Properties of Cast Iron Produced with a Limited Share of Pig Iron in the Charge
Abstract
1. Introduction
2. Materials and Methods
- Nikon ECLIPSE LV150 metallographic microscope equipped with a Nikon DS-Fi1-U2 camera (Nikon Corporation, Tokyo, Japan);
- ZEISS SUPRA 35 high-resolution (Carl Zeiss AG, Oberkochen, Germany) scanning electron microscope, equipped with an EDS chemical composition analysis system;
- LECO GDS500A emission spectrometer (LECO Corporation, St. Joseph, MI, USA);
- LECO CS-125 analyzer (LECO Corporation, St. Joseph, MI, USA), enabling precise determination of the carbon and sulfur concentrations.
- Liquidus temperature (Tliq)—the temperature at which the first solid particles form, marking the beginning of solidification. For alloys with compositions deviating from the eutectic point, a liquidus temperature appears on the cooling curve: it corresponds to the austenite liquidus for hypoeutectic cast irons and to the graphite liquidus for hypereutectic cast irons. In strictly eutectic alloys, the liquidus temperature is not defined as a single point but rather as part of the eutectic reaction region [18];
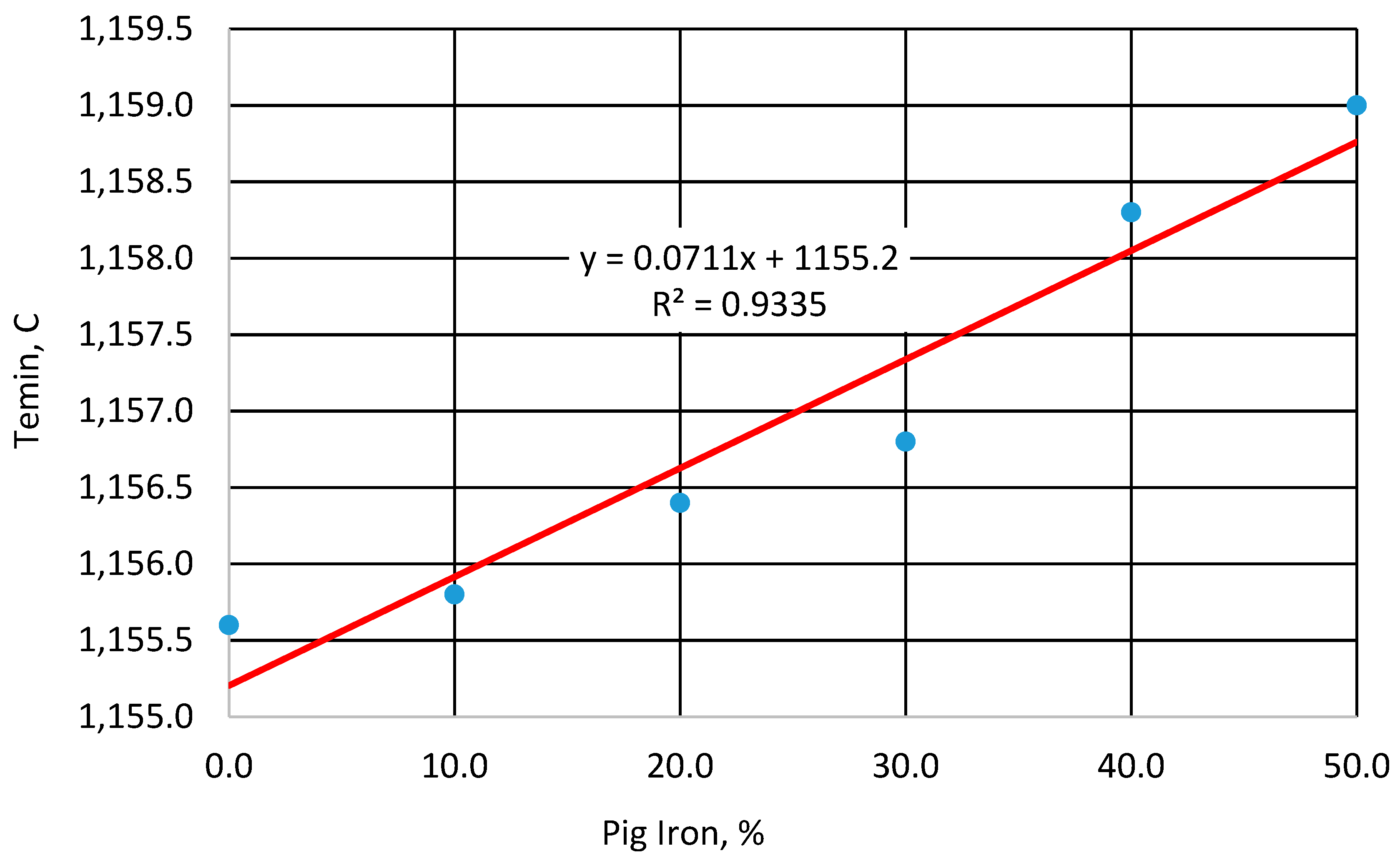
- Minimum eutectic temperature (Temin)—the lowest temperature reached during eutectic solidification. At this point, the latent heat of crystallization equals the heat released during cooling. This is the most important indicator of cast iron nucleation. The higher the Temin, the better the nucleation potential. It is commonly accepted that below 1135 °C, nucleation is low and there is a high risk of primary carbides forming in the casting. Between 1135 °C and 1145 °C, nucleation is considered optimal, and the risk of primary carbides appears only in thin-walled castings. Above 1145 °C, nucleation is very good and there is no risk of primary carbide formation [10,18,19,20];
- Maximum eutectic temperature (Temax)—the highest temperature during the eutectic transformation [18];
- Solidus temperature (Tsolidus)—the temperature at which the solidification process ends [18];
- Recalescence (Rec)—the difference between the maximum and minimum eutectic temperatures. The optimal value of recalescence for gray cast iron is between 4 and 9 °C, and for ductile iron it ranges from 2 to 5 °C. A low Rec value may indicate a low level of graphite expansion, which can lead to porosity formation. This value should be assessed in relation to the nucleation level and the current position in the Fe-C phase diagram. A high Rec value is associated with a high level of graphite expansion and may cause mold distortion [10,18,19,20].
