Scheme 1.
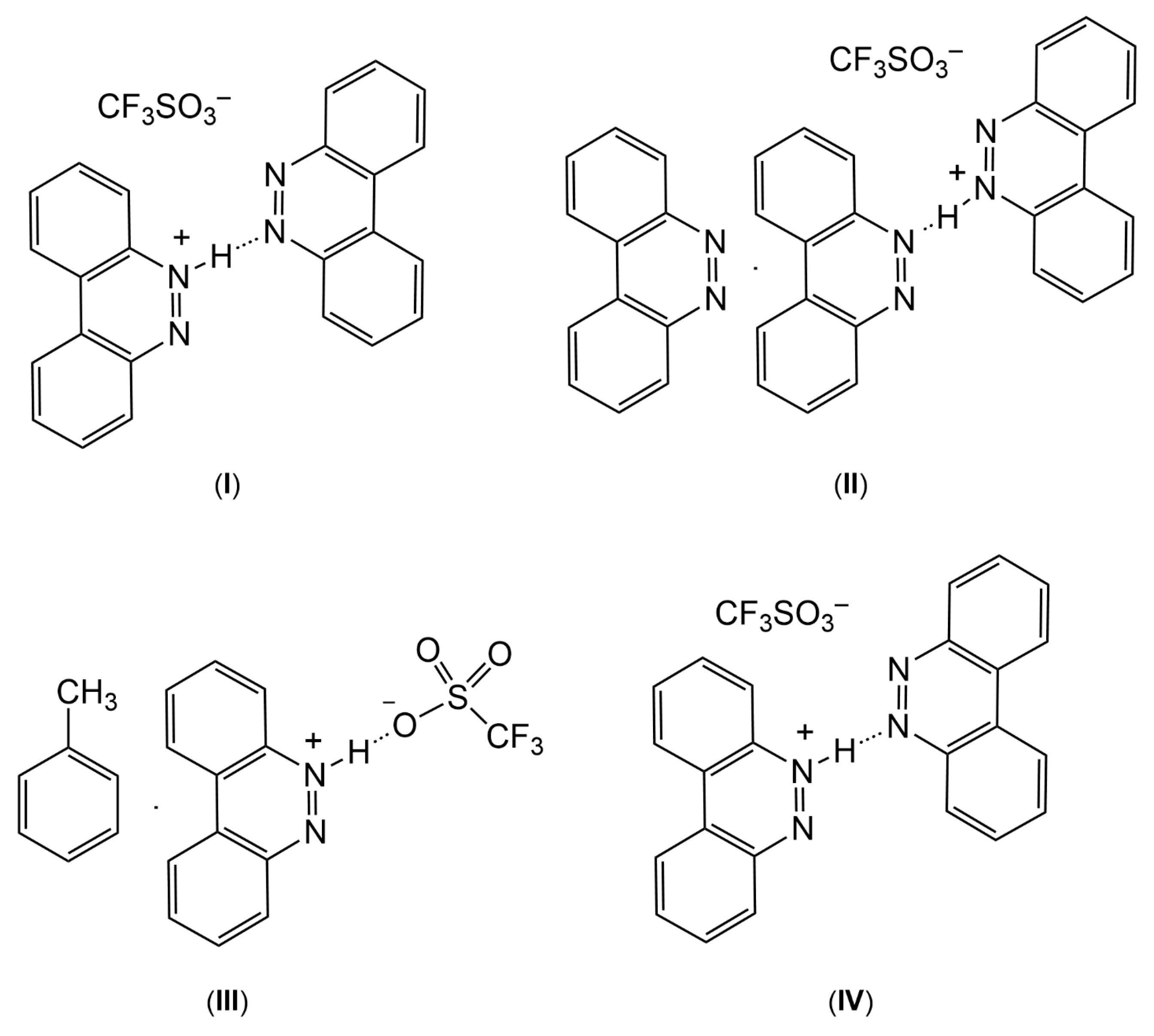
Chemical representations and composition of benzo[c]cinnolinium trifluoromethanesulfonate salts isolated from this study. Compounds I and IV are polymorphic.
Scheme 1.
Chemical representations and composition of benzo[c]cinnolinium trifluoromethanesulfonate salts isolated from this study. Compounds I and IV are polymorphic.
Scheme 2.
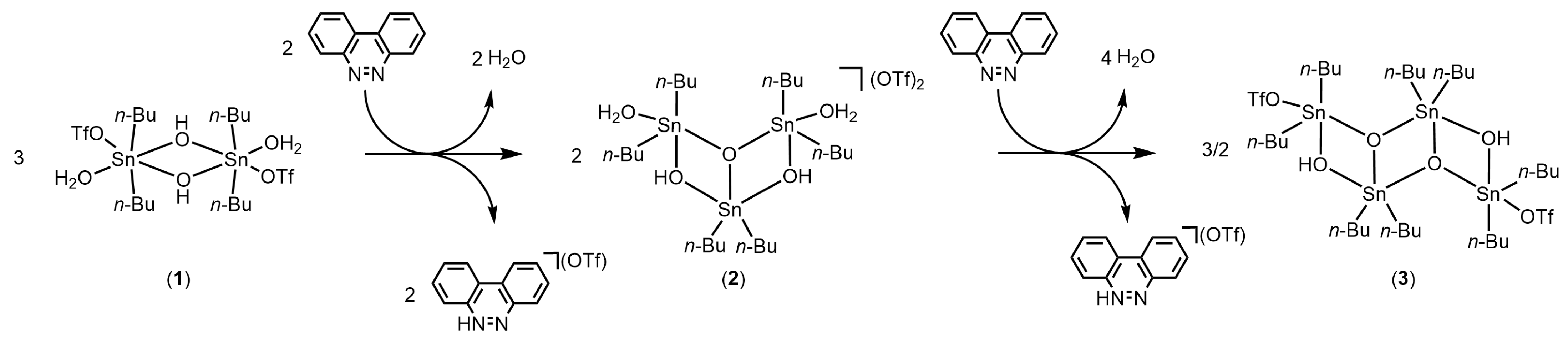
Possible mechanism leading to the formation of [C12H9N2]+[CF3SO3]− from 1 (OTf = OSO2CF3).
Scheme 2.
Possible mechanism leading to the formation of [C12H9N2]+[CF3SO3]− from 1 (OTf = OSO2CF3).
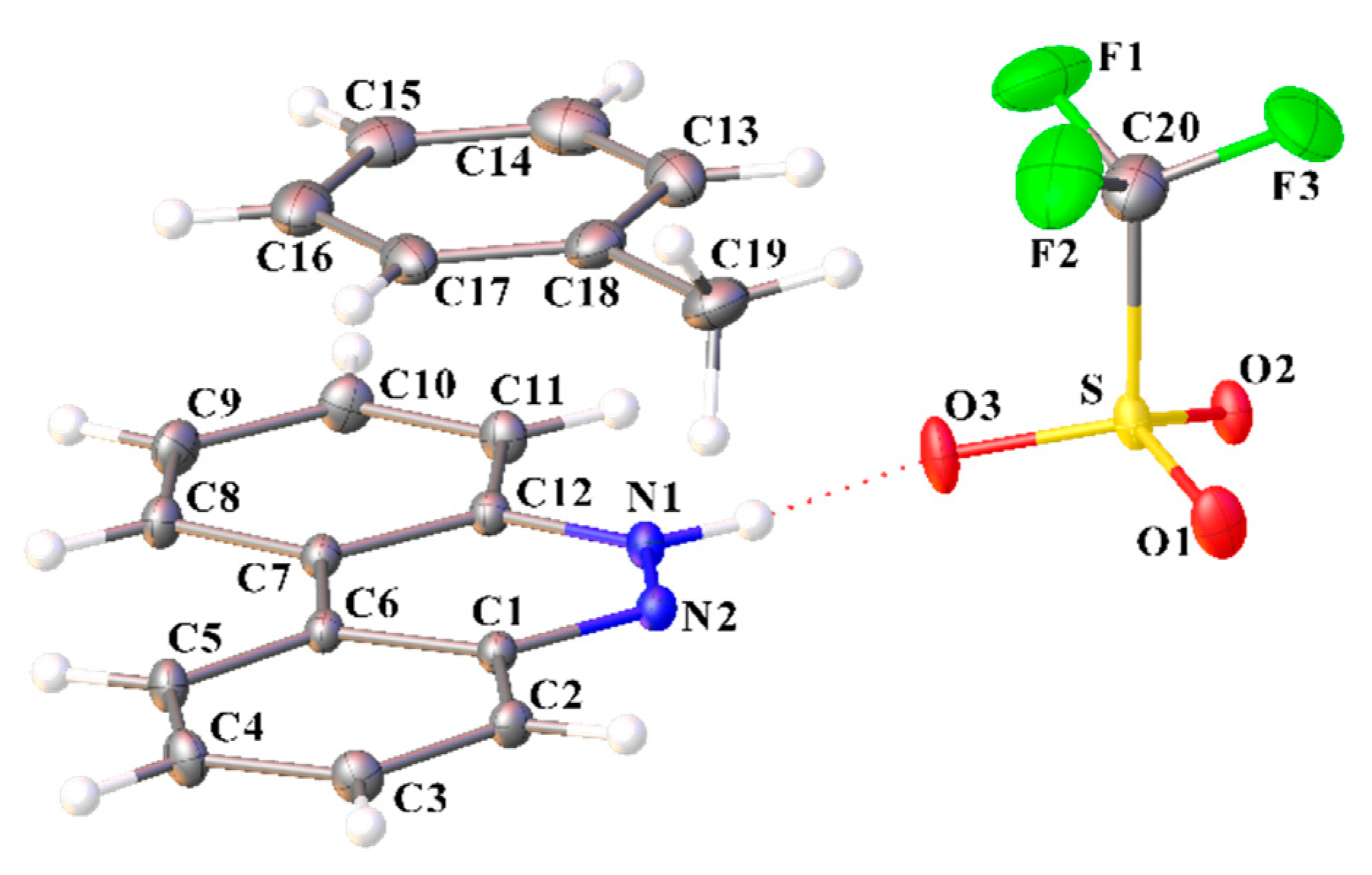
Figure 1.
