The Effect of Powder-to-Flux Ratio and Heating Duration on the Microstructure and Corrosion Resistance of WO3 Nanoparticle-Reinforced Sn–20Bi Coatings on Low-Carbon Steel
Abstract
1. Introduction
2. Materials and Methods
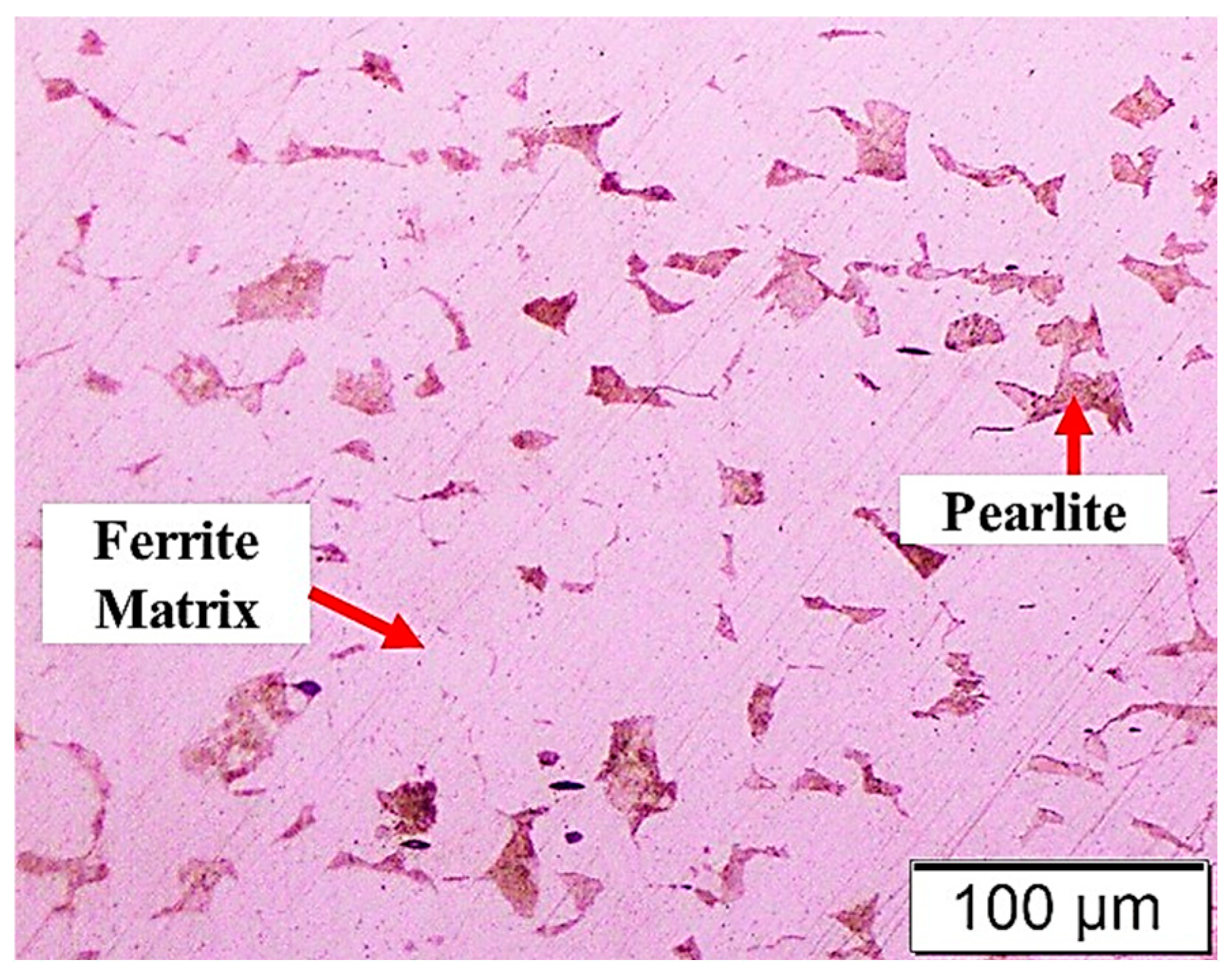
2.1. Substrate Preparation
2.2. Coating Preparation
2.3. Coating Application and Post-Treatment
2.4. Sample Characterization
2.5. Corrosion Testing
3. Results and Discussion
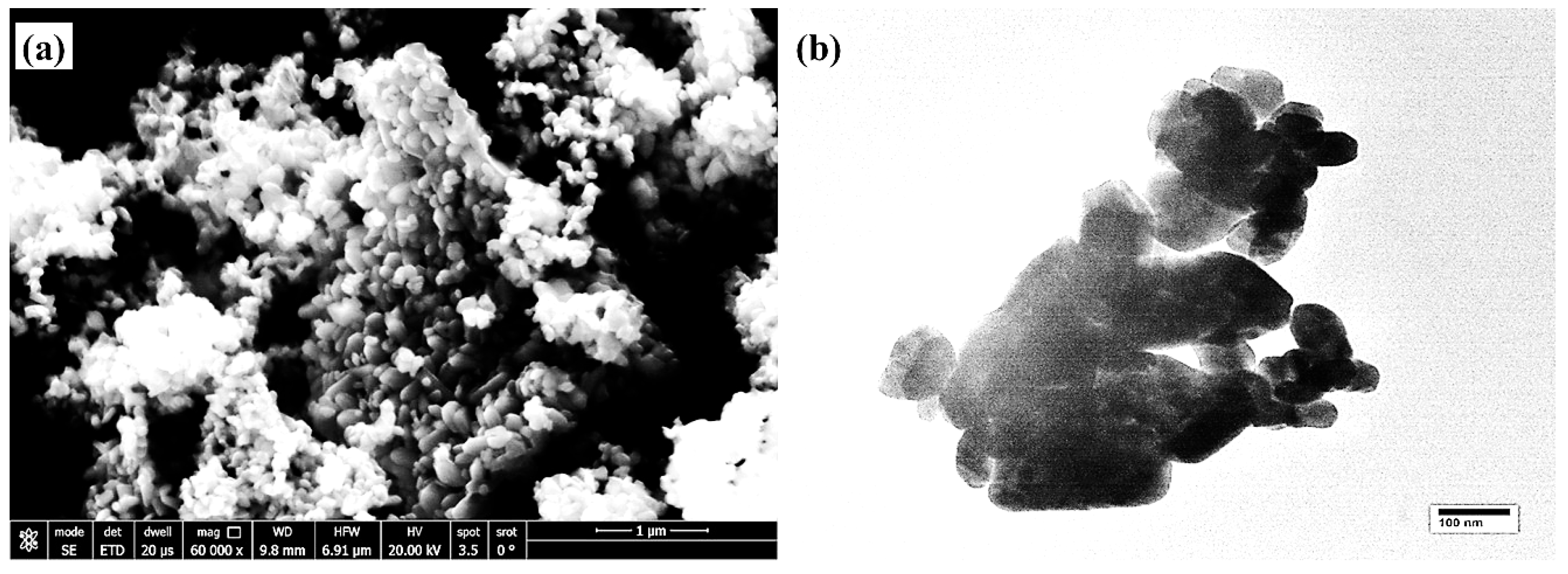
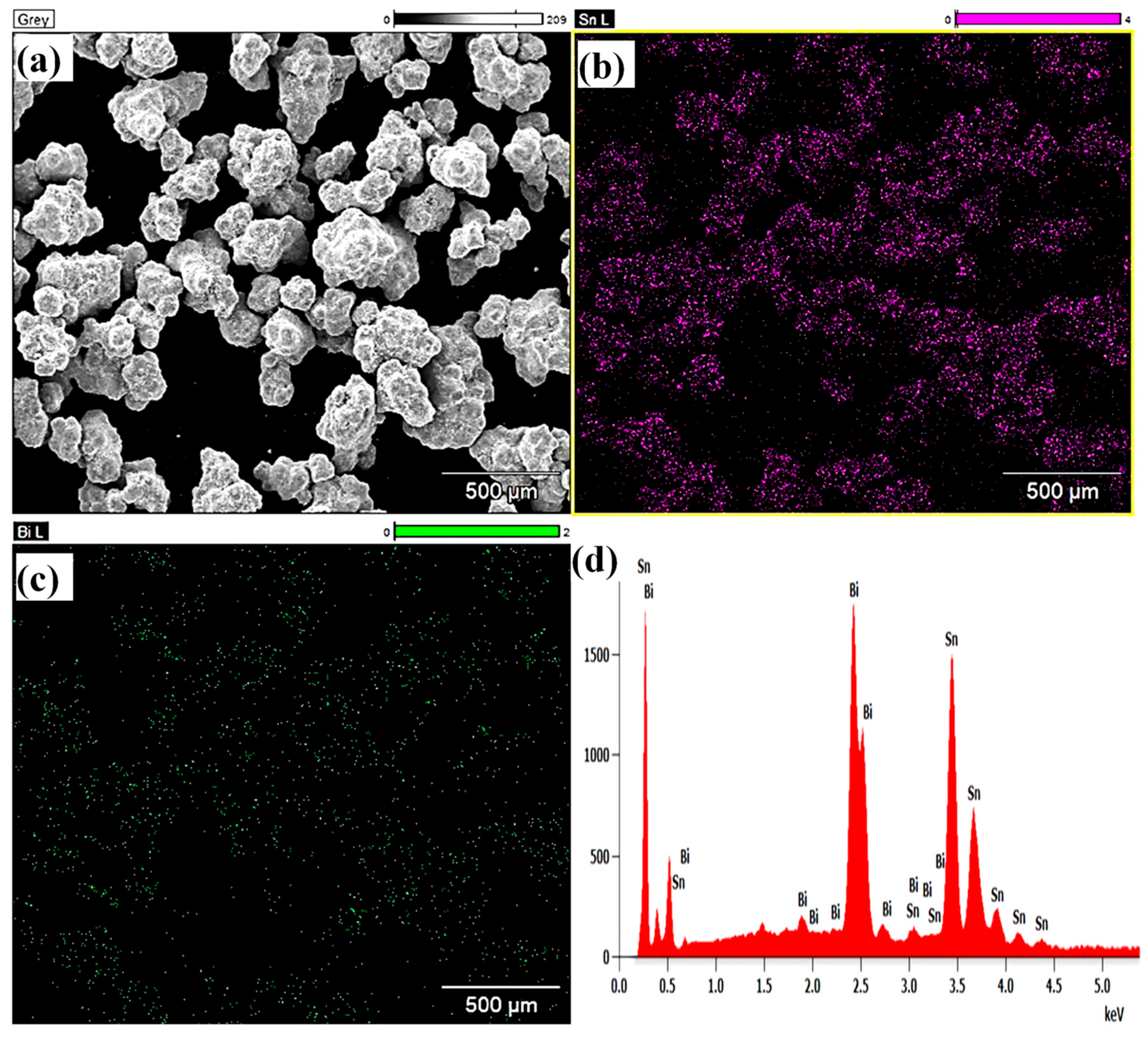
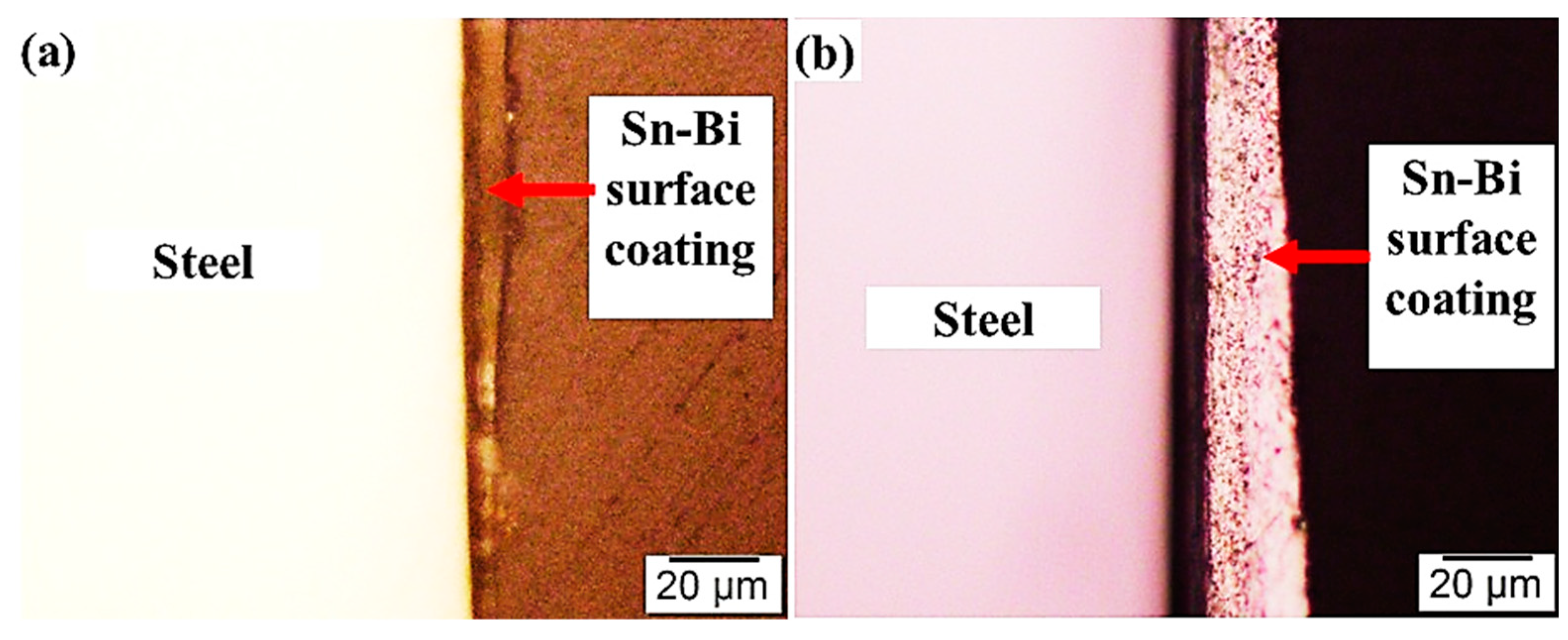
3.1. Microstructures of the Coating Surface and Coating/LCS Interface
3.2. Corrosion Measurements
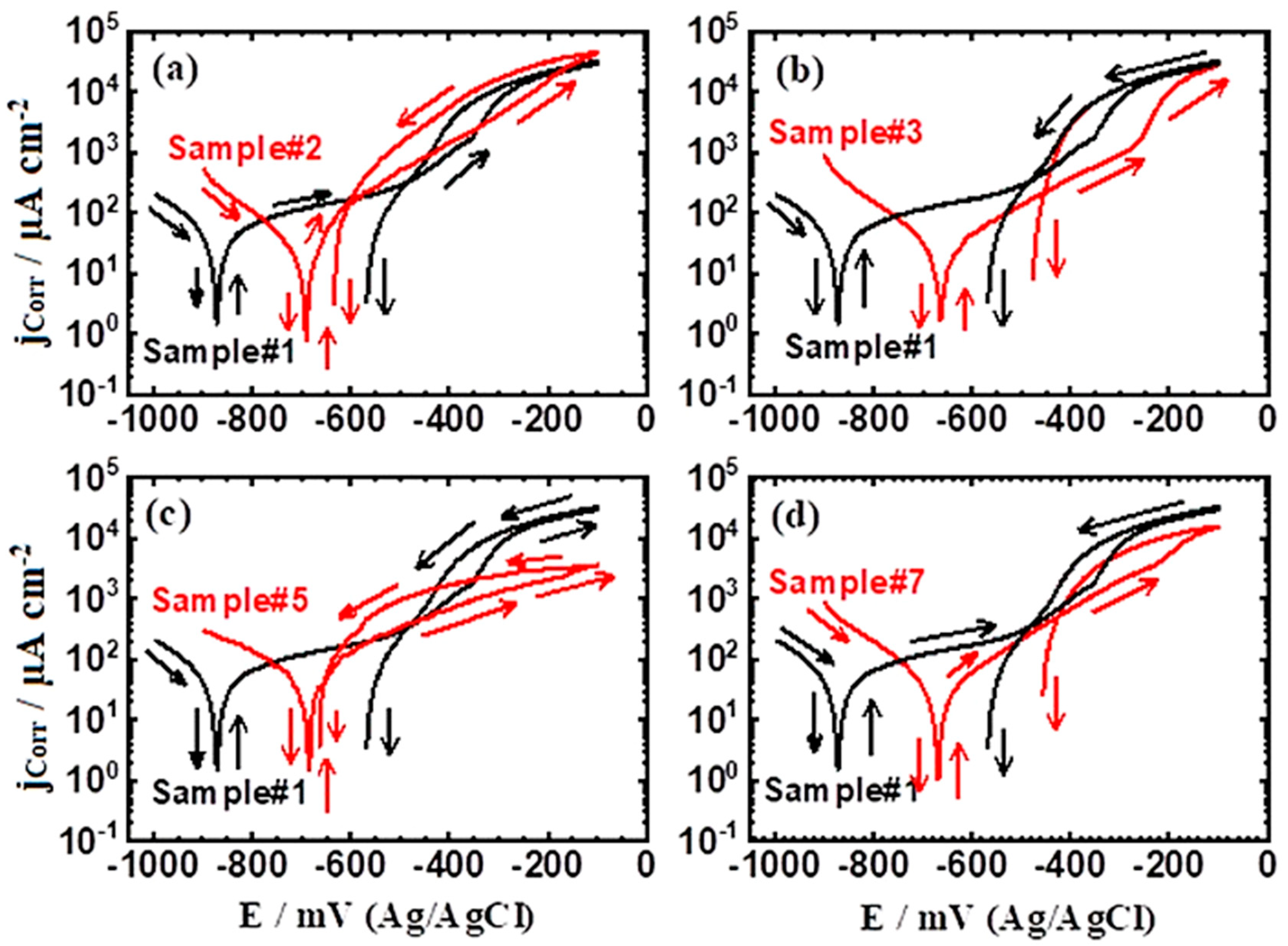
3.2.1. CPP Measurements
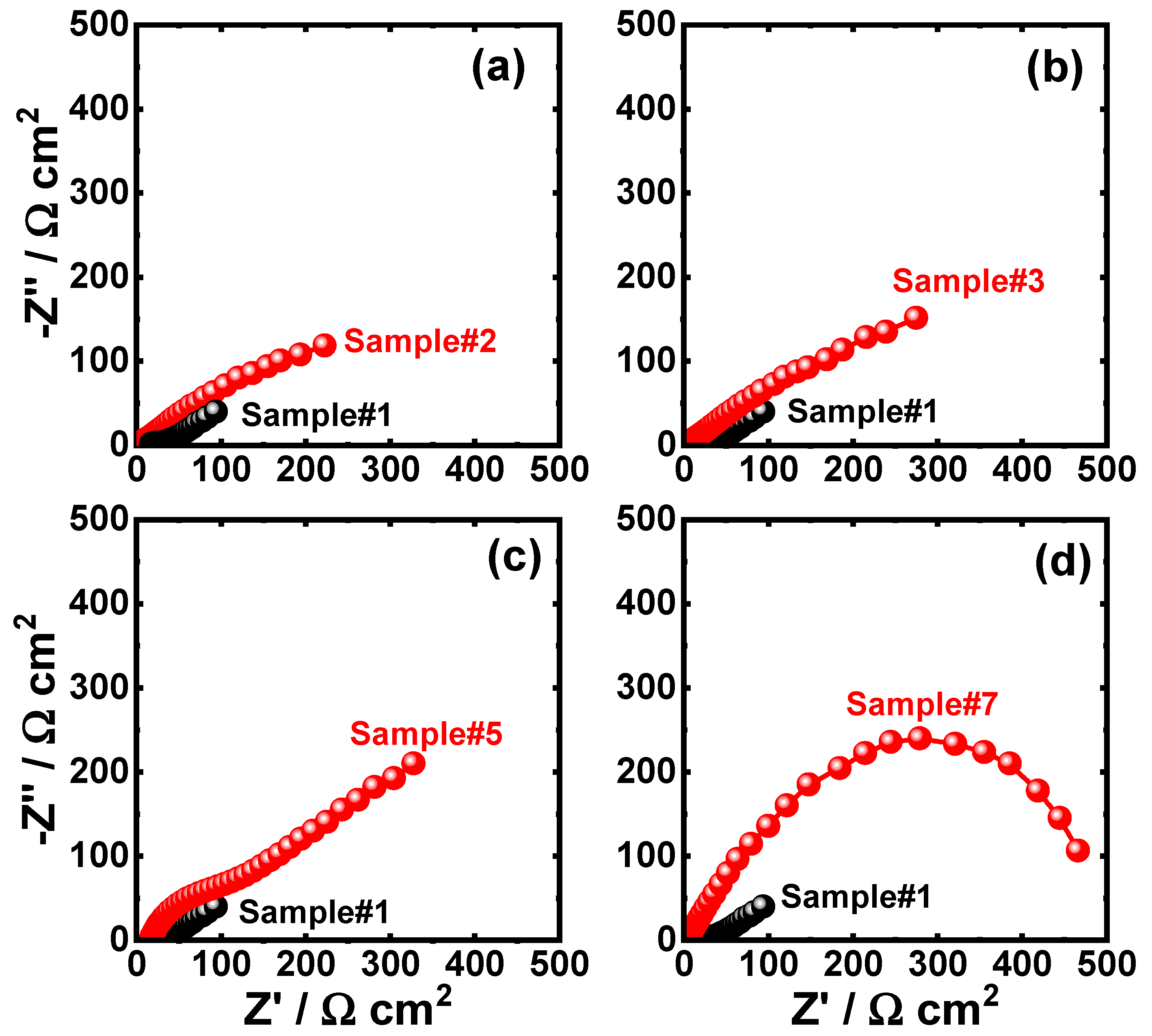
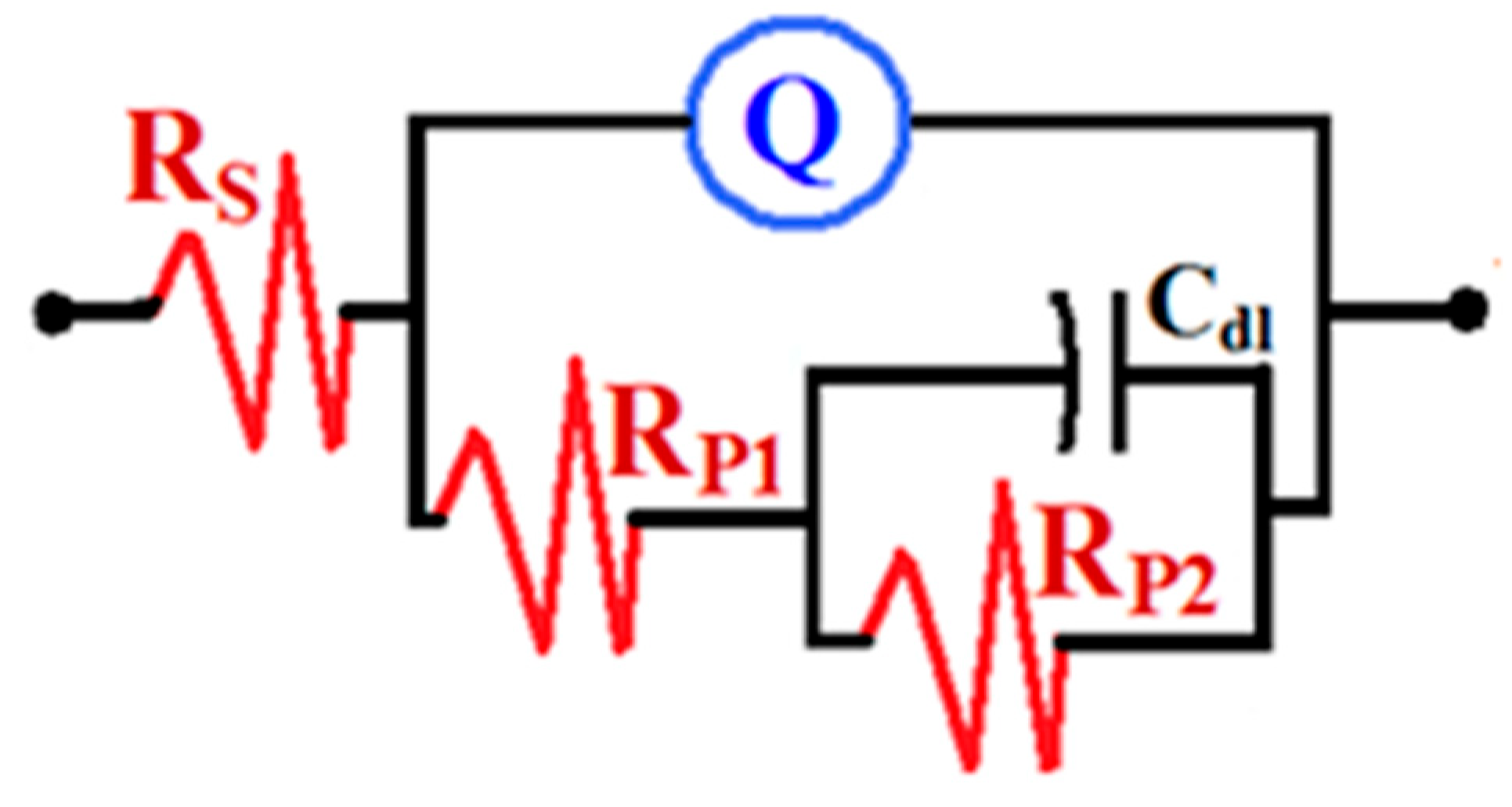
3.2.2. EIS Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion mechanisms and microstructure development in pack aluminizing of Ni-based alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Chintada, V.B.; Koona, R.; Bahubalendruni, M.V.A.R. State of Art Review on Nickel Based Electroless Coatings and Materials. J. Bio-Tribo-Corros. 2021, 7, 134. [Google Scholar] [CrossRef]