3. Results
3.1. Chemical Composition
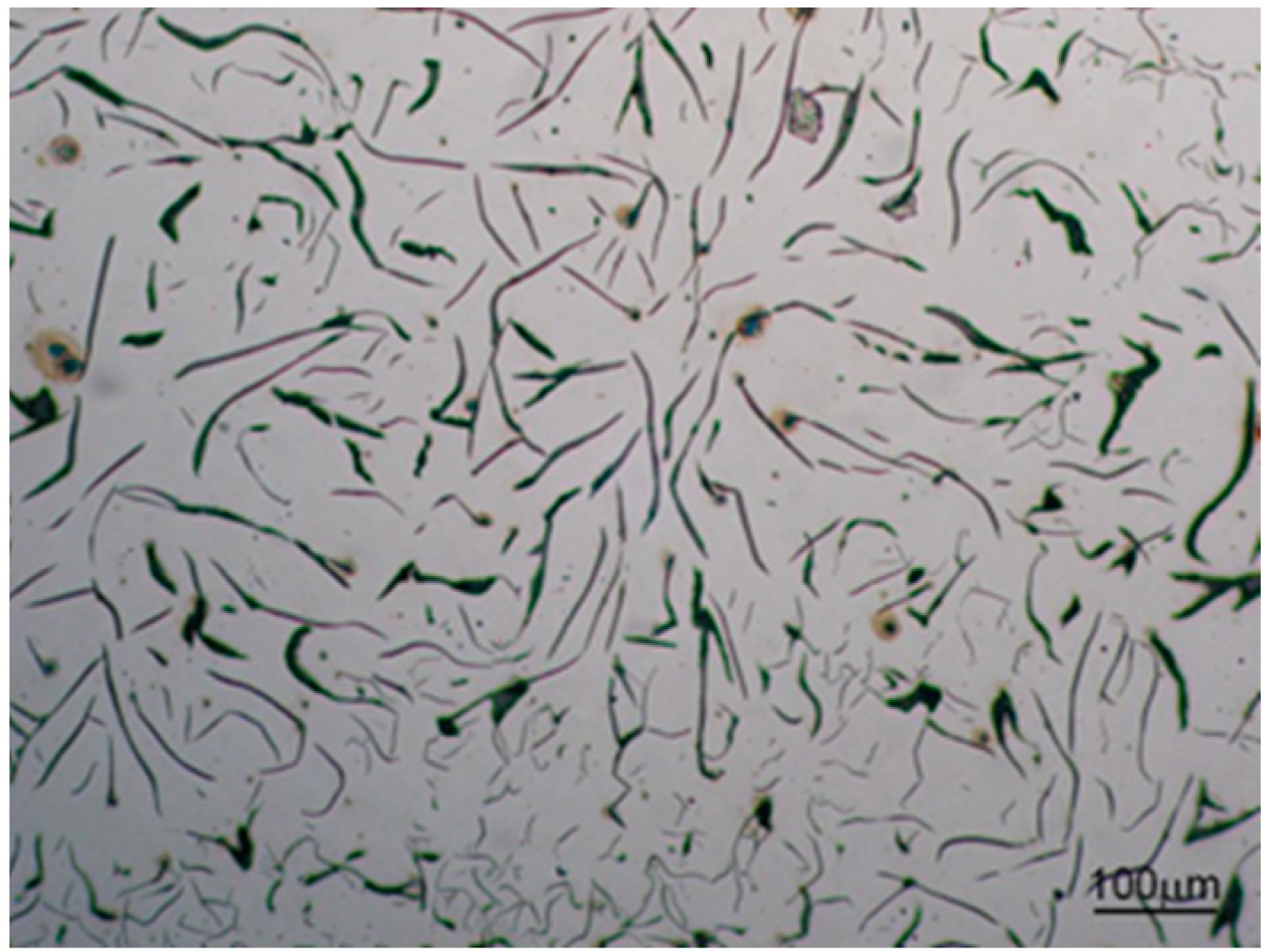
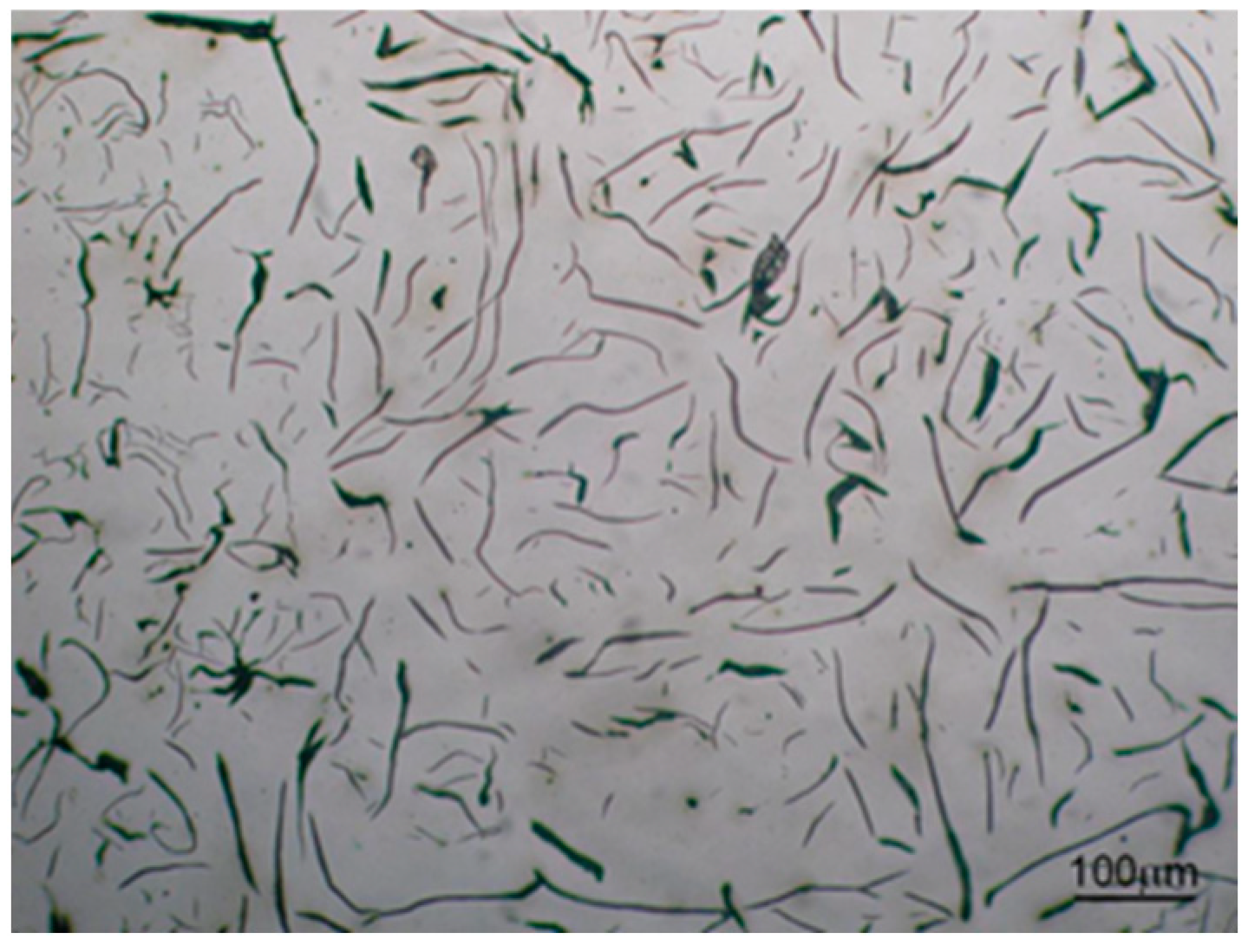
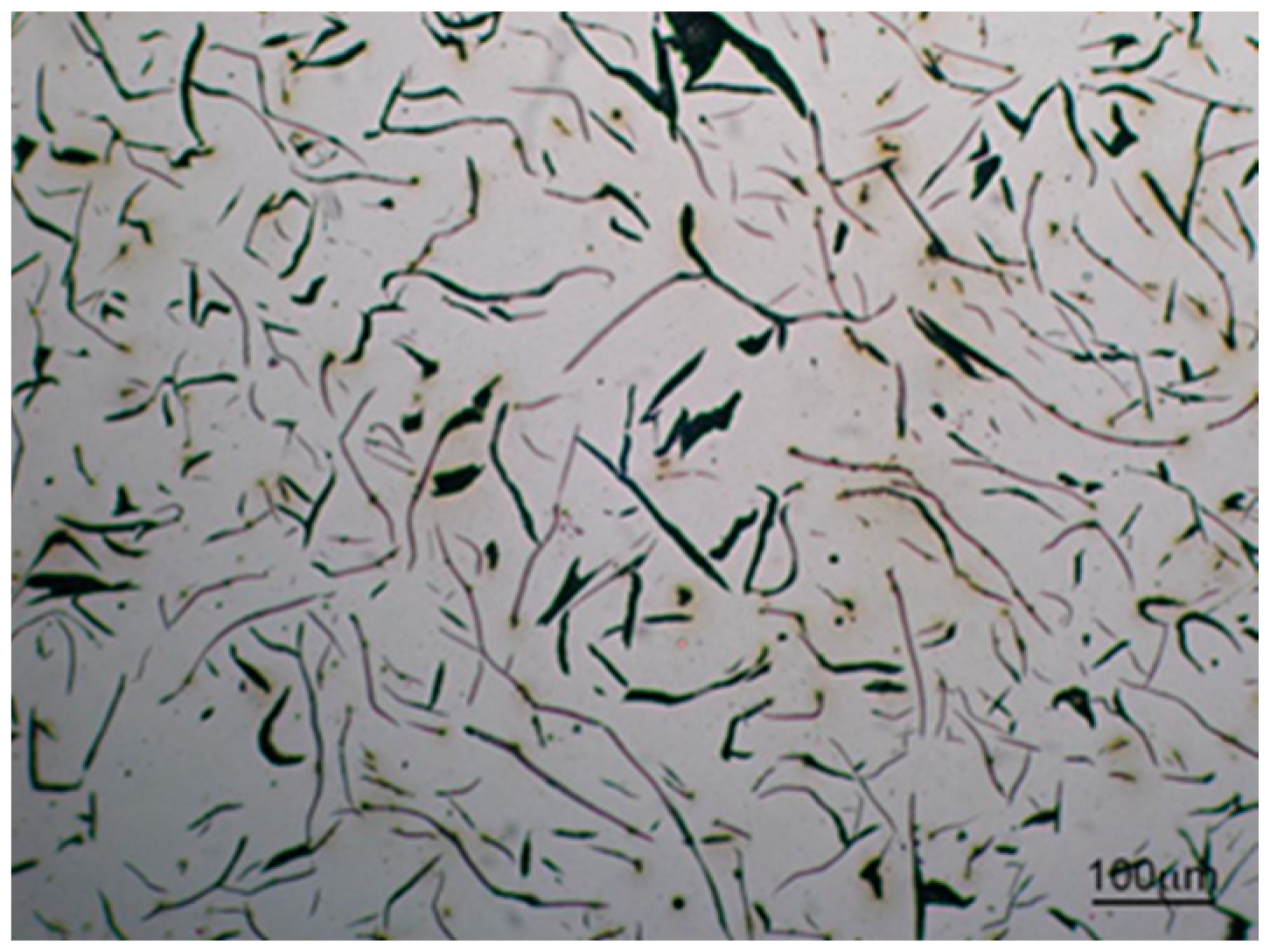
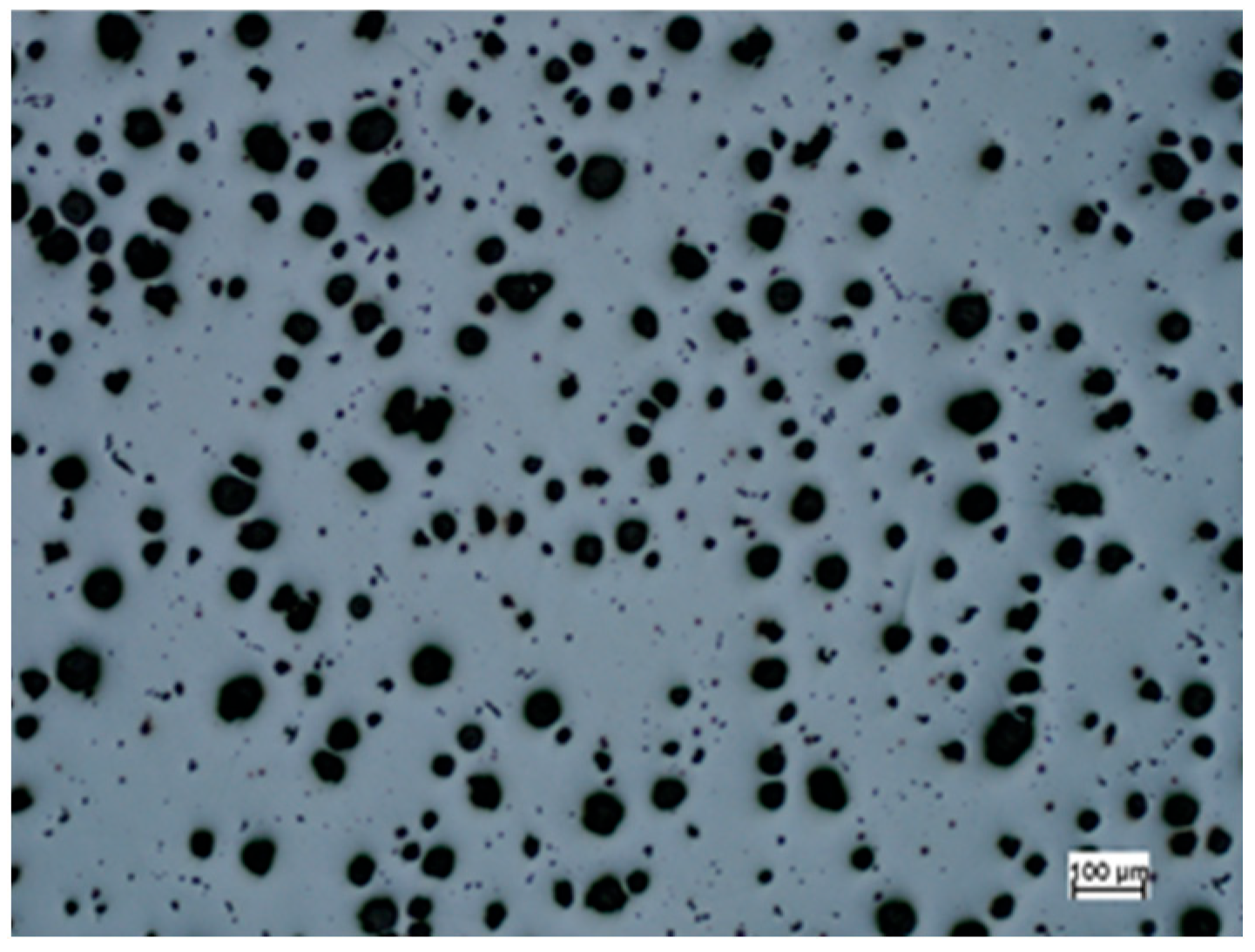
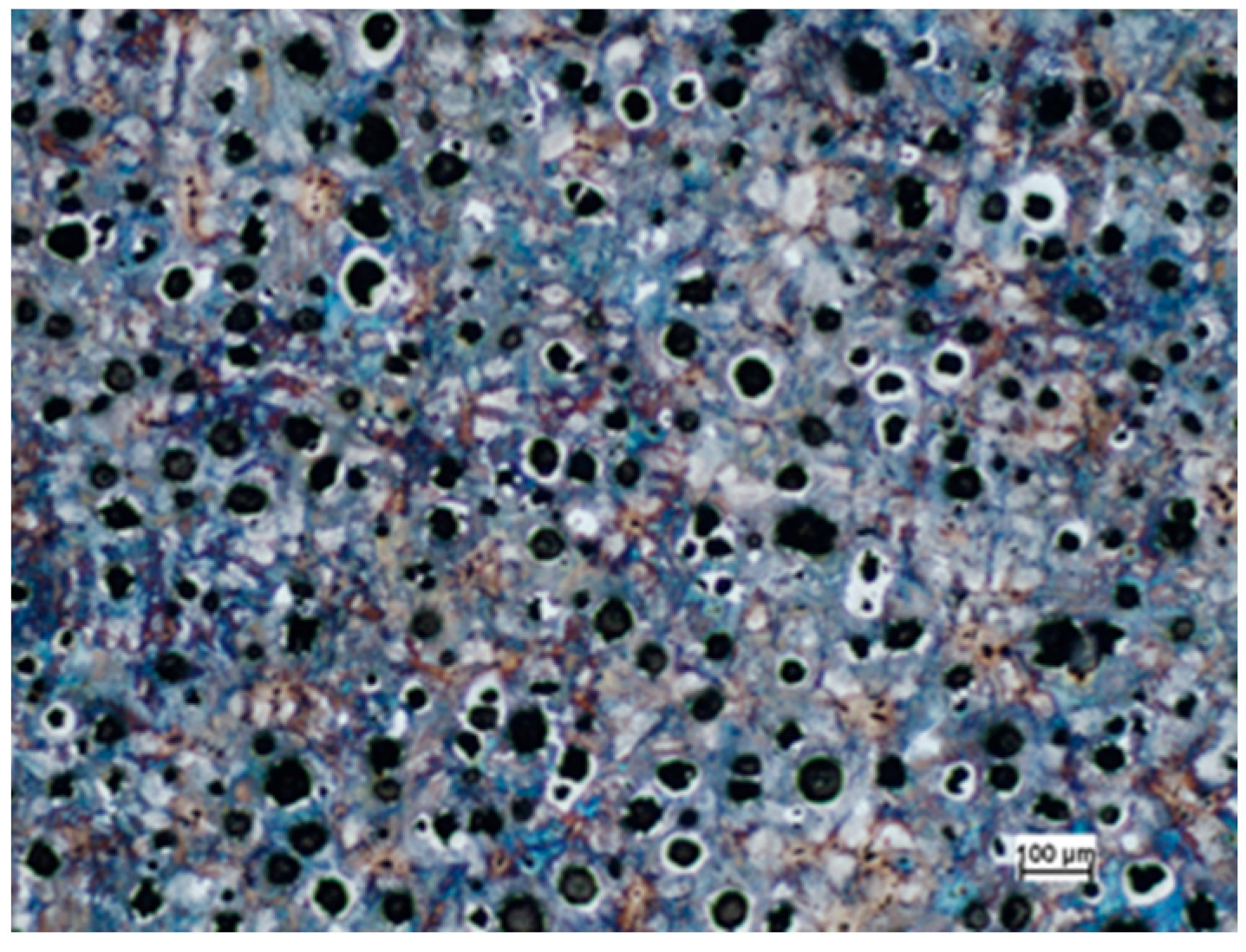
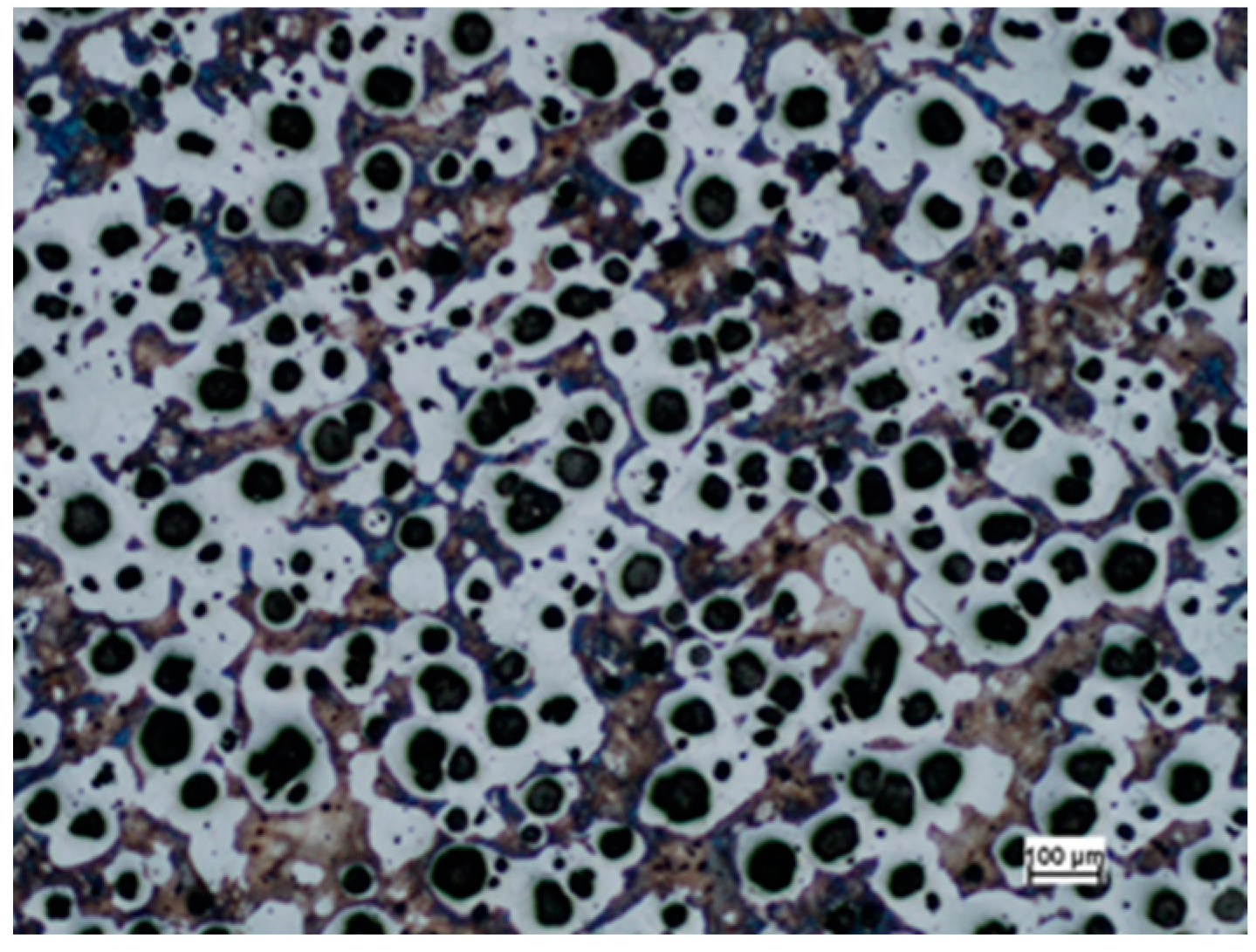
3.2. Microstructure
3.3. Cooling Curve Analysis
4. Conclusions
- It is possible to produce good-quality gray and ductile cast iron without the use of pig iron in the charge;
- Regardless of the applied charge composition, high values of the minimum eutectic transformation temperature (Temin > 1135 °C is necessary) are achieved for unmodified cast iron for the temperature range from 115.6 °C to 1159 °C, indicating a sufficient level of graphite nucleation. An increase in pig iron content from 0 to 50% led to a rise in Temin of only 4.3 °C, which allows for a reduction in the amount of inoculant needed to ensure solidification in the stable Fe–C system;
- The modification process allows an increase in the Temin temperature in the range of 18–26 °C;
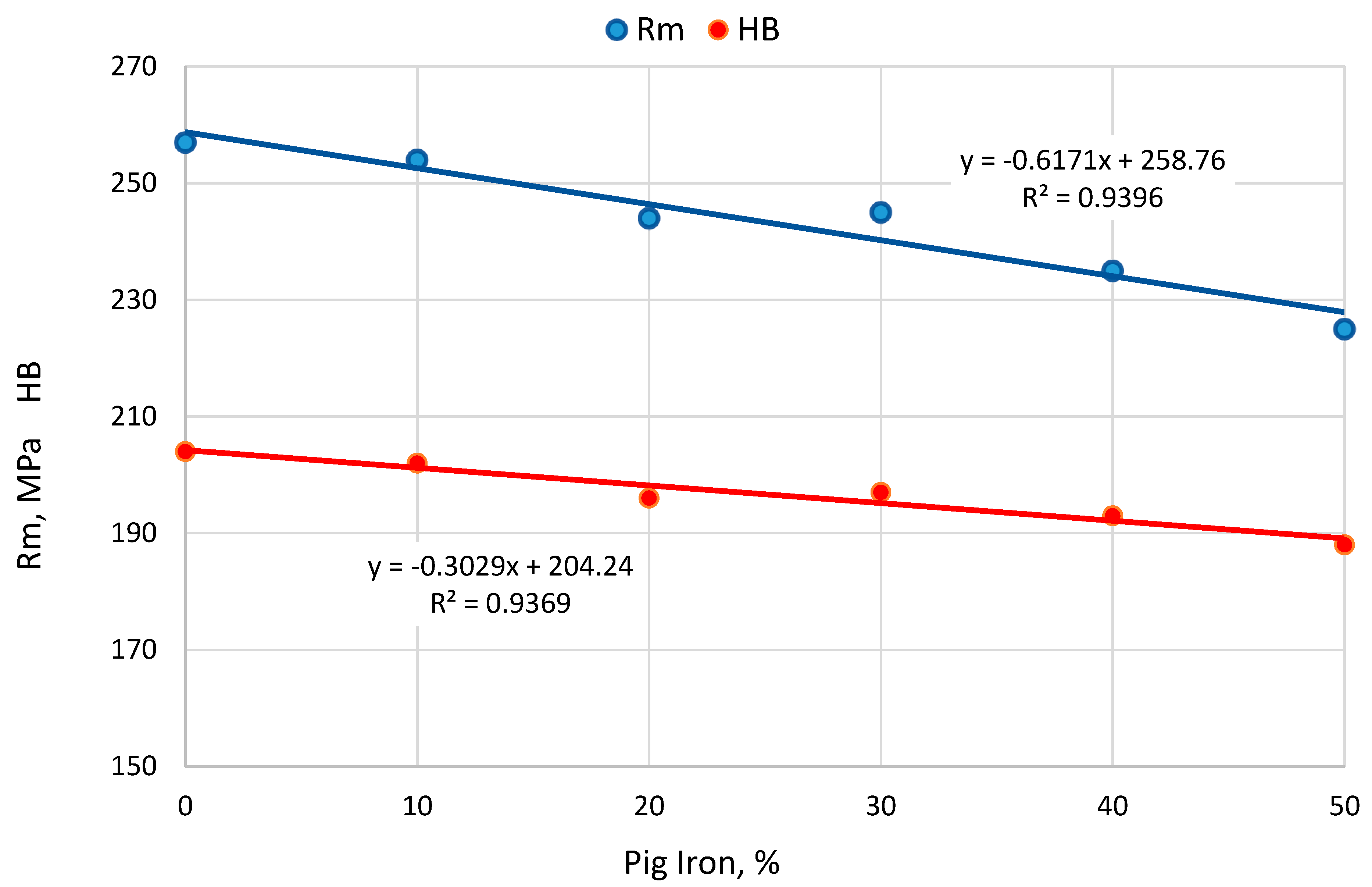
- Increasing the pig iron content in the charge from 0 to 50% causes a decrease in tensile strength and HB hardness by approximately 10%. This most likely results from a reduction in the Mn content in the alloy, which is due to the lower Mn content in pig iron compared to steel scrap.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dwulat, R.; Janerka, K.; Grzesiak, K. Influence of Charge Materials on the Metallurgical Quality of Gray Cast Iron. Arch. Foundry Eng. 2023, 23, 66–71. [Google Scholar] [CrossRef]