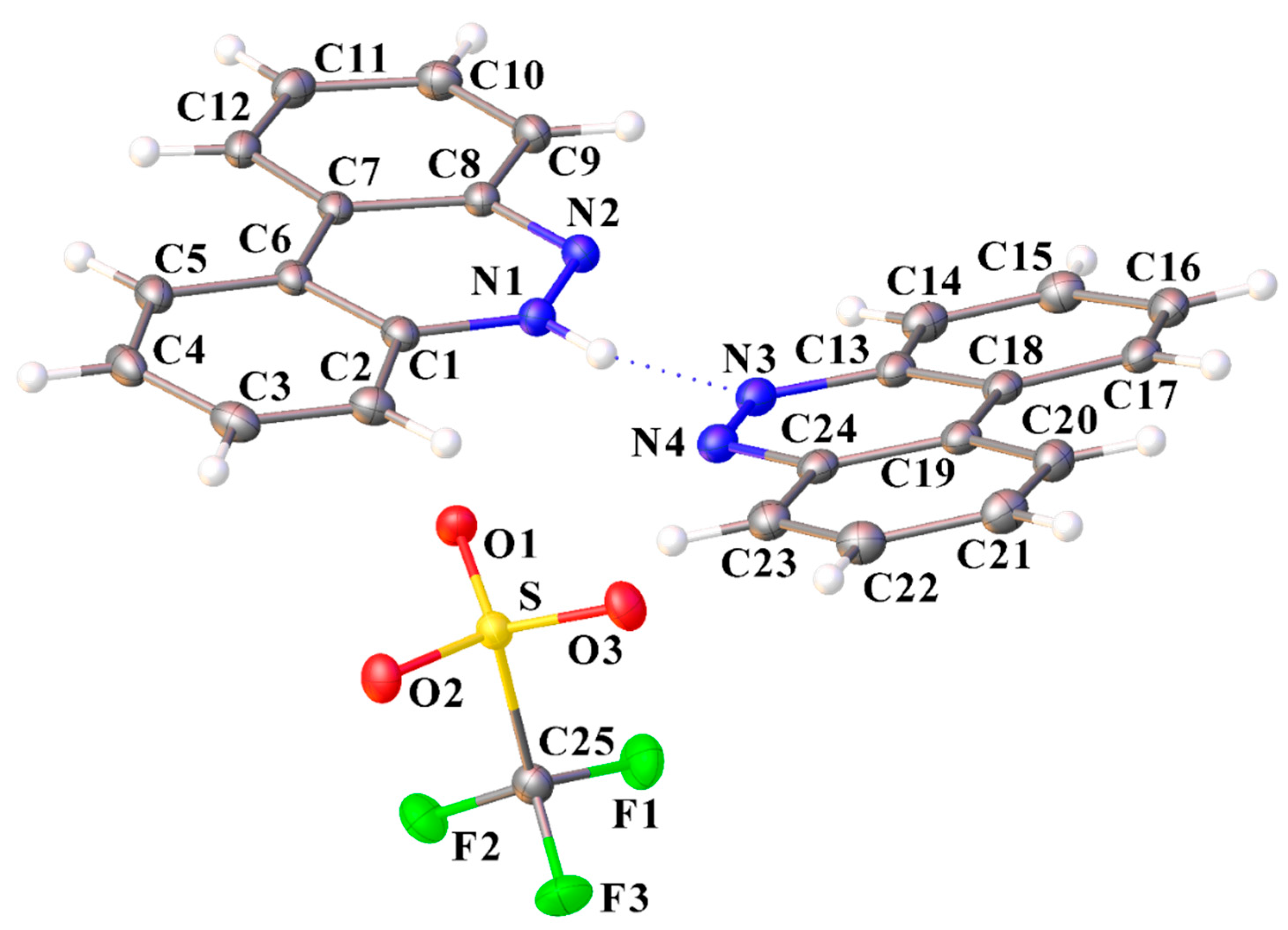
Molecular structure of [C12H9N2][CF3SO3]·(C12H8N2) (I) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white.
Figure 1.
Molecular structure of [C12H9N2][CF3SO3]·(C12H8N2) (I) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white.
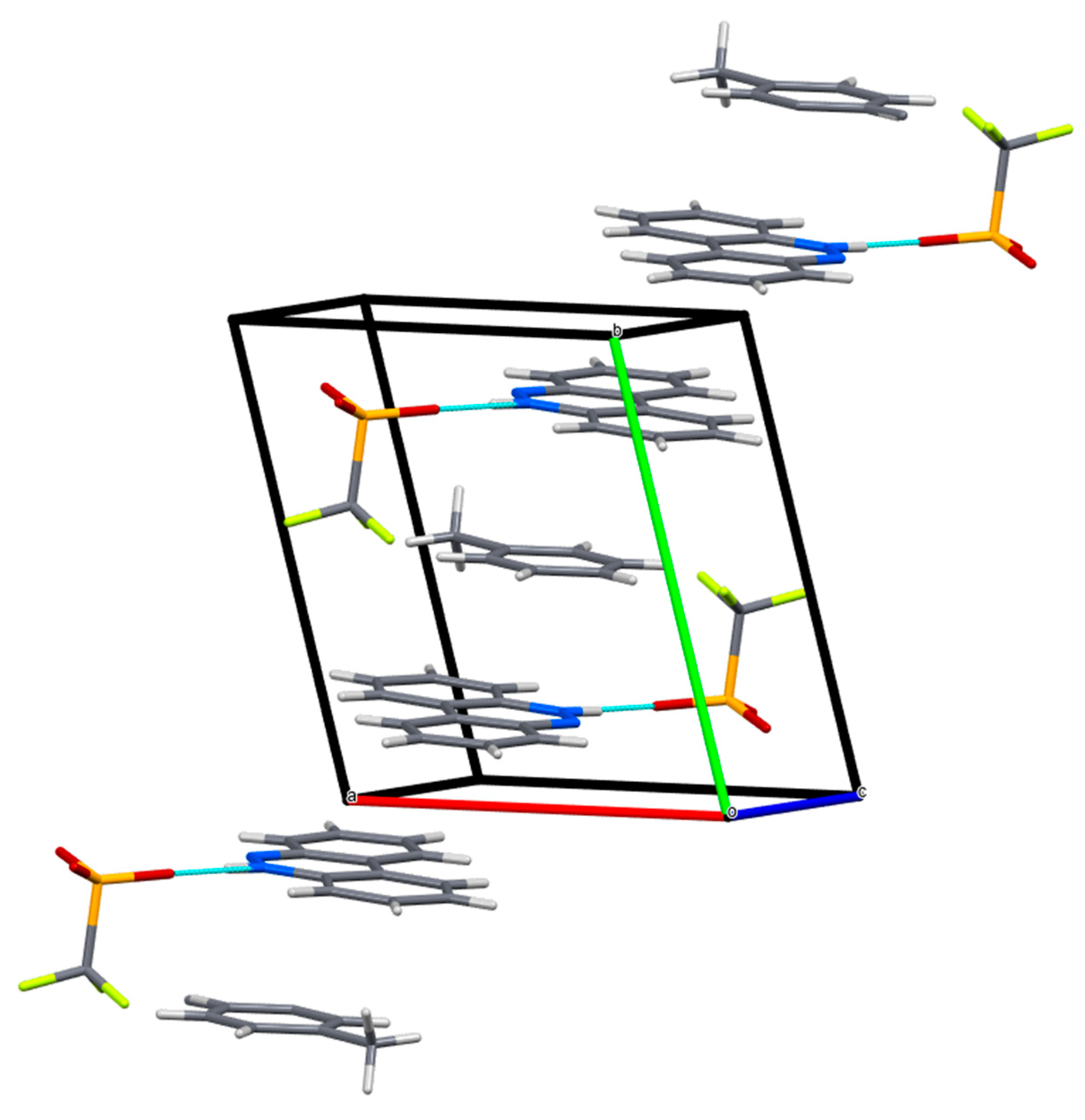
Figure 2.
