Enhancement of Ethylene-Butene Terpolymer Performance via Carbon Nanotube-Induced Nanodispersion of Montmorillonite Layers
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Preparation of Modified SMMT
2.3. Preparation of EBT Composites
2.4. Characterization
3. Results and Discussion
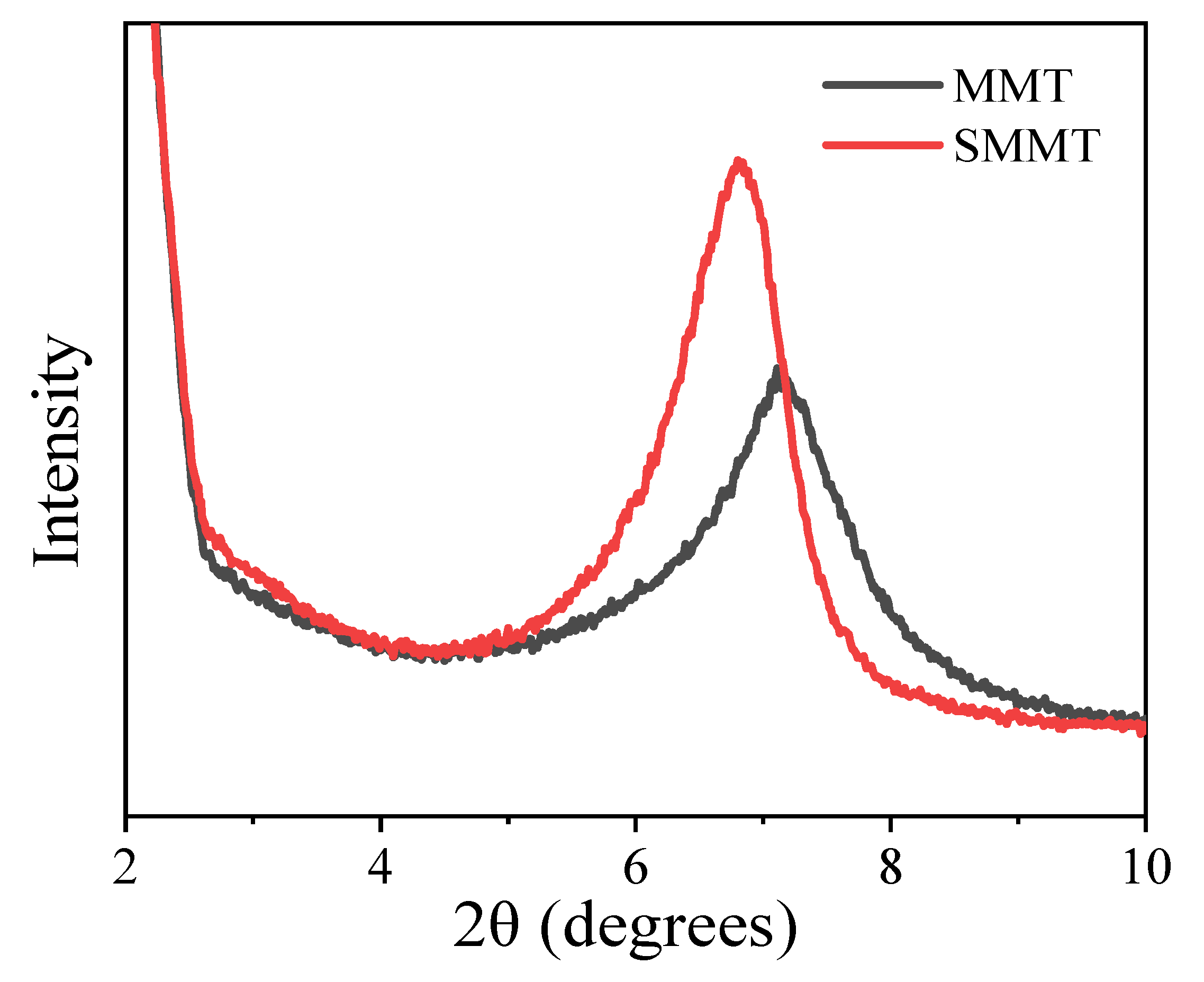
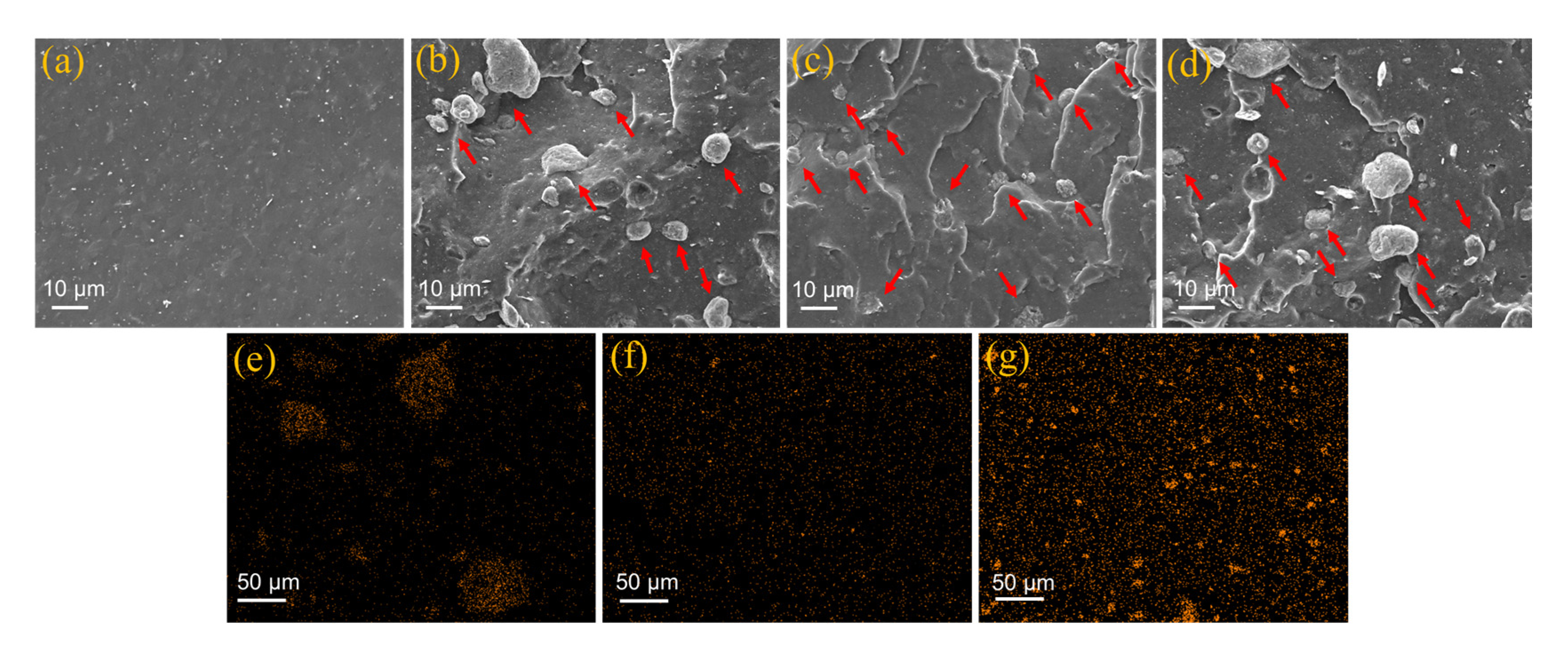
3.1. Structure and Morphology
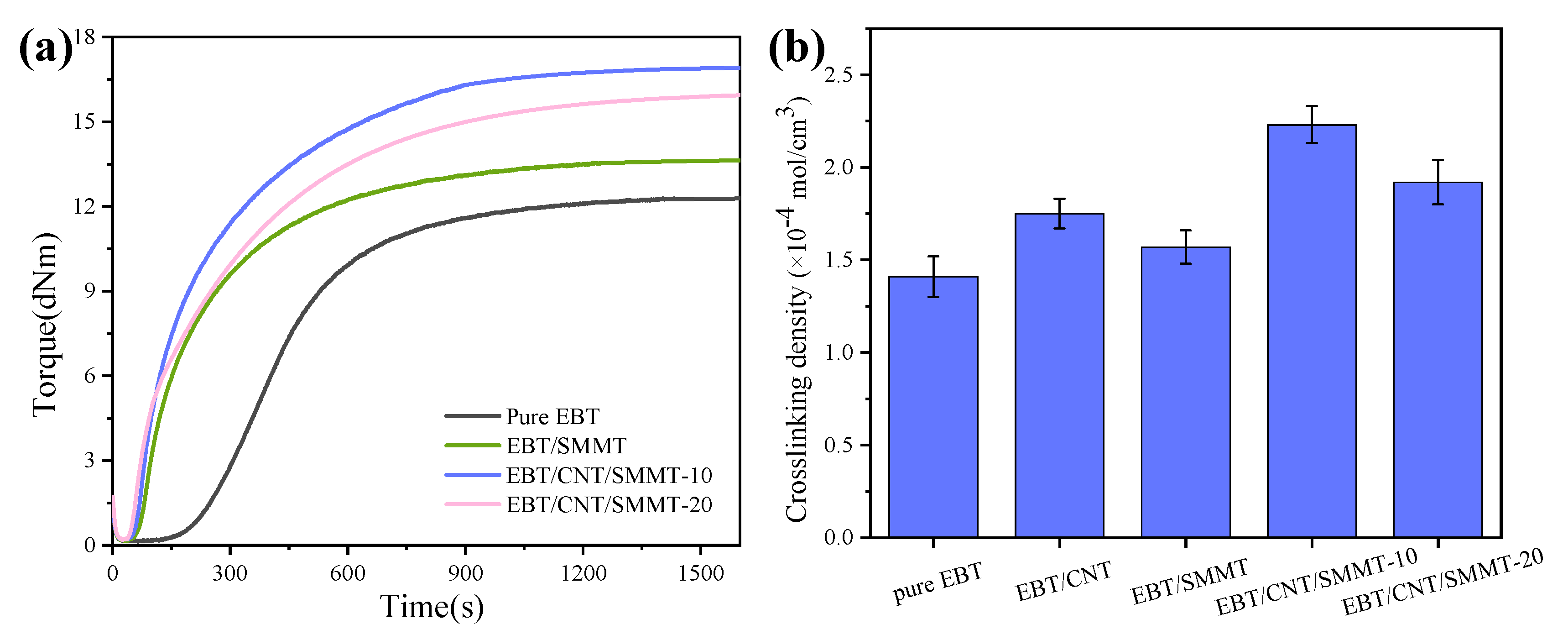
3.2. Vulcanization Performances
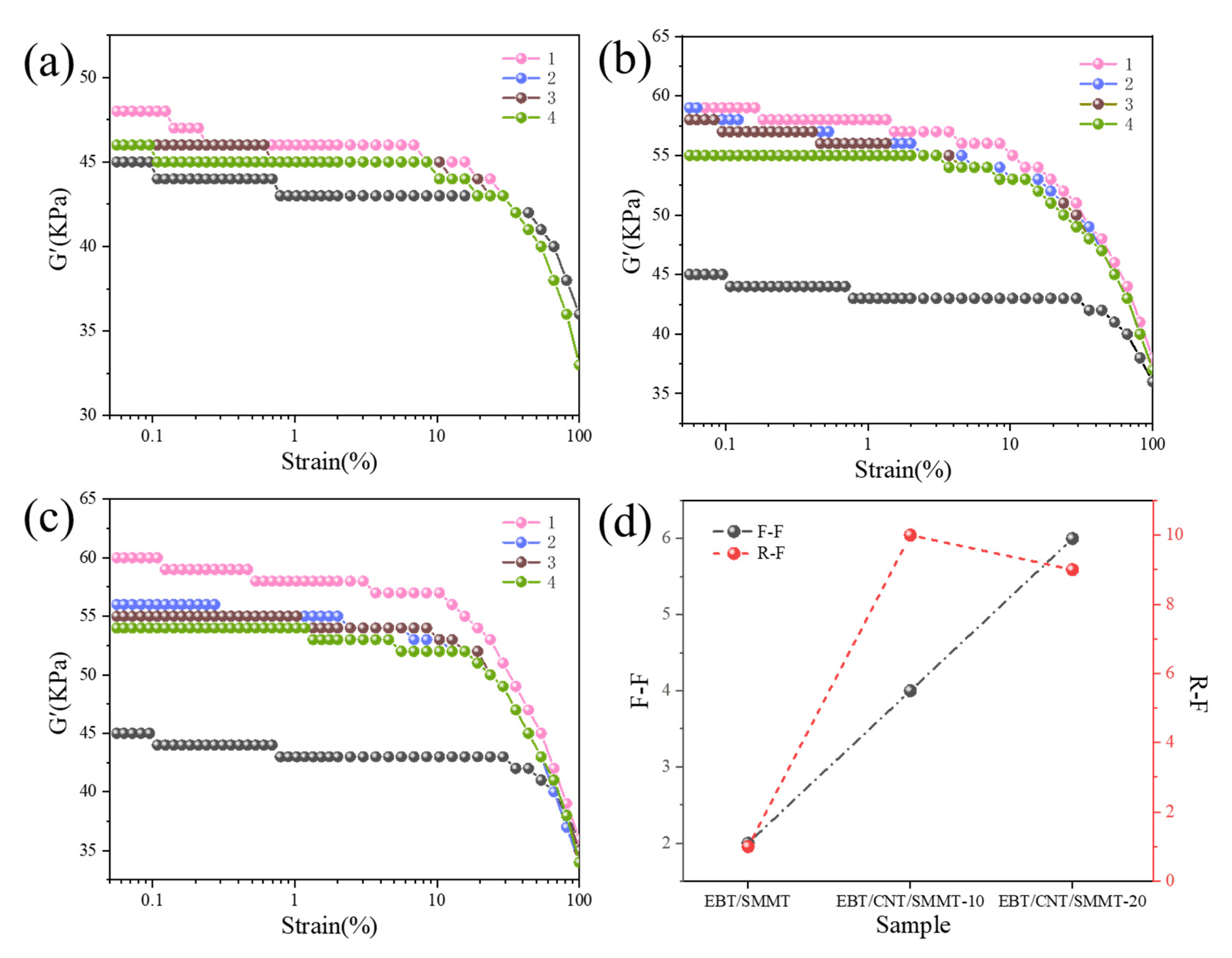
