Maintaining Glycerol-Based Hexagonal Structures by Crosslinkers for High Permeability Nanofiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LLC Formation and Crosslinking
2.3. Characterizations
3. Results and Discussion
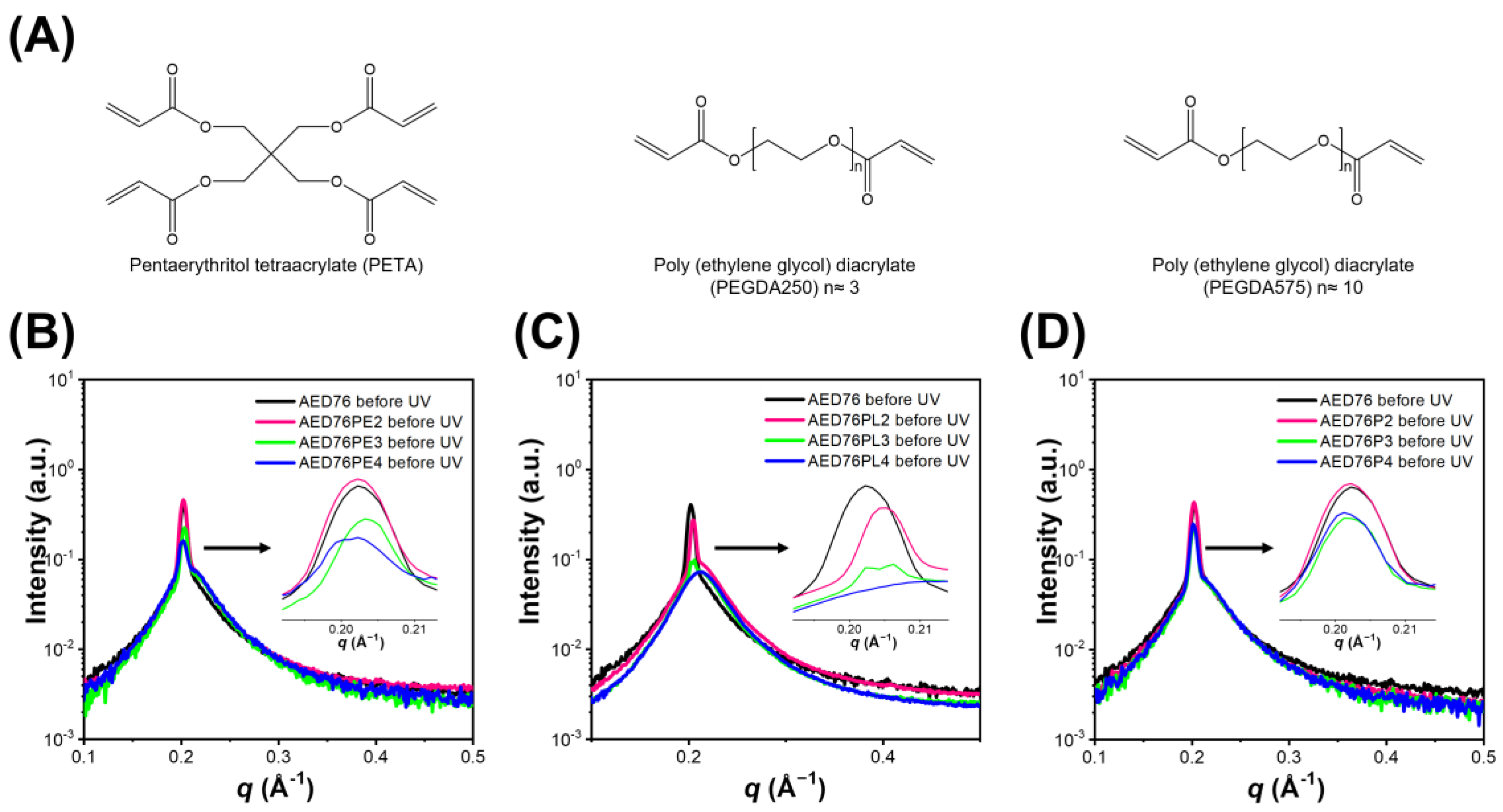
3.1. The Effect of Crosslinkers on the Interfacial Stability of the HLLC Mesophases Before Polymerization
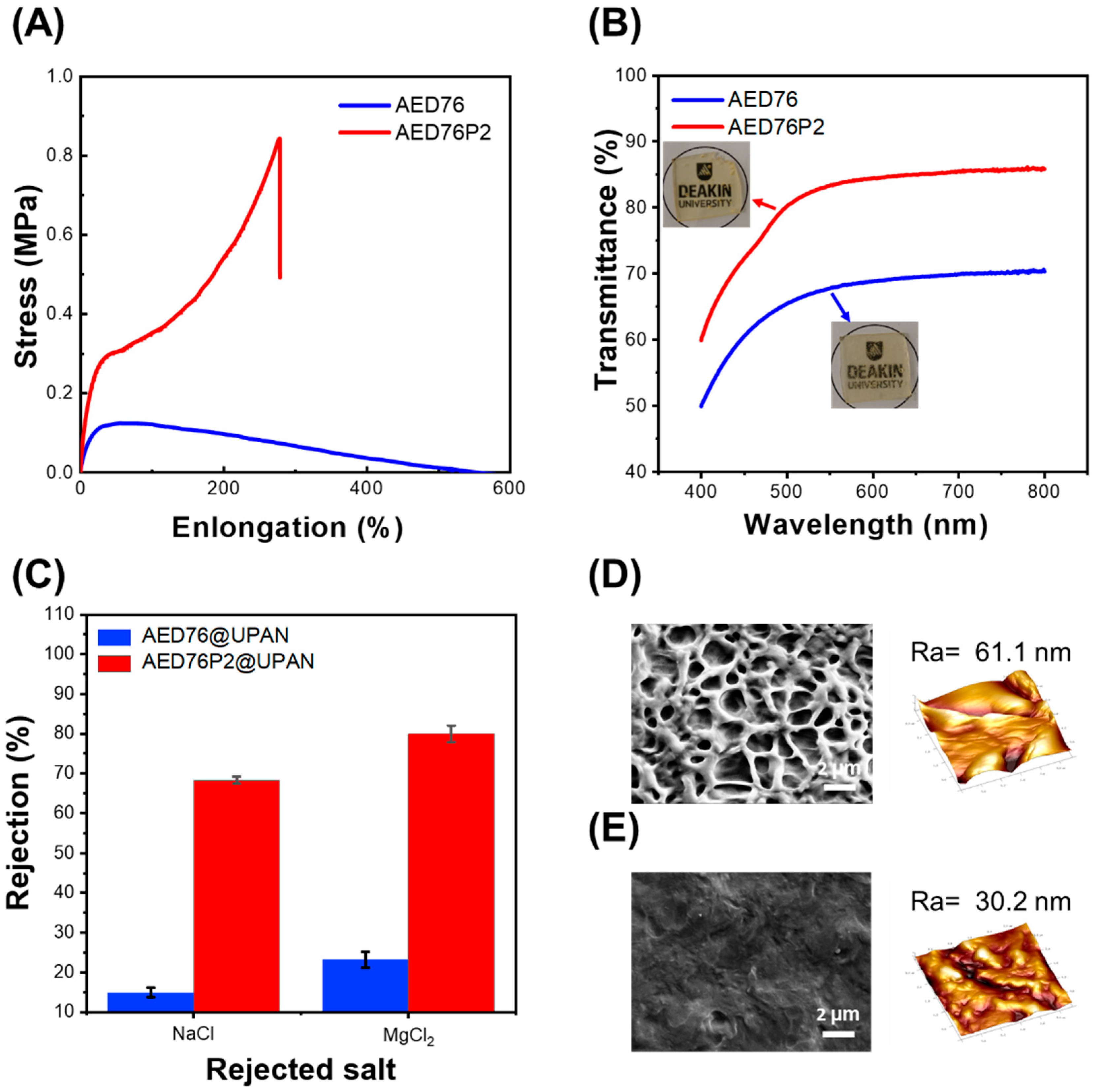
3.2. The Role of Crosslinkers in the Retention of Hexagonal Structures and Other Properties of the Glycerol-Based HLLC-Templated Membranes
4. Conclusions
- The poly (ethylene glycol) diacrylate (PEGDA) with ten ethylene glycol units exhibits high hydrophilicity, which minimizes the interference with the interfacial stability of hexagonal mesophases and enhances the concentration accommodation of crosslinkers.