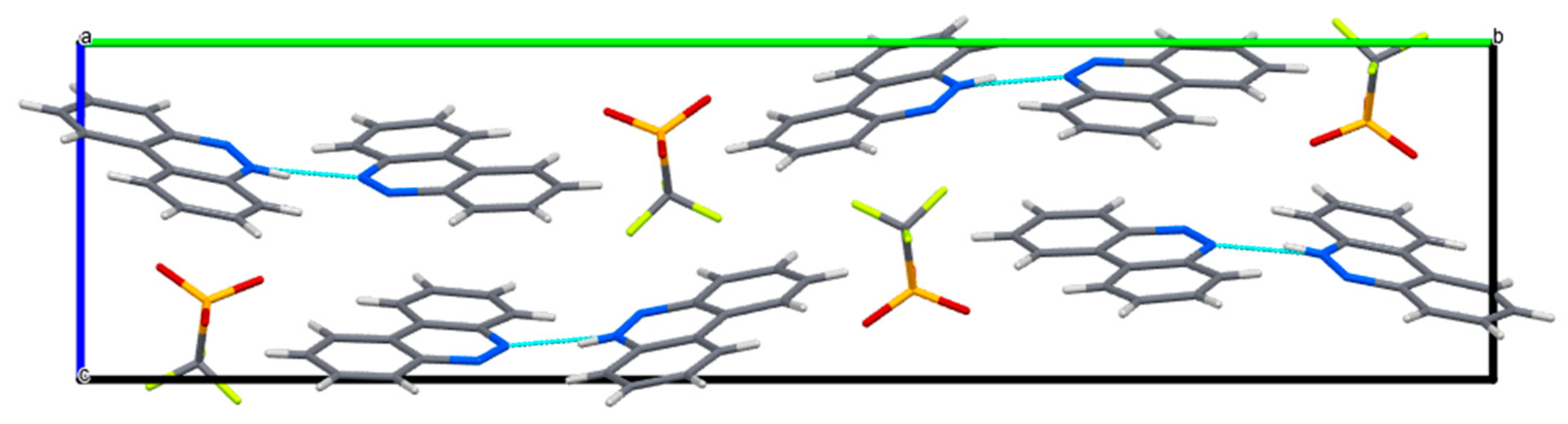
The crystal packing of I in the unit cell (MERCURY representation).
Figure 2.
The crystal packing of I in the unit cell (MERCURY representation).
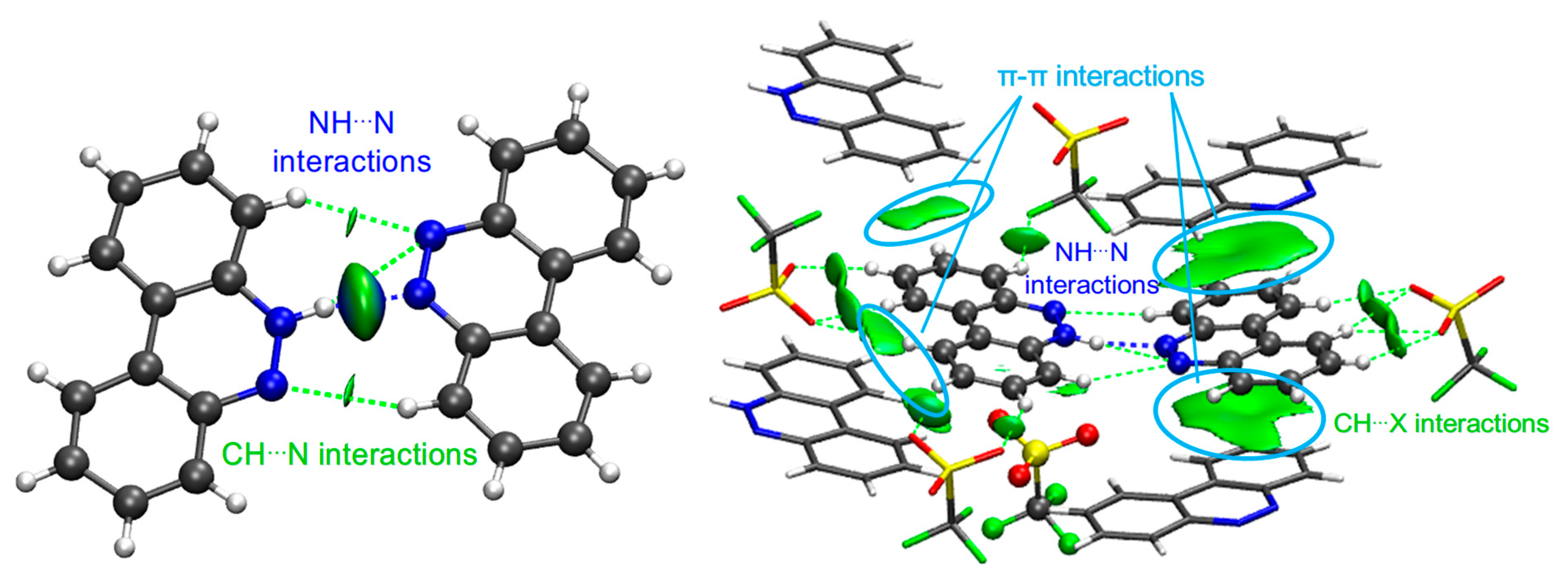
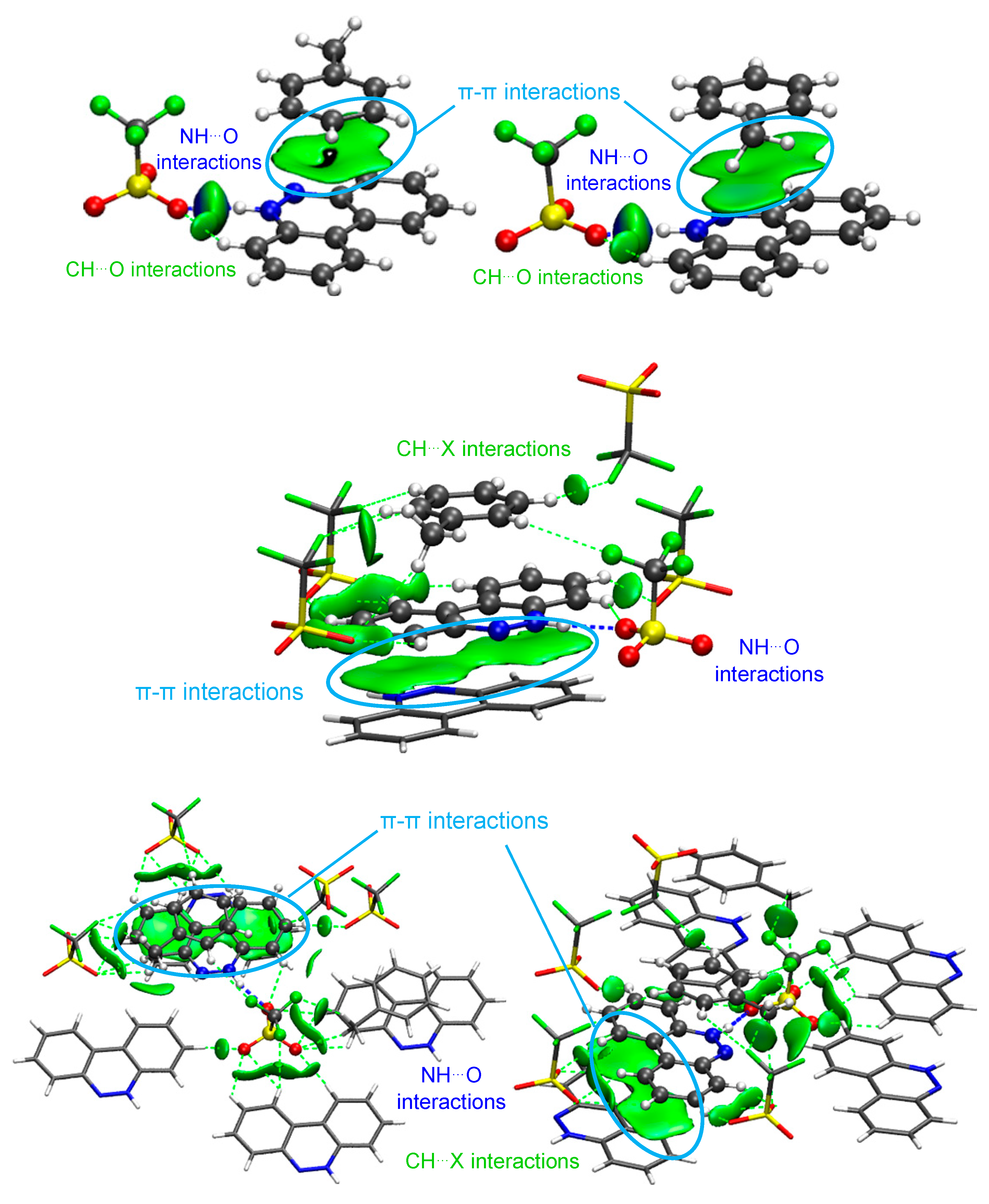
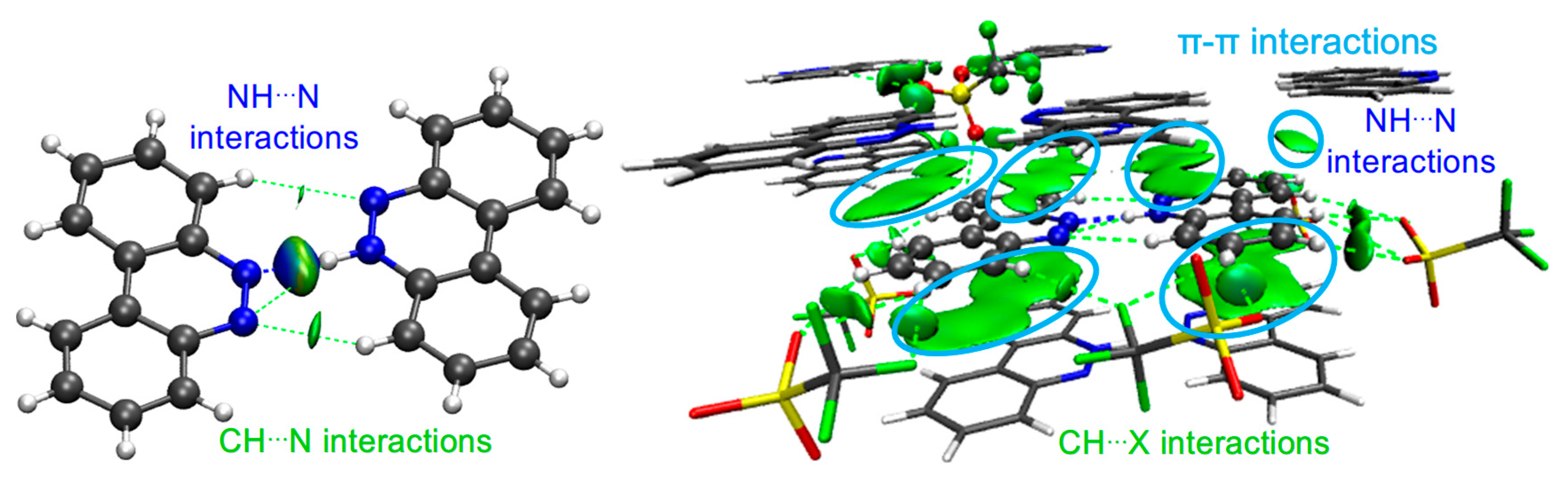
Figure 3.
