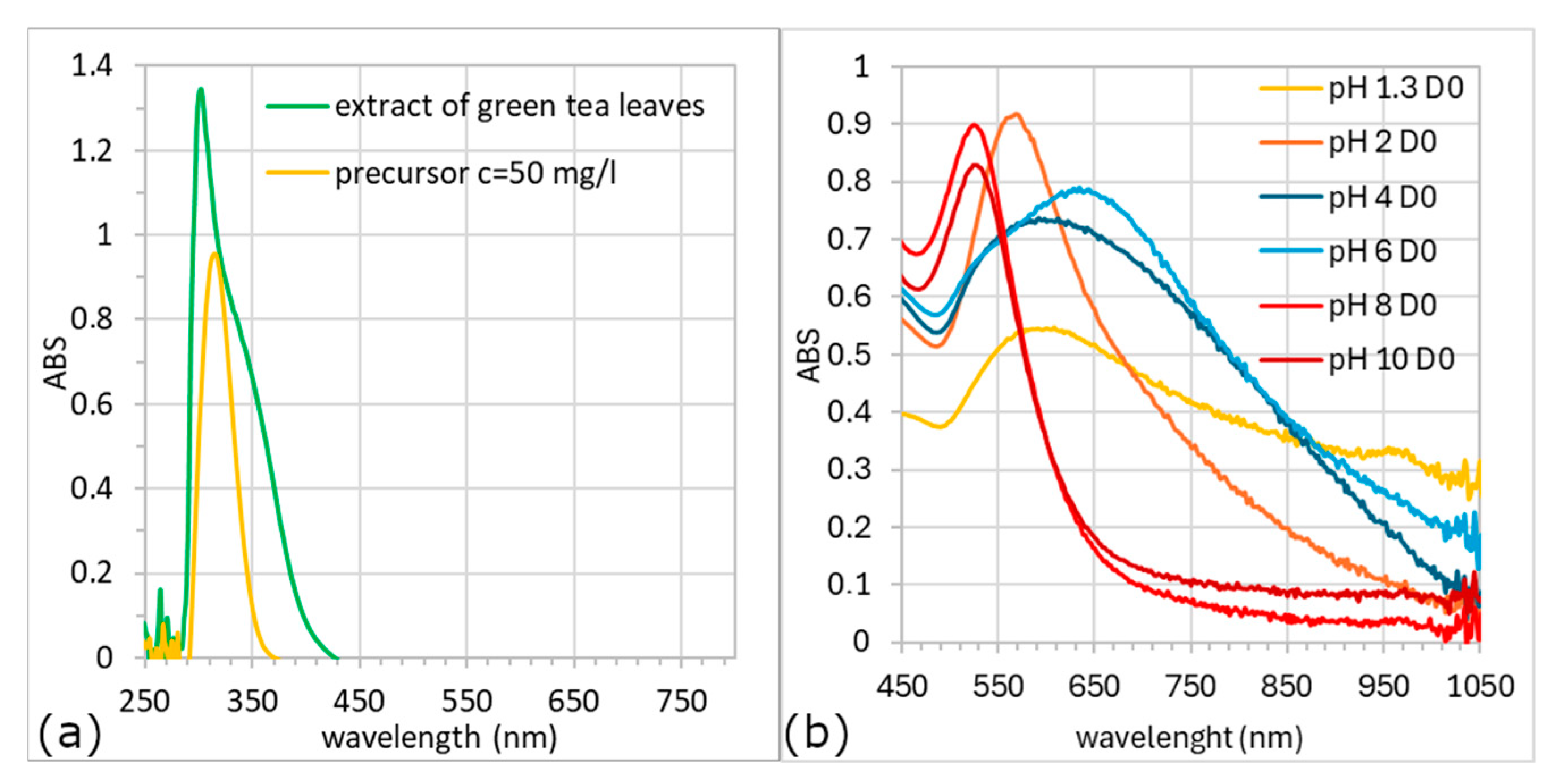
3.2. UV-Vis Analysis
- , —intensity of the transmitted and incident light (W)
- —molar absorption coefficient (L/mol·cm)
- c—concentration in (mol/L)
- l—the length of the light path in (cm)
- A—absorbance
- Type I—tall and narrow spectra; ABSmax ~530 nm; colloids in the shades of violet–red (solutions with pH 8 and 10),
- Type II—ABSmax ~580 nm; beige and violet–beige solutions (pH 1.3 and 2),
- Type III—broad spectrum; ABSmax of more than 600 nm; colloids in the shades of blue (pH 4 and 6).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Aziz, F.; Ihsan, A.; Nazir, A.; Ahmad, I.; Bajwa, S.Z.; Rehman, A.; Diallo, A.; Khan, W.S. Novel route synthesis of porous and solid gold nanoparticles for investigating their comparative performance as contrast agent in computed tomography scan and effect on liver and kidney function. Int. J. Nanomed. 2017, 12, 1555–1563. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. Honeybee pollen assisted biosynthesis of nanogold and its application as catalyst in reduction of 4-nitrophenol. Heliyon 2022, 8, e10191. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Abd-Elrahman, N.K. Physical methods for preparation of nanomaterials, their characterization and applications: A review. J. Umm Al-Qura Univ. Appl. Sci. 2025, 11, 356–377. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of Various Size Gold Nanoparticles by Chemical Reduction Method with Different Solvent Polarity. Nanoscale Res. Lett. 2020, 15, 140. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Santoyo-Salzar, J.; Rangel-Cortes, E.; Goya, G.F.; Cardozo-Mata, V.; Pescador-Rojas, J.A. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultrason. Sonochem. 2021, 70, 105274. [Google Scholar] [CrossRef]
- Raghunandan, D.; Bedre, M.D.; Basavaraja, S.; Sawle, B.; Manjunath, S.Y.; Venkataraman, A. Rapid biosynthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf. B Biointerfaces 2010, 79, 235–240. [Google Scholar] [CrossRef]
- Philip, D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 807–810. [Google Scholar] [CrossRef]
- Palaniswamy, S.; Bepari, A.; Rajan, S.; Assiri, R.A.; Shibu, A.; Narayasamy, A.; Krishnamoorthy, K. Biosynthesis of gold nanoparticles from Martynia annua aqueous leaves extract, its antioxidant potential and antimicrobial ability against selected wound pathogens. Inorg. Chem. Comm. 2024, 70, 113241. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Siksa, P.; Kováčová, M.; Bureš, R.; Tkáčiková, Ľ.; Džunda, R.; Tampubolon, I.O.; Balážová, Ľ.; Baláž, M. Lavender-mediated solvent-free biomechanochemical synthesis of antibacterially active Ag/AgCl nanoparticles using a Taguchi design. Adv. Nat. Sci. Nanosci. Nanotechnol. 2025, 16, 015018. [Google Scholar] [CrossRef]
- Nehl, C.L.; Liao, H.; Hafner, J.H. Optical Properties of Star-Shaped Gold Nanoparticles. Nano Lett. 2006, 6, 683–688. [Google Scholar] [CrossRef]
- Silvestri, A.; Lay, L.; Psaro, R.; Polito, L.; Evangelisti, C. Fluidic Manufacture of Star-Shaped Gold Nanoparticles. Chemistry 2017, 23, 9732–9735. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef]
- Barbosa, S.; Agrawal, A.; Rodríguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-Marzán, L.M. Tuning Size and Sensing Properties in Colloidal Gold Nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef]