- Dwulat, R.; Janerka, K. Evaluation of the metallurgical quality of nodular cast iron in the production conditions of a foundry. J. Manuf. Mater. Process. 2023, 7, 18. [Google Scholar] [CrossRef]
- Riposan, I.; Chisamera, M.; Stan, S. Enhanced Quality in Electric Melt Grey Cast Irons. ISIJ Int. 2013, 53, 1683–1695. [Google Scholar] [CrossRef]
- Riposan, I.; Chisamera, M.; Stan, S. Performance of heavy ductile iron castings for windmills. China Foundry 2010, 7, 163–170. [Google Scholar]
- Futas, P.; Pribulova, A.; Petrik, J.; Blasko, P.; Junakova, A.; Sabik, V. Metallurgical Quality of Cast Iron Made from Steel Scrap and Possibilities of Its Improvement. Metals 2023, 13, 27. [Google Scholar] [CrossRef]
- Futas, P.; Pribulova, A.; Fedorko, G.; Molnar, V. Influence of Steel Scrap in the Charge on the Properties of Gray Cast Iron. ISIJ Int. 2017, 57, 374–379. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Horie, H.; Kowata, T.; Koike, K.; Shimizu, K. Influence of Steel Scrap on Microstructure and Mechanical Properties of Spheroidal Graphite Cast Iron. Mater. Trans. 2003, 44, 1419–1424. [Google Scholar] [CrossRef]
- Karsay, S.I. Ductile Iron I, Production: The State of the Art; Quebec Iron and Titanium Corporation: Quebec City, QC, Canada, 1976. [Google Scholar]
- Chişamera, M.; Ripoşan, I.; Stan, S.; Ecob, C.; Grasmo, G.; Wilkinson, D. Preconditioning of Electrically Melted Grey Cast Irons. U.P.B. Sci. Bull. Ser. B 2009, 71, 115–126. [Google Scholar]