NCI isosurfaces for I ((left): between BCCH and BCC; (right): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
Figure 3.
NCI isosurfaces for I ((left): between BCCH and BCC; (right): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
Figure 4.
Molecular structure of [C12H9N2][CF3SO3]·(C12H8N2)2 (II) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white.
Figure 4.
Molecular structure of [C12H9N2][CF3SO3]·(C12H8N2)2 (II) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white.
Figure 5.
The crystal packing of II in the unit cell (MERCURY representation).
Figure 5.
The crystal packing of II in the unit cell (MERCURY representation).
Figure 6.
NCI isosurfaces for II ((left): between BCCH and BCC; (right): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
Figure 6.
NCI isosurfaces for II ((left): between BCCH and BCC; (right): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
Figure 7.
Molecular structure of [C12H9N2][CF3SO3]·(C7H8) (III) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white.
Figure 7.
Molecular structure of [C12H9N2][CF3SO3]·(C7H8) (III) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white.
Figure 8.
The crystal packing of III in the unit cell (Mercury representation).
Figure 8.
The crystal packing of III in the unit cell (Mercury representation).
Figure 9.
NCI isosurfaces for III ((top): between BCCH and BCC, with the two conformations of the toluene; (middle): with other closed molecules; (bottom): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
Figure 9.
NCI isosurfaces for III ((top): between BCCH and BCC, with the two conformations of the toluene; (middle): with other closed molecules; (bottom): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
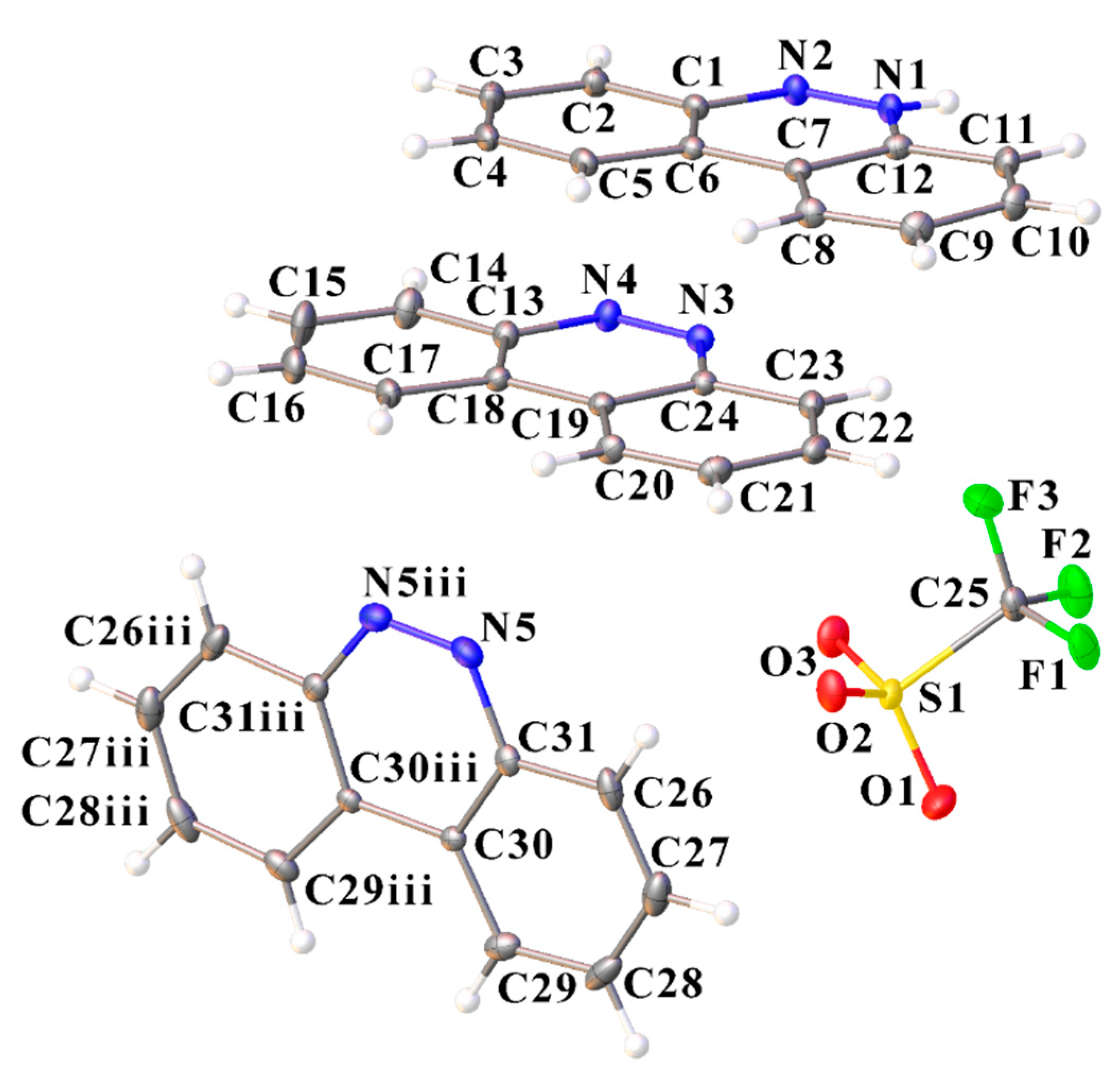
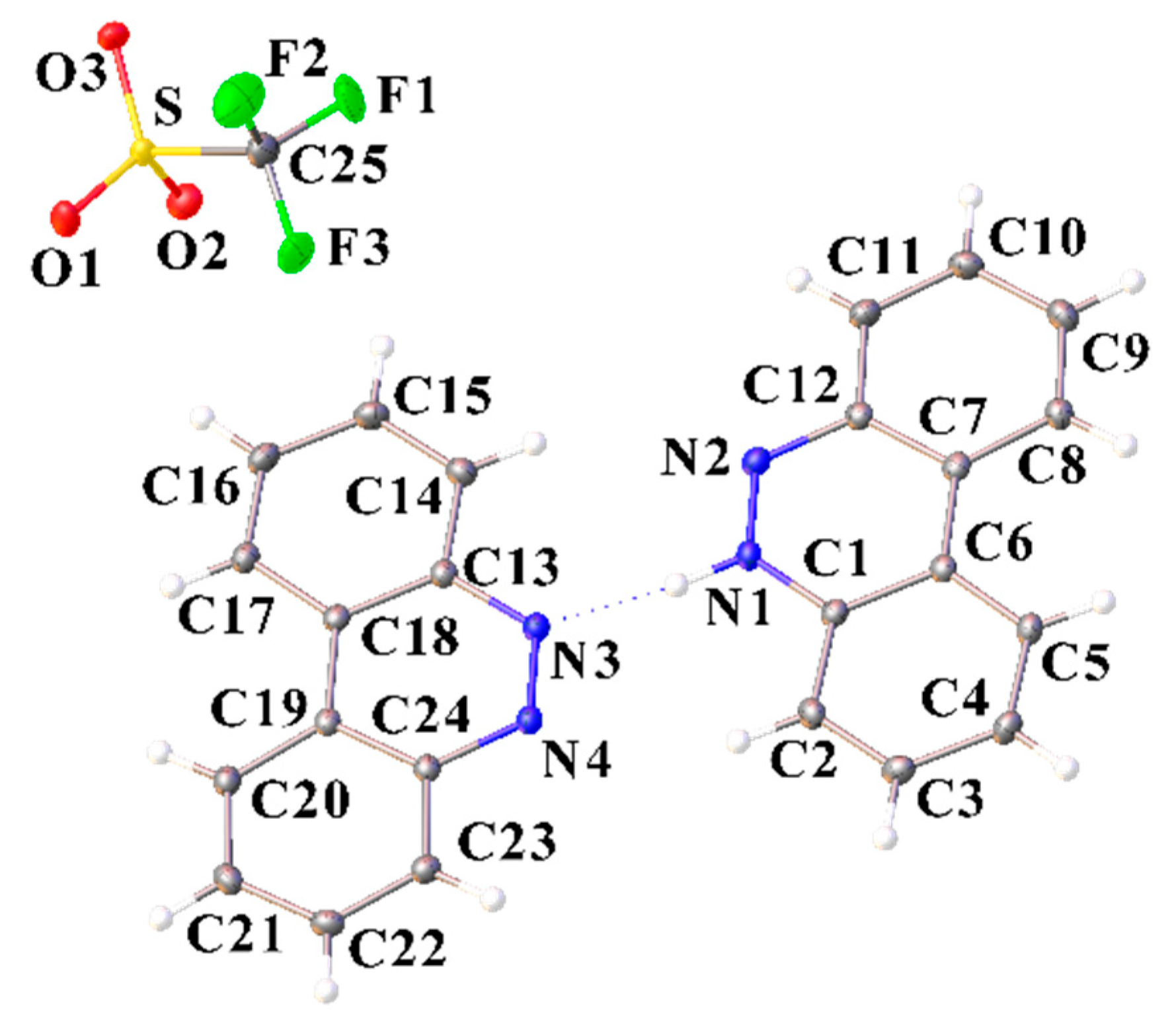
Figure 10.