- The hydrophilic PEGDA, with a long chain but low concentration of reactive groups, better connects the cylinders and retains the hexagonal structures compared to the hydrophobic one with shorter ethylene glycol units but a high concentration of reactive groups.
- The well-retained glycerol hexagonal structured nanofiltration membranes exhibit a remarkable pure water permeability of 40 L m−2 h−1 bar−1 µm, which is attributed to the strong hygroscopic effect of glycerol and the crumpled surface of membranes due to the flexible nature of the system plasticized by glycerol. The permeability of the membranes is significantly higher than that of commercial membranes [29,30] and compares favorably to similar systems [2].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Kim, D.; Dong, R.; Feng, X.; Osuji, C.O. Tunable organic solvent nanofiltration in self-assembled membranes at the sub–1 nm scale. Sci. Adv. 2022, 8, eabm5899. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Imran, Q.; Zhang, Y.; Sixdenier, L.; Lu, X.; Kaufman, G.; Gabinet, U.; Kawabata, K.; Elimelech, M.; Osuji, C.O. Precise nanofiltration in a fouling-resistant self-assembled membrane with water-continuous transport pathways. Sci. Adv. 2019, 5, eaav9308. [Google Scholar] [CrossRef] [PubMed]
- Dierking, I.; Martins Figueiredo Neto, A. Novel trends in lyotropic liquid crystals. Crystals 2020, 10, 604. [Google Scholar] [CrossRef]
- Gu, S.; Yuan, B.; Bai, B.; Tong, X.; O’Dell, L.A.; Wang, D.; Kong, L.; Wang, G. Towards a high-flux separation layer from hexagonal lyotropic liquid crystals for thin-film composite membranes. Membranes 2021, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; de Campo, L.; O’Dell, L.A.; Zhang, L.; Zhang, J.; Knott, R.; Zhang, J.; Yang, J.; Lynch, P.A.; Li, Y. Maintaining hexagonal structures through interfacial positioning of crosslinkers for nanofiltration. J. Colloid Interface Sci. 2025, 683, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Saadat, Y.; Tabatabaei, S.M.; Kim, K.; Foudazi, R. Thermoresponsive antifouling ultrafiltration membranes from mesophase templating. J. Membr. Sci. 2023, 684, 121861. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, R.; Gabinet, U.R.; Poling-Skutvik, R.; Kim, N.K.; Lee, C.; Imran, O.Q.; Feng, X.; Osuji, C.O. Rapid fabrication by lyotropic self-assembly of thin nanofiltration membranes with uniform 1 nanometer pores. ACS Nano 2021, 15, 8192–8203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.-X.; Li, Q.; Jia, R.; Tang, Q.; Huang, H.; Zhang, Y.; Feng, X. Highly Ordered Gyroid Nanostructured Polymers: Facile Fabrication by Polymerizable Pluronic Surfactants. ACS Macro Lett. 2024, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.M.; Wiesenauer, B.R.; Hatakeyama, E.S.; Barton, J.L.; Noble, R.D.; Gin, D.L. Glycerol-based bicontinuous cubic lyotropic liquid crystal monomer system for the fabrication of thin-film membranes with uniform nanopores. Chem. Mater. 2012, 24, 4005–4007. [Google Scholar] [CrossRef]
- Auvray, X.; Petipas, C.; Anthore, R.; Rico, I.; Lattes, A. X-ray diffraction study of mesophases of cetyltrimethylammonium bromide in water, formamide, and glycerol. J. Phys. Chem. 2002, 93, 7458–7464. [Google Scholar] [CrossRef]
