Magnesia Partially Stabilized Zirconia/Hydroxyapatite Biocomposites: Structural, Morphological and Microhardness Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Mg-PSZ Preparation
2.2. HAP Preparation
2.3. Biocomposites Preparation
2.4. Characterization Methods
3. Results and Discussion
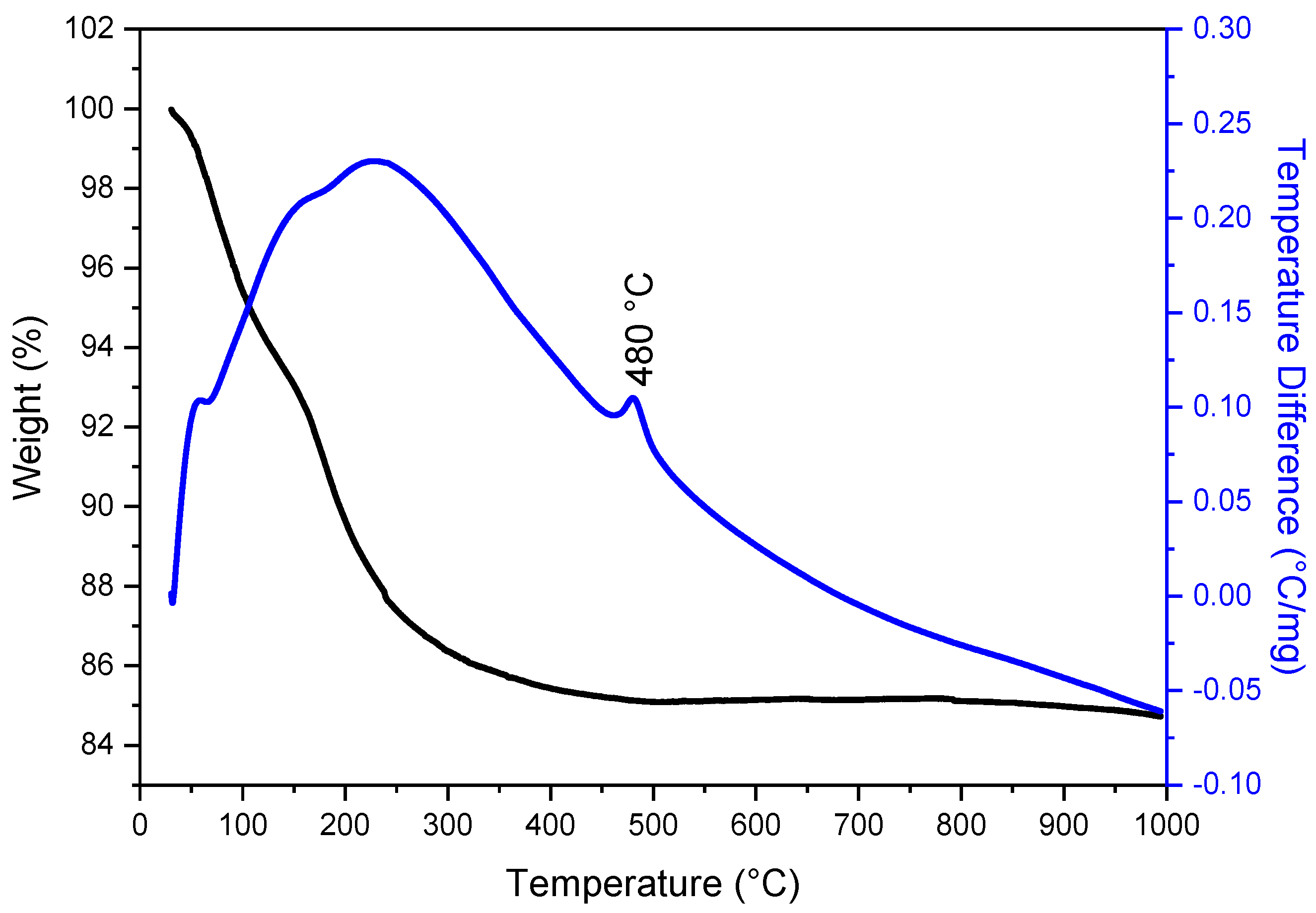
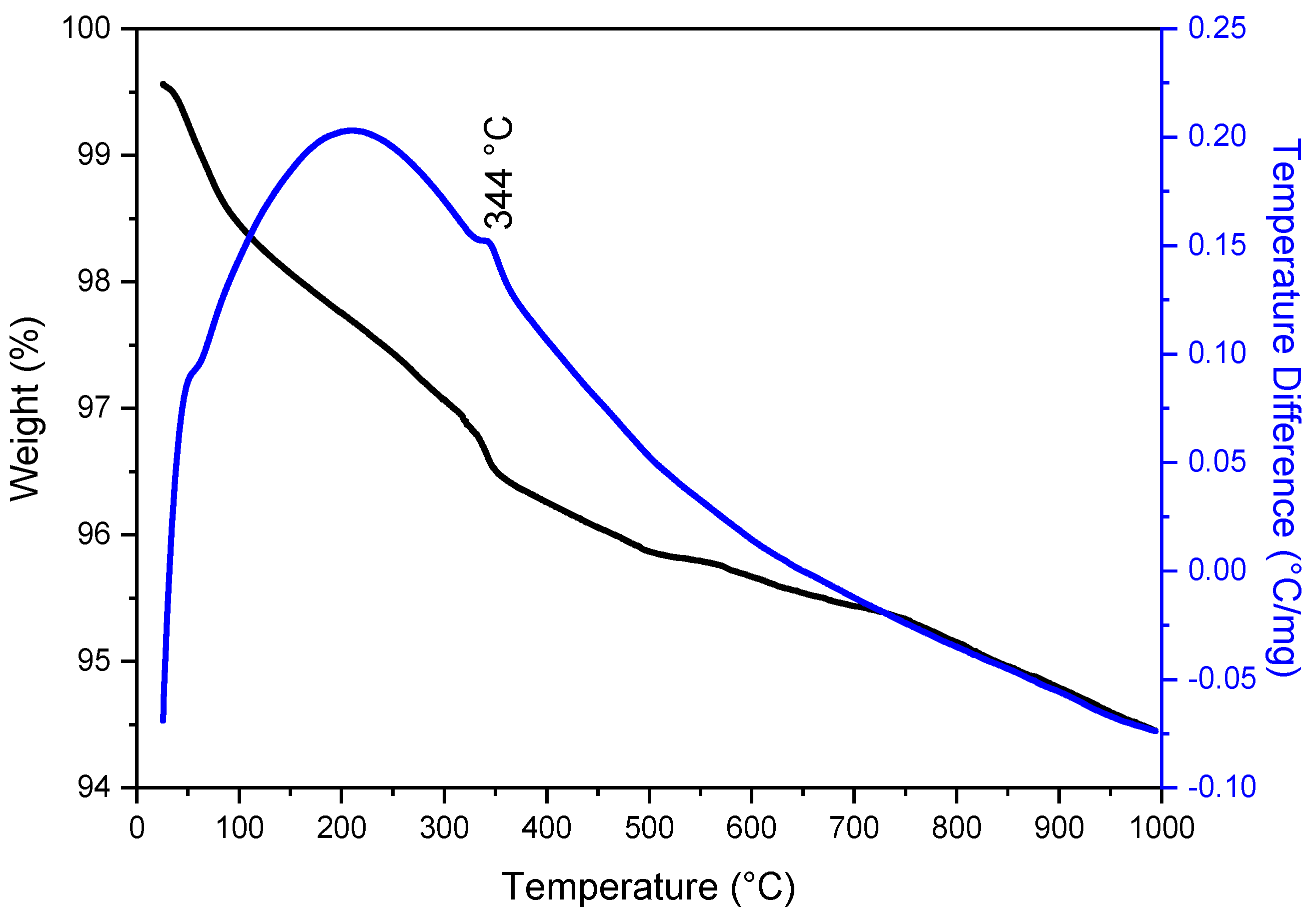
3.1. Thermal Analysis
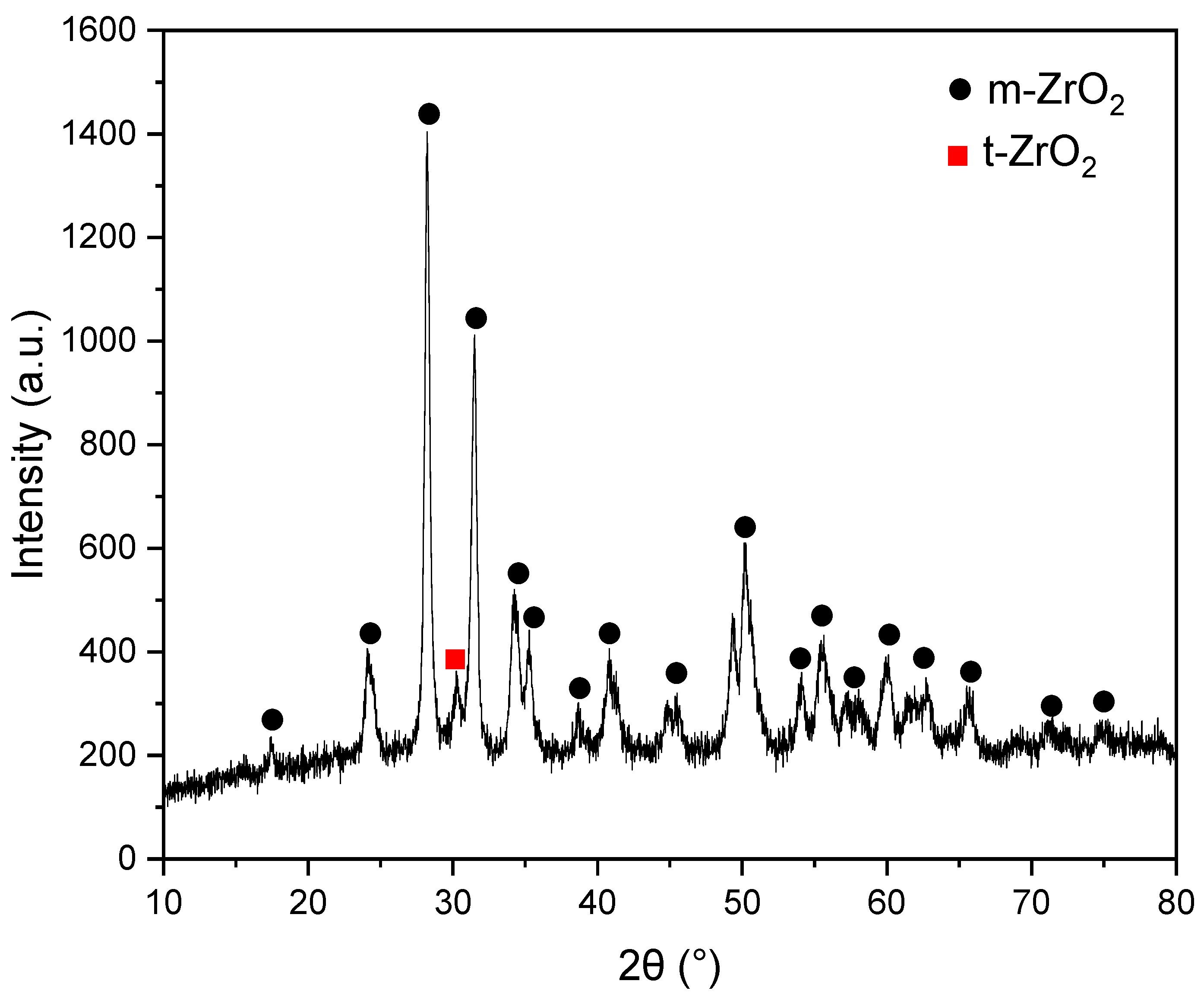
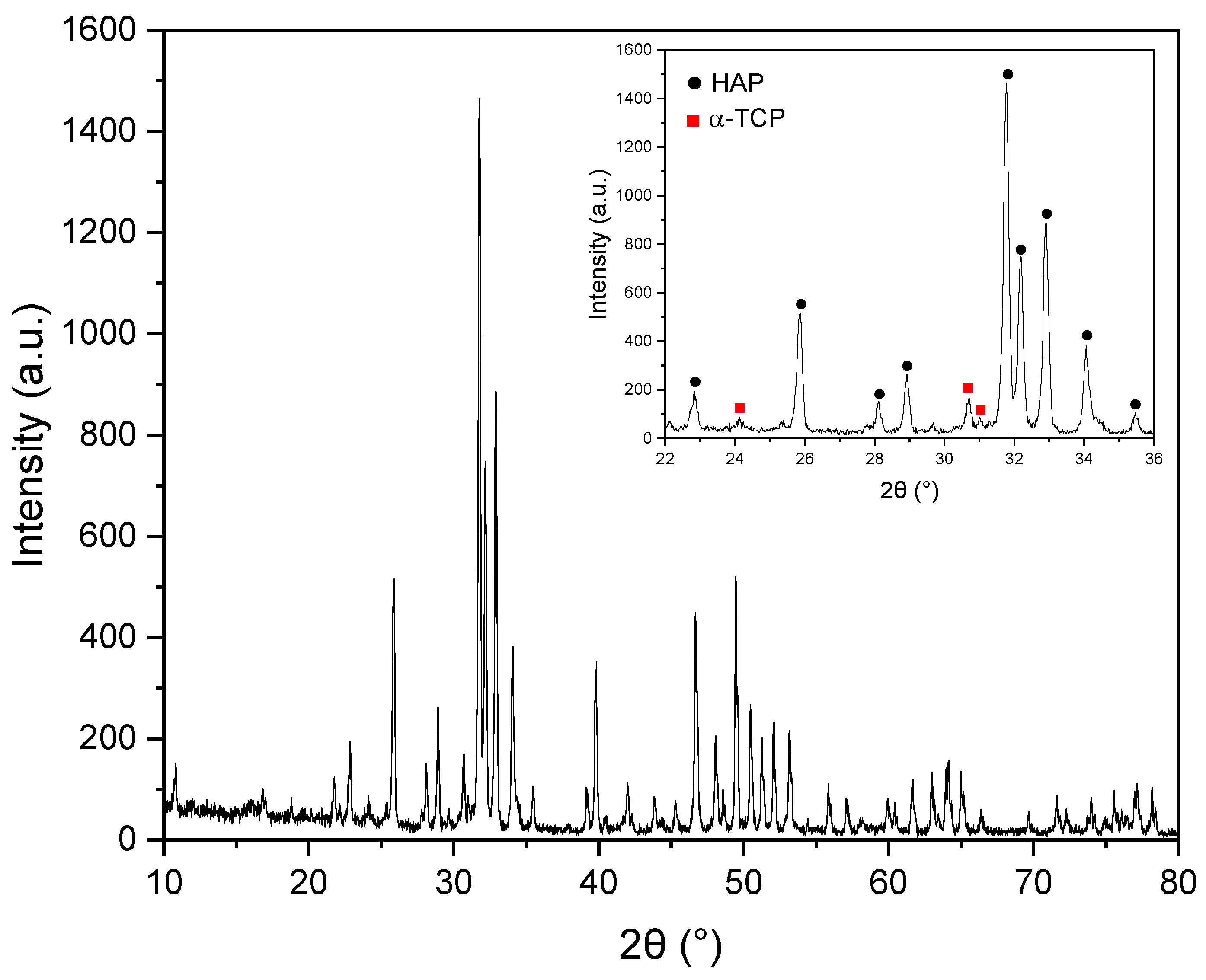
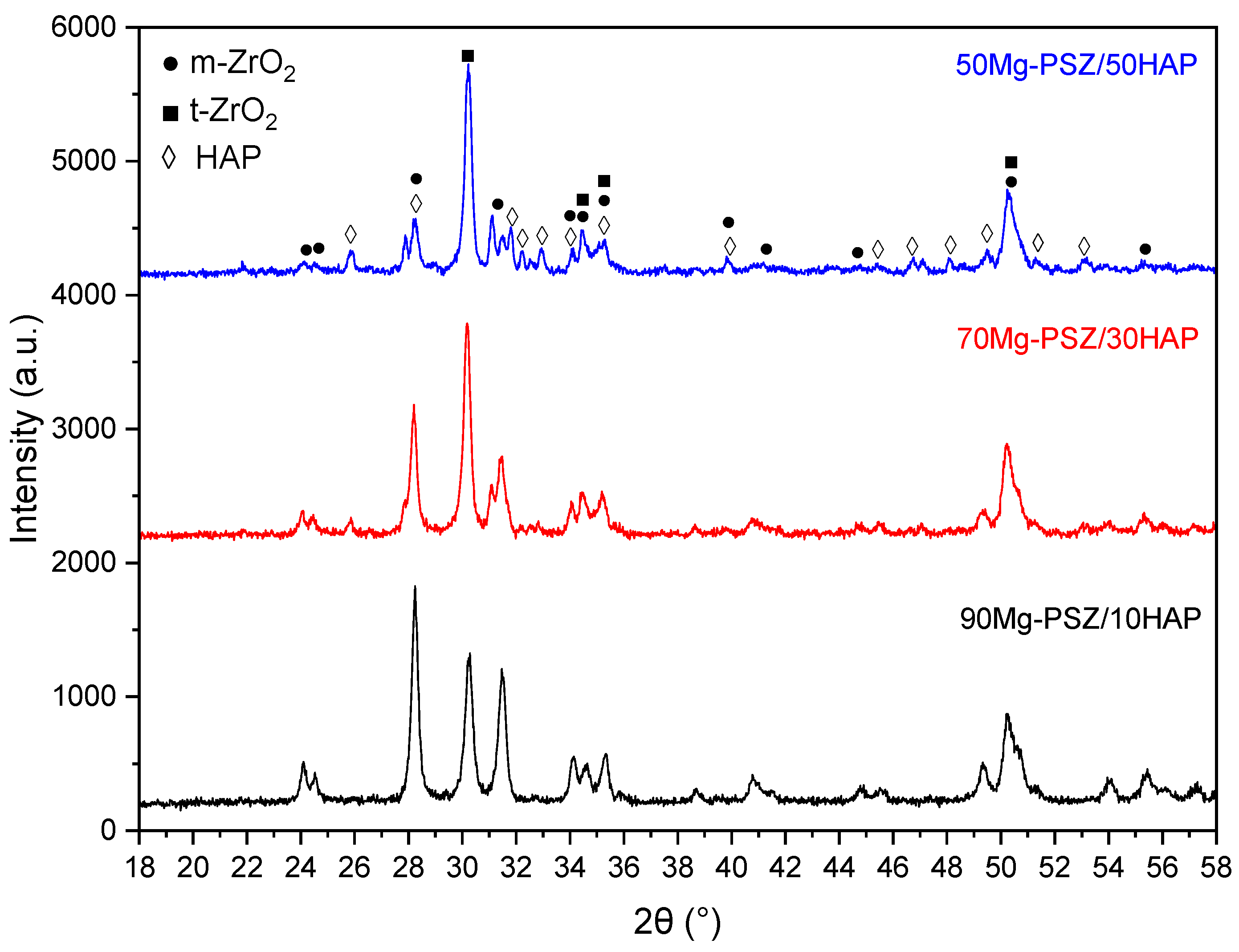
3.2. Structural Analyses
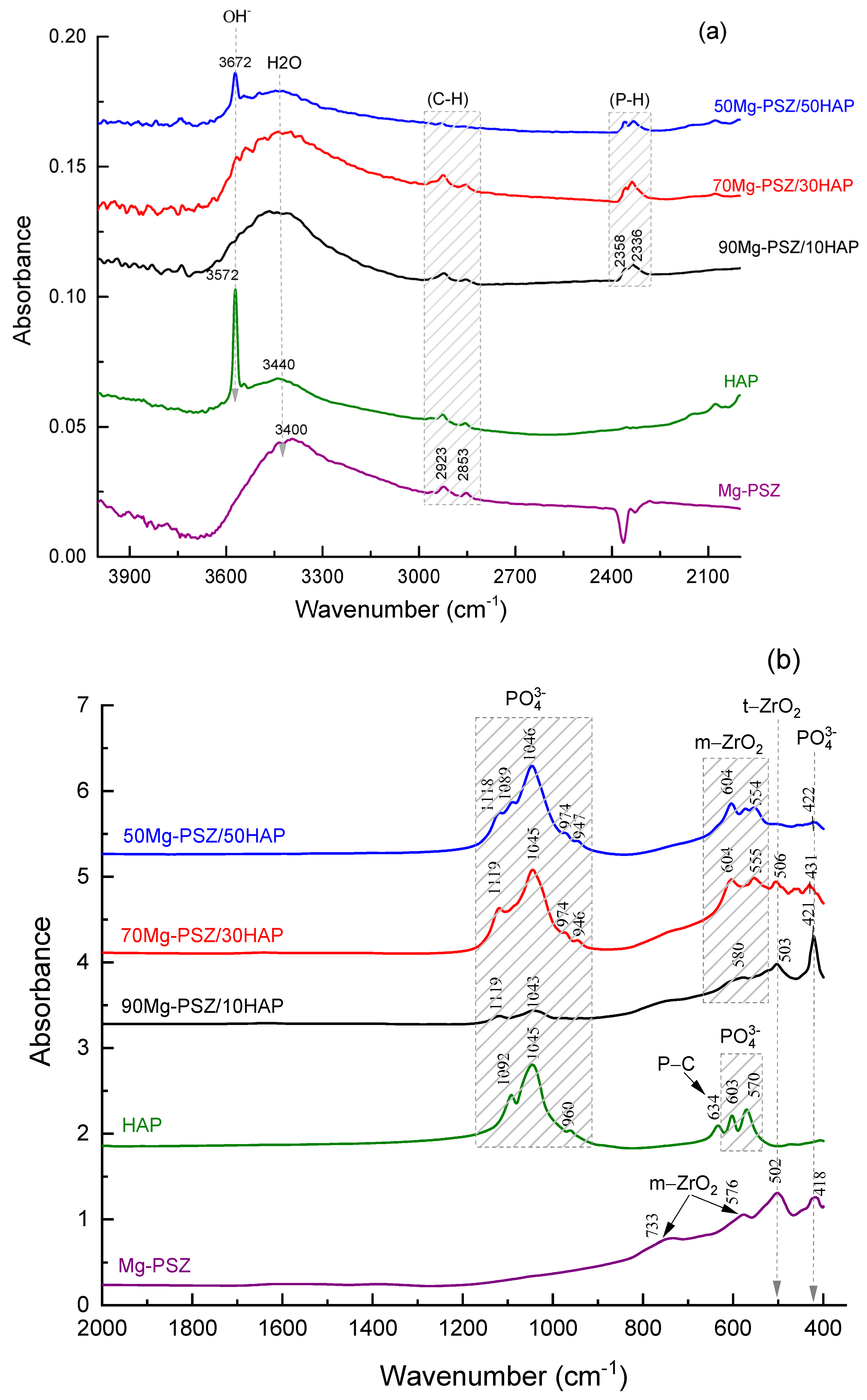
3.3. FTIR Spectroscopy Investigation
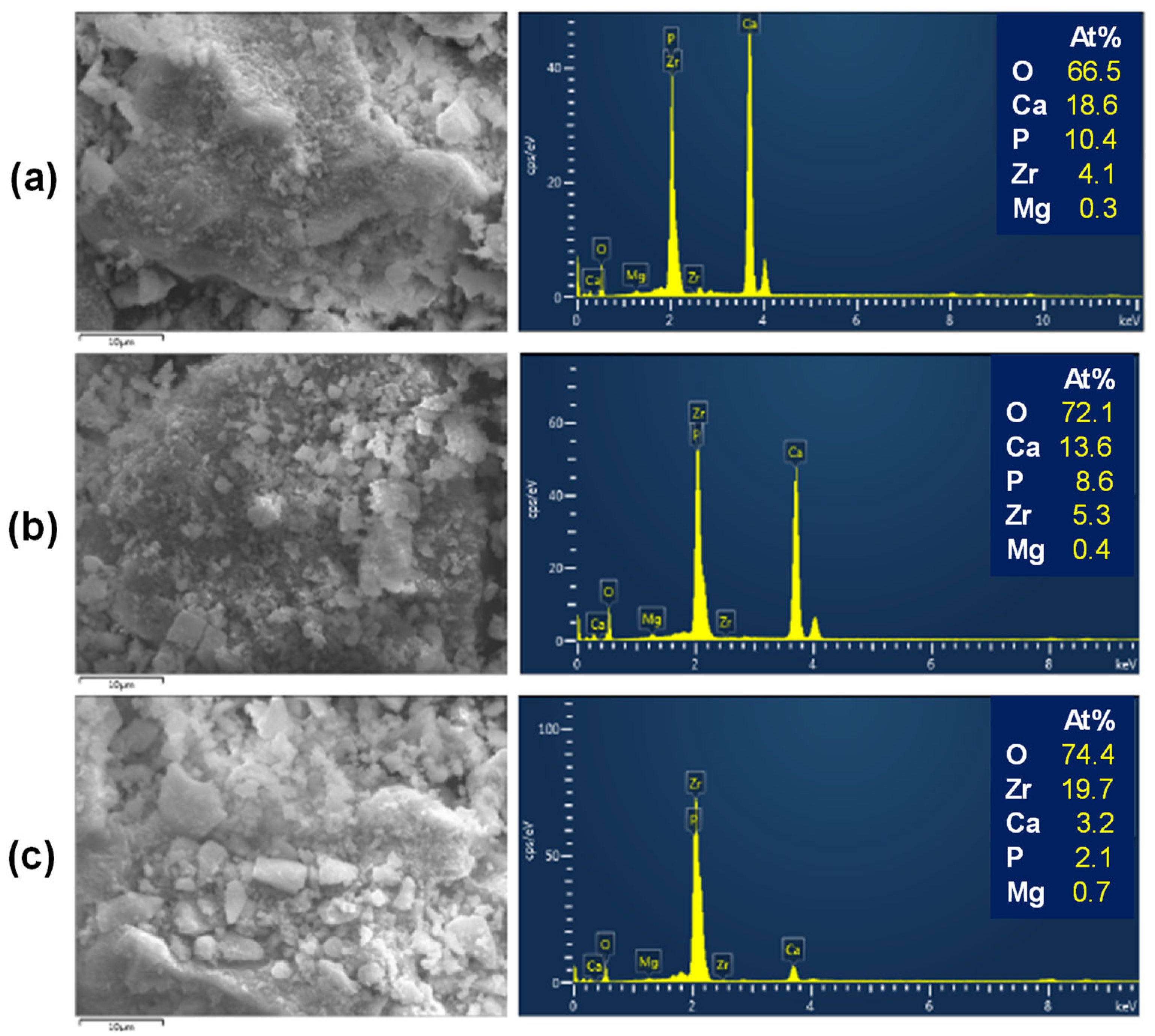
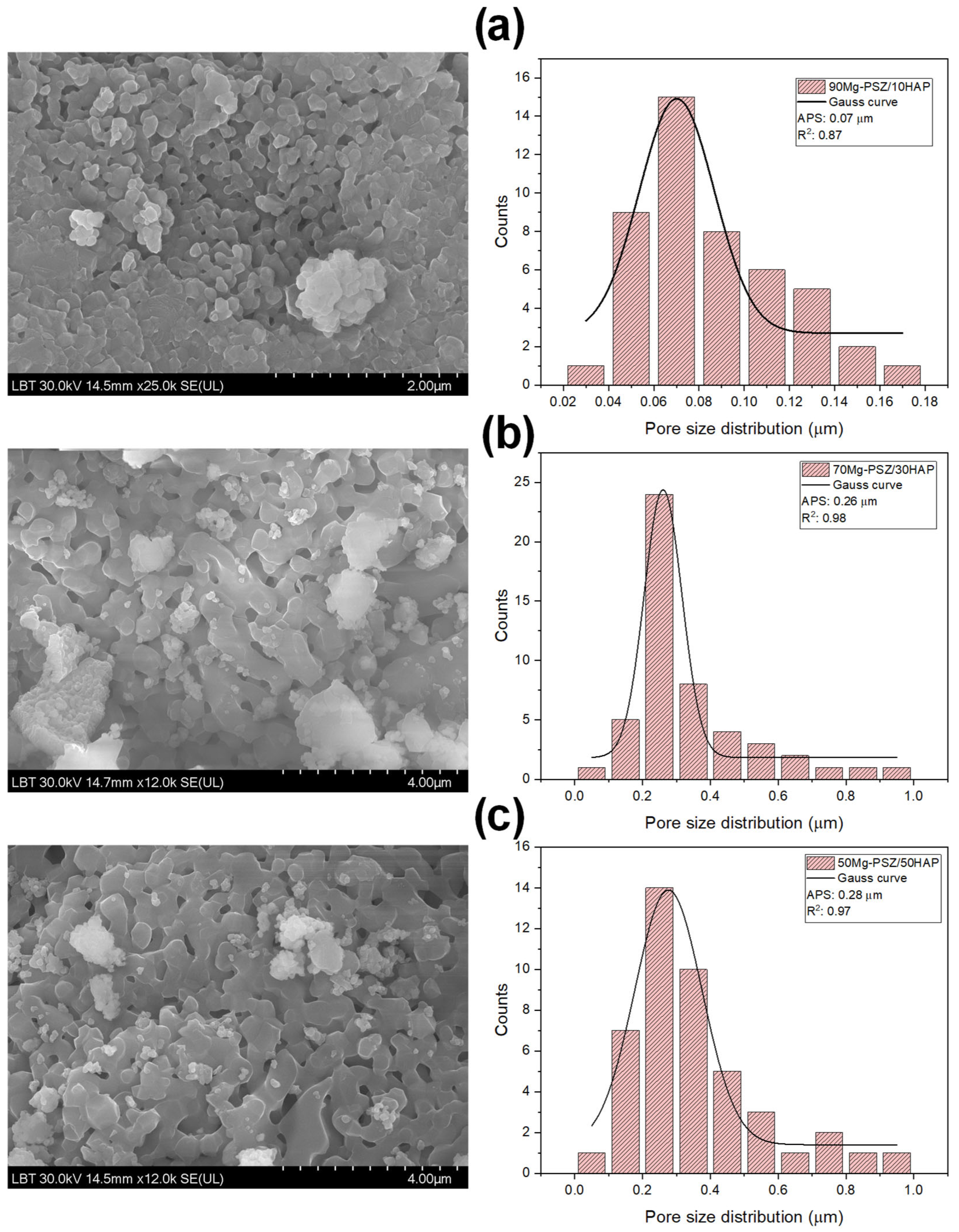
3.4. Morphological Analyses
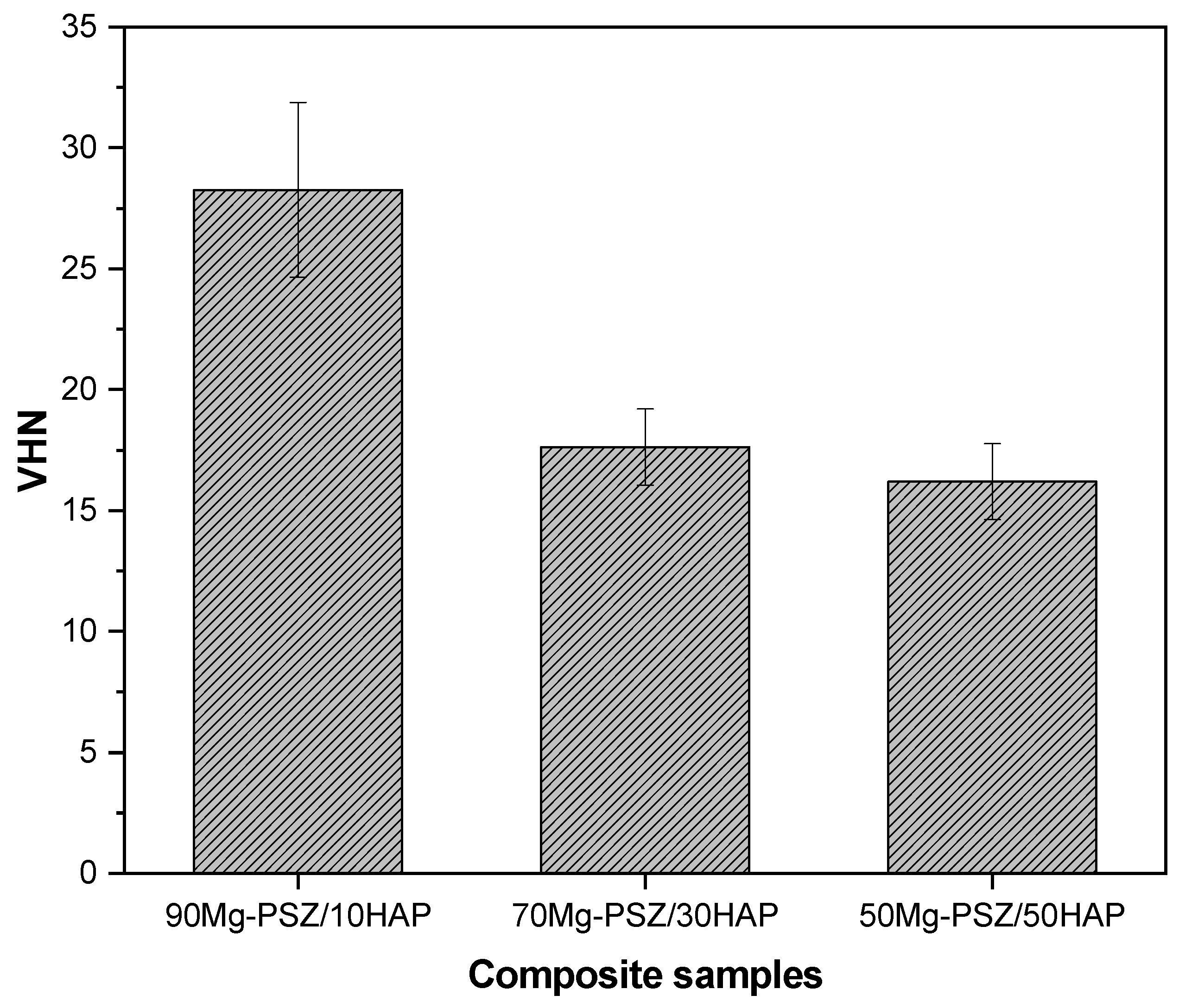
3.5. Vickers Microhardness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Xu, L.; Guo, M.; Li, Z.; Xu, Z.; Ye, J.; Li, W.; Wei, S. Effects of calcination temperature on grain growth and phase transformation of nano-zirconia with different crystal forms prepared by hydrothermal method. J. Mater. Res. Technol. 2022, 19, 4003–4017. [Google Scholar] [CrossRef]
- Guo, M.; Wang, G.; Zhao, Y.; Li, H.; Tang, K.; Zhao, Y.; Burgess, K. Preparation of Nano-ZrO2 powder via a microwave-assisted hydrothermal method. Ceram. Int. 2021, 47, 12425–12432. [Google Scholar] [CrossRef]
- Hussain, A.; Gautam, C.; Jafri, A.; Mishra, V.K.; Madheshiya, A.; Gautam, A.; Singh, M.K.; Gautam, R.K.; Gupta, M.; Arshad, M.; et al. Formation of multifunctional ZrO2–MgO-hBN nanocomposite for enhanced bone regeneration and E coli bacteria filtration applications. Ceram. Int. 2020, 46, 23006–23020. [Google Scholar] [CrossRef]