3.3. R-F and F-F Interaction Analysis
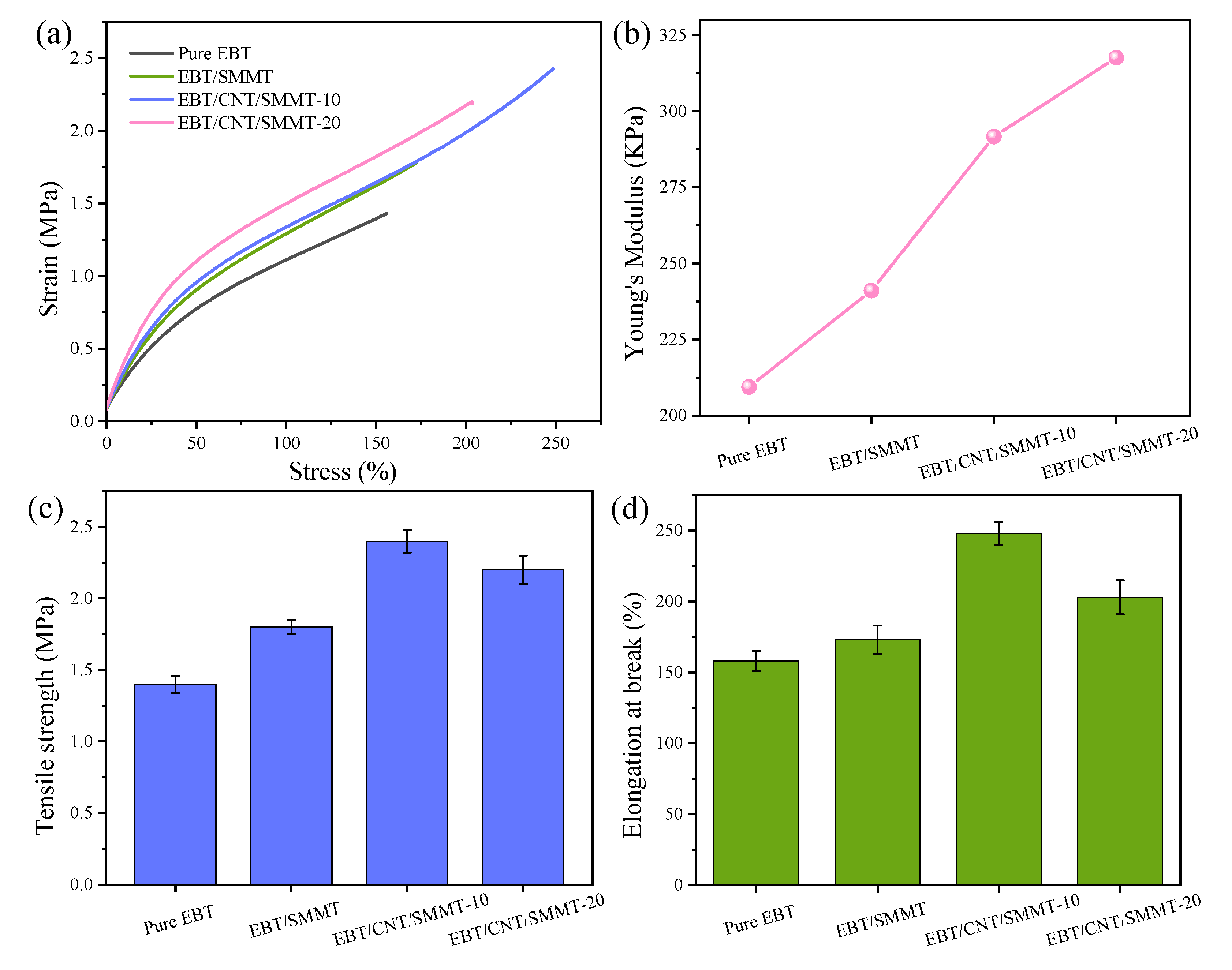
3.4. Mechanical Properties
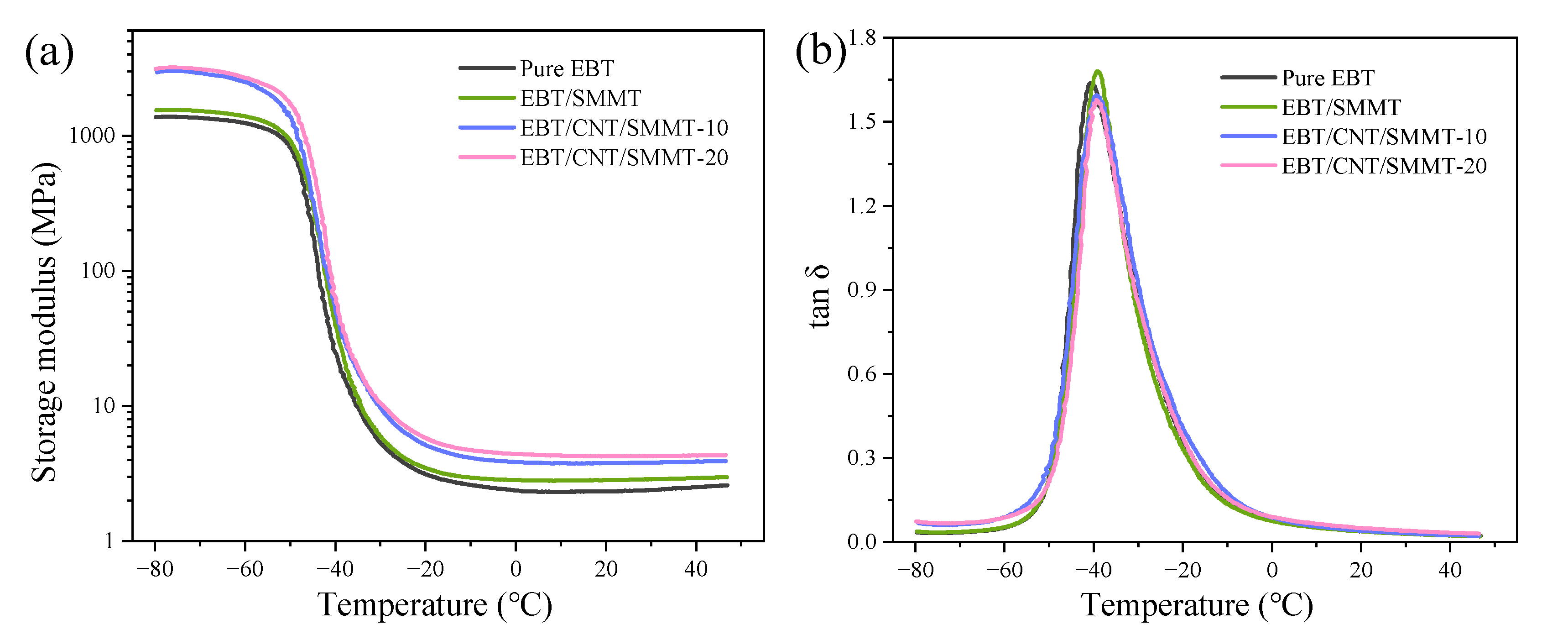
3.5. Dynamic Mechanical Analysis (DMA)
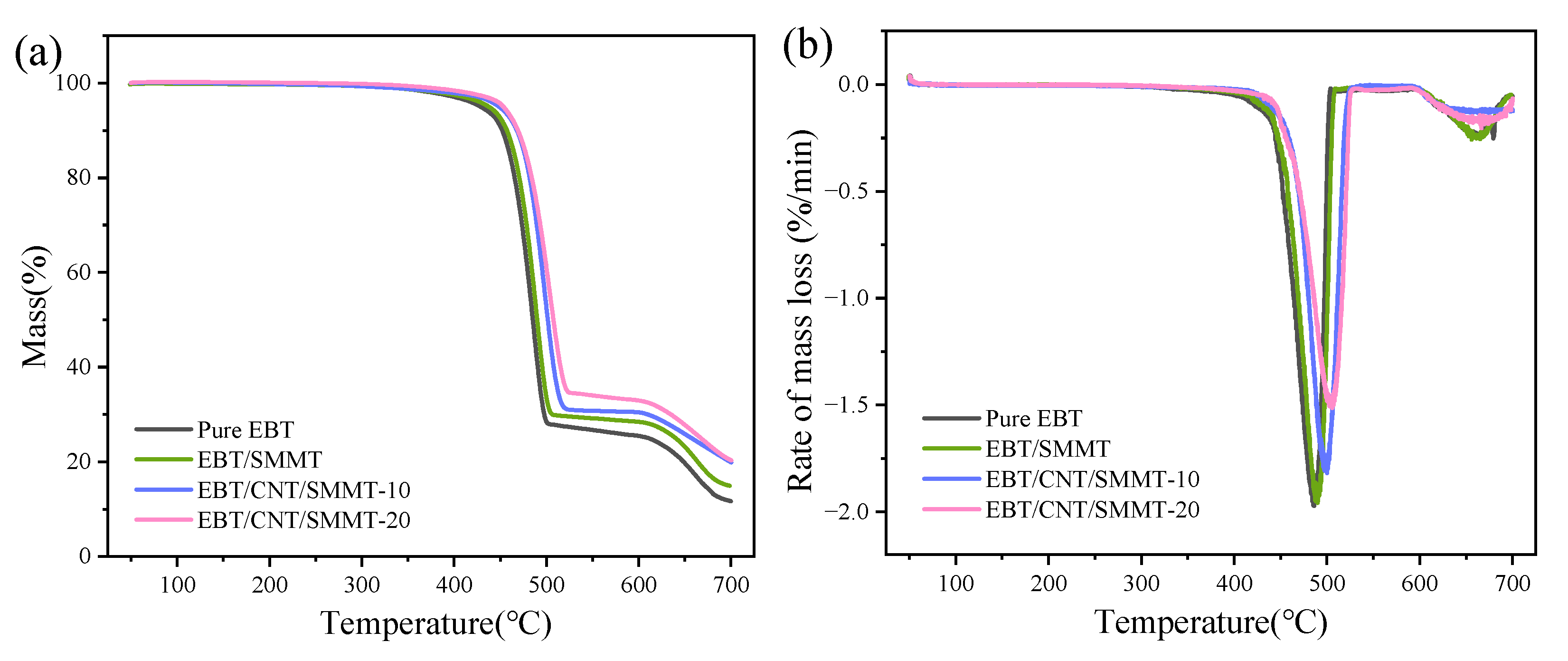
3.6. Thermogravimetric Analysis (TGA)
3.7. Surface Wettability and Water Absorption Performance Tests
4. Conclusions
- This study investigates the performance regulation of EBT rubber-based composites with CNT/SMMT hybrid fillers. Experiments show that when