Lab- and Large-Scale Hydrothermal Synthesis of Vanadium Dioxide Thermochromic Powder
Abstract
1. Introduction
2. Materials and Methods
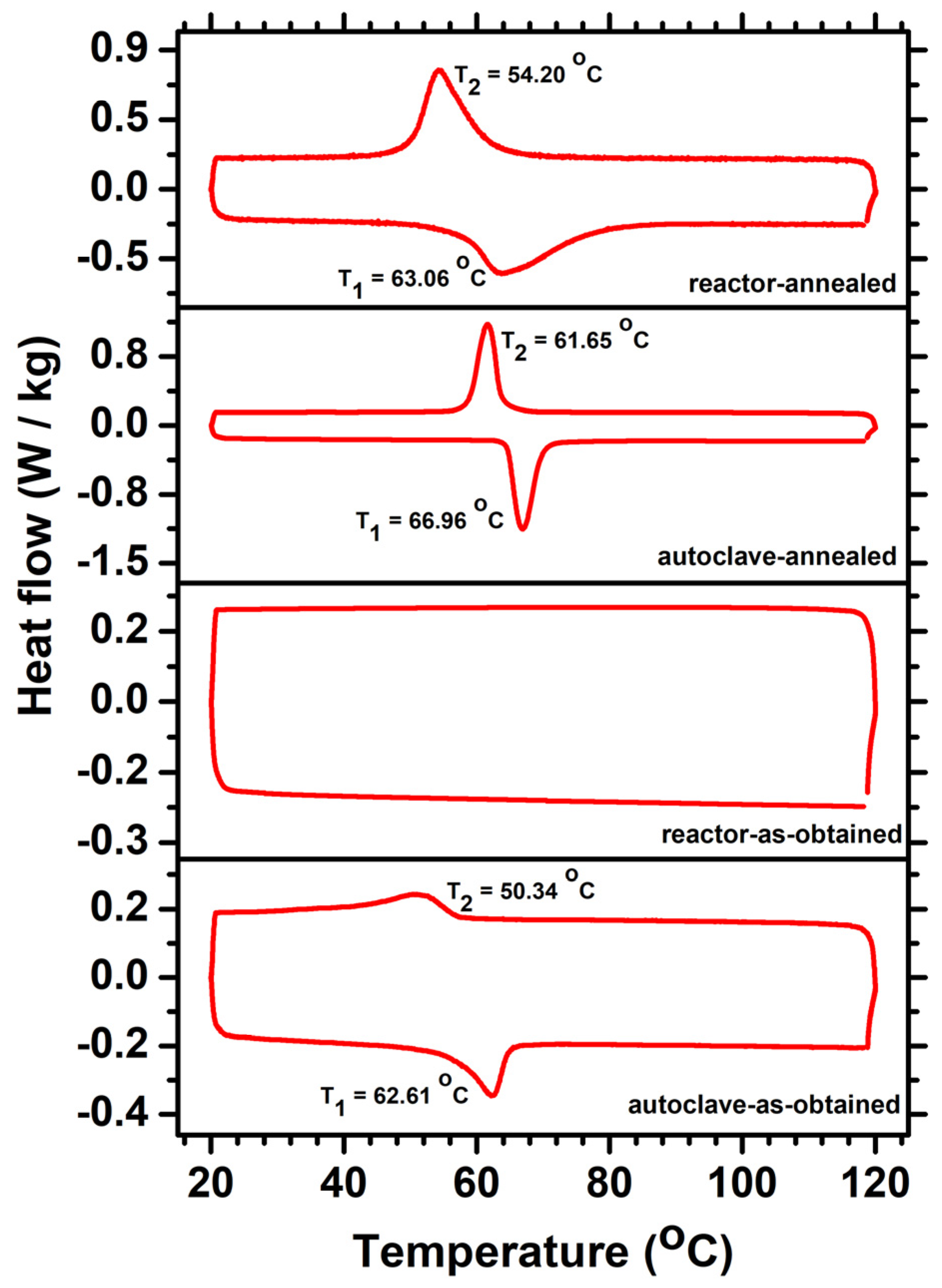
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morin, F.J. Oxides Which Show a Metal-to-Insulator Transition at the Neel Temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Chudnovskii, F.A.; Stefanovich, G.B. Metal-Insulator Transition in Disordered VO2. J. Solid State Chem. 1992, 143, 137–143. [Google Scholar] [CrossRef]
- Verleur, H.W.; Barker, A.S.; Berglund, C.N. Optical Properties of VO2 between 0.25 and 5 EV. Rev. Mod. Phys. 1968, 40, 737. [Google Scholar] [CrossRef]
- Singh, S.; Anand, J.K.; Chitnis, U.; Garg, S.; Arora, K.; Goswami, A.; Singh, R. Controlling the Mott-Peierls Transition in Epitaxial VO2 (M1) Film Grown by PLD for near-IR Photodetection. J. Appl. Phys. 2025, 137, 054501. [Google Scholar] [CrossRef]
- Zouini, M.; Chaib, A.B.; Mdaa, A.; el Khattabi, E.M. Information Restitution in the Optical Memories Using a Thin Layer of the Vanadium Dioxide. J. Eng. Sci. Technol. Rev. 2018, 11, 26–34. [Google Scholar] [CrossRef]
- Ligmajer, F.; Kejík, L.; Tiwari, U.; Qiu, M.; Nag, J.; Konečný, M.; Šikola, T.; Jin, W.; Haglund, R.F.; Appavoo, K.; et al. Epitaxial VO2 Nanostructures: A Route to Large-Scale, Switchable Dielectric Metasurfaces. ACS Photonics 2018, 5, 2561–2567. [Google Scholar] [CrossRef]
- Kutepov, M.E.; Kaydashev, V.E.; Stryukov, D.V.; Konstantinov, A.S.; Mikheykin, A.S.; Nikolskiy, A.V.; Kozakov, A.T.; Morozov, A.D.; Kashchenko, M.A.; Alymov, G.V.; et al. Optimizing Deposition Regimes to Fabricate VO2/TiO2/c-Al2O3 Thin Films for Active Metasurfaces. J. Adv. Dielectr. 2023, 14, 2340011. [Google Scholar] [CrossRef]
- Yang, R.X.; Zhang, Q.C.; Cai, J.S.; Cao, X.; Yang, Q.; Song, Y.Z.; Zhang, W. Unique Polysulfide Reaction on VO2 for Restraining Shuttle Effect in Soft-Packaged Li–S Pouch Cells. Rare Met. 2024, 43, 2842–2850. [Google Scholar] [CrossRef]
- Evans, G.P.; Powell, M.J.; Johnson, I.D.; Howard, D.P.; Bauer, D.; Darr, J.A.; Parkin, I.P. Room Temperature Vanadium Dioxide–Carbon Nanotube Gas Sensors Made via Continuous Hydrothermal Flow Synthesis. Sens. Actuators B Chem. 2018, 255, 1119–1129. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Liu, J.; Hu, M. Room Temperature CH4 Sensing Properties of Au Decorated VO2 Nanosheets. Mater. Lett. 2016, 184, 92–95. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Lv, M.L.; Yang, M.; Ma, W.X.; Zhang, G.; Yu, Y.Z.; Wu, Y.Q.; Li, H.B.; Liu, D.Z.; Yang, Y.S. Application of New Energy Thermochromic Composite Thermosensitive Materials of Smart Windows in Recent Years. Molecules 2022, 27, 1638. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.; Ribeiro, R.M.; Teixeira, V. Synthesis and Characterization of VO2-Based Thermochromic Thin Films for Energy-Efficient Windows. Nanoscale Res. Lett. 2011, 6, 301. [Google Scholar] [CrossRef] [PubMed]
- Madida, I.G.; Simo, A.; Sone, B.; Maity, A.; Kana Kana, J.B.; Gibaud, A.; Merad, G.; Thema, F.T.; Maaza, M. Submicronic VO2-PVP Composites Coatings for Smart Windows Applications and Solar Heat Management. Sol. Energy 2014, 107, 758–769. [Google Scholar] [CrossRef]
- Hoffmann, S.; Lee, E.S.; Clavero, C. Solar Energy Materials & Solar Cells Examination of the Technical Potential of Near-Infrared Switching Thermochromic Windows for Commercial Building Applications. Sol. Energy Mater. Sol. Cells 2014, 123, 65–80. [Google Scholar] [CrossRef]
- Savorianakis, G.; Rousseau, C.; Battie, Y.; Naciri, A.E.; Maes, B.; Voué, M. Surface & Coatings Technology Optical Anisotropy of Nanostructured Vanadium Dioxide Thermochromic Thin Films Synthesized by Reactive Magnetron Sputtering Combined with Glancing Angle Deposition. Surf. Coat. Technol. 2025, 502, 131938. [Google Scholar]
- Li, M.; Fang, C.; Cheng, Y.; Zhang, X.; Han, H.; Zhao, J.; Zhang, Y. A Facile Pathway to Fabricate VO2(M) Nanoparticles via Sol-Gel Method for Flexible Thermochromic Films with Efficient Infrared Stealth. Vacuum 2024, 221, 112885. [Google Scholar] [CrossRef]
- Basso, M.; Colusso, E.; Carraro, C.; Kalha, C.; Riaz, A.A.; Bombardelli, G.; Napolitani, E.; Chen, Y.; Jasieniak, J.; Ratcliff, L.E.; et al. Rapid Laser-Induced Low Temperature Crystallization of Thermochromic VO2 Sol-Gel Thin Films. Appl. Surf. Sci. 2023, 631, 157507. [Google Scholar] [CrossRef]
- Wang, J.; Dai, Y.; Yu, J.; Wang, Y.; Chen, D. Electrical Properties of Single-Crystal VO2(M) by RF Magnetron Sputtering. J. Mater. Sci. Mater. Electron. 2024, 35, 1998. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Li, X.; Wang, Y.; Dong, X.; Huang, G.; Mei, Y. Low-Temperature Growth of High-Quality VO2 Epitaxial Film on c-Plane Sapphire by Reactive Magnetron Sputtering. Appl. Phys. Lett. 2024, 125, 071904. [Google Scholar] [CrossRef]