- Huo, C.; Khoshnamvand, M.; Liu, P.; Liu, C.; Yuan, C.-G. Rapid mediated biosynthesis and quantification of AuNPs using persimmon (Diospyros Kaki, L.f) fruit extract. J. Mol. Struc. 2018, 1178, 366–374. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Adaila, K.; Omar, A.A. Effect of pH in the Biosynthesis of Gold Nanoparticles: A Review. Afr. J. Adv. Pure Appl. Sci. 2023, 2, 360–368. Available online: https://aaasjournals.com/index.php/ajapas/index (accessed on 1 May 2025).
- Hadi, H.T.; Ibrahim, O.M.S. Green Synthesis and Characterization of Gold Nanoparticles Using Crushed Clove Buds (Syzygium aromaticum) Oil Extracted by Hydrodistillation. J. Res. Pharm. 2024, 28, 1883–1891. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.-K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859. [Google Scholar] [CrossRef]
- Ariski, R.T.; Lee, K.K.; Kim, Y.; Lee, C.-S. The impact of pH and temperature on the green gold nanoparticles preparation using Jeju Hallabong peel extract for biomedical applications. RSC Adv. 2024, 14, 14582–14592. [Google Scholar] [CrossRef]
- Baesso, A.S.; Silva, D.J.; Soares, A.K.; Paula, M.M.S.; Gonzalez de Cademartori, P.H. Biosynthesis of gold nanoparticles using papaya seed extract for the functionalization of nanocellulose membranes. Ind. Crops Prod. 2023, 197, 116601. [Google Scholar] [CrossRef]
- Patra, S.; Golder, A.K.; Uppaluri, R.V. Green synthesis of carbon dots from mature green tea leaves for label-free fluorescence sensing of chromium(VI). Opt. Mater. 2024, 154, 115767. [Google Scholar] [CrossRef]
- Sahu, S.K.; Mansoori, A.; Jana, S.K.; Kumar, A.; Ghorai, T.K. Biosynthesis of silver nanoparticles using green tea aqueous leaf extract and their biological and chemotherapeutic activity. J. Molec. Struct. 2025, 1320, 139690. [Google Scholar] [CrossRef]
- Datta, D.; Deepak, K.S.; Das, B. Progress in the synthesis, characterisation, property enhancement techniques and application of gold nanoparticles: A review. MRS Commun. 2022, 12, 700–715. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Chaurasia, A.K.; Singh, R.K.; Kumar, A. Synthesis, optimization, characterization, antioxidant and anti-cancerous activity analysis of nanoparticles IA-AuNPs synthesized using leaf extract of soil grown Ipomoea aquatica. J. Mol. Struct. 2025, 1327, 141212. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Fawzy, M.; Hosny, M. Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Appl. Nanosci. 2023, 13, 3149–3160. [Google Scholar] [CrossRef]
- Rahman, A.U.; Khan, A.U.; Yuan, Q.; Wei, Y.; Ahmad, A.; Ullah, S.; Khan, Z.U.H.; Shams, S.; Tariq, M.; Ahmad, W. Tuber extract of Arisaema flavum eco-benignly and effectively synthesize silver nanoparticles: Photocatalytic and antibacterial response against multidrug resistant engineered E. coli QH4. J. Photochem. Photobiol. B Biol. 2019, 193, 31–38. [Google Scholar] [CrossRef]
- Meng, X.-H.; Li, N.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. Plant Resources, Chemical Constituents and Bioactivities of Tea Plants from the Genus Camellia Section Thea. J. Agric. Food Chem. 2018, 67, 5318–5349. [Google Scholar] [CrossRef]
