The Effect of Spark Current Tuning on the Formation of Cu Nanoparticles Synthesized by Spark Ablation in Nitrogen Atmosphere
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- -
- The XRD spectra consist of broad peaks where we assume that nitride and oxide compounds coexist; in particular, two contributions from the Cu3N nitride phase are detected at 2θ = 22.6° (100) and 2θ = 40° (111).
- -
- The Raman analysis shows good agreement with the XRD results, recording peaks at a Raman shift assigned to the Cu-N stretching mode and additionally to the characteristic vibration mode of the monoclinic CuO crystal.
- -
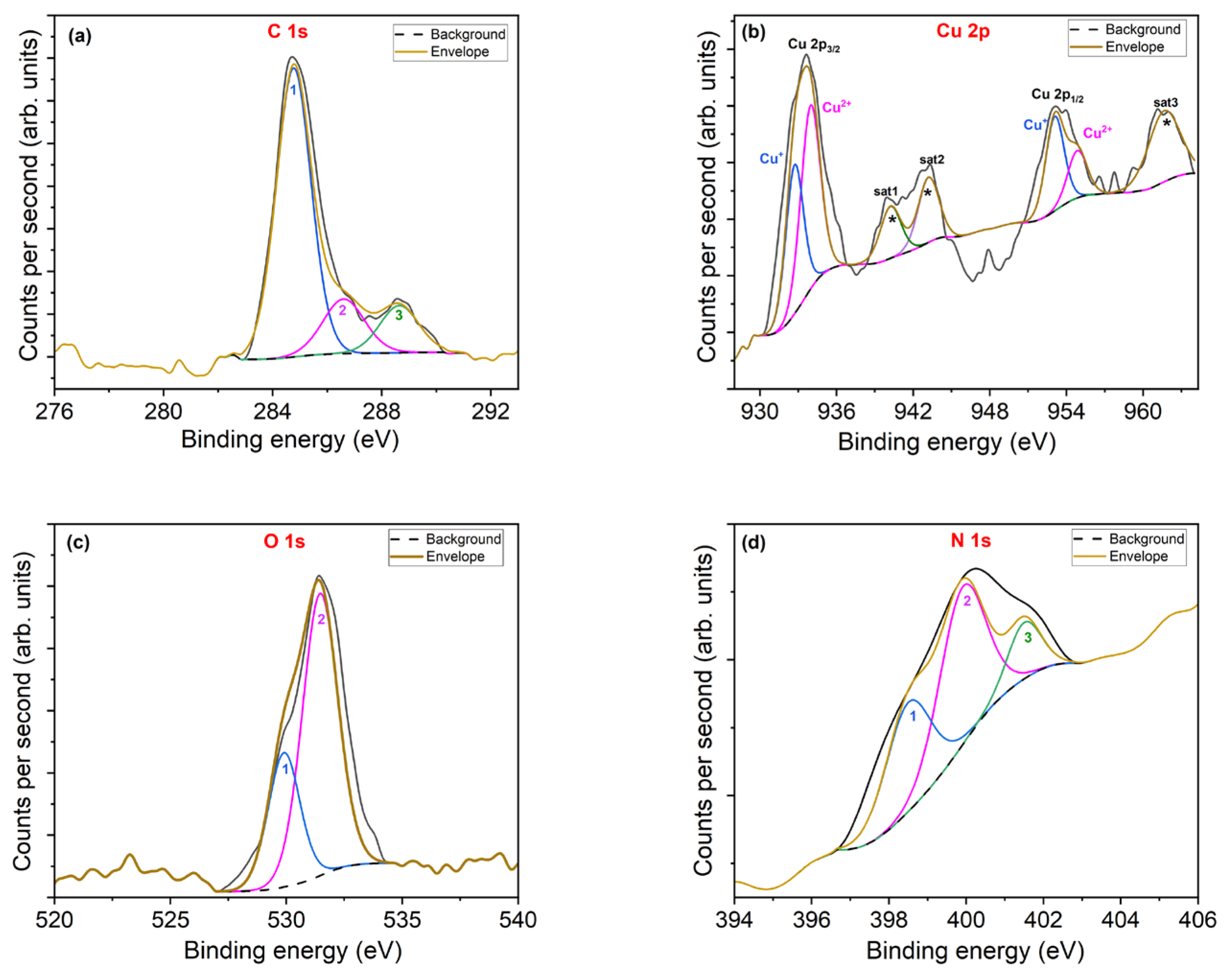
- The surface chemistry studied by XPS confirms the presence of copper, nitrogen and oxygen in the samples, and the binding energies of the peaks are attributable to Cu-N and Cu-O bonds.