- Gaskell, J.M.; Afzaal, M.; Sheel, D.W.; Yates, H.M.; Delfanazari, K.; Muskens, O.L. Optimised Atmospheric Pressure CVD of Monoclinic VO2 Thin Films with Picosecond Phase Transition. Surf. Coat. Technol. 2016, 287, 160–165. [Google Scholar] [CrossRef]
- Alie, D.; Gedvilas, L.; Wang, Z.; Tenent, R.; Engtrakul, C.; Yan, Y.; Shaheen, S.E.; Dillon, A.C.; Ban, C. Direct Synthesis of Thermochromic VO2 through Hydrothermal Reaction. J. Solid State Chem. 2014, 212, 237–241. [Google Scholar] [CrossRef]
- Li, W.; Ji, S.; Li, Y.; Huang, A.; Luo, H.; Jin, P. Synthesis of VO2 Nanoparticles by a Hydrothermal-Assisted Homogeneous Precipitation Approach for Thermochromic Applications. RSC Adv. 2014, 4, 13026–13033. [Google Scholar] [CrossRef]
- Karahan, O.; Tufani, A.; Unal, S.; Misirlioglu, I.B.; Menceloglu, Y.Z.; Sendur, K. Synthesis and Morphological Control of VO2 Nanostructures via a One-Step Hydrothermal Method. Nanomaterials 2021, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kuno, M.; Yamada, Y. Fast Synthesis of Monoclinic VO2 Nanoparticles by Microwave-Assisted Hydrothermal Method and Application to High-Performance Thermochromic Film. Sol. Energy Mater. Sol. Cells 2023, 255, 112311. [Google Scholar] [CrossRef]
- Mawazzan, M.A.; Rabinal, M.K. Rapid Single-Step Hydrothermal Synthesis of Phase Pure VO2 (M) with Tailored Morphology. ChemistrySelect 2024, 9, e202402971. [Google Scholar] [CrossRef]
- Lu, D.; Li, D.; Wang, Y.; Zhang, Y.; Yu, Z.; Xi, X. Microwave Hydrothermal Preparation of VO2. Phys. Status Solidi Appl. Mater. Sci. 2024, 221, 2300432. [Google Scholar] [CrossRef]
- Khaled, K.; Berardi, U.; Schlaf, M.; Soldatov, D.V. Hydrothermal Synthesis and Energy Saving Potential of Thermochromic VO 2 Nanocomposite Window Coatings in Temperate North American Climates. Sol. Energy 2025, 291, 113411. [Google Scholar] [CrossRef]
- Guo, D.; Ling, C.; Wang, C.; Wang, D.; Li, J.; Zhao, Z.; Wang, Z.; Zhao, Y.; Zhang, J.; Jin, H. Hydrothermal One-Step Synthesis of Highly Dispersed M-Phase VO2 Nanocrystals and Application to Flexible Thermochromic Film. ACS Appl. Mater. Interfaces 2018, 10, 28627–28634. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Dehiya, B.S.; Umar, A.; Goyat, M.S. Phase Modulation in Nanocrystalline Vanadium Di-Oxide (VO2) Nanostructures Using Citric Acid via One Pot Hydrothermal Method. Ceram. Int. 2019, 45, 18452–18461. [Google Scholar] [CrossRef]