- Auvray, X.; Perche, T.; Anthore, R.; Petipas, C.; Rico, I.; Lattes, A. Structure of Lyotropic Phases Formed by Sodium Dodecyl-Sulfate in Polar-Solvents. Langmuir 1991, 7, 2385–2393. [Google Scholar] [CrossRef]
- Dörfler, H.; Senst, A. Influence of glycerol on the formation of lyotropic mesophases—Microscopic texture observations for determining preliminary phase diagrams of binary K-soap/glycerol systems. Colloid Polym. Sci. 1993, 271, 173–189. [Google Scholar] [CrossRef]
- Li, P.; Reinhardt, M.I.; Dyer, S.S.; Moore, K.E.; Imran, O.Q.; Gin, D.L. Effects of structural modification of (alkyldiene-imidazolium bromide)-based gemini monomers on the formation of the lyotropic bicontinuous cubic phase. Soft Matter 2021, 17, 9259–9263. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.A.; Schenkel, M.R.; Wiesenauer, B.R.; Gin, D.L. Alkyl-bis(imidazolium) salts: A new amphiphile platform that forms thermotropic and non-aqueous lyotropic bicontinuous cubic phases. Chem. Commun. 2013, 49, 9407–9409. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.H.; Yue, X.; Yi, S.J.; Li, Q.T.; Chen, X. Unique lamellar lyotropic liquid crystal phases of nonionic phytosterol ethoxylates in glycerol. RSC Adv. 2015, 5, 101393–101400. [Google Scholar] [CrossRef]
- Shukla, R.K.; Raina, K.K. Effect of viscosity, pH and physicochemical parameters of solvent on the aggregation and dielectric behaviour of lyotropic liquid crystals binary mixtures. J. Mol. Liq. 2018, 250, 71–79. [Google Scholar] [CrossRef]
- Atkin, R.; Bobillier, S.M.C.; Warr, G.G. Propylammonium Nitrate as a Solvent for Amphiphile Self-Assembly into Micelles, Lyotropic Liquid Crystals, and Microemulsions. J. Phys. Chem. B 2010, 114, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Guàrdia, E.; Martí, J.; Padró, J.A.; Saiz, L.; Komolkin, A.V. Dynamics in hydrogen bonded liquids: Water and alcohols. J. Mol. Liq. 2002, 96–97, 3–17. [Google Scholar] [CrossRef]
- Hajizadeh, M.; Golub, M.; Bektas, I.; Rusevich, L.L.; Embs, J.P.; Lohstroh, W.; Paulsen, H.; Pieper, J. Modulation of Protein Dynamics by Glycerol in Water-Soluble Chlorophyll-Binding Protein (WSCP). Crystals 2025, 15, 569. [Google Scholar] [CrossRef]
- Saadat, Y.; Imran, O.Q.; Osuji, C.O.; Foudazi, R. Lyotropic liquid crystals as templates for advanced materials. J. Mater. Chem. A 2021, 9, 21607–21658. [Google Scholar] [CrossRef]
- Arora, B.; Tandon, R.; Attri, P.; Bhatia, R. Chemical crosslinking: Role in protein and peptide science. Curr. Protein Pept. Sci. 2017, 18, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Liu, C.; Lu, Z.; Li, M.; Hurren, C.; Wang, D. Photopolymerized multifunctional sodium alginate-based hydrogel for antibacterial and coagulation dressings. Int. J. Biol. Macromol. 2024, 260, 129428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Garvey, C.J.; Zhang, J.; O’Dell, L.A.; Krause-Heuer, A.M.; Forsyth, M.; Darwish, T.A.; Miloš, S.; Kong, L. Evolution of structural dimensions in mesoporous template precursor from hexagonal lyotropic liquid crystals. J. Phys. Condens. Matter 2019, 32, 075101. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.C.; Kilpatrick, P.K. Using deuterium NMR lineshapes to analyze lyotropic liquid crystalline phase transitions. Langmuir 1992, 8, 1679–1687. [Google Scholar] [CrossRef]