- Stan, S.; Chisamera, M.; Riposan, I.; Barstow, M. Application of thermal analysis to monitor the quality of hypoeutectic cast irons during solidification in sand and metal moulds. J. Therm. Anal. Calorim. 2012, 110, 1185–1192. [Google Scholar] [CrossRef]
- Riposan, I.; Chisamera, M.; Stan, S. Application of cooling curve analysis in solidification pattern and structure control of grey cast irons. J. Therm. Anal. Calorim. 2018, 132, 1017–1028. [Google Scholar] [CrossRef]
- Cojocaru, A.M.; Riposan, I.; Stan, S. Solidification influence in the control of inoculation effects in ductile cast irons by thermal analysis. J. Therm. Anal. Calorim. 2019, 138, 2131–2143. [Google Scholar] [CrossRef]
- Stefanescu, D.M.; Suarez, R.; Kim, S.B. 90 years of thermal analysis as a control tool in the melting of cast iron. China Foundry 2020, 17, 69–84. [Google Scholar] [CrossRef]
- Chisamera, M.; Riposan, I.; Stan, S.; Stefan, E.; Costache, G. Thermal analysis control of in-mould and ladle inoculated grey cast irons. China Foundry 2015, 6, 145–151. [Google Scholar]
- Erturka, S.O.; Kumruoglub, L.C.; Ozel, A. Determination of Feederless Casting Limits by Thermal Analysis in Cast Iron. Acta Phys. Pol. Ser. A 2017, 131, 370–373. [Google Scholar] [CrossRef]
- Seidu, S.O. Thermal Analysis of Preconditioned Ductile Cast Iron. Int. J. Curr. Eng. Technol. 2013, 3, 813–818. [Google Scholar]