- -
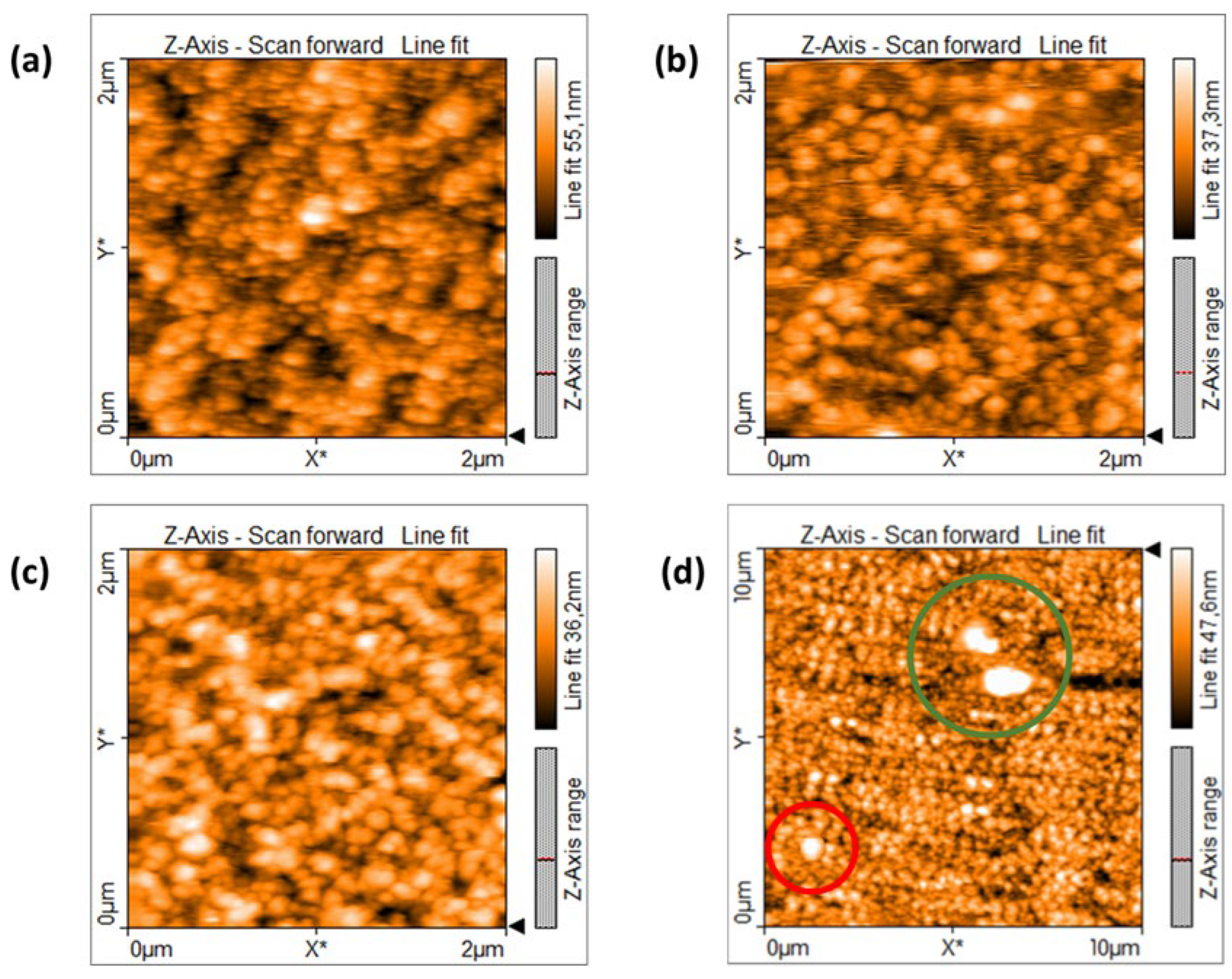
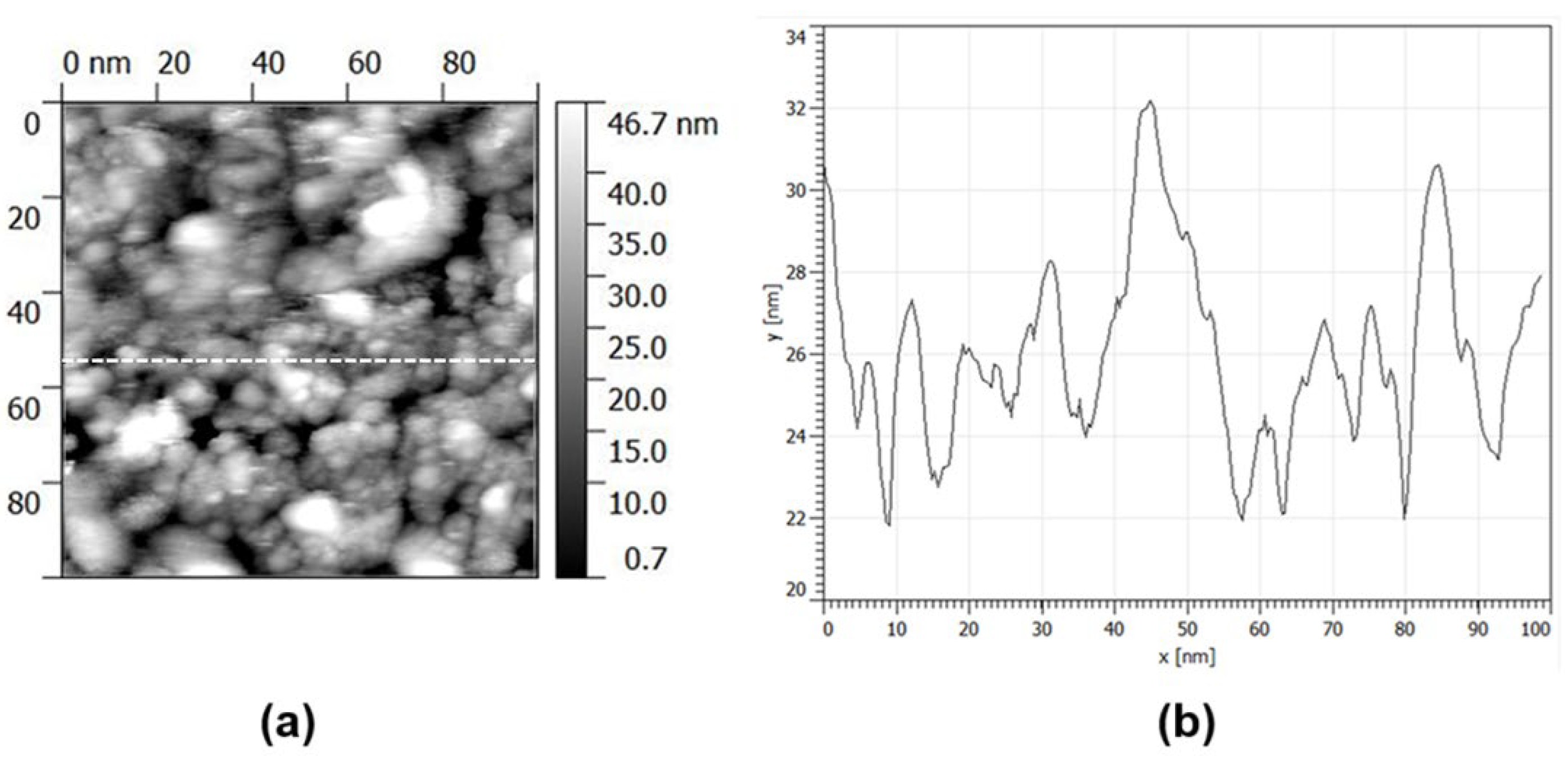
- AFM and STM scans show the co-presence of single NP and NP aggregates; NP size is almost the same around 6.6 nm, while aggregates become larger by increasing the spark current.