- Firouzi, A.; Atef, F.; Oertli, A.; Stucky, G.; Chmelka, B. Alkaline lyotropic silicate—Surfactant liquid crystals. J. Am. Chem. Soc. 1997, 119, 3596–3610. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Hill, A.J.; She, F.H.; Thornton, A.W.; Hoang, M.; Kong, L.X. Structure retention in cross-linked poly (ethylene glycol) diacrylate hydrogel templated from a hexagonal lyotropic liquid crystal by controlling the surface tension. Soft Matter 2012, 8, 2087–2094. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, L.; de Campo, L.; Knott, R.; O’Dell, L.A.; Zhang, J.; Liu, K.; Li, X.; Yang, J.; Lynch, P.A. Interfacial design of support substrate for a continuous mesophase-templated active layer with adjustable pore size. Colloids Surf. A 2024, 698, 134569. [Google Scholar] [CrossRef]
- Kéba Diawara, C.; Paugam, L.; Pontié, M.; Pierre Schlumpf, J.; Jaouen, P.; Quéméneur, F. Influence of chloride, nitrate, and sulphate on the removal of fluoride ions by using nanofiltration membranes. Sep. Sci. Technol. 2005, 40, 3339–3347. [Google Scholar] [CrossRef]
- Cooper, J.; Ye, Y.; Razmjou, A.; Chen, V. High-Value Organic Acid Recovery from First-Generation Bioethanol Dunder Using Nanofiltration. Ind. Eng. Chem. Res. 2020, 59, 11940–11952. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.Y.; Helmer, B.; Chung, T.-S. Thin-film composite membranes and formation mechanism of thin-film layers on hydrophilic cellulose acetate propionate substrates for forward osmosis processes. Ind. Eng. Chem. Res. 2012, 51, 10039–10050. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Fabrication and characterization of a surface-patterned thin film composite membrane. J. Membr. Sci. 2014, 452, 11–19. [Google Scholar] [CrossRef]
- Mondal, S.M.; Chaudhary, A. Sustainable Development of an Ultrafiltration Membrane from Banana Pseudo Stem for Use in Water Filtration; Jaypee University of Information Technology: Solan, India, 2023. [Google Scholar]



| Sample | Q100 (Å−1) | D100 (Å) | Dinter (Å) | R (Å) | Rw (Å) | S (Å2) |
|---|---|---|---|---|---|---|
| AED76 before UV | 0.2022 | 31.0583 | 35.8631 | 16.7495 | 2.3639 | 76.6915 |
| AED76P2 before UV | 0.2022 | 31.0583 | 35.8631 | 16.7313 | 2.4003 | 76.7750 |
| AED76PE2 before UV | 0.2022 | 31.0583 | 35.8631 | 16.3392 | 3.1847 | 78.6177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; O’Dell, L.A.; Kong, L. Maintaining Glycerol-Based Hexagonal Structures by Crosslinkers for High Permeability Nanofiltration. Crystals 2025, 15, 664. https://doi.org/10.3390/cryst15070664
Gu S, O’Dell LA, Kong L. Maintaining Glycerol-Based Hexagonal Structures by Crosslinkers for High Permeability Nanofiltration. Crystals. 2025; 15(7):664. https://doi.org/10.3390/cryst15070664
Chicago/Turabian StyleGu, Senlin, Luke A. O’Dell, and Lingxue Kong. 2025. "Maintaining Glycerol-Based Hexagonal Structures by Crosslinkers for High Permeability Nanofiltration" Crystals 15, no. 7: 664. https://doi.org/10.3390/cryst15070664
APA StyleGu, S., O’Dell, L. A., & Kong, L. (2025). Maintaining Glycerol-Based Hexagonal Structures by Crosslinkers for High Permeability Nanofiltration. Crystals, 15(7), 664. https://doi.org/10.3390/cryst15070664