- Kołakowski, J.; Brzeżański, M.; Burdzy, D.; Sobieraj, J.; Urbanowicz, M.; Paruch, T.; Janerka, K. The effect of successive stages of the melting process on the nucleation of ductile cast iron. Arch. Foundry Eng. 2023, 23, 61–67. [Google Scholar] [CrossRef]
- Stefan, E.; Riposan, I.; Chisamera, M. Application of thermal analysis in solidification pattern control of La-inoculated grey cast irons. J. Therm. Anal. Calorim. 2019, 138, 2491–2503. [Google Scholar] [CrossRef]
- Seidu, S.O.; Riposan, I. Thermal analysis of inoculated ductile irons. U.P.B. Sci. Bull. Ser. B 2011, 73, 241–254. [Google Scholar]
- Chisamera, M.; Stan, S.; Riposan, I.; Stefan, E.; Costache, G. Thermal analysis of inoculated grey cast irons. Ann. “Dunarea de Jos” Univ. Galati. Fascicle IX. Metall. Mater. Sci. 2008, 2, 11–16. [Google Scholar]
- Stefanescu, D.M. Analysis of the rationale and accuracy of the use of carbon Equivalent and thermal analysis in the quality control of cast iron. Int. J. Met. 2022, 16, 1057–1078. [Google Scholar] [CrossRef]
- Available online: http://www.proservicetech.it/itacax-thermal-analysis-final-iron-quality-control (accessed on 14 March 2025).
- Available online: https://www.novacast.se/product/atas/ (accessed on 17 March 2025).





| Melt No. | Steel Scrap kg | Pig Iron kg | Recarburizer g | FeSi75 g |
|---|---|---|---|---|
| 1_GCI | 10.37 | - | 376 | 350 |
| 2_GCI | 11.47 | - | 520 | 290 |
| 3_GCI | 14.15 | - | 522 | 421 |
| 4_GCI | 11.59 | - | 440 | 300 |
| 5_GCI | 10.04 | - | 370 | 350 |
| 6_GCI | 10.32 | - | 385 | 350 |