Molecular structure of [C12H9N2][CF3SO3]·(C12H9N2) (IV) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white. Selected bond lengths (A) and angles (°): N4–C24–C23 116.40 (13), C13–C18–C19 116.89 (13), C24–C19–C18 116.93 (14), O1–S–O2 114.64 (8), O1–S–O3 115.24 (8), O2–S–O3 114.68 (8), O1–S–C25 102.22 (8), O2–S–C25 103.79 (9), O3–S–C25 103.97 (8).
Figure 10.
Molecular structure of [C12H9N2][CF3SO3]·(C12H9N2) (IV) showing the atom labeling scheme (ORTEP view). Color code: sulfur, yellow; oxygen, red; nitrogen, blue; fluoride, green; carbon, grey; hydrogen, white. Selected bond lengths (A) and angles (°): N4–C24–C23 116.40 (13), C13–C18–C19 116.89 (13), C24–C19–C18 116.93 (14), O1–S–O2 114.64 (8), O1–S–O3 115.24 (8), O2–S–O3 114.68 (8), O1–S–C25 102.22 (8), O2–S–C25 103.79 (9), O3–S–C25 103.97 (8).
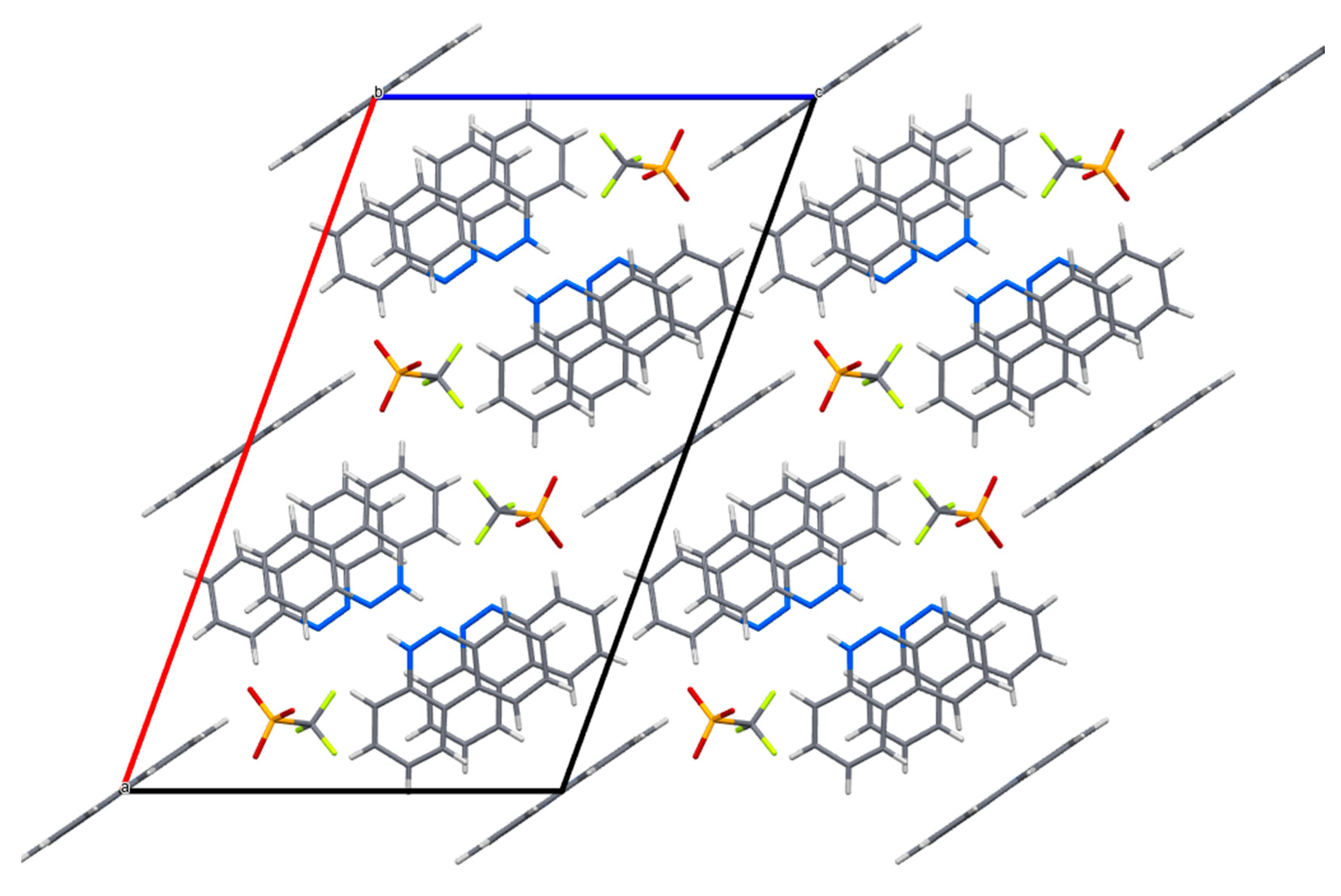
Figure 11.
The crystal packing of salt IV in the unit cell (MERCURY representation).
Figure 11.
The crystal packing of salt IV in the unit cell (MERCURY representation).