- Xiang, W.; Le Drogoff, B.; Chaker, M. An Innovative Method to Achieve Large-Scale High-Quality VO2 Thin Films: Oxidation of Vanadium Nitride Material Deposited by Sputtering. Appl. Surf. Sci. 2023, 633, 157607. [Google Scholar] [CrossRef]
- Rezek, J.; Szelwicka, J.; Vlček, J.; Čerstvý, R.; Houška, J.; Fahland, M.; Fahlteich, J. Transfer of the Sputter Technique for Deposition of Strongly Thermochromic VO2-Based Coatings on Ultrathin Flexible Glass to Large-Scale Roll-to-Roll Device. Surf. Coat. Technol. 2022, 442, 128273. [Google Scholar] [CrossRef]
- Zouridi, L.; Gagaoudakis, E.; Mantsiou, E.; Dragani, T.; Maragaki, X.; Aperathitis, E.; Kiriakidis, G.; Binas, V. The Effect of Additives on the Hydrothermal Synthesis and Thermochromic Performance of Monoclinic Vanadium Dioxide Powder. Oxygen 2022, 2, 410–423. [Google Scholar] [CrossRef]
- ASTM G173-03 Standard Tables of Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on a 37° Tilted Surface. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2003.
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; Wiley: New York, NY, USA, 2000; ISBN 978-0471399186. [Google Scholar]
- Wang, C.; Xu, H.; Wang, C.; Liu, T.; Yang, S.; Nie, Y.; Guo, X.; Ma, X.; Jiang, X. Preparation of VO2 (M) Nanoparticles with Exemplary Optical Performance from VO2 (B) Nanobelts by Ball Milling. J. Alloys Compd. 2021, 877, 159888. [Google Scholar] [CrossRef]
- Jin, W.; Park, K.; Cho, J.Y.; Bae, S.H.; Siyar, M.; Jang, H.; Park, C. Thermochromic Properties of ZnO/VO2/ZnO Films on Soda Lime Silicate Glass Deposited by RF Magnetron Sputtering. Ceram. Int. 2023, 49, 10437–10444. [Google Scholar] [CrossRef]
- Ho, H.C.; Lai, Y.C.; Chen, K.; Dao, T.D.; Hsueh, C.H.; Nagao, T. High Quality Thermochromic VO2 Films Prepared by Magnetron Sputtering Using V2O5 Target with In Situ Annealing. Appl. Surf. Sci. 2019, 495, 143436. [Google Scholar] [CrossRef]
- Barimah, E.K.; Boontan, A.; Steenson, D.P.; Jose, G. Infrared Optical Properties Modulation of VO2 Thin Film Fabricated by Ultrafast Pulsed Laser Deposition for Thermochromic Smart Window Applications. Sci. Rep. 2022, 12, 11421. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, J.; Shi, F.; Dong, Y.; Jiang, S. The Thermochromic Characteristics of Zn-Doped VO2 That Were Prepared by the Hydrothermal and Post-Annealing Process and Their Polyurethane Composite Films. Ceram. Int. 2021, 47, 15631–15638. [Google Scholar] [CrossRef]