| 7_GCI | 14.12 | - | 522 | 415 |
| 8_GCI | 11.57 | - | 450 | 300 |
| 9_GCI | 11.43 | - | 460 | 300 |
| 10PI_GCI | 2.95 | 9.41 | - | 260 |
| Melt No. | Steel Scrap kg | Pig Iron kg | Recarburizer g | FeSi75 g |
|---|---|---|---|---|
| 1_DI | 10.0 | - | 400 | 372 |
| 2_DI | 9.8 | - | 400 | 275 |
| 3_DI | 9.6 | - | 400 | 275 |
| 4_DI | 10.2 | - | 400 | 276 |
| 5_DI | 10.2 | - | 460 | 276 |
| 6_DI | 10.3 | - | 465 | 270 |
| 7_DI | 10.0 | - | 520 | 265 |
| 8PI_DI | 1.50 | 10 | - | 281 |
| Melt No. | C % | Si % | Mn % | P % | S % |
|---|---|---|---|---|---|
| 1_GCI | 2.84 | 2.14 | 0.49 | 0.035 | 0.031 |
| 2_GCI | 3.55 | 1.90 | 0.56 | 0.025 | 0.020 |
| 3_GCI | 3.26 | 2.24 | 0.40 | 0.033 | 0.017 |
| 4_GCI | 3.30 | 1.96 | 0.46 | 0.033 | 0.024 |
| 5_GCI | 3.46 | 2.13 | 0.48 | 0.026 | 0.024 |
| 6_GCI | 3.16 | 2.14 | 0.48 | 0.022 | 0.032 |
| 7_GCI | 3.34 | 2.12 | 0.37 | 0.029 | 0.028 |
| 8_GCI | 3.34 | 1.96 | 0.48 | 0.043 | 0.047 |
| 9_GCI | 3.25 | 1.97 | 0.51 | 0.022 | 0.021 |
| 10PI_GCI | 3.34 | 1.98 | 0.33 | 0.045 | 0.035 |
| Melt No. | C % | Si % | Mn % | P % | S % |
|---|---|---|---|---|---|
| 1_DI | 3.59 | 3.17 | 0.46 | 0.017 | 0.004 |
| 2_DI | 3.68 | 2.88 | 0.48 | 0.018 | 0.006 |
| 3_DI | 3.79 | 2.72 | 0.39 | 0.007 | 0.006 |
| 4_DI | 3.80 | 2.55 | 0.54 | 0.020 | 0.001 |
| 5_DI | 3.55 | 2.53 | 0.47 | 0.018 | 0.002 |
| 6_DI | 3.75 | 2.40 | 0.54 | 0.015 | 0.009 |
| 7_DI | 3.74 | 2.31 | 1.08 | 0.042 | 0.018 |
| 8PI_DI | 3.62 | 3.20 | 0.10 | 0.041 | 0.009 |