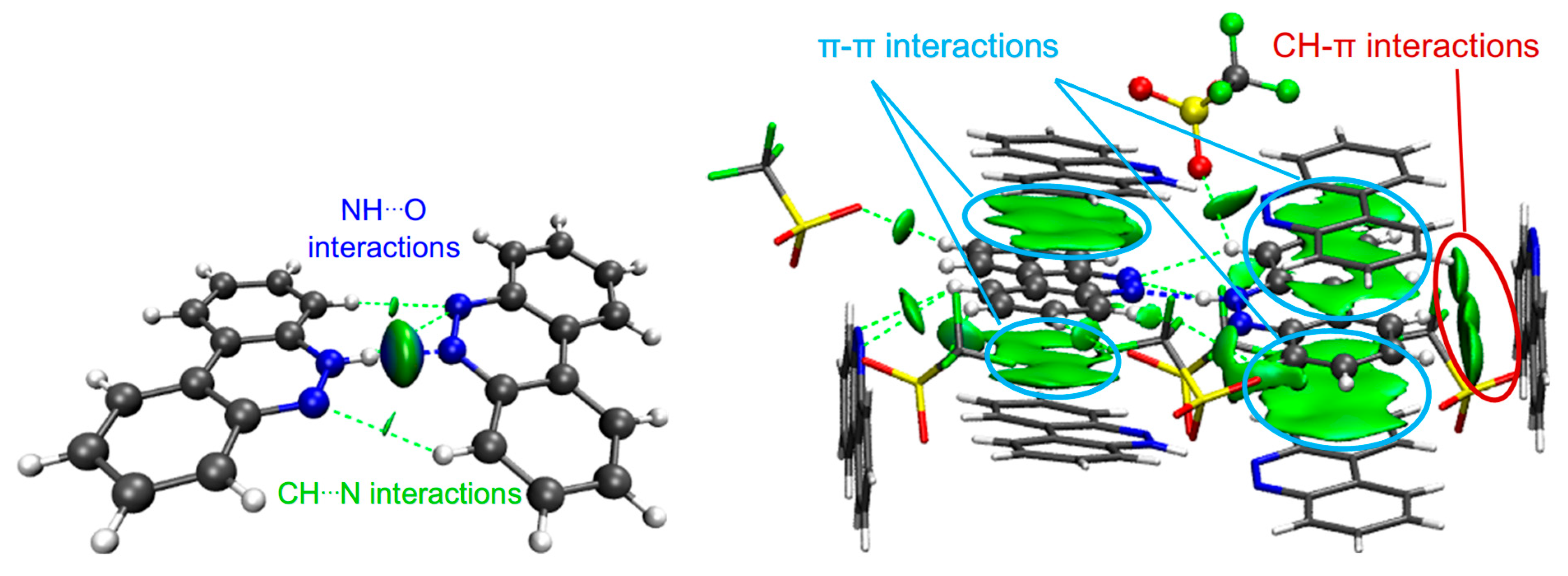
Figure 12.
NCI isosurfaces for IV ((left): between BCCH and BCC); (right): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
Figure 12.
NCI isosurfaces for IV ((left): between BCCH and BCC); (right): with other cells). Atom color code: C in gray, O in red, N in blue, F in green, H in white, S in yellow.
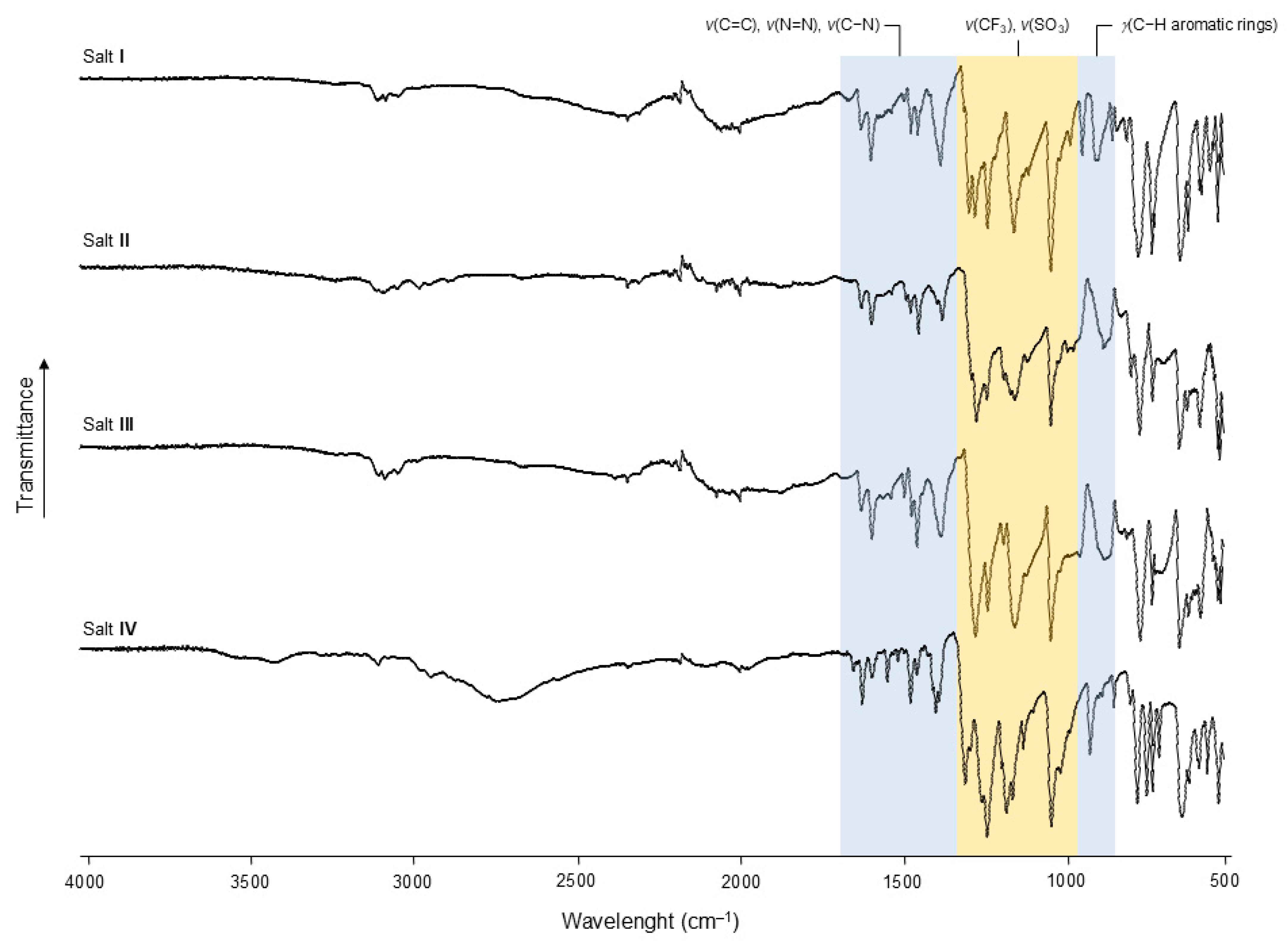
Figure 13.
FT-IR spectra (ATR mode) of salts I–IV highlighting characteristic bands related to BCCH (blue banner) and CF3SO3− (yellow banner).
Figure 13.
FT-IR spectra (ATR mode) of salts I–IV highlighting characteristic bands related to BCCH (blue banner) and CF3SO3− (yellow banner).
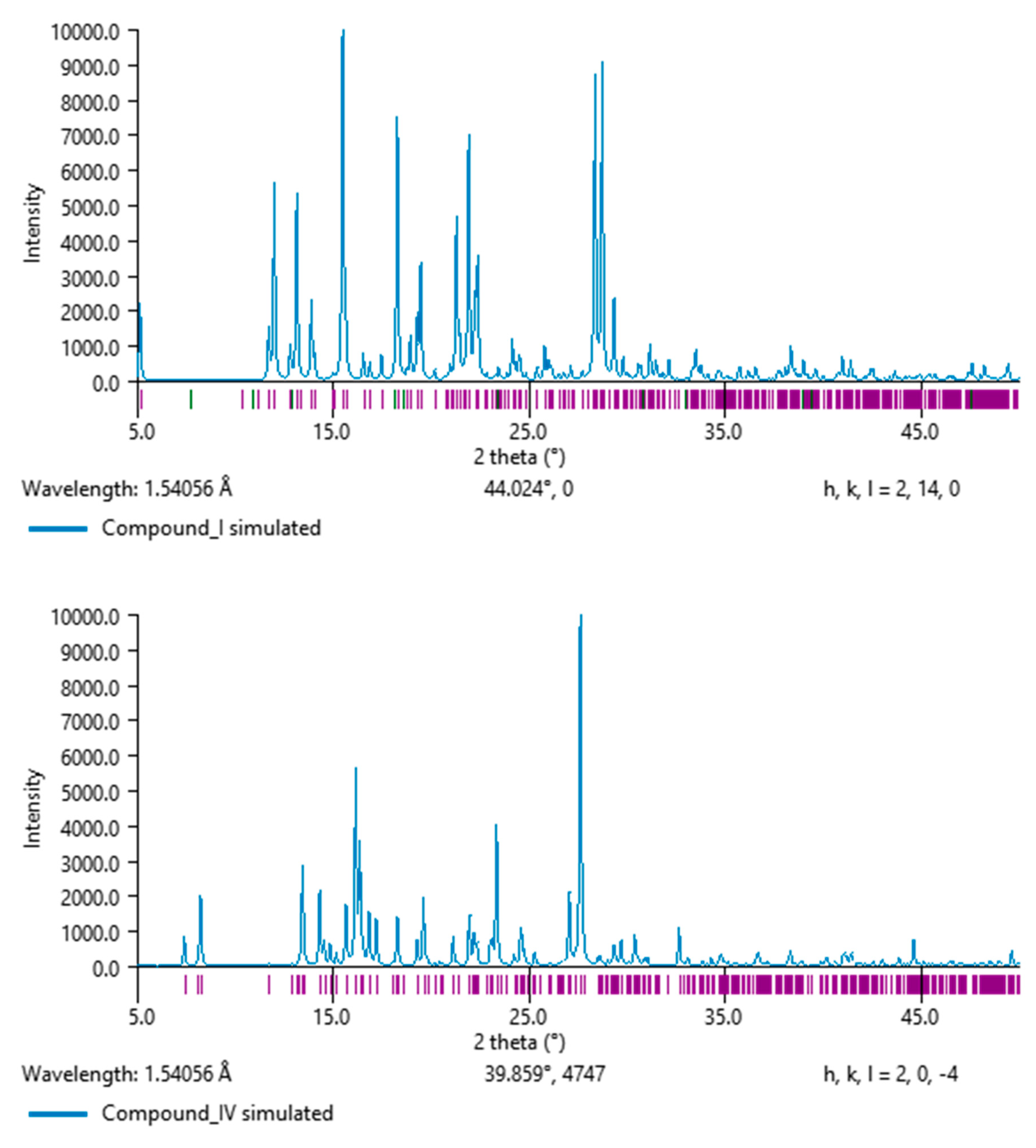
Figure 14.