| Sample | 2θ (deg) | Phase/Crystalline Planes | dhkl (nm) |
|---|---|---|---|
| Autoclave As-obtained | 14.85 | VO2(A)/(110) | 0.596 |
| 25.35 | V6O13/(110) | 0.351 | |
| 27.80 | VO2(M)/(011) | 0.320 | |
| 29.95 | VO2(A)/(220) | 0.298 | |
| 33.30 | VO2(A)/(212) | 0.268 | |
| 37.05 | VO2(B)/(-112) | 0.242 | |
| 55.51 | VO2(M)/(211) | 0.165 | |
| Reactor As-obtained | 23.95 | VO2(B)/(201) | 0.371 |
| 30.45 | V6O13/(400) | 0.293 | |
| 36.90 | VO2(M)/(20-2) | 0.243 | |
| 42.00 | VO2(M)/(21-2) | 0.214 | |
| 55.16 | VO2(M)/(21-3) | 0.166 | |
| Autoclave Annealed | 25.30 | V6O13/(110) | 0.351 |
| 26.80 | V6O13/(003) | 0.332 | |
| 27.80 | VO2(M)/(011) | 0.320 | |
| Reactor Annealed | 27.35 | V6O13/(111) | 0.325 |
| 28.30 | VO2(B)/(-202) | 0.315 | |
| 36.90 | VO2(M)/(20-2) | 0.243 |
| Method | Material | TC (°C) | ΔTrIR (%) | ΔTrsol (%) | Trlum (%) | Ref. |
|---|---|---|---|---|---|---|
| Thermal Oxidation (large-scale) | VO2 | - | 69.3 (@2000 nm) | - | - | [30] |
| Sputtering (large-scale) | W:VO2 | 22 | - | 10 | 46 | [31] |
| Sputtering | VO2 | - | - | 7 | 30 | [36] |
| Sputtering | VO2 | 62 | 74 (@2500 nm) | 5.87 | 38.77 | [37] |
| Pulsed Laser Deposition | VO2 | 60 | 66.26 (@2600 nm) | - | - | [38] |
| Sol–Gel | VO2 | 72 | - | 8.9 | 56.4 | [16] |
| Hydrothermal | VO2 | 64 | 7.27 | 53.68 | [39] | |
| Microwave Hydrothermal | VO2 | 50 | 20.73 | 55.04 | [24] | |
| Hydrothermal Synthesis (large-scale) | VO2 | 54 | 17 (@2000 nm) | 9 | 44 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagaoudakis, E.; Mantsiou, E.; Zouridi, L.; Aperathitis, E.; Binas, V. Lab- and Large-Scale Hydrothermal Synthesis of Vanadium Dioxide Thermochromic Powder. Crystals 2025, 15, 668. https://doi.org/10.3390/cryst15080668
Gagaoudakis E, Mantsiou E, Zouridi L, Aperathitis E, Binas V. Lab- and Large-Scale Hydrothermal Synthesis of Vanadium Dioxide Thermochromic Powder. Crystals. 2025; 15(8):668. https://doi.org/10.3390/cryst15080668
Chicago/Turabian StyleGagaoudakis, Emmanouil, Eleni Mantsiou, Leila Zouridi, Elias Aperathitis, and Vasileios Binas. 2025. "Lab- and Large-Scale Hydrothermal Synthesis of Vanadium Dioxide Thermochromic Powder" Crystals 15, no. 8: 668. https://doi.org/10.3390/cryst15080668
APA StyleGagaoudakis, E., Mantsiou, E., Zouridi, L., Aperathitis, E., & Binas, V. (2025). Lab- and Large-Scale Hydrothermal Synthesis of Vanadium Dioxide Thermochromic Powder. Crystals, 15(8), 668. https://doi.org/10.3390/cryst15080668