| Melt No. | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| GCI1_0PI | 3.27 | 2.25 | 0.854 | 0.030 | 0.022 |
| GCI2_10PI | 3.48 | 3.06 | 0.734 | 0.036 | 0.019 |
| GCI3_20PI | 3.38 | 2.12 | 0.732 | 0.035 | 0.022 |
| GCI4_30PI | 3.32 | 2.36 | 0.695 | 0.034 | 0.011 |
| GCI5_40PI | 3.53 | 1.81 | 0.641 | 0.046 | 0.022 |
| GCI6_50PI | 3.56 | 1.99 | 0.597 | 0.046 | 0.021 |
| Melt No. | Tliquidus °C | Temin °C | Temax °C | Tsolidus °C | Rec °C |
|---|---|---|---|---|---|
| 1_GCI | 1235.4 | 1144.5 | 1148.5 | 1103.7 | 4.0 |
| 2_GCI | 1198.5 | 1147.6 | 1148.5 | 1094.4 | 0.9 |
| 3_GCI | 1192.3 | 1156.2 | 1157.0 | 1099.4 | 0.8 |
| 4_GCI | 1200.5 | 1156.6 | 1157.1 | 1112.2 | 0.5 |
| 5_GCI | 1204.8 | 1150.9 | 1152.8 | 1113.7 | 1.9 |
| 6_GCI | 1199.0 | 1145.5 | 1150.6 | 1087.5 | 5.1 |
| 7_GCI | 1197.0 | 1150.8 | 1152.7 | 1113.7 | 1.9 |
| 8_GCI | 1191.7 | 1148.3 | 1148.5 | 1103.6 | 0.2 |
| 9_GCI | 1184.1 | 1152.9 | 1157.3 | 1116.3 | 4.4 |
| 10PI_GCI | 1203.7 | 1150.5 | 1153.3 | 1107.5 | 2.8 |
| Melt No. | Tliquidus °C | Temin °C | Temax °C | Tsolidus °C | Rec °C |
|---|---|---|---|---|---|
| 1_DI | 1149.7 | 1133.1 | 1143.4 | 1080.9 | 10.3 |
| 2_DI | 1148.1 | 1115.8 | 1123.2 | 1069.2 | 7.4 |
| 3_DI | 1152.1 | 1124.4 | 1129.2 | 1092.4 | 4.8 |
| 4_DI | 1160.4 | 1133.4 | 1141.3 | 1094.2 | 7.9 |
| 5_DI | 1186.7 | 1124.6 | 1128.4 | 1095.4 | 3.8 |
| 6_DI | 1144.6 | 1129.7 | 1143.1 | 1086.7 | 13.4 |
| 7_DI | 1159.3 | 1133.2 | 1139.4 | 1098.6 | 6.2 |
| 8PI_DI | 1142.4 | 1133.1 | 1145.6 | 1084.5 | 12.5 |