- -
- SEM observations confirm AFM morphologies, showing the presence of agglomerates together with NP aggregates with increasing size by increasing Ispark.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CuN | Copper Nitride |
| NPs | Nanoparticles |
| XRD | X-Ray Diffraction |
| SEM | Scanning Electron Microscope |
| STM | Scanning Tunnelling Microscope |
| AFM | Atomic Force Microscope |
| RMS | Root Mean Square |
| UHV | Ultra-High Vacuum |
References
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, L.V.; Rana, S.S. A critical review of various synthesis methods of nanoparticles and their applications in biomedical, regenerative medicine, food packaging, and environment. Discov. Appl. Sci. 2024, 6, 371. [Google Scholar] [CrossRef]
- Kohut, A.; Galbacs, G.; Marton, Z.; Geretovszky, Z. Characterization of a copper spark discharge plasma in argon atmosphere used for nanoparticle generation. Plasma Sources Sci. Technol. 2017, 26, 045001. [Google Scholar] [CrossRef]
- Siampani, M.; Lazanas, A.C.; Spyrou, K.; Prodromidis, M.I. Eco-friendly spark-generated CoxOy nanoparticle-modifed graphite screen-printed sensing surfaces for the determination of H2O2 in energy drinks. Microchim. Acta 2024, 191, 150. [Google Scholar] [CrossRef]
- Mouhtadi, S.; Jurin, F.E.; Buron, C.C.; Pochard, I. Tuning Thin Film Thickness and Porosity with Layer-by-Layer Submicronic Particles Assembly. Chem. Nano Mat. 2024, 10, e202400404. [Google Scholar] [CrossRef]
- van Ginkel, H.J.; Vollebregt, S.; Schimd-Ott, A.; Zhang, G.Q. Mass and density determination of porous nanoparticle films using a quartz crystal microbalance. Nanotechnology 2022, 33, 485704. [Google Scholar] [CrossRef]
- Available online: https://vsparticle.com/products/research-tools/vsp-p1-nanoprinter (accessed on 20 May 2025).
- Majerič, P. Nanostructured materials, structures and mechanical properties, processing and applications. Metall. Mater. Data 2024, 2, 15–22. [Google Scholar] [CrossRef]
- Paredes, P.; Rauwel, E.; Rauwel, P. Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials. Nanomaterials 2022, 12, 2218. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lee, P.I.; Sakalley, S.; Wen, C.K.; Cheng, W.C.; Sun, H.; Chen, S.C. Enhanced Electrical Properties of Copper Nitride Films Deposited via High Power Impulse Magnetron Sputtering. Nanomaterials 2022, 12, 2814. [Google Scholar] [CrossRef]