- Ha Pham, T.T.; Dien, N.D.; Vu, X.H. Facile synthesis of silver/gold alloy nanoparticles for ultra-sensitive rhodamine B detection. RSC Adv. 2021, 11, 21475–21488. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2022, 3, 792. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Mizwari, Z.M.; Mohammadi-Aghdam, S.; Ahmadi, S.; Ebrahimzadeh, M.A.; Mortazavi-Derazkola, S. Optimization and evaluation of anticancer, antifungal, catalytic, and antibacterial activities: Biosynthesis of spherical-shaped gold nanoparticles using Pistacia vera hull extract (AuNPs@PV). Arab. J. Chem. 2023, 16, 104423. [Google Scholar] [CrossRef]
- Lukman, A.I.; Gong, B.; Marjo, C.E.; Roessner, U.; Harris, A.T. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J. Colloid Interface Sci. 2011, 353, 433–444. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Miličevič, A. Flavonoid Oxidation Potentials and Antioxidant Activities-Theoretical Models Based on Oxidation Mechanisms and Related Changes in Electronic Structure. Int. J. Mol. Sci. 2024, 25, 5011. [Google Scholar] [CrossRef]
- Timoszyk, A.; Grochowalska, R. Mechanism and Antibacterial Activity of Gold Nanoparticles (AuNPs) Functionalized with Natural Compounds from Plants. Pharmaceutics 2022, 14, 2599. [Google Scholar] [CrossRef]

| Reference | Reducing Agent/Plant Source | Synthesis Method/Conditions | Shape of AuNPs | Size Range |
|---|---|---|---|---|
| [4] | Honeybee pollen | Green synthesis | Mostly spherical | 7–42 nm |
| [5] | Various (overview) | Multiple methods (review) | Various (spheres, rods, and cages) | 1–100+ nm |
| [9] | Clove bud extract | Macerated extract, room temp | Irregular | 5–100 nm |
| [10] | Mangifera indica leaf extract | Rapid green synthesis | Spherical | ~18 nm |
| [11] | Martynia annua leaf extract | Room temp biosynthesis | Spherical | 21 nm |
| [15] | Seed-mediated growth | High-yield synthesis | Short rods | 20–100 nm length |
| [16] | Seed-mediated growth | Size tuning with silver ions | Nanostars | 40–150 nm |
| [17] | Diospyros kaki fruit extract | Biosynthesis | Various shapes | 20–100+ nm |
| [18] | Review (multiple plant types) | Focus on the pH effect | Various shapes | 15–90 nm |
| [20] | Magnolia kobus, Diospyros kaki | Biological synthesis | Various shapes | 5–300 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velgosova, O.; Mikulková, Z.; Lisnichuk, M. The Influence of Precursor pH on the Synthesis and Morphology of AuNPs Synthesized Using Green Tea Leaf Extract. Crystals 2025, 15, 682. https://doi.org/10.3390/cryst15080682
Velgosova O, Mikulková Z, Lisnichuk M. The Influence of Precursor pH on the Synthesis and Morphology of AuNPs Synthesized Using Green Tea Leaf Extract. Crystals. 2025; 15(8):682. https://doi.org/10.3390/cryst15080682
Chicago/Turabian StyleVelgosova, Oksana, Zuzana Mikulková, and Maksym Lisnichuk. 2025. "The Influence of Precursor pH on the Synthesis and Morphology of AuNPs Synthesized Using Green Tea Leaf Extract" Crystals 15, no. 8: 682. https://doi.org/10.3390/cryst15080682
APA StyleVelgosova, O., Mikulková, Z., & Lisnichuk, M. (2025). The Influence of Precursor pH on the Synthesis and Morphology of AuNPs Synthesized Using Green Tea Leaf Extract. Crystals, 15(8), 682. https://doi.org/10.3390/cryst15080682