Powder X-ray diffraction pattern simulation feature of salt
I (
up) and salt
IV (
bottom) polymorphs (PXRD in MERCURY [
48]).
Figure 14.
Powder X-ray diffraction pattern simulation feature of salt
I (
up) and salt
IV (
bottom) polymorphs (PXRD in MERCURY [
48]).
Table 1.
Crystallographic and refinement data for salts I–IV.
Table 1.
Crystallographic and refinement data for salts I–IV.
| Compound | I | II | III | IV |
|---|
| Empirical formula | [C12H9N2][CF3SO3]·C12H8N2 | [C12H9N2][CF3SO3]·(C12H8N2)2 | [C12H9N2][CF3SO3]·½(C7H8) | [C12H9N2][CF3SO3]·C12H8N2 |
| Dcalc/g cm−3 | 1.510 | 1.519 | 1.519 | 1.520 |
| μ/mm−1 | 0.207 | 0.191 | 0.248 | 0.208 |
| Formula weight | 510.48 | 600.59 | 752.70 | 510.48 |
| Color | clear light yellow | clear light colorless | clear light yellow | clear light yellow |
| Shape | prism-shaped | prism-shaped | prism-shaped | prism-shaped |
| Size/mm3 | 0.15 × 0.08 × 0.03 | 0.60 × 0.15 × 0.08 | 0.43 × 0.28 × 0.25 | 0.25 × 0.17 × 0.08 |
| T/K | 115 | 115 | 115 | 115 |
| Crystal system | monoclinic | monoclinic | triclinic | triclinic |
| Flack parameter | | 0.06 (10) | | |
| Space group | P21/c | C2 | P−1 | P−1 |
| a/Å | 8.0422 (2) | 26.0765 (11) | 8.9256 (5) | 7.6998 (3) |
| b/Å | 34.2479 (11) | 6.8956 (2) | 10.0000 (5) | 12.3988 (5) |
| c/Å | 8.6981 (2) | 15.5443 (6) | 10.0028 (6) | 13.3783 (5) |
| a/° | 90 | 90 | 85.326 (3) | 63.178 (2) |
| b/° | 110.424 (1) | 110.061 (1) | 74.238 (2) | 81.436 (2) |
| g/° | 90 | 90 | 73.233 (3) | 78.890 (2) |
| V/Å3 | 2245.10 (11) | 2625.49 (17) | 822.70 (8) | 1115.63 (8) |
| Z | 4 | 4 | 1 | 2 |
| Z’ | 1 | 1 | 0.5 | 1 |
| Wavelength/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Radiation type | MoKa | Mo Ka | Mo Ka | MoKa |
| Qmin/° | 2.379 | 1.663 | 2.468 | 1.859 |
| Qmax/° | 27.469 | 27.522 | 27.436 | 27.439 |
| Measured Refl’s. | 9050 | 5887 | 6778 | 7835 |
| Indep’t Refl’s | 5075 | 5887 | 3656 | 5001 |
| Refl’s I ≥ 2 s(I) | 4487 | 5685 | 3517 | 4434 |
| Rint | 0.0189 | 0.019 | 0.0152 | 0.0161 |
| Parameters | 325 | 388 | 252 | 325 |
| Restraints | 0 | 1 | 0 | 0 |
| Largest peak | 0.311 | 0.272 | 0.376 | 0.291 |
| Deepest hole | −0.397 | −0.259 | −0.393 | −0.372 |
| GooF | 1.127 | 1.083 | 1.075 | 1.063 |
| wR2 (all data) | 0.1016 | 0.0938 | 0.0919 | 0.1040 |
| wR2 | 0.0975 | 0.0911 | 0.0903 | 0.0974 |
| R1 (all data) | 0.0539 | 0.0404 | 0.0362 | 0.0494 |
| R1 | 0.0461 | 0.0380 | 0.0347 | 0.0417 |
| CCDC deposition no. | 2449220 | 2449221 | 2449222 | 2449223 |
Table 2.
Selected bond lengths (A) and angles (°) for I.
Table 2.
Selected bond lengths (A) and angles (°) for I.
| N1–N2 | 1.293 (2) | C13–C18 | 1.409 (3) |
| N1–C1 | 1.374 (2) | C18–C19 | 1.436 (3) |
| N2–C8 | 1.361 (2) | S–O1 | 1.4438 (14) |
| C1–C6 | 1.409 (3) | S–O2 | 1.4423 (14) |
| C6–C7 | 1.435 (3) | S–O3 | 1.4447 (14) |
| C7–C8 | 1.425 (2) | S–C25 | 1.826 (2) |
| N3–N4 | 1.291 (2) | C25–F1 | 1.342 (2) |
| N3–C13 | 1.389 (2) | C25–F2 | 1.338 (2) |
| N4–C24 | 1.386 (2) | C25–F3 | 1.339 (2) |
| C19-C24 | 1.409 (3) | | |
| N2–N1–C1 | 126.54 (16) | N3–C13–C14 | 116.90 (17) |
| N1–N2–C8 | 117.09 (15) | N4–C24–C19 | 123.03 (17) |
| N1–C1–C6 | 118.79 (16) | N4–C24–C23 | 116.33 (17) |
| N1–C1–C2 | 118.71 (17) | O2–S–O1 | 115.12 (8) |
| C1–C6–C7 | 117.19 (16) | O2–S–O3 | 115.35 (8) |
| C12–C7–C6 | 124.71 (16) | O1–S–O3 | 114.99 (9) |
| N4–N3–C13 | 121.90 (16) | O2–S–C25 | 102.88 (9) |
| N3–N4–C24 | 119.47 (16) | O1–S–C25 | 103.11 (9) |
| N3–C13–C18 | 121.90 (17) | O3–S–C25 | 102.76 (9) |
Table 3.
Geometry of hydrogen bonds and short contacts for I.
Table 3.
Geometry of hydrogen bonds and short contacts for I.
| Interaction | D a···A (Å) | H···A b (Å) | D-H···A (°) |
|---|
| N1−H···N3 | 2.721 (2) | 1.8530 (17) | 168.34 (11) |
| N1−H···N4 | 3.439 (2) | 2.6052 (17) | 158.49 (12) |
| C2−H···N4 | 3.509 (3) | 2.6665 (17) | 148.08 (14) |
| C14−H···N2 | 3.509 (3) | 2.6793 (17) | 146.16 (15) |
| C5−H···O1 i | 3.648 (3) | 2.7044 (14) | 172.54 (11) |
| C12−H···O1 i | 3.399 (2) | 2.4522 (15) | 174.48 (11) |
| C11−H···O2 i | 3.281 (3) | 2.6045 (14) | 128.43 (15) |
| C4−H···O1 ii | 3.265 (3) | 2.3893 (16) | 153.18 (12) |
| C3−H···O3 ii | 3.448 (3) | 2.6092 (17) | 147.41 (14) |
| C17−H···O3 iii | 3.419 (3) | 2.4743 (14) | 173.06 (10) |
| C20−H···O2 iii | 3.251 (3) | 2.5895 (14) | 127.05 (13) |
| C20−H···O3 iii | 3.498 (3) | 2.5523 (16) | 174.13 (11) |
| C21−H···O2 iii | 3.315 (3) | 2.7164 (14) | 121.64 (16) |
| C9−H···F2 iv | 3.353 (2) | 2.6077 (13) | 135.66 (12) |
Table 4.
Selected bond lengths (A) and angles (°) for II.
Table 4.
Selected bond lengths (A) and angles (°) for II.
| N1–N2 | 1.294 (3) | C13–C18 | 1.418 (3) |
| C1–N2 | 1.375 (3) | C18–C19 | 1.432 (4) |
| C1–C6 | 1.419 (3) | O1–S1 | 1.431 (3) |
| C12–N1 | 1.376 (3) | O2–S1 | 1.444 (2) |
| C6–C7 | 1.435 (4) | O3–S1 | 1.439 (2) |
| C7–C12 | 1.414 (3) | C25–F1 | 1.338 (3) |
| N3–N4 | 1.290 (3) | C25–F2 | 1.328 (3) |
| C24–N3 | 1.382 (3) | C25–F3 | 1.329 (4) |
| C13–N4 | 1.374 (3) | C25–S1 | 1.825 (3) |
| C19–C24 | 1.411 (3) | C31–N5 | 1.377 (4) |
| F2–C25–F1 | 106.7 (2) | O1–S1–O3 | 115.51 (17) |
| F2–C25–F3 | 108.0 (3) | O1–S1–O2 | 115.06 (17) |
| F3–C25–F1 | 108.0 (3) | O3–S1–O2 | 114.29 (13) |
| F2–C25–S1 | 111.4 (2) | O1–S1–C25 | 103.83 (15) |
| F3–C25–S1 | 111.2 (2) | O2–S1–C25 | 103.29 (14) |
| F3–C25–F1 | 107.1 (3) | O3–S1–C25 | 102.45 (14) |
Table 5.