- Ngashangua, S.; Vasanthavel, S.; Ponnilavan, V.; Kannan, S. Effect of MgO additions on the phase stability and degradation ability in ZrO2-Al2O3 composite systems. Ceram. Int. 2015, 41, 3814–3821. [Google Scholar] [CrossRef]
- Keerthana, L.; Sakthivel, C.; Prabha, I. MgO-ZrO2 mixed nanocomposites: Fabrication methods and applications. Mater. Today Sustain 2019, 3–4, 100007. [Google Scholar] [CrossRef]
- Bizo, L.; Mureşan-Pop, M.; Barabás, R.; Barbu-Tudoran, L.; Berar, A. In Vitro Degradation of Mg-Doped ZrO2 Bioceramics at the Interface with Xerostom® Saliva Substitute Gel. Materials 2023, 16, 2680. [Google Scholar] [CrossRef] [PubMed]
- Cojan, C.A.; Barabás, R.; Mureșan-Pop, M.; Bizo, L. Comparative Phase Evolution, Morphological and Optical Analysis of Partially Stabilized Zirconia Ceramics. Studia UBB Chem. 2024, LXIX, 7–17. [Google Scholar] [CrossRef]
- Godwin, E.R.; Kumar I, P.A.; Mathows, J.; Govindasamy, C.; Al-Numair, K.S.; Sana, S.S.; Chandrasekaran, K.; Arulselvan, P. Biomedical potential of green-engineered chitosan-magnesium oxide nanoparticles: An in vitro study on antibacterial and anticancer activities. Ceram. Int. 2024, 50, 39775–39786. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium Oxide (MgO) Nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Cho, J.; Yang, B.; Shen, C.; Wang, H.; Zhang, X. Micromechanical properties and microstructure evolution of magnesia partially stabilized zirconia prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2023, 43, 1098–1107. [Google Scholar] [CrossRef]