- Amaya, A.; Piamba, O.; Olaya, J. Improvement of Corrosion Resistance for Gray Cast Iron in Palm Biodiesel Application Using Thermoreactive Diffusion Niobium Carbide (NbC) Coating. Coatings 2018, 8, 216. [Google Scholar] [CrossRef]
- Guan, Z.; Qian, J.; Qin, H.; Hou, J.; Zhou, Y.; Xie, Z.; Yu, F.; Chen, Y.; Wan, G.; Zeng, H. Osteogenerative and corrosion-decelerating teriparatide-mediated strontium–zinc phosphate hybrid coating on biodegradable zinc–copper alloy for orthopaedic applications. Mater. Today Commun. 2024, 39, 109010. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Zhang, J.; Yang, P.; An, M. Studies on the enhanced properties of nanocrystalline Zn–Ni coatings from a new alkaline bath due to electrolyte additives. RSC Adv. 2015, 5, 58199–58210. [Google Scholar] [CrossRef]
- Anwar, S.; Zhang, Y.; Khan, F. Electrochemical behaviour and analysis of Zn and Zn–Ni alloy anti-corrosive coatings deposited from citrate baths. RSC Adv. 2018, 8, 28861–28873. [Google Scholar] [CrossRef]
- Li, Y.J.; Liang, T.Q.; Ao, R.; Zhao, H.; Chen, X.; Zeng, J. Oxidation resistance of iron-based coatings by supersonic arc spraying at high temperature. Surf. Coat. Technol. 2018, 347, 99–112. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, F.; Wu, Y.; Le, J.; Liu, L.; He, M.; Zhu, J.; Hu, W. Protective diffusion coatings on magnesium alloys: A review of recent developments. J. Alloy. Compd. 2012, 520, 11–21. [Google Scholar] [CrossRef]
- Yu, L.; Xiao, C.; Jiang, W.; Li, W.; Ni, S.; Song, M. Effects of aluminizing on the microstructure and wear resistance of AISI 321 steel. Surf. Coat. Technol. 2024, 483, 130753. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, K.; Zou, Y.C.; Cai, H.; Wang, Y.M.; Ouyang, J.H.; Li, X.W. Microstructure and high temperature oxidation resistance property of packing Al cementation on Ti–Al–Zr alloy. Surf. Coat. Technol. 2019, 374, 1051–1058. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Chen, F.-S. Thermoreactive deposition/diffusion coating of chromium carbide by contact-free method. Mater. Chem. Phys. 2005, 91, 192–199. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Chromium and vanadium carbide and nitride coatings obtained by TRD techniques on UNI 42CrMoS4 (AISI 4140) steel. Surf. Coat. Technol. 2016, 286, 319–326. [Google Scholar] [CrossRef]