Geometry of hydrogen bonds and short contacts for II.
Table 5.
Geometry of hydrogen bonds and short contacts for II.
| Interaction | D a···A (Å) | H···A b (Å) | D-H···A (°) |
|---|
| C20−H···N5 | 3.479 (4) | 2.758 (3) | 133.33 (18) |
| C21−H···O3 | 3.201 (4) | 2.423 (2) | 139.08 (19) |
| N1−H···N3 i | 2.668 (3) | 1.820 (2) | 173.7 (2) |
| N1−H···N4 i | 3.341 (3) | 2.559 (2) | 153.39 (18) |
| C23 i−H i···N2 | 3.544 (4) | 2.714 (2) | 146.37 (19) |
| C11−H···N4 i | 3.481 (4) | 2.656 (2) | 145.6 (2) |
| C11−H···O1 ii | 3.245 (4) | 2.606 (3) | 124.9 (2) |
| C16−H···O2 iii | 3.474 (3) | 2.536 (2) | 169.3 (3) |
| C22−H···F2 iv | 3.537 (4) | 2.6341 (2) | 159.73 (19) |
| C23−H···F1 iv | 3.482 (3) | 2.757 (2) | 133.72 (19) |
| C29 v−H v ···N5 | 3.409 (4) | 2.478 (3) | 166.7 (2) |
| C2−H2···F1 vi | 3.295 (4) | 2.546 (2) | 136.0 (2) |
Table 6.
Selected bond lengths (A) and angles (°) for III.
Table 6.
Selected bond lengths (A) and angles (°) for III.
| N1–N2 | 1.2916 (14) | O2–S | 1.4354 (9) |
| C1–N2 | 1.3590 (15) | O3–S | 1.4510 (9) |
| C1–C6 | 1.4257 (15) | C20–S | 1.8232 (18) |
| C6–C7 | 1.4283 (16) | C20–F1 | 1.324 (2) |
| C7–C12 | 1.4083 (16) | C20–F2 | 1.327 (2) |
| C12–N1 | 1.3727 (15) | C20–F3 | 1.329 (2) |
| O1–S | 1.4336 (10) | | |
| N1–N2–C1 | 116.87 (10) | O2–S–C20 | 103.88 (7) |
| N2–N1–C12 | 126.83 (10) | O3–S–C20 | 101.96 (7) |
| C1–C6–C7 | 117.50 (11) | F1–C20–F2 | 107.08 (15) |
| C12–C7–C6 | 117.23 (10) | F1–C20–F3 | 108.35 (16) |
| O1–S–O2 | 115.80 (6) | F2–C20–F3 | 107.80 (15) |
| O1–S–O3 | 115.33 (6) | F1–C20–S | 111.38 (12) |
| O2–S–O3 | 113.69 (6) | F2–C20–S | 110.80 (14) |
| O1–S–C20 | 103.75 (8) | F3–C20–S | 111.26 (12) |
Table 7.
Geometry of hydrogen bonds and short contacts for III.
Table 7.
Geometry of hydrogen bonds and short contacts for III.
| Interaction | D·a··A (Å) | H···A b (Å) | D-H···A (°) |
|---|
| N1−H···O3 | 2.6802 (14) | 1.8007 (10) | 167.17 (8) |
| C11−H···O3 | 3.2581 (17) | 2.5621 (11) | 130.29 (9) |
| C13−H···F2 | 3.459 (6) | 2.7058 (14) | 136.7 (4) |
| C2−H···O2 i | 3.3048 (17) | 2.6864 (10) | 123.29 (8) |
| C3−H···O2 i | 3.3259 (18) | 2.7314 (10) | 121.34 (9) |
| C19−H···O2 i | 3.755 (3) | 2.8195 (9) | 159.8 (2) |
| C3−H···O3 i | 3.4962 (17) | 2.6038 (10) | 156.64 (8) |
| C17−H···F1 i | 3.7668 (7) | 3.0179 (17) | 136.8 (4) |
| C19−H···O2 i | 3.755 (3) | 2.8195 (9) | 159.8 (2) |
| C4−H···O1 ii | 3.3474 (18) | 2.6938 (12) | 126.52 (9) |
| C5−H···O1 ii | 3.3629 (17) | 2.7384 (11) | 123.97 (8) |
| C5−H···O2 ii | 3.4246 (18) | 2.4772 (10) | 175.01 (8) |
| C8−H···O2 ii | 3.4892 (16) | 2.5444 (9) | 172.94 (8) |
| C10−H···O1 iii | 3.2846 (18) | 2.4665 (12) | 144.23 (9) |
| C14−H···F1 iv | 3.399 (7) | 2.6169 (17) | 139.9 (4) |
| C16−H···F2 v | 3.443 (7) | 2.7662 (14) | 128.9 (4) |
Table 8.
Selected bond lengths (A) and angles (°) for IV.
Table 8.
Selected bond lengths (A) and angles (°) for IV.
| N1–N2 | 1.2941 (18) | C18–C19 1.434 (2) |
| N3–N4 | 1.2971 (18) | C13–C18 1.410 (2) |
| N1–C1 | 1.3729 (19) | S–O1 1.4381 (12) |
| N2–C12 | 1.365 (2) | S–O2 1.4420 (13) |
| C1–C6 | 1.416 (2) | S–O3 1.4440 (12) |
| C6–C7 1.428 (2) | S–C25 1.8258 (18) | |
| C7–C12 1.423 (2) | F1–C25 1.331 (2) | |
| N3–C13 1.3824 (19) | F2–C25 1.333 (2) | |
| N4–C24 1.3793 (19) | F3–C25 1.331 (2) | |
| C19–C24 1.415 (2) | | |
| N1–N2–C12 117.37 (13) | N4–C24–C23 | 116.40 (13) |
| N2–N1–C1 126.36 (13) | C13–C18–C19 | 116.89 (13) |
| C1–C6–C7 117.44 (14) | C24–C19–C18 | 116.93 (14) |
| C12–C7–C6 117.46 (14) | O1–S–O2 | 114.64 (8) |
| N3–N4–C24 119.56 (13) | O1–S–O3 | 115.24 (8) |
| N3-C13–C18 121.67 (14) | O2–S–O3 | 114.68 (8) |
| N3–C13–C14 117.14 (14) | O1–S–C25 | 102.22 (8) |
| N4–N3–C13 122.12(13) | O2–S–C25 | 103.79(9) |
| N4–C24–C19 122.79(14) | O3–S–C25 | 103.97(8) |
Table 9.
Geometry of hydrogen bonds and short contacts for IV.
Table 9.
Geometry of hydrogen bonds and short contacts for IV.
| Interaction | D a···A (Å) | H···A b (Å) | D-H···A (°) |
|---|
| N1−H···N4 | 3.4188 (19) | 2.5190 (13) | 157.67 (10) |
| N1−H···N3 | 2.713 (2) | 1.7648 (14) | 173.33 (10) |
| C2−H···N4 | 3.427 (2) | 2.5668 (14) | 150.62 (12) |
| C3−H···O1 i | 3.317 (2) | 2.4173 (14) | 157.83 (11) |
| C3−H···F3 i | 3.648 (2) | 2.9239 (14) | 134.00 (12) |
| C4−H···O1 ii | 3.140 (2) | 2.5252 (14) | 122.51 (11) |
| C5−H···O1 ii | 3.175 (2) | 2.6109 (15) | 118.40 (11) |
| C5−H···O3 ii | 3.570 (2) | 2.6360 (14) | 167.75 (10) |
| C8−H···O3 ii | 3.676 (2) | 2.7338 (14) | 171.70 (10) |
| C9−H···O2 iii | 3.301 (2) | 2.7173 (14) | 120.36 (12) |
| C10−H···O2 iii | 3.274 (2) | 2.6799 (13) | 121.20 (11) |
| C11−H···F2 iv | 3.518 (3) | 2.6665 (17) | 149.51 (11) |
| C14−H···N2 | 3.574 (2) | 2.7226 (15) | 149.51 (12) |
| C16−H···O2 | 3.246 (2) | 2.659 (13) | 120.50 (11) |
| C17−H···O2 v | 3.571 (2) | 2.6764 (14) | 157.13 (10) |
| C20−H···O2 v | 3.343 (2) | 2.7258 (16) | 162.41 (10) |
| C21−H···O3 vi | 3.306 (2) | 2.6782 (13) | 124.14 (12) |
| C22−H···O3 vi | 3.303 (2) | 2.6812 (13) | 123.60 (12) |
| C22−H···F1 vi | 3.495 (2) | 2.5838 (14) | 160.72 (11) |
| C23−H···F3 i | 3.598 (2) | 2.6684 (15) | 166.07 (10) |