Diamond-like Cage Motifs in {Cu6(StBu)4} Complexes with Pyridines
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. SCXRD
2.3. DFT Calculations
2.4. Preparation of “{Cux(StBu)y}” Stock Solution
2.5. Synthesis of [Cu6(StBu)4(2-Me-py)5(CH3CN)(NO3)](NO3) (1)
2.6. Synthesis of [Cu6(StBu)4(Me3py)4(NO3)2]·3.5CH3CN (2a) and [Cu6(StBu)4(Me3py)5(NO3)](NO3)·5CH3CN (2b)
2.7. Synthesis of (NHEt3)[Cu6(StBu)4(CH3CN)3(NO3)3]·H2O (3)
2.8. Synthesis of [Cu6(StBu)4(2-Br-py)4(NO3)2]·2-Br-py (4)
2.9. Synthesis of [Cu6(StBu)4(3-Br-py)6][Cu6(StBu)4(CH3CN)6](NO3)4·9CH3CN (5)
2.10. Synthesis of [Cu6(StBu)4(3-Cl-py)6][Cu6(StBu)4(CH3CN)6](NO3)4·5CH3CN (6)
3. Results and Discussion
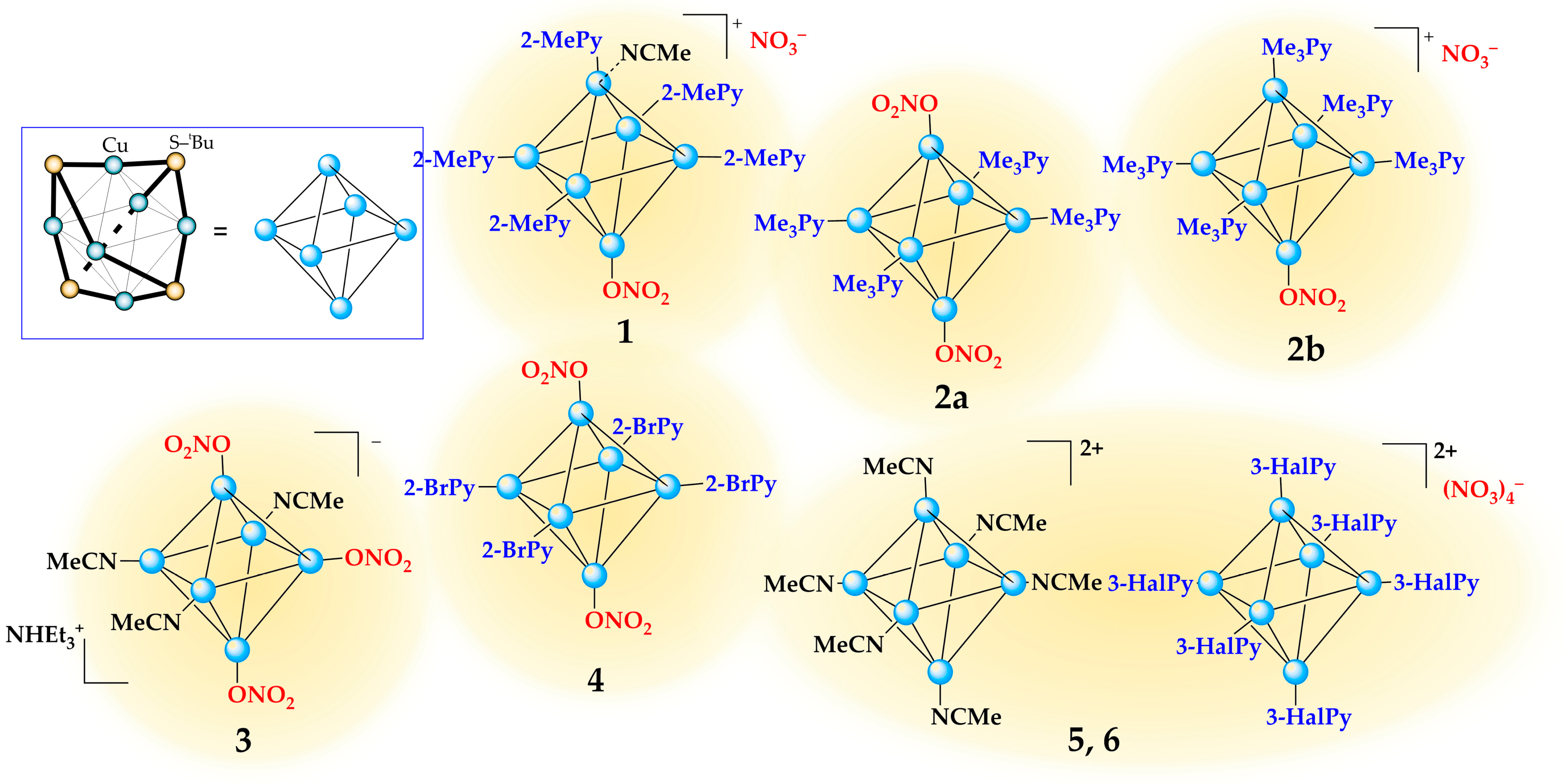
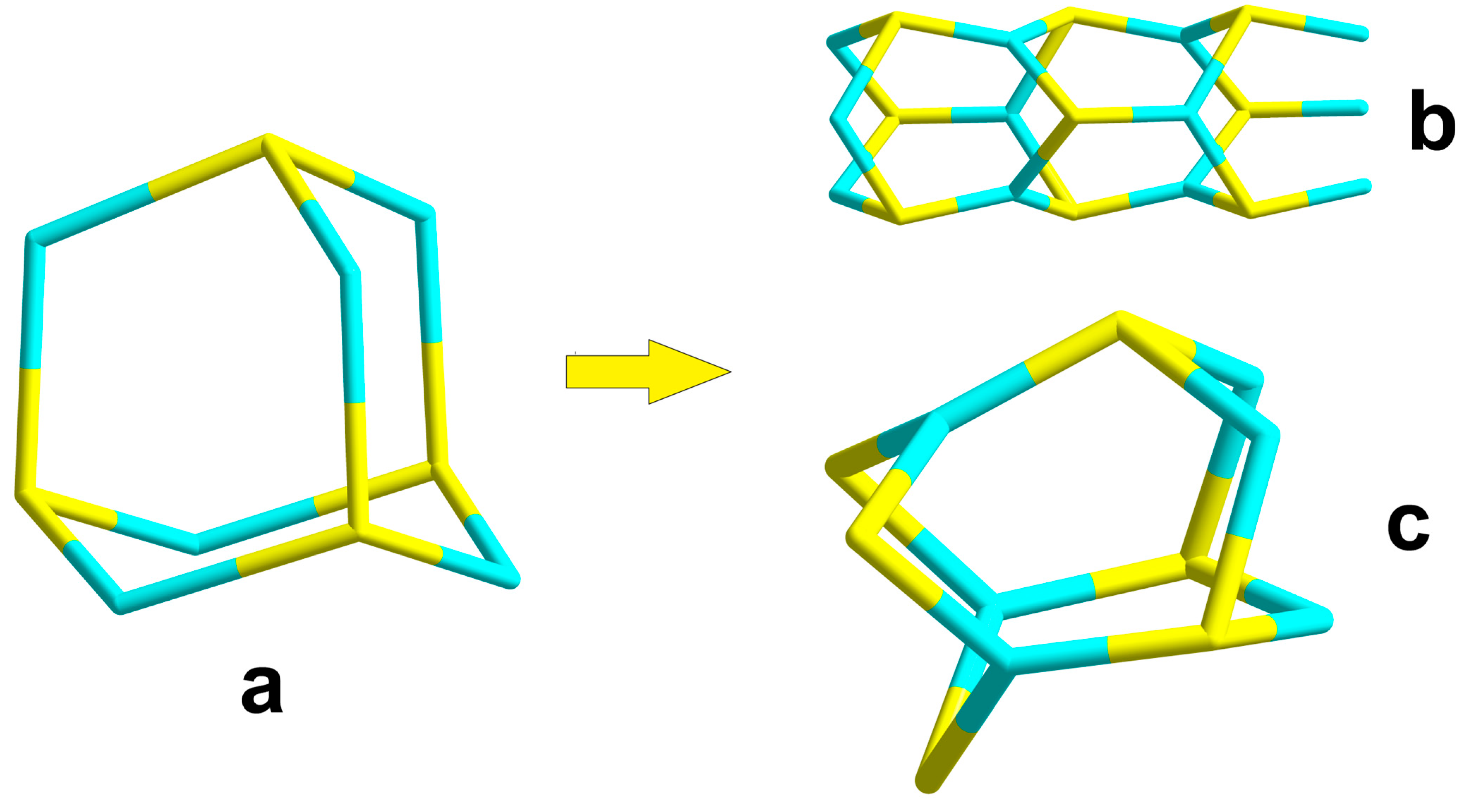
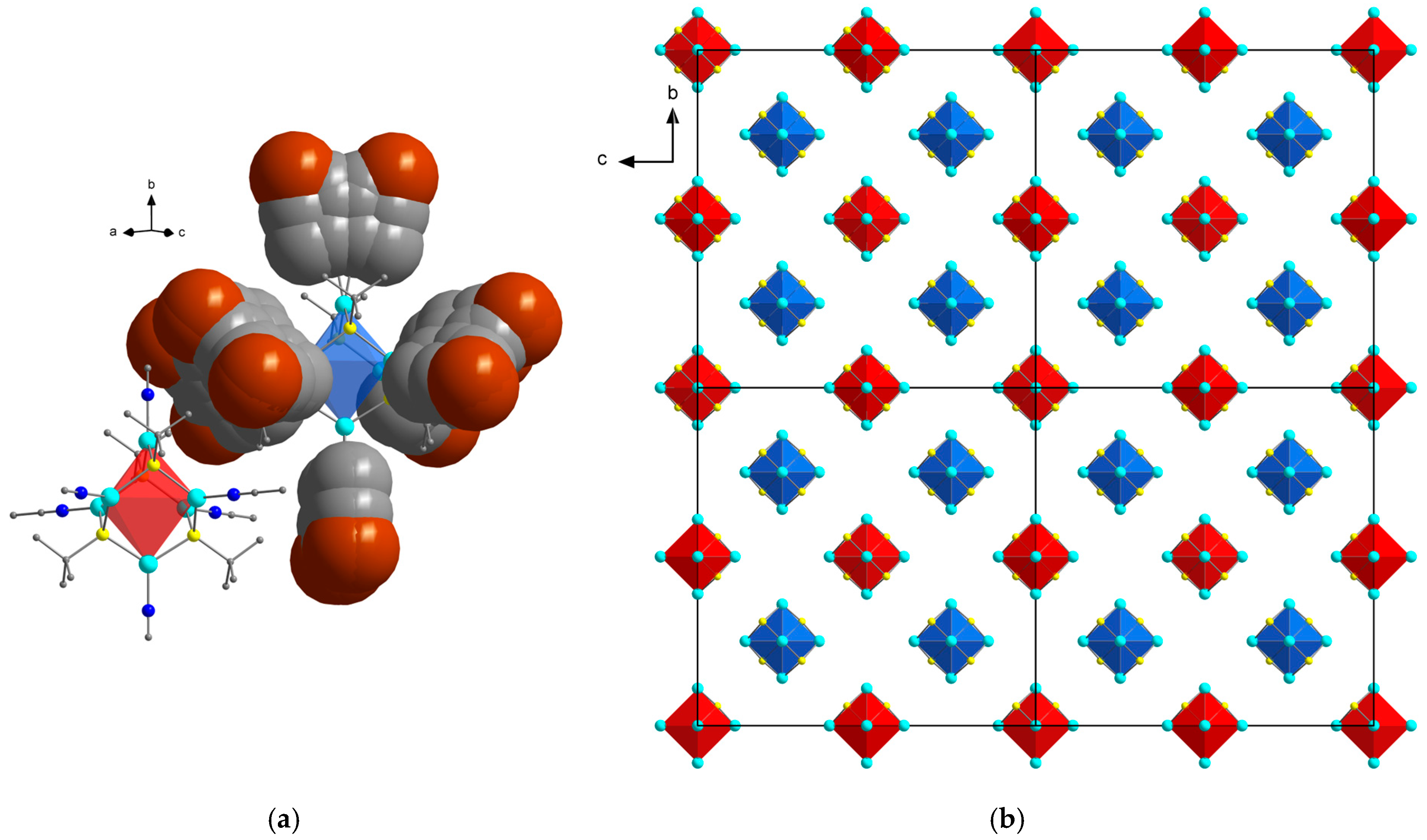
3.1. Synthesis and Crystal Structure
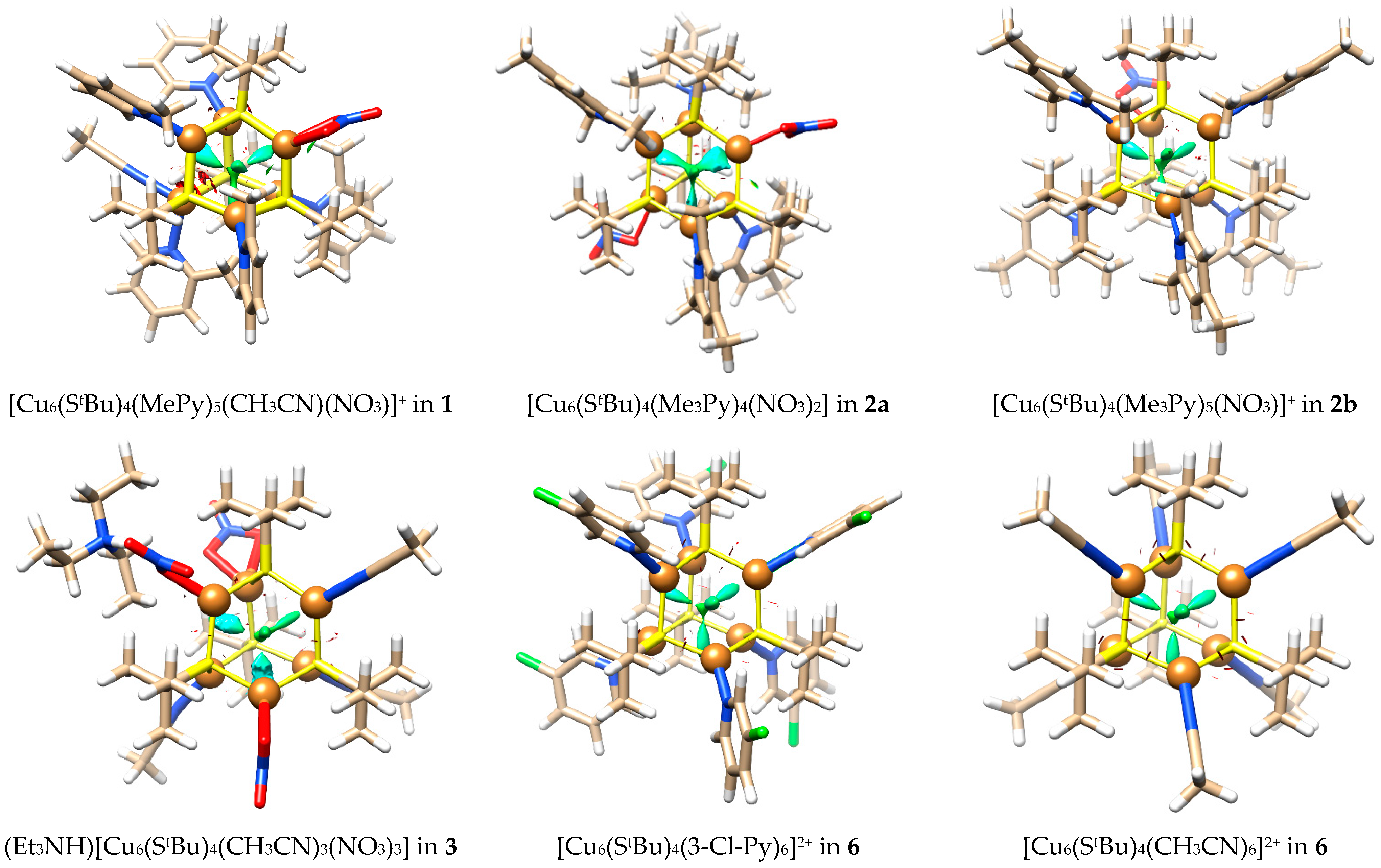
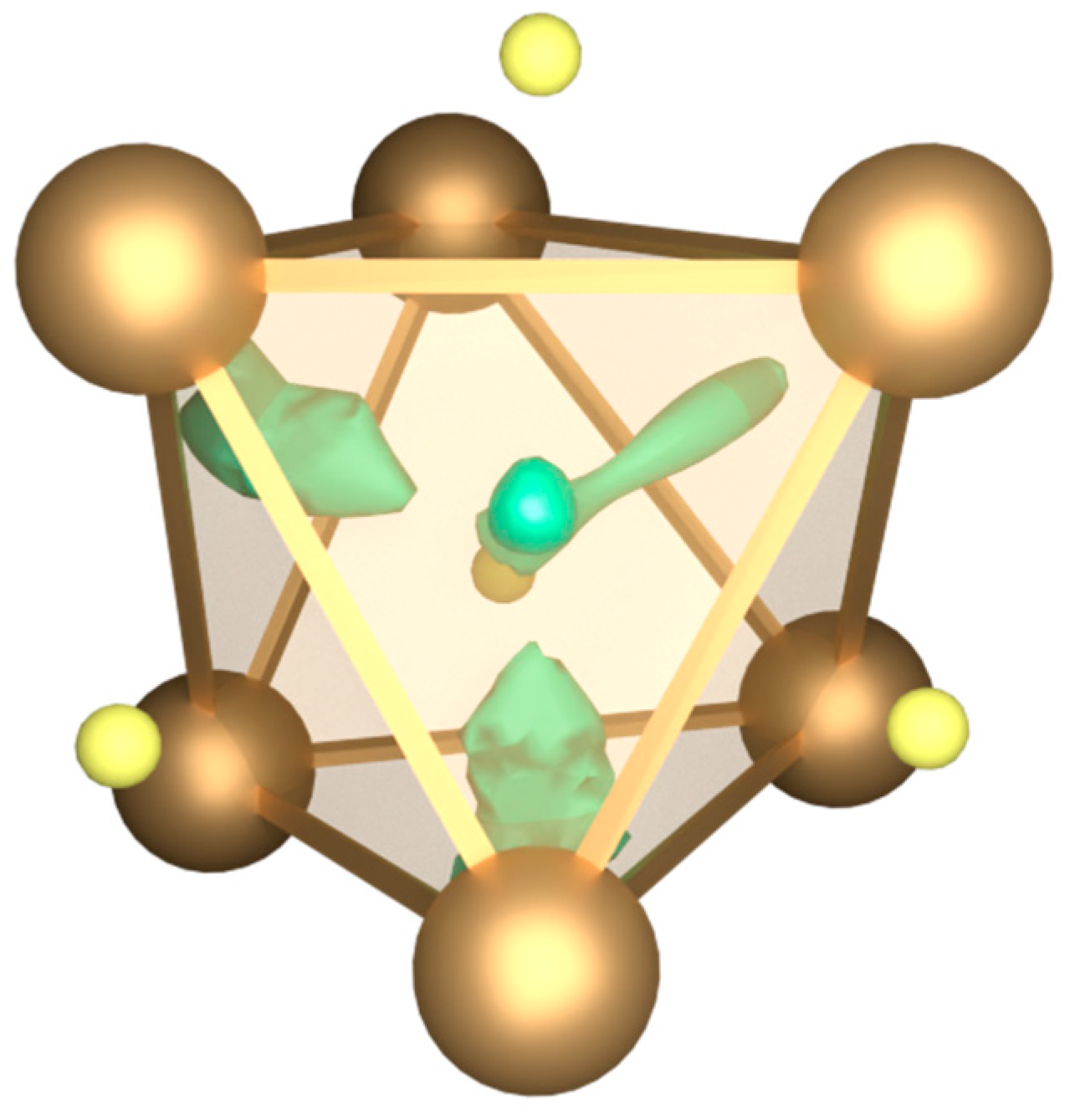
3.2. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCXRD | Single Crystal X-ray Diffraction |
| QTAIM | Quantum Theory of Atoms in Molecules |
| NCI | Noncovalent Interactions |
| RDG | Reduced Density Gradient |
| RCP | Ring Critical Point |
| CCP | Cage Critical Point |
References
- Wang, Z.; Gupta, R.K.; Luo, G.; Sun, D. Recent Progress in Inorganic Anions Templated Silver Nanoclusters: Synthesis, Structures and Properties. Chem. Rec. 2020, 20, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Baghdasaryan, A.; Bürgi, T. Copper nanoclusters: Designed synthesis, structural diversity, and multiplatform applications. Nanoscale 2021, 13, 6283–6340. [Google Scholar] [CrossRef]
- Liu, L.-J.; Zhang, M.-M.; Deng, Z.; Yan, L.-L.; Lin, Y.; Phillips, D.L.; Yam, V.W.-W.; He, J. NIR-II emissive anionic copper nanoclusters with intrinsic photoredox activity in single-electron transfer. Nat. Commun. 2024, 15, 4688. [Google Scholar] [CrossRef]
- Sagadevan, A.; Murugesan, K.; Bakr, O.M.; Rueping, M. Copper nanoclusters: Emerging photoredox catalysts for organic bond formations. Chem. Commun. 2024, 60, 13858–13866. [Google Scholar] [CrossRef]
- Brocha Silalahi, R.P.; Jo, Y.; Liao, J.; Chiu, T.; Park, E.; Choi, W.; Liang, H.; Kahlal, S.; Saillard, J.; Lee, D.; et al. Hydride-containing 2-Electron Pd/Cu Superatoms as Catalysts for Efficient Electrochemical Hydrogen Evolution. Angew. Chem. Int. Ed. 2023, 62, e202301272. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, M.; Palladino, P.; Scarano, S.; Minunni, M. Copper nanoclusters and their application for innovative fluorescent detection strategies: An overview. Sens. Actuators Rep. 2022, 4, 100108. [Google Scholar] [CrossRef]
- Skvortsova, S.V.; Verkhov, F.K.; Nikolaenkova, E.B.; Rakhmanova, M.I.; Kokina, T.E.; Sukhikh, T.S.; Shekhovtsov, N.A.; Bushuev, M.B. Interplay of the Cu⋯Cu distance and coordination geometry as a factor affecting the quantum efficiency in dimeric copper(i) halide complexes with derivatives of 4-pyrazolylpyrimidine-2-thiol. Dalton Trans. 2025, 54, 9000–9015. [Google Scholar] [CrossRef]
- Liao, P.; Shi, D.; Liao, J.; Liu, C.W.; Artem’ev, A.V.; Kuimov, V.A.; Gusarova, N.K.; Trofimov, B.A. Facile Self-Assembly Synthesis and Characterization of Diselenophosphinato Octanuclear Cu I Clusters Inscribed in a Twelve-Vertex Selenium Polyhedron. Eur. J. Inorg. Chem. 2012, 2012, 4921–4929. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Liu, C.W. Recent progress in dichalcophosphate coinage metal clusters and superatoms. Chem. Commun. 2023, 59, 7182–7195. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Doronina, E.P.; Rakhmanova, M.I.; Hei, X.; Stass, D.V.; Tarasova, O.A.; Bagryanskaya, I.Y.; Samsonenko, D.G.; Novikov, A.S.; Nedolya, N.A.; et al. A family of CuI-based 1D polymers showing colorful short-lived TADF and phosphorescence induced by photo-and X-ray irradiation. Dalton Trans. 2023, 52, 4017–4027. [Google Scholar] [CrossRef]
- Davydova, M.P.; Berezin, A.S.; Samsonenko, D.G.; Artem’ev, A.V. Cu(I) complexes designed on 2-pyrimidylphosphine and 1,4-dicyanobenzene: Synthesis and thermally activated delayed fluorescence. Inorg. Chim. Acta 2021, 521, 120347. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Rakhmanova, M.I.; Hei, X.; Samsonenko, D.G.; Stass, D.V.; Bagryanskaya, I.Y.; Ryzhikov, M.R.; Fedin, V.P.; Li, J.; Artem’ev, A.V. A new subclass of copper(i) hybrid emitters showing TADF with near-unity quantum yields and a strong solvatochromic effect. Chem. Commun. 2023, 59, 2923–2926. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Lin, Y.-F.; Nain, A.; Huang, Y.-F.; Chang, H.-T. A critical review of copper nanoclusters for monitoring of water quality. Sens. Actuators Rep. 2021, 3, 100026. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, Z.; Zhao, Y.; Yu, M.; Jiang, F.; Chen, L.; Hong, M. A copper(I) thiolate coordination polymer with thermochromic and mechanochromic luminescence. Inorg. Chem. Commun. 2022, 140, 109432. [Google Scholar] [CrossRef]
- Veselska, O.; Podbevšek, D.; Ledoux, G.; Fateeva, A.; Demessence, A. Intrinsic triple-emitting 2D copper thiolate coordination polymer as a ratiometric thermometer working over 400 K range. Chem. Commun. 2017, 53, 12225–12228. [Google Scholar] [CrossRef]
- Liang, X.-Q.; Gupta, R.K.; Li, Y.-W.; Ma, H.-Y.; Gao, L.-N.; Tung, C.-H.; Sun, D. Structural Diversity of Copper(I) Cluster-Based Coordination Polymers with Pyrazine-2-thiol Ligand. Inorg. Chem. 2020, 59, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Y.; Zhang, L.; He, W.-M.; Yang, L.; Zhang, C.; Wang, Z.-Y.; Zhang, R.; Chen, J.-H.; Wang, S.; Zang, S.-Q.; et al. High-performance primary explosives derived from copper thiolate cluster-assembled materials for micro-initiating device. Chem. Eng. J. 2020, 389, 124455. [Google Scholar] [CrossRef]
- Liu, C.W.; Staples, R.J.; Fackler, J.P. Copper(I) 1,1-dithiolate cluster transformations. Synthesis of [Bu4N]6[Cu6(S,i-MNT)6], i-MNT=[S2CC(CN)2]−, from [Bu4N]4[Cu8(i-MNT)6] with sulfur. Reaction of the cyclic hexanuclear complex with phosphine to give the tetrahedral [Bu4N]4[Cu4(i-MNT)4] which. Coord. Chem. Rev. 1998, 174, 147–177. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Lam, C.-H.; Fung, W.K.-M.; Cheung, K.-K. Syntheses, Photophysics, and Photochemistry of Trinuclear Copper(I) Thiolate and Hexanuclear Copper(I) Selenolate Complexes: X-ray Crystal Structures of [Cu6(μ-dppm)4(μ3-SePh)4](BF4)2 and [Cu6{μ-(Ph2P)2NH}4(μ3-SePh)4](BF4)2. Inorg. Chem. 2001, 40, 3435–3442. [Google Scholar] [CrossRef]
- Baghdasaryan, A.; Besnard, C.; Lawson Daku, L.M.; Delgado, T.; Burgi, T. Thiolato Protected Copper Sulfide Cluster with the Tentative Composition Cu74S15(2-PET)45. Inorg. Chem. 2020, 59, 2200–2208. [Google Scholar] [CrossRef]
- Bühler, R.; Wolf, R.M.; Gemel, C.; Stephan, J.; Deger, S.N.; Kahlal, S.; Fischer, R.A.; Saillard, J.-Y. Cuprophilic Interactions in Polymeric [Cu10O2(Mes)6]n. Inorg. Chem. 2024, 63, 17617–17625. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Filippova, E.A.; Petrov, P.A.; Sukhikh, T.S.; Sokolov, M.N.; Abramov, P.A. Crystal structure of [Cu3(StBu)3]n. Zhurnal Struct. Khimii 2025, 66, 147696. [Google Scholar]
- Volchek, V.V.; Berezin, A.S.; Sokolov, M.N.; Abramov, P.A. Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies. Inorganics 2022, 10, 225. [Google Scholar] [CrossRef]
- Maiti, B.K.; Pal, K.; Sarkar, S. Flexible Cu I –Thiolate Clusters with Relevance to Metallothioneins. Eur. J. Inorg. Chem. 2007, 2007, 5548–5555. [Google Scholar] [CrossRef]
- Dance, I.G.; Guerney, P.J.; Rae, A.D.; Scudder, M.L. Planar bridging thiolate in (Ph3P)2Cu(μ-SPh)2Cu(PPh3)2. Inorg. Chem. 1983, 22, 2883–2887. [Google Scholar] [CrossRef]
- Gao, X.; He, S.; Zhang, C.; Du, C.; Chen, X.; Xing, W.; Chen, S.; Clayborne, A.; Chen, W. Single Crystal Sub-Nanometer Sized Cu6(SR)6 Clusters: Structure, Photophysical Properties, and Electrochemical Sensing. Adv. Sci. 2016, 3, 1600126. [Google Scholar] [CrossRef] [PubMed]
- Morozov, I.V.; Serezhkin, V.N.; Troyanov, S.I. Modes of coordination and stereochemistry of the NO3− anions in inorganic nitrates. Russ. Chem. Bull. 2008, 57, 439–450. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, T.S.; Sokolov, M.N.; Abramov, P.A. Diamond-like Cage Motifs in {Cu6(StBu)4} Complexes with Pyridines. Crystals 2025, 15, 607. https://doi.org/10.3390/cryst15070607
Sukhikh TS, Sokolov MN, Abramov PA. Diamond-like Cage Motifs in {Cu6(StBu)4} Complexes with Pyridines. Crystals. 2025; 15(7):607. https://doi.org/10.3390/cryst15070607
Chicago/Turabian StyleSukhikh, Taisiya S., Maxim N. Sokolov, and Pavel A. Abramov. 2025. "Diamond-like Cage Motifs in {Cu6(StBu)4} Complexes with Pyridines" Crystals 15, no. 7: 607. https://doi.org/10.3390/cryst15070607
APA StyleSukhikh, T. S., Sokolov, M. N., & Abramov, P. A. (2025). Diamond-like Cage Motifs in {Cu6(StBu)4} Complexes with Pyridines. Crystals, 15(7), 607. https://doi.org/10.3390/cryst15070607