the CNT/SMMT mass ratio is optimized to 1:5, the comprehensive properties of EBT composites are improved in multiple dimensions: Young’s modulus increases by 40.4%, tensile strength by 71.4%, and elongation at break remains at 250%, addressing the strength–toughness trade-off of traditional rigid fillers. In terms of thermal stability, the maximum decomposition temperature rises from 486.2 °C to 505.8 °C, while environmental resistance is significantly enhanced, with the water contact angle increasing from 88.1° to 117.5° and water absorption rate dropping below 0.2%. The study also indicates that an excessive SMMT:CNT ratio leads to filler aggregation, deteriorating the properties of EBT composites.
- SEM observations and four-cycle strain-scanning analysis reveal that the introduction of CNTs significantly improves the dispersion uniformity and interfacial compatibility of SMMT in the EBT matrix, enhancing F-F and F-R interactions. This promotes the formation of a denser rigid network and a 1D CNT/2D SMMT interpenetrating network, boosting the modulus and strength of EBT composites. Meanwhile, CNT/SMMT hybrid fillers facilitate uniform vulcanization reactions, forming a more stable crosslinked network and further optimizing network load-bearing capacity. Additionally, the “molecular spring” effect from stearic acid intercalation endows the material with unique strength-ductility synergy, maintaining elongation at break through lamellar sliding while increasing modulus.
- This material system demonstrates clear application values in fields such as building waterproof sealing materials (adapting to structural deformation and resisting rainwater penetration), automotive component seals (withstanding high temperatures in engine compartments and reducing costs), protective casings for outdoor electronic devices (resisting performance degradation in humid-heat environments), and moisture-proof encapsulation for transformers (combining moisture resistance and heat tolerance).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPDM | Ethylene propylene diene monomer |
| EBT | Ethylene-butene-terpolymer |
| CNTs | Carbon nanotubes |
| MWCNT | Multi-walled carbon nanotube |
| MMT | Montmorillonite |
| SMMT | Stearic acid-modified montmorillonite |
| 0D/1D/2D | Zero-dimensional/one-dimensional/three-dimensional |
| ZnO | Zinc oxide |
| SA | Stearic acid |
| TMTD | Tetramethylthiuram disulfide |
| MDR | Rotorless rheometer |
| Tc10 | Scorch time |
| Tc90 | Optimal vulcanization time |
| ML | Minimum torque |
| MH | Maximum torque |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| D-RPA | Dynamic rubber processing analysis |
| DMA | Dynamic mechanical analysis |
| TGA | Thermogravimetric analysis |
| F-F | Fillers and Fillers |
| F-R | Fillers and Rubber |
References
- Mahmoud, D.S.; Said, A.A.; Abd El-Kader, A.E.; El-Sabbagh, S.H. Enhanced physicomechanical performance of nitrile rubber composites by utilizing eco-friendly natural oil as a plasticizer. J. Vinyl Addit. Technol. 2022, 28, 894–906. [Google Scholar] [CrossRef]
- Liang, R.; Yu, W.; Shen, J.; Zhang, Z.; Lin, Z.; Wang, J.; Jia, L.; Chen, F.; Shangguan, Y.; Zheng, Q. β-Crystals aided greater energy absorbing ethylene–propylene rubber in polypropylene blends for outstanding low-temperature toughness. Polym. Test. 2025, 142, 108670. [Google Scholar] [CrossRef]
- Bao, S.; Zhu, L.; Wang, H.; Luo, H.; Chen, F.; Yu, W.; Zhang, Z.; Zhuang, X.; Wu, Q.; Shangguan, Y.; et al. Synergistic effect of compound rubber and SiO2 nanoparticles on low-temperature toughening and balanced stiffness-toughness of random copolymer polypropylene nanocomposites. Compos. Sci. Technol. 2023, 241, 110210. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, S.; Ma, X. Expansion of high-performance specialty elastomers (I)—Ethylene propylene rubber, acrylate elastomers, and ethylene-butylene rubber. Rubber Ind. 2021, 68, 705–717. [Google Scholar]
- Xiao, C.; Tan, L.; Yu, H.; Chen, X.; Liu, Q. Preparation and performance of ternary ethylene-propylene rubber/trans-1,4-polybutadiene rubber/ethylene-vinyl acetate rubber composites for high-speed railway vibration isolation pads. Synth. Rubber Ind. 2022, 45, 380–385. [Google Scholar]
- Sayfo, P.; Pirityi, D.Z.; Pölöskei, K. Characterization of graphene-rubber nanocomposites: A review. Mater. Today Chem. 2023, 29, 101397. [Google Scholar] [CrossRef]
- Li, C.; Chen, H.; Zhang, L.; Zhong, J. Electrical properties of carbon nanotube/liquid metal/rubber nanocomposites. AIP Adv. 2020, 10, 102003. [Google Scholar] [CrossRef]
- Wu, C.M.; Hsieh, W.Y.; Cheng, K.B.; Lai, C.C.; Lee, K.C. Barrier properties of layered-silicate reinforced ethylene-propylenediene monomer/chloroprene rubber nanorubbers. Nanomaterials 2018, 8, 314. [Google Scholar] [CrossRef]
- Bakhshizade, A.; Ghasemi-Ghalebahman, A.; Hajimousa, M.A. Development of novel models for fatigue life assessment of natural rubber/styrene-butadiene rubber/nanoclay nanocomposite. Polym. Compos. 2024, 45, 1851–1871. [Google Scholar] [CrossRef]
- Polgar, L.M.; Criscitiello, F.; Van Essen, M.; Araya-Hermosilla, R.; Migliore, N.; Lenti, M.; Raffa, P.; Picchioni, F.; Pucci, A. Thermoreversibly cross-linked EPM rubber nanocomposites with carbon nanotubes. Nanomaterials 2018, 8, 58. [Google Scholar] [CrossRef]
- Jose, T.; Moni, G.; Raju, A.J.; George, J.J.; George, S.C. Multifunctional multi-walled carbon nanotube reinforced natural rubber nanocomposites. Ind. Crops Prod. 2017, 105, 63–73. [Google Scholar] [CrossRef]
- Kim, H.I.; Wang, M.; Lee, S.K.; Kang, J.; Nam, J.D.; Ci, L.; Suhr, J. Tensile properties of millimeter-long multi-walled carbon nanotubes. Sci. Rep. 2017, 7, 9512. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ramos, I.; Rosas, G.; López-Sosa, L.B.; Zárate-Medina, J. Dispersion of carbon nanotubes using nonylphenol commercial surfactant. Microsc. Microanal. 2019, 25, 2412–2413. [Google Scholar] [CrossRef]
- Ong, W.; Gao, X.; Gui, D.; Cai, X.; Tan, G.; Liu, J. Preparation of liquid crystal 4’-allyloxy-biphenyl-4-ol functionalized MWCNTs and their application on improving mechanical and thermal properties of silicon resin. Polym. Eng. Sci. 2016, 56, 1118–1124. [Google Scholar]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N.; et al. Mechanical performance and applications of CNTs reinforced polymer composites—A review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef]
- Frank, B.P.; Goodwin, D.G., Jr.; Bohutskyi, P.; Phan, D.C.; Lu, X.; Kuwama, L.; Bouwer, E.J.; Fairbrother, D.H. Influence of polymer type and carbon nanotube properties on carbon nanotube/polymer nanocomposite biodegradation. Sci. Total Environ. 2020, 742, 140512. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Kaminskii, V.V.; Iturralde, P.; Chumak, M.A. Advances in Electrically and Thermally Conductive Functional Nanocomposites Based on Carbon Nanotubes. Polymers 2025, 17, 71. [Google Scholar] [CrossRef]