- Mysore, T.H.M.; Patil, A.Y.; Hegde, C.; Sudeept, M.A.; Kumar, R.; Soudagar, M.E.M.; Fattah, I.M.R. Apatite insights: From synthesis to biomedical applications. Eur. Polym. J. 2024, 209, 112842. [Google Scholar] [CrossRef]
- Liu, C.; Xu, M.; Wang, Y.; Yin, Q.; Hu, J.; Chen, H.; Sun, Z.; Liu, C.; Li, X.; Zhou, W.; et al. Exploring the potential of hydroxyapatite-based materials in biomedicine: A comprehensive review. Mater. Sci. Eng. R Rep. 2024, 161, 100870. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 27, 9721. [Google Scholar] [CrossRef]
- Liu, W.; Cheong, N.; He, Z.; Zhang, T. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. J. Funct. Biomater. 2025, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, V.S.; Paramonova, E.V.; Avakyan, L.A.; Eremina, N.V.; Makarova, S.V.; Bulina, N.V. Effect of Magnesium Substitution on Structural Features and Properties of Hydroxyapatite. Materials 2023, 16, 5945. [Google Scholar] [CrossRef] [PubMed]
- Rehner, A.M.G.; Moldoveanu, E.-T.; Niculescu, A.-G.; Bîclesanu, F.C.; Pangică, A.M.; Grumezescu, A.M.; Croitoru, G.-A. Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings. J. Compos. Sci. 2025, 9, 140. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials-A Systematic Review of the Current Scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Slósarczyk, A.; Paszkiewicz, Z. Mechanical properties of HAp–ZrO2 composites. J. Eur. Ceram. Soc. 2006, 26, 1481–1488. [Google Scholar] [CrossRef]
- Barabás, R.; Fort, C.I.; Turdean, G.L.; Bizo, L. Influence of HAP on the Morpho-Structural Properties and Corrosion Resistance of ZrO2-Based Composites for Biomedical Applications. Crystals 2021, 11, 202. [Google Scholar] [CrossRef]