- Yamada, N.; Maruya, K.; Yamaguchi, Y.; Cao, X.; Ninomiya, Y. P- to n-Type Conversion and Nonmetal−Metal Transition of Lithium-Inserted Cu3N Films. Chem. Mater. 2015, 27, 8076–8083. [Google Scholar] [CrossRef]
- Rodríguez-Tapiador, M.I.; Merino, J.; Jawhari, T.; Muñoz-Rosas, A.L.; Bertomeu, J.; Fernández, S. Power effect on the properties of copper nitride films as solar absorber deposited in pure nitrogen atmosphere. Appl. Res. 2024, 3, e202200105. [Google Scholar] [CrossRef]
- Hussein, H.S. The state of the art of nanomaterials and its applications in energy saving. Bull. Natl. Res. Cent. 2023, 47, 7. [Google Scholar] [CrossRef]
- Sahoo, D.; Naik, R. Nanoscience and Nanotechnologies for Photovoltaics. In Handbook of Energy Materials; Gupta, R., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Oni, A.M.; Mohsin, A.S.M.; Rahman, M.M.; Bhuian, M.B.H. A comprehensive evaluation of solar cell technologies, associated loss mechanisms, and efficiency enhancement strategies for photovoltaic cells. Energy Rep. 2024, 11, 3345–3366. [Google Scholar] [CrossRef]
- Nakamura, T.; Hiyoshi, N.; Hayashi, H.; Ebina, T. Preparation of plate-like copper nitride nanoparticles from a fatty acid copper(II) salt and detailed observations by high resolution transmission electron microscopy and high-angle annular dark-field scanning transmission electron microscopy. Mater. Lett. 2015, 139, 271–274. [Google Scholar] [CrossRef]
- Feng, J.; Biskos, G.; Schmidt-Ott, A. Toward industrial scale synthesis of ultrapure singlet nanoparticles with controllable sizes in a continuous gas-phase process. Sci. Rep. 2015, 5, 15788. [Google Scholar] [CrossRef]
- Figueira, C.A.; Rosario, G.D.; Pugliese, D.; Rodríguez-Tapiador, M.I.; Fernández, S. Effect of Argon on the Properties of Copper Nitride Fabricated by Magnetron Sputtering for the Next Generation of Solar Absorbers. Materials 2022, 15, 8973. [Google Scholar] [CrossRef]
- Trivedi, A.; Thakarda, J.; Chavda, N.; Agrawal, Y.K.; Maity, P. A new route towards selective synthesis of supported Cu2O and CuO nanoparticles under extremely mild condition. Nano-Struct. Nano-Objects 2016, 6, 34–38. [Google Scholar] [CrossRef]
- Devaraj, P.; Peranantham, P.; Jeyachandran, Y.L. Oxidation behavior of copper nitride thin films deposited by direct current magnetron sputtering. J. Mater. Sci. Mater. Electron. 2021, 32, 27899–27912. [Google Scholar] [CrossRef]