- Posadas, P.; Bernal-Ortega, P.; Bernal, M.M.; Nogales, A.; Navarro, R.; Valentín, J.L. From nanoscale to macroscale characterization of sulfur-modified oxidized carbon nanotubes in styrene butadiene rubber compounds. ACS Omega 2024, 9, 31669–31683. [Google Scholar] [CrossRef]
- Kumar, S. Investigating effect of CNT agglomeration in CNT/polymer nanocomposites using multiscale finite element method. Mech. Mater. 2023, 184, 104706. [Google Scholar] [CrossRef]
- Pumchusak, J.; Tha, N.; Keawsujai, W.; Chaiwan, P. Effect of organo-modified montmorillonite nanoclay on mechanical, thermo-mechanical, and thermal properties of carbon fiber-reinforced phenolic composites. Polymers 2021, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J. Natural rubber/dendrimer modified montmorillonite nanocomposites: Mechanical and flame-retardant properties. Materials 2017, 11, 41. [Google Scholar] [CrossRef]
- Song, Y.; Lin, G.; Zhang, L.; Geng, C.; Li, Q.; Wang, H.; Liu, F.; Liang, Z.; Li, Y. Synergistic effect of hybrid montmorillonite materials on the wear resistance of natural rubber/butadiene rubber composites. J. Appl. Polym. Sci. 2022, 139, e52464. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Pan, L.; Xu, N.; Huang, H.; Li, T. Compatibilization of poly(lactic acid)/ethylene-propylene-diene rubber blends by using organic montmorillonite as a compatibilizer. J. Appl. Polym. Sci. 2016, 133, 46. [Google Scholar] [CrossRef]
- Cao, J.; Du, B.; Fan, Z. Effect of short-term high temperature on the microstructure and electrical properties of blended polypropylene cable insulation. Guangdong Electr. Power 2024, 37, 111–118. [Google Scholar]
- Fu, Q.; Peng, L.; Li, Z.; Lin, M.; Zhang, L.; Xie, S.; Hou, Y.; Kong, X.; Du, B. Development and challenges of environmentally friendly dry transformer insulation materials. Guangdong Electr. Power 2024, 37, 69–83. [Google Scholar]
- Liu, H.; Yang, L.; Liu, X.; Cao, J.P.; Zhang, J.; Luo, Z.; Gao, Z. Silicon dioxide nanoparticle decorated graphene with excellent dispersibility in natural rubber composites via physical mixing for application in green tires. Compos. Part B Eng. 2023, 258, 110700. [Google Scholar] [CrossRef]
- Damampai, K.; Pichaiyut, S.; Stöckelhuber, K.W.; Das, A.; Nakason, C. Ferric ions crosslinked epoxidized natural rubber filled with carbon nanotubes and conductive carbon black hybrid fillers. Polymers 2022, 14, 4392. [Google Scholar] [CrossRef]
- Siriwas, T.; Pichaiyut, S.; Susoff, M.; Petersen, S.; Nakason, C. Enhancing curing, mechanical, and electrical properties of epoxidized natural rubber nanocomposites with graphene and carbon nanotubes hybrid fillers. J. Mater. Sci. 2023, 58, 15676–15695. [Google Scholar] [CrossRef]
- Kitisavetjit, W.; Nakaramontri, Y.; Pichaiyut, S.; Wisunthorn, S.; Nakason, C.; Kiatkamjornwong, S. Influences of carbon nanotubes and graphite hybrid filler on properties of natural rubber nanocomposites. Polym. Test. 2020, 93, 106981. [Google Scholar] [CrossRef]
- Pichaiyut, S.; Kitisavetjit, W.; Nakason, C. Synergistic effects of graphite and carbon nanotube hybrid fillers on key properties of epoxidized natural rubber nanocomposites. J. Polym. Res. 2023, 30, 412. [Google Scholar] [CrossRef]
- Fernandes, F.M.; Ruiz-Hitzky, E. Assembling nanotubes and nanofibres: Cooperativeness in sepiolite–carbon nanotube materials. Carbon 2014, 72, 296–303. [Google Scholar] [CrossRef]
- Sun, M. Molecular Dynamics Simulations on Enhancement of Mechanical Properties of clay Using Carbon Nanotubes. Ph.D. Dissertation, Monash University, Clayton, Australia, 2014. [Google Scholar]
- Lan, Y.F.; Lin, J.J. Observation of carbon nanotube and clay micelle-like microstructures with dual dispersion properties. J. Phys. Chem. A 2009, 113, 8654–8659. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Q.; Mao, Y. Sonication-driven dispersion of multiwalled carbon nanotubes in water with the aid of Na+-montmorillonite. J. Dispers. Sci. Technol. 2022, 43, 598–604. [Google Scholar] [CrossRef]
- Ivanoska-Dacikj, A.; Bogoeva-Gaceva, G.; Bužarovska, A. Clay improved dispersion of carbon nanotubes in different solvents. Contributions Sec. Nat. Math. Biotech. Sci. MASA 2017, 36, 5–10. [Google Scholar] [CrossRef][Green Version]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Wießner, S.; Fischer, D.; Reuter, U.; Heinrich, G. ‘Expanded organoclay’ assisted dispersion and simultaneous structural alterations of multiwall carbon nanotube (MWCNT) clusters in natural rubber. Compos. Sci. Technol. 2015, 107, 36–43. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Li, D.; Sun, W.; Li, Z.; Liang, Y.; Fu, Q.; Tang, N.; Zhang, B.; Huang, F.; et al. Investigation of the Mechanical and Thermal Properties of MWCNT/SiC-Filled Ethylene–Butene–Terpolymer Rubber. Crystals 2025, 15, 539. [Google Scholar] [CrossRef]
- GB/T 16584; Determination of Curing Characteristics of Rubber by Rotorless Rheometer (S). National Standards of the People’s Republic of China: Beijing, China, 1996.