- Antoniac, I. Bioceramics and Biocomposites: From Research to Clinical Practice; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Fan, H.; Wang, L.; Dou, R. Properties of zirconia-doped hydroxyapatite ceramics prepared by vat photopolymerization additive manufacturing technology and microwave sintering. Ceram. Int. 2025. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Liu, S.; Dong, X.; Li, Y.; Zhang, H.; Yang, Z.; Su, Q.; Huang, W.; Zheng, W.; et al. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mat. Sci. Eng. C-Mater. 2019, 105, 110054. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Pang, Y.; Li, E.; Zhang, J.Y.; Xu, D. Preparation and characterisation of zirconia/hydroxyapatite bioactive composites as potential dental implants. J. Mater. Sci. Mater. Eng. 2024, 19, 43. [Google Scholar] [CrossRef]
- Sivaperumal, V.R.; Mani, R.; Polisetti, V.; Aruchamy, K.; Oh, T. Synthesis of Hydroxyapatite (HAp)-Zirconia Nanocomposite Powder and Evaluation of Its Biocompatibility: An In Vitro Study. Appl. Sci. 2022, 12, 11056. [Google Scholar] [CrossRef]
- Mohammad, M.; Taha, S.; Shahatha, A. Preparation of Hydroxyapatite/Zirconia Porous Composites via Polymeric Sponge Method and Study the Physical and Bioactivity Properties. IOP Conf. Ser. Mater. Sci. Eng. 2020, 757, 012046. [Google Scholar] [CrossRef]
- Zaw, L.H.; Nurazreena, A.; Aye Aye, T.; Ahmad-Fauzi, M.N. Characterization of CaO-ZrO2 Reinforced Hap Biocomposite for Strength and Toughness Improvement. Procedia Chem. 2016, 19, 510–516. [Google Scholar] [CrossRef]