- Fan, X.S.; Yang, Z.G.; Xia, Z.X.; Zhang, C.; Che, H.Q. The microstructure evolution of VC coatings on AISI H13 and 9Cr18 steel by thermo-reactive deposition process. J. Alloys Compd. 2010, 505, 15–18. [Google Scholar] [CrossRef]
- Castillejo, F.E.; Marulanda, D.M.; Olaya, J.J.; Alfonso, J.E. Wear and corrosion resistance of niobium–chromium carbide coatings on AISI D2 produced through TRD. Surf. Coat. Technol. 2014, 254, 104–111. [Google Scholar] [CrossRef]
- Ros-Yañez, T.; Houbaert, Y.; Gómez Rodríguez, V. High-silicon steel produced by hot dipping and diffusion annealing. J. Appl. Phys. 2002, 91, 7857–7859. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H. Microstructure development and mechanical properties of quenching and partitioning (Q&P) steel and an incorporation of hot-dipping galvanization during Q&P process. Mater. Sci. Eng. A 2013, 586, 100–107. [Google Scholar] [CrossRef]
- Lou, D.C.; Akselsen, O.M.; Onsqien, M.I.; Solberg, J.K.; Berget, J. Surface modification of steel and cast iron to improve corrosion resistance in molten aluminium. Surf. Coat. Technol. 2006, 200, 5282–5288. [Google Scholar] [CrossRef]
- Galyon, G.T. A History of Tin Whisker Theory: 1946 to 2004. In Proceedings of the SMTAI International Conference 2004, Chicago, IL, USA, 26–28 September 2024. Technical Report from IBM eSG Group to iNEMI. [Google Scholar]
- Baated, A.; Hamasaki, K.; Kim, S.S.; Kim, K.S.; Suganuma, K. Whisker Growth Behavior of Sn and Sn Alloy Lead-Free Finishes. J. Electron. Mater. 2011, 40, 2278–2289. [Google Scholar] [CrossRef]
- Singh, A.K.; Drunka, R.; Smits, K.; Vanags, M.; Iesalnieks, M.; Joksa, A.A.; Blumbergs, I.; Steins, I. Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles. Crystals 2023, 13, 508. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Ramadan, M.; Sherif, E.-S.M.; Hafez, K.M.; Subhani, T.; Fathy, N.; Alghamdi, A.S.; Khedr, M.H. Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating. Crystals 2022, 12, 332. [Google Scholar] [CrossRef]
- Davoodi, F.; Akbari-Kharaji, E.; Danaee, I.; Zaarei, D.; Shishesaz, M. Corrosion Behavior of Epoxy/Polysulfide Coatings Incorporated with Nano-CeO2 Particles on Low Carbon Steel Substrate. Corrosion 2022, 78, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Fathy, N.; Hafez, K.M.; Abdulaziz, F.; Abdelraouf, R.E.; Ramadan, M. Development of Hypoeutectic SnBi Alloy Solder Reinforced with WO3 Nanoparticles for Connecting Cu Substrates via Thermal Bonding. Sci. Rep. 2025, 15, 6849. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Khaliq, A.; Hafez, K.M.; Alghamdi, A.S.; Fathy, N.; Harraz, F.A.; Ayadi, B.; Abdel Halim, K.S. Super Bonding Strength of Al2O3 Nanoparticles Reinforced Sn Interlayer Steel/Aluminum Bimetal Casting. Crystals 2022, 12, 324. [Google Scholar] [CrossRef]
- Diouf, P.; Jones, A. Investigation of Bond Strength in Centrifugal Lining of Babbitt on Cast Iron. Metall. Mater. Trans. A 2010, 41, 603–609. [Google Scholar] [CrossRef]
- Ramadan, M.; Hafez, K.M.; Alghamdi, A.S.; Ayadi, B.; Abdel Halim, K.S. Novel Approach for Using Ductile Iron as Substrate in Bimetallic Materials for Higher Interfacial Bonding Bearings. Int. Met. Cast. 2021, 11, 145. [Google Scholar]
- Horvath, B.; Illes, B.; Shinohara, T. Growth of Intermetallics between Sn/Ni/Cu, Sn/Ag/Cu and Sn/Cu Layered Structures. Thin Solid Films 2014, 556, 345–353. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Li, N.; Wang, R. Growth Behavior of Intermetallic Compounds in Fe/Sn Diffusion Couples. J. Mater. Sci. Mater. Electron. 2019, 30, 12639–12646. [Google Scholar] [CrossRef]