- Arulkumar, E.; Thanikaikarasan, S.; Tesfie, N. Influence of Deposition Parameters for Cu2O and CuO Thin Films by Electrodeposition Technique: A Short Review. J. Nanomater. 2023, 2023, 8987633. [Google Scholar] [CrossRef]
- Rodríguez-Tapiador, M.I.; Asensi, J.M.; Roldán, M.; Merino, J.; Bertomeu, J.; Fernández, S. Copper Nitride: A Versatile Semiconductor with Great Potential for Next-Generation Photovoltaics. Coatings 2023, 13, 1094. [Google Scholar] [CrossRef]
- Rodríguez-Tapiador, M.I.; Merino, J.; Jawhari, T.; Muñoz-Rosas, A.L.; Bertomeu, J.; Fernández, S. Impact of the RF Power on the Copper Nitride Films Deposited in a Pure Nitrogen Environment for Applications as Eco-Friendly Solar Absorber. Materials 2023, 16, 1508. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, A.; Paul, A.M.; Nivetha, R.; Gothandapani, K.; Gopal, T.S.; Jacob, G.; Muthuramamoorty, M.; Pandiaraj, S.; Alodhayb, A.; Kim, S.Y.; et al. Development of Cu3N electrocatalyst for hydrogen evolution reaction in alkaline medium. Sci. Rep. 2022, 12, 2004. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Chang, Y.-H. Growth without Post-annealing of Monoclinic VO2 Thin Film by Atomic Layer Deposition Using VCl4 as Precursor. Coatings 2018, 8, 431. [Google Scholar] [CrossRef]
- Mahendra, G.; Malathi, R.; Kedhareswara, S.P.; Lakshmi-Narayana, A.; Dhananjaya, M.; Guruprakash, N.; Hussain, O.M.; Mauger, A.; Julien, C.M. RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Appl. Nano 2021, 2, 46–66. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Olszok, V.; Bierwirth, M.; Weber, A.P. Interaction of Reactive Gases with Platinum Aerosol Particles at Room Temperature: Effects on Morphology and Surface Properties. Nanomaterials 2021, 11, 2266. [Google Scholar] [CrossRef]
- Beverina, M.; Sanchez-Cortes, S.; Schabes, F.I.; Zapata, J.; Arias Cassara, M.L.; Tuttolomondo, M.E. Spectroscopic characterization (Raman and infrared) of Aloe maculata from the north Argentina region. Vib. Spectrosc. 2022, 122, 103423. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Gallardo-Vega, C.; De la Cruz, W. Study of the structure and electrical properties of the copper nitride thin films deposited by pulsed laser deposition. App. Surf. Sci. 2006, 252, 8001–8004. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.X.; Han, J.; Hu, K.; Chen, J.S. Self-Supported Sheets-on-Wire CuO@Ni(OH)2/Zn(OH)2 Nanoarrays for High-Performance Flexible Quasi-Solid-State Supercapacitor. Processes 2021, 9, 680. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo-Phao, L.F.E.; Kolokoto, T.; Zimuwandeyi, M.; Gqoba, S.S.; Mubiayi, K.P.; Moloto, M.J.; Van Wyk, J.; Moloto, N. Elucidating the effect of precursor decomposition time on the structural and optical properties of copper(i) nitride nanocubes. RSC Adv. 2020, 10, 34231–34246. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Sanchez, L.; Martin, F.; Ramos-Barrado, J.R.; Sanchez, M. Use of low-temperature nanostructured CuO thin films deposited by spray-pyrolysis in lithium cells. Thin Solid Films 2005, 474, 133–140. [Google Scholar] [CrossRef]
- Guerrero-Araque, D.; Acevedo-Peña, P.; Ramírez-Ortega, D.; Lartundo-Rojas, L.; Gómez, R. SnO2–TiO2 structures and the effect of CuO, CoO metal oxide on photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2017, 92, 1531–1539. [Google Scholar] [CrossRef]
- Miura, A.; Takei, T.; Kumada, N. Synthesis of Cu3N from CuO and NaNH2. J. Asian Ceram. Soc. 2014, 2, 326–328. [Google Scholar] [CrossRef]
- Fan, X.; Wu, Z.; Li, H.; Geng, B.; Li, C.; Yan, P. Morphology and thermal stability of Ti-doped copper nitride films. J. Phys. D Appl. Phys. 2007, 40, 3430–3435. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. Photoelectron Spectroscopic Investigation of Nitrogen-Doped Titania Nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Aghajani, S.; Accardo, A.; Tichem, M. Aerosol Direct Writing and Thermal Tuning of Copper Nanoparticle Patterns as Surface-Enhanced Raman Scattering Sensors. ACS Appl. Nano Mater. 2020, 3, 5665–5675. [Google Scholar] [CrossRef]
- Ternero, P.; Sedrpooshan, M.; Wahlqvist, D.; Meuller, B.O.; Ek, M.; Hubner, J.-M.; Westerstrom, R.; Messing, M.E. Effect of the carrier gas on the structure and composition of Co–Ni bimetallic nanoparticles generated by spark ablation. J. Aerosol Sci. 2023, 170, 106146. [Google Scholar] [CrossRef]
- Maisser, A.; Barmpounis, K.; Holm, S.; Attoui, M.; Schmidt-Ott, A.; Kangasluoma, J.; Biskos, G. Characterization of atmospheric-pressure spark generated atomic silver and gold clusters by time-of-flight mass spectrometry. J. Aerosol Sci. 2021, 156, 105780. [Google Scholar] [CrossRef]
- Hagen, F.P.; Rinkenburger, A.; Günther, J.; Bockhorn, H.; Niessner, R.; Suntz, R.; Loukou, A.; Trimis, D.; Haisch, C. Spark discharge-generated soot: Varying nanostructure and reactivity against oxidation with molecular oxygen by synthesis conditions. J. Aerosol Sci. 2020, 143, 105530. [Google Scholar] [CrossRef]
- Preger, C.; Bulbucan, C.; Meuller, B.O.; Ludvigsson, L.; Kostanyan, A.; Muntwiler, M.; Deppert, K.; Westerstrom, R.; Messing, M.E. Controlled oxidation and self-passivation of bimetallic Magnetic FeCr and FeMn aerosol nanoparticles. J. Phys. Chem. C 2019, 123, 16083–16090. [Google Scholar] [CrossRef]
- Kohut, A. Hydrogen-assisted spark generation of silver nanoparticles: The effect of hydrogen content on the signal intensity in surface-enhanced Raman spectroscopy. J. Aerosol Sci. 2023, 167, 106090. [Google Scholar] [CrossRef]
- Giannakopoulos, K.; Lasithiotakis, M.; Karakasis, C.; Gini, M.; Gardelis, S.; Karakasiliotis, I.; Mouti, N.; Xesfyngi, Y.; Manolis, G.K.; Georgoutsou-Spyridonos, M.; et al. Spark Discharge Aerosol-Generated Copper-Based Nanoparticles: Structural & Optical Properties; Application on the Antiviral (SARS-CoV-2) and Antibacterial Improvement of Face Masks. ChemPlusChem 2024, 89, e202400194. [Google Scholar] [CrossRef]
- Radovanović-Perić, F.; Burtscher, M.; Panžić, I.; Kiener, D.; Mandić, V. Novel ambient-condition solid-state synthesis route of nanocrystalline TiN thin films via spark plasma ablation deposition. Ceram. Int. 2025, 51, 5576–5582. [Google Scholar] [CrossRef]
- Loizidis, C.; Petallidou, K.C.; Maisser, A.; Bezantakos, S.; Pfeiffer, T.V.; Schmidt-Ott, A.; Biskos, G. Insights into the enhancement of nanoparticle production throughput by atmospheric-pressure spark ablation. Aerosol Sci. Technol. 2024, 58, 1421–1431. [Google Scholar] [CrossRef]
- Petallidou, K.C.; Schmidt-Ott, A.; Biskos, G. Stability of nanoparticle production by atmospheric-pressure spark ablation. Aerosol Sci. Technol. 2024, 58, 1079–1088. [Google Scholar] [CrossRef]
- Feng, J.; Guo, X.; Ramlawi, N.; Pfeiffer, T.V.; Geutjens, R.; Basak, S.; Nirschl, H.; Biskos, G.; Zandbergen, H.W.; Schmidt-Ott, A. Green manufacturing of metallic nanoparticles: A facile and universal approach to scaling up. J. Mater. Chem. A 2016, 4, 11222–11227. [Google Scholar] [CrossRef]
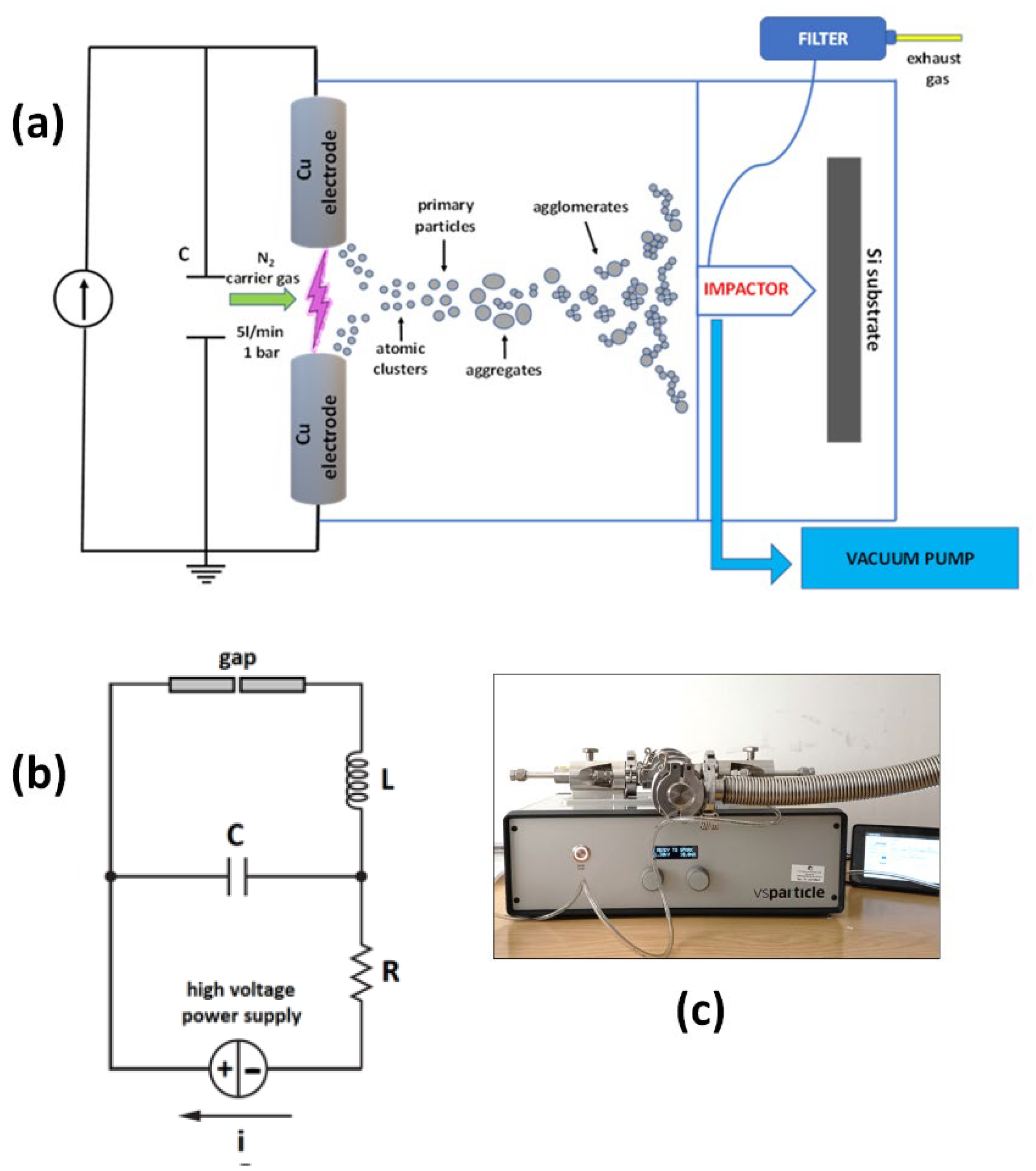
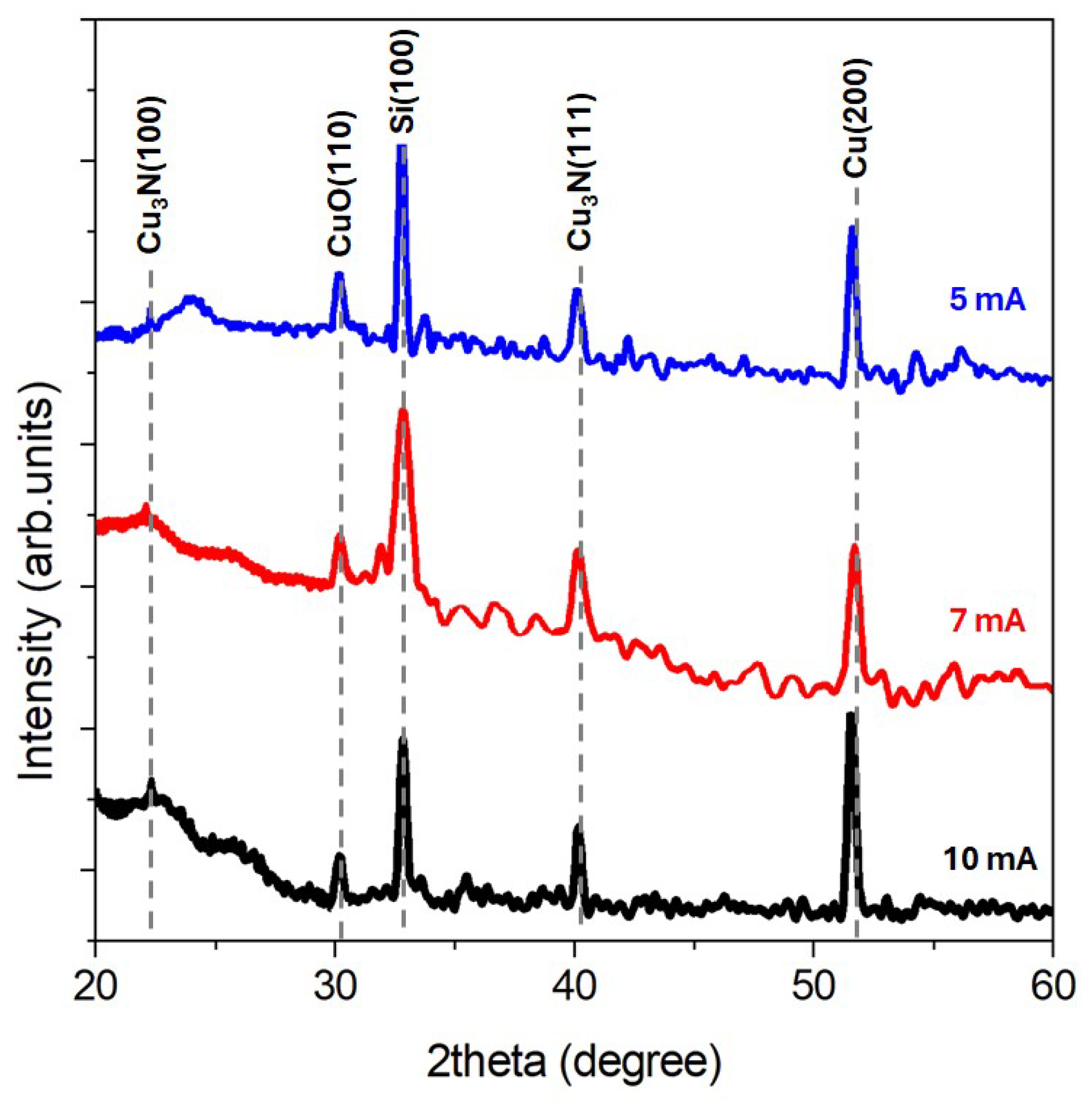
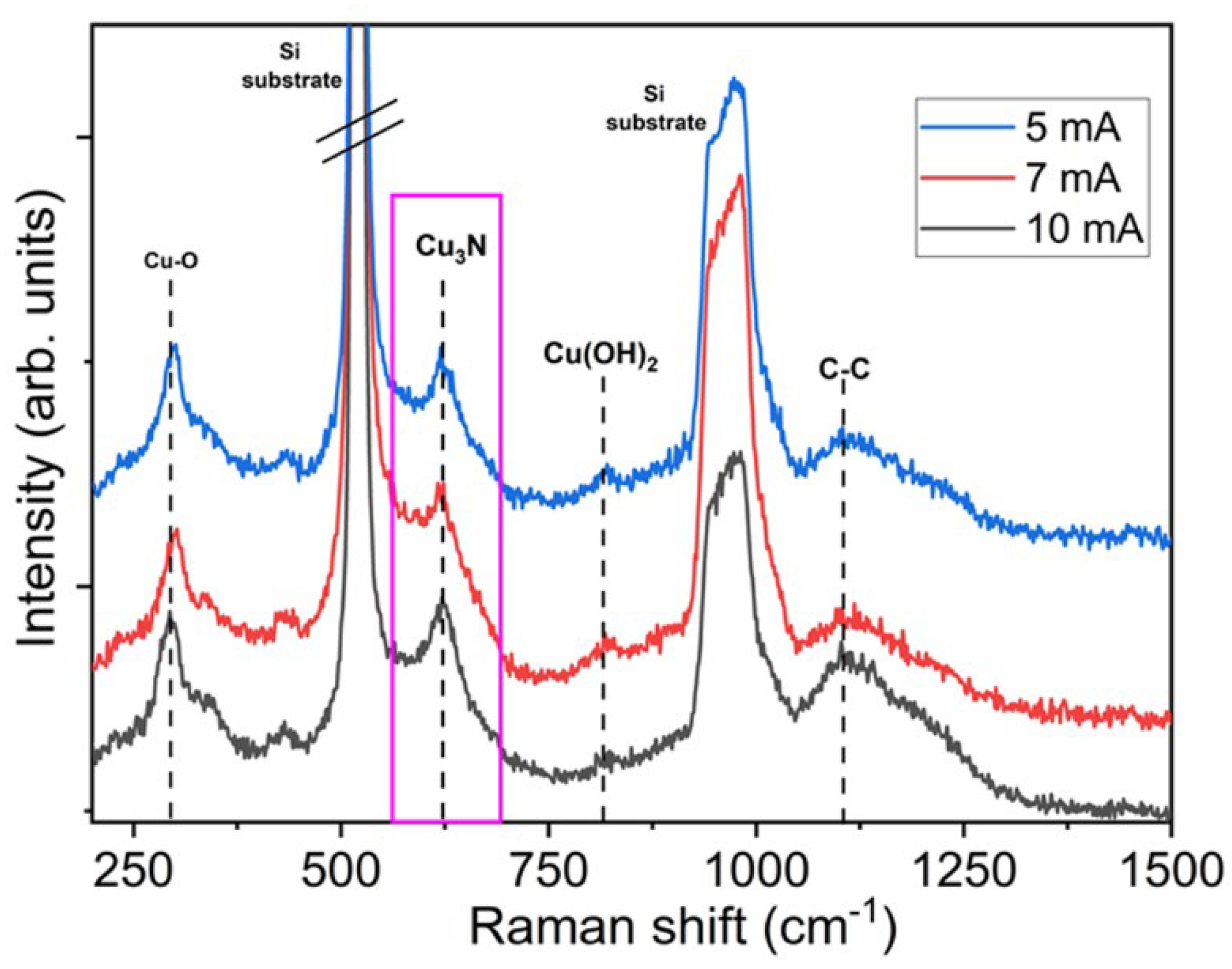
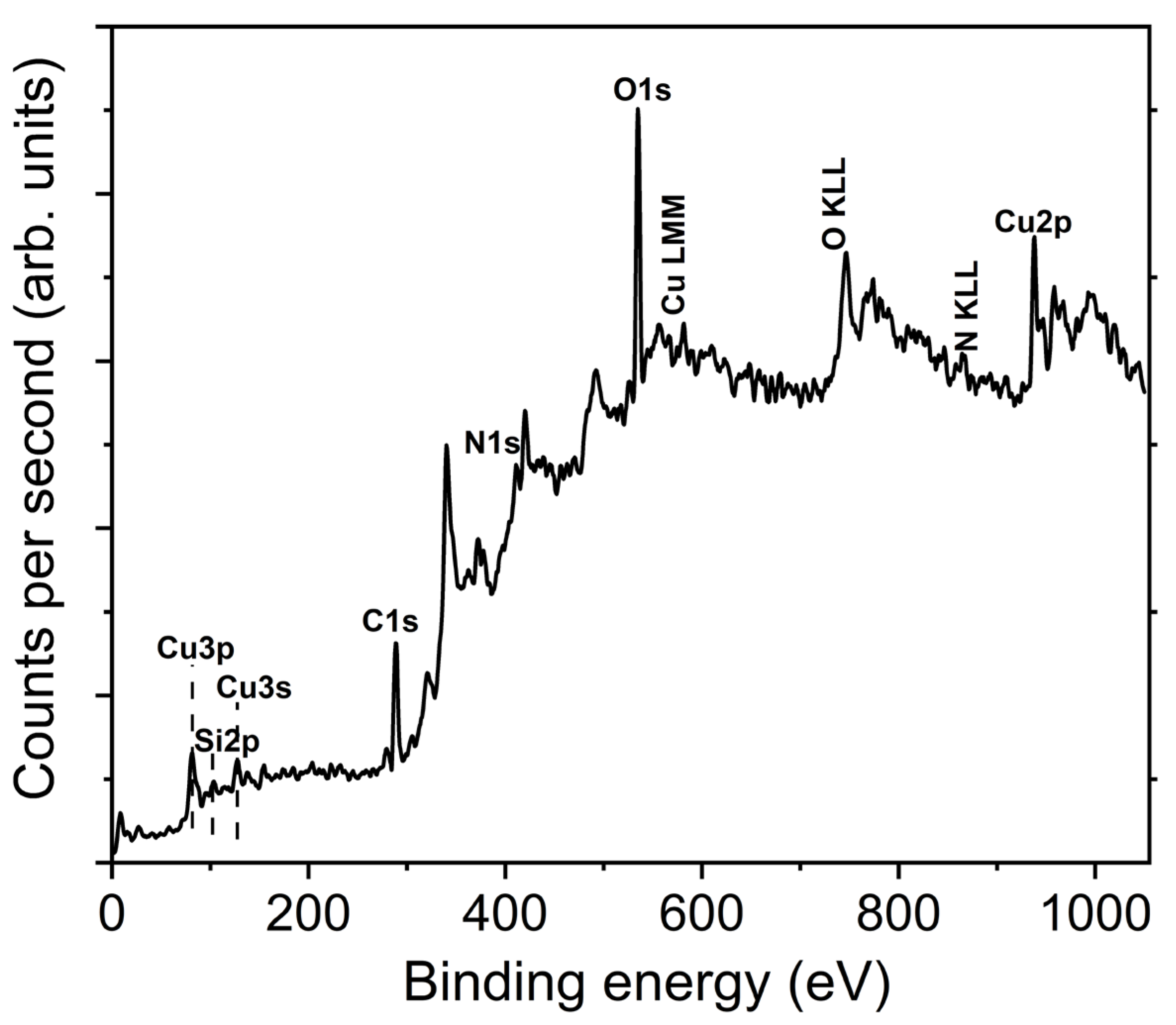

| Parameters | Values |
|---|---|
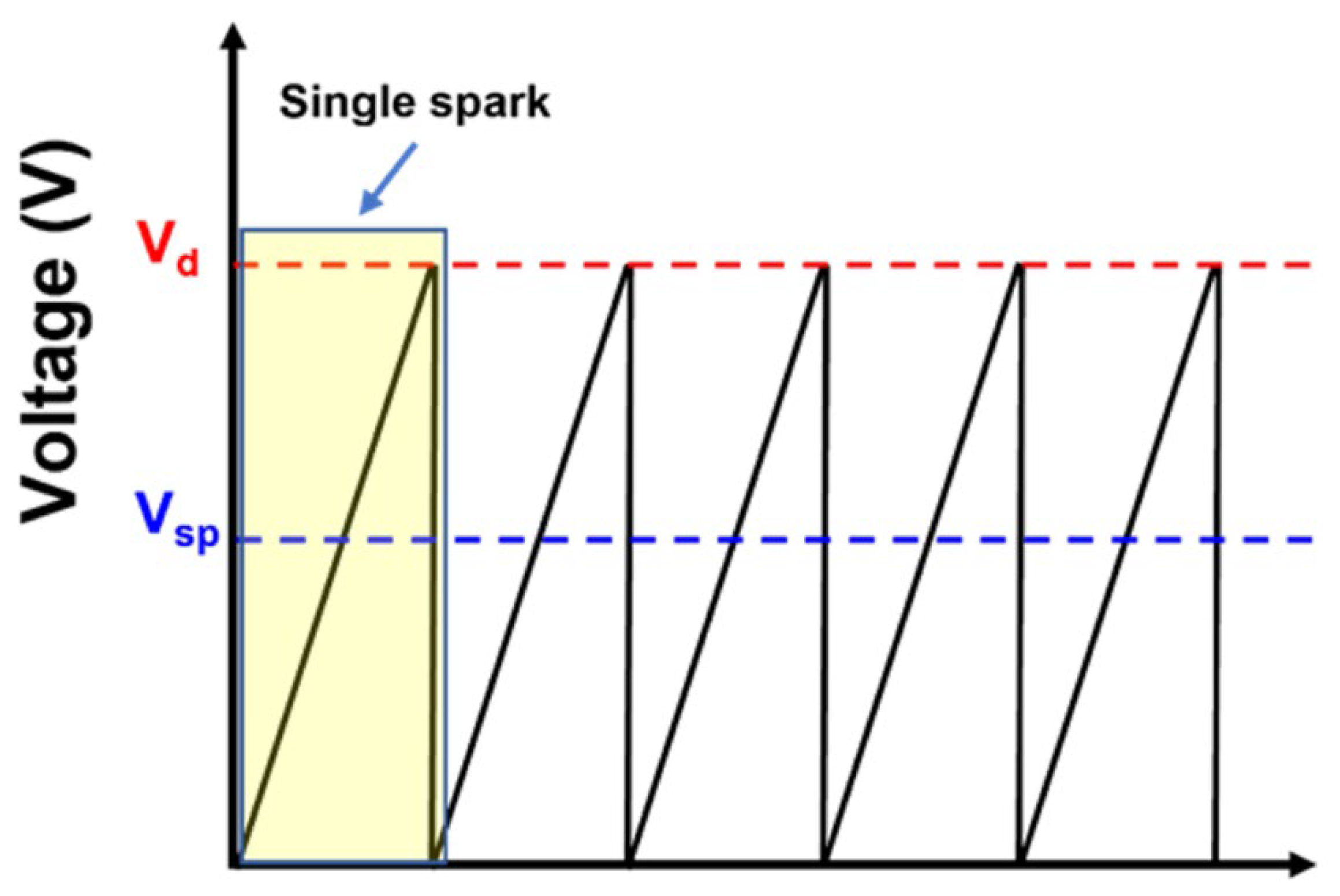
| Vsp | 1.3 kV |
| N2 flux | 5 L/min |
| Deposition time | 300 s |
| Ispark (tuned) | 5-7-10 mA |
| Espark | 47.5 mJ |
| BE (eV) | FWHM | AREA | %AREA | |
|---|---|---|---|---|
| C1s | ||||
| 1 | 284.8 | 1.5 | 2202 | 70.8 |
| 2 | 286.6 | 1.8 | 499 | 16 |
| 3 | 288.6 | 1.7 | 406 | 13.1 |
| Cu2p3/2 | ||||
| Cu+ | 932.8 | 1.6 | 2234 | 18.8 |
| Cu2+ | 933.9 | 1.8 | 3165 | 25 |
| sat1 | 940.2 | 1.9 | 959 | 7.8 |
| sat2 | 943.2 | 1.9 | 1191 | 9.8 |
| Cu2p1/2 | ||||
| Cu+ | 953.1 | 1.7 | 1536 | 12.9 |
| Cu2+ | 954.9 | 1.8 | 814 | 6.9 |
| sat3 | 961.8 | 3 | 2095 | 18 |
| O1s | ||||
| 1 | 529.9 | 1.8 | 4457 | 70.2 |
| 2 | 531.5 | 1.6 | 1894 | 29.8 |
| N1s | ||||
| 1 | 398.7 | 1.4 | 241 | 35.1 |
| 2 | 400 | 1.4 | 353 | 51.4 |
| 3 | 401.6 | 1 | 92 | 13.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signore, M.A.; Della Torre, A.; Serra, A.; Manno, D.; Rinaldi, R.; Mazzeo, M.; Francioso, L.N.; Velardi, L. The Effect of Spark Current Tuning on the Formation of Cu Nanoparticles Synthesized by Spark Ablation in Nitrogen Atmosphere. Crystals 2025, 15, 587. https://doi.org/10.3390/cryst15070587
Signore MA, Della Torre A, Serra A, Manno D, Rinaldi R, Mazzeo M, Francioso LN, Velardi L. The Effect of Spark Current Tuning on the Formation of Cu Nanoparticles Synthesized by Spark Ablation in Nitrogen Atmosphere. Crystals. 2025; 15(7):587. https://doi.org/10.3390/cryst15070587
Chicago/Turabian StyleSignore, Maria Assunta, Antonio Della Torre, Antonio Serra, Daniela Manno, Rosaria Rinaldi, Marco Mazzeo, Luca Nunzio Francioso, and Luciano Velardi. 2025. "The Effect of Spark Current Tuning on the Formation of Cu Nanoparticles Synthesized by Spark Ablation in Nitrogen Atmosphere" Crystals 15, no. 7: 587. https://doi.org/10.3390/cryst15070587
APA StyleSignore, M. A., Della Torre, A., Serra, A., Manno, D., Rinaldi, R., Mazzeo, M., Francioso, L. N., & Velardi, L. (2025). The Effect of Spark Current Tuning on the Formation of Cu Nanoparticles Synthesized by Spark Ablation in Nitrogen Atmosphere. Crystals, 15(7), 587. https://doi.org/10.3390/cryst15070587