- GB/T 531.1; Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness—Part 1: Durometer Method (Shore Hardness) (S). National Standards of the People’s Republic of China: Beijing, China, 2008.
- GB/T 528; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties (S). National Standards of the People’s Republic of China: Beijing, China, 2009.
- GB/T 40396; Test Method for Glass Transition Temperature of Polymer Matrix Composites by Dynamic Mechanical Analysis (DMA) (S). National Standards of the People’s Republic of China: Beijing, China, 2021.
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–521. [Google Scholar] [CrossRef]
- Wang, M.; Yong, Z. Control of basic aggregate structure: Application and mechanism analysis in nitrile rubber composite materials. Polym. Compos. 2025, 46, 156–168. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Liu, A. Constructing conductive network using 1D and 2D conductive fillers in porous poly(aryl ether nitrile) for EMI shielding. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130414. [Google Scholar] [CrossRef]
- Wang, W.; Hou, G.; Zheng, Z.; Wang, L.; Liu, J.; Wu, Y.; Zhang, L.; Lyulin, A. Designing polymer nanocomposites with a semi-interpenetrating or interpenetrating network structure: Toward enhanced mechanical properties. Phys. Chem. Chem. Phys. 2017, 19, 15808–15820. [Google Scholar] [CrossRef]
- Tang, C.; Xiang, L.; Su, J.; Wang, K.; Yang, C.; Zhang, Q.; Fu, Q. Largely improved tensile properties of chitosan film via unique synergistic reinforcing effect of carbon nanotube and clay. J. Phys. Chem. B 2008, 112, 3876–3881. [Google Scholar] [CrossRef] [PubMed]


| Pure EBT | EBT/SMMT | EBT/CNT/SMMA-10 | EBT/CNT/SMMA-20 | |
|---|---|---|---|---|
| CNT/phr | 0 | 0 | 2 | 2 |
| SMMT/phr | 0 | 10 | 10 | 20 |
| ML (dNm) | MH (dNm) | MH-ML (dNm) | Tc10 (min) | Tc90 (min) | |
|---|---|---|---|---|---|
| Pure EBT | 0.12 | 12.30 | 12.18 | 4.02 | 12.66 |
| EBT/SMMT | 0.16 | 13.55 | 13.39 | 1.36 | 9.90 |
| EBT/CNT/SMMT-10 | 0.21 | 16.91 | 16.70 | 1.20 | 11.41 |
| EBT/CNT/SMMT-20 | 0.19 | 15.92 | 15.73 | 0.99 | 12.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, J.; Li, D.; Sun, W.; Li, Z.; Liang, Y.; Fu, Q.; Tang, N.; Zhang, B.; Huang, F.; et al. Enhancement of Ethylene-Butene Terpolymer Performance via Carbon Nanotube-Induced Nanodispersion of Montmorillonite Layers. Crystals 2025, 15, 612. https://doi.org/10.3390/cryst15070612
Zhang L, Liu J, Li D, Sun W, Li Z, Liang Y, Fu Q, Tang N, Zhang B, Huang F, et al. Enhancement of Ethylene-Butene Terpolymer Performance via Carbon Nanotube-Induced Nanodispersion of Montmorillonite Layers. Crystals. 2025; 15(7):612. https://doi.org/10.3390/cryst15070612
Chicago/Turabian StyleZhang, Li, Jianming Liu, Duanjiao Li, Wenxing Sun, Zhi Li, Yongchao Liang, Qiang Fu, Nian Tang, Bo Zhang, Fei Huang, and et al. 2025. "Enhancement of Ethylene-Butene Terpolymer Performance via Carbon Nanotube-Induced Nanodispersion of Montmorillonite Layers" Crystals 15, no. 7: 612. https://doi.org/10.3390/cryst15070612
APA StyleZhang, L., Liu, J., Li, D., Sun, W., Li, Z., Liang, Y., Fu, Q., Tang, N., Zhang, B., Huang, F., Fan, X., Wei, Y., Bai, P., & Wang, Y. (2025). Enhancement of Ethylene-Butene Terpolymer Performance via Carbon Nanotube-Induced Nanodispersion of Montmorillonite Layers. Crystals, 15(7), 612. https://doi.org/10.3390/cryst15070612