- Vaynman, S.; McKeown, S.A. Energy-Based Methodology for the Fatigue Life Prediction of Solder Materials. IEEE Trans. Compon. Hybrids Manuf. Technol. 1993, 16, 317–322. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, S.M.; Li, Z.H.; Hou, C.J.; Li, G.Y. Morphological Evolution and Growth Kinetics of Interfacial Cu6Sn5 and Cu3Sn Layers in Low-Ag Sn–0.3Ag–0.7Cu–xMn/Cu Solder Joints During Isothermal Ageing. J. Electron. Mater. 2018, 47, 5913–5929. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M. Kinetics of Isothermal Reactive Diffusion Between Solid Fe and Liquid Al. J. Mater. Sci. 2010, 45, 5676–5684. [Google Scholar] [CrossRef]
- Suzuki, T.; Kajihara, M. Kinetics of Isothermal Reactive Diffusion Between Solid Cu and Liquid Sn. J. Electron. Mater. 2018, 47, 18–26. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Y.; Li, Y.; Liu, Y.; Wang, Y. Effect of Fe2O3 Nanoparticles Size on the Properties of Sn–1.0Ag–0.5Cu Nano-Composite Solders and Joints. J. Alloy. Compd. 2016, 662, 272–282. [Google Scholar] [CrossRef]