| Melt No. | Tliquidus °C | Temin °C | Temax °C | Tsolidus °C | Rec °C |
|---|---|---|---|---|---|
| GCI1_0PI | 1203.0 | 1155.6 | 1158.8 | 1121.2 | 3.2 |
| GCI2_10PI | 1183.9 | 1155.8 | 1158.2 | 1125.3 | 2.4 |
| GCI3_20PI | 1192.2 | 1156.4 | 1158.9 | 1114.6 | 2.5 |
| GCI4_30PI | 1161.0 | 1156.8 | 1158.8 | 1119.4 | 2.0 |
| GCI5_40PI | 1164.2 | 1158.3 | 1164.7 | 1122.4 | 4.4 |
| GCI6_50PI | 1177.9 | 1159.0 | 1161.4 | 1125.5 | 2.4 |
| Inoculant | Temin [°C] | Tsolidus [°C] | Rec [°C] |
|---|---|---|---|
| No inoculation | 1125 | 1084 | 9.9 |
| FeSi-based inoculant | 1143 | 1095 | 7.1 |
| Ce-based inoculant | 1149 | 1103 | 5.6 |
| Ba-based inoculant | 1144 | 1098 | 7.4 |
| Bi-based inoculant | 1151 | 1100 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janerka, K.; Jezierski, J. Properties of Cast Iron Produced with a Limited Share of Pig Iron in the Charge. Crystals 2025, 15, 614. https://doi.org/10.3390/cryst15070614
Janerka K, Jezierski J. Properties of Cast Iron Produced with a Limited Share of Pig Iron in the Charge. Crystals. 2025; 15(7):614. https://doi.org/10.3390/cryst15070614
Chicago/Turabian StyleJanerka, Krzysztof, and Jan Jezierski. 2025. "Properties of Cast Iron Produced with a Limited Share of Pig Iron in the Charge" Crystals 15, no. 7: 614. https://doi.org/10.3390/cryst15070614
APA StyleJanerka, K., & Jezierski, J. (2025). Properties of Cast Iron Produced with a Limited Share of Pig Iron in the Charge. Crystals, 15(7), 614. https://doi.org/10.3390/cryst15070614