- Yeongjun, S.; Shiori, N.; Tomoyo, G.; Sunghun, C.; Tohru, S. Densification of hydroxyapatite/zirconia nanocomposites fabricated via low-temperature mineralization sintering process and their mechanical properties. Sci. Rep. 2025, 15, 2479. [Google Scholar] [CrossRef]
- Gautam, A.; Gautam, C.; Mishra, M.; Sahu, S.; Nanda, R.; Kisan, B.; Gautam, R.K.; Prakash, R.; Sharma, K.; Singh, D.; et al. Synthesis, structural, mechanical, and biological properties of HAp-ZrO2-hBN biocomposites for bone regeneration applications. Ceram. Int. 2021, 47, 30203–30220. [Google Scholar] [CrossRef]
- Es-saddik, M.; Laasri, S.; Taha, M.; Laghzizil, A.; Guidara, A.; Chaari, K.; Bouaziz, J.; Hajjaji, A.; Nunzi, J.M. Effect of the surface chemistry on the stability and mechanical properties of the Zirconia-Hydroxyapatite bioceramic. Surf. Interfaces 2021, 23, 100980. [Google Scholar] [CrossRef]
- Kumar, V.A.; Rama Murty Raju, P.; Ramanaiah, N.; Siriyala, R. Effect of ZrO2 content on the mechanical properties and microstructure of HAp/ZrO2 nanocomposites. Ceram. Int. 2018, 44, 10345–10351. [Google Scholar] [CrossRef]
- Ayoub, G.; Veljovic, D.; Lezaja Zebic, M.; Miletic, V.; Palcevskis, E.; Petrovic, R.; Janackovic, D. Composite nanostructured hydroxyapatite/yttrium stabilized zirconia dental inserts-The processing and application as dentin substitutes. Ceram. Int. 2018, 44, 18200–18208. [Google Scholar] [CrossRef]
- Matsumoto, T.J.; An, S.-H.; Ishimoto, T.; Nakano, T.; Matsumoto, T.; Imazato, S. Zirconia-hydroxyapatite composite material with micro porous structure. Dent. Mater. 2011, 27, e205–e212. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhai, Y.; Li, X.; Li, Y.; Wang, K. Coprecipitation Synthesis of MgO-Doped ZrO2 Nano Powder. J. Am. Ceram. Soc. 2006, 89, 3577–3581. [Google Scholar] [CrossRef]
- Yuan, L.; Xiang, D.; Yu, J. Effect of solvents on the properties of co-precipitated MgO-ZrO2 nano powders. J. Ceram. Process. Res. 2013, 14, 517–520. [Google Scholar] [CrossRef]
- Barabás, R.; de Souza Ávila, E.; Ladeira, L.O.; Mosqueira Antônio, L.; Tötös, R.; Simedru, D.; Bizo, L.; Cadar, O. Graphene Oxides/Carbon Nanotubes–Hydroxyapatite Nanocomposites for Biomedical Applications. Arab. J. Sci. Eng. 2020, 45, 219–227. [Google Scholar] [CrossRef]
- Barabás, R.; Deemter, D.; Katona, G.; Batin, G.; Barabás, L.; Bizo, L.; Cadar, O. Comparative study on physicochemical and mechanical characterization of new nanocarbon-based hydroxyapatite nanocomposites. Turk. J. Chem. 2019, 43, 809–824. [Google Scholar] [CrossRef]
- Barabás, R.; Czikó, M.; Dékány, I.; Bizo, L.; Bogya, E.S. Comparative study of particle size analysis of hydroxyapatite-based nanomaterials. Chem. Pap. 2013, 67, 1414–1423. [Google Scholar] [CrossRef]
- Bizo, L.; Sabo, K.; Barábas, R.; Katona, G.; Barbu-Tudoran, L.; Berar, A. Structural, morphological and dissolution properties of ZrO2-based biocomposites for dental applications. Studia UBB Chem. 2020, 1, 137–148. [Google Scholar] [CrossRef]
- Murray, M.G.S.; Wang, J.; Ponton, C.B.; Marquis, P.M. An improvement in processing of hydroxyapatite ceramics. J. Mater. Sci. 1995, 30, 3061–3074. [Google Scholar] [CrossRef]
- Ficai, A.; Albu, M.G.; Birsan, M.; Sonmez, M.; Ficai, D.; Trandafir, V.; Andronescu, E. Collagen hydrolysate based collagen/hydroxyapatite composite materials. J. Mol. Struct. 2013, 1037, 154–159. [Google Scholar] [CrossRef]
- Senra, M.R.; Barbosa de Lima, R.; de Holanda Saboya Souza, D.; de Fátima Vieira Marques, M.; Neves Monteiro, S. Thermal characterization of hydroxyapatite or carbonated hydroxyapatite hybrid composites with distinguished collagens for bone graft. J. Mater. Res. Technol. 2020, 9, 7190–7200. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Baev, S.G.; Matvienko, A.A.; Gerasimov, K.B.; Logutenko, O.A.; Bystrov, V.S. A Study of Thermal Stability of Hydroxyapatite. Minerals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Jamil, M.; Elouahli, A.; Abida, F.; Assaoui, J.; Gourri, E.; Hatim, Z. Apatitic calcium phosphate/montmorillonite nano-biocomposite: In-situ synthesis, characterization and dissolution properties. Heliyon 2022, 8, e10042. [Google Scholar] [CrossRef] [PubMed]
- Kantana, W.; Jarupoom, P.; Pengpat, K.; Eitssayeam, S.; Tunkasiri, T.; Rujijanagul, G. Properties of hydroxyapatite/zirconium oxide nanocomposites. Ceram. Int. 2013, 39, S379–S382. [Google Scholar] [CrossRef]
- Hashimoto, K.; Baba, M.; Shibata, H.; Yoshida, K. Enhanced sinterability and mechanical strength of beta-type tricalcium phosphates ceramics through reaction sintering with hydroxyapatite. J. Eur. Ceram. Soc. 2025, 45, 117023. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Youn, H.-J.; Hong, K.S.; Chang, B.-S.; Lee, C.-K.; Chung, S.-S. An improvement in sintering property of β-tricalcium phosphate by addition of calcium pyrophosphate. Biomaterials 2002, 23, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Allam, M.A.; Moharram, M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 83, 56–60. [Google Scholar] [CrossRef]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Iwasa, M.; Kotobuki, N.; Tanaka, T.; Hirose, M.; Ohgushi, H.; Jiang, D. Fabrication of Hydroxyapatite-Zirconia Composites for Orthopedic Applications. J. Am. Ceram. Soc. 2006, 89, 3348–3355. [Google Scholar] [CrossRef]
| Sample ID | Mg-PSZ (wt.%) | HAP (wt.%) |
|---|---|---|
| 90Mg-PSZ/10HAP | 90 | 10 |
| 70Mg-PSZ/30HAP | 70 | 30 |
| 50Mg-PSZ/50HAP | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizo, L.; Bot, A.-L.; Mureșan-Pop, M.; Barbu-Tudoran, L.; Cojan, C.A.; Barabás, R. Magnesia Partially Stabilized Zirconia/Hydroxyapatite Biocomposites: Structural, Morphological and Microhardness Properties. Crystals 2025, 15, 608. https://doi.org/10.3390/cryst15070608
Bizo L, Bot A-L, Mureșan-Pop M, Barbu-Tudoran L, Cojan CA, Barabás R. Magnesia Partially Stabilized Zirconia/Hydroxyapatite Biocomposites: Structural, Morphological and Microhardness Properties. Crystals. 2025; 15(7):608. https://doi.org/10.3390/cryst15070608
Chicago/Turabian StyleBizo, Liliana, Adriana-Liana Bot, Marieta Mureșan-Pop, Lucian Barbu-Tudoran, Claudia Andreea Cojan, and Réka Barabás. 2025. "Magnesia Partially Stabilized Zirconia/Hydroxyapatite Biocomposites: Structural, Morphological and Microhardness Properties" Crystals 15, no. 7: 608. https://doi.org/10.3390/cryst15070608
APA StyleBizo, L., Bot, A.-L., Mureșan-Pop, M., Barbu-Tudoran, L., Cojan, C. A., & Barabás, R. (2025). Magnesia Partially Stabilized Zirconia/Hydroxyapatite Biocomposites: Structural, Morphological and Microhardness Properties. Crystals, 15(7), 608. https://doi.org/10.3390/cryst15070608