- Mohammadnejad, M.; Habibolahzadeh, A.; Yousefpour, M. Effect of Nano-Oxide Addition on Corrosion Performance of Hot Dip Zinc Coating. Prot. Met. Phys. Chem. Surf. 2016, 52, 100–103. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Han, J.P.; Li, Y. Effect of Tin on Corrosion Resistance and Formability of Tin Bearing Ferritic Stainless Steel. Mater. Res. Innov. 2014, 18, 9–11. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the Formation and Growth of Intermetallic Phases during Interdiffusion between Low Carbon Steel and Aluminum Alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In Situ Raman Spectroscopy and Electrochemical Techniques for Studying Corrosion and Corrosion Inhibition of Iron in Sodium Chloride Solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Ahmed, A.H. Alleviation of Iron Corrosion in Chloride Solution by N,N′-bis[2-Methoxynaphthylidene]amino]oxamide as a Corrosion Inhibitor. Crystals 2021, 11, 1516. [Google Scholar] [CrossRef]
- Yao, J.L.; Ren, B.; Huang, Z.F.; Cao, P.G.; Gu, R.A.; Tian, Z.-Q. Extending Surface Raman Spectroscopy to Transition Metals for Practical Applications IV. A Study on Corrosion Inhibition of Benzotriazole on Bare Fe Electrodes. Electrochim. Acta 2003, 48, 1263–1271. [Google Scholar] [CrossRef]
- Sliem, M.H.; Radwan, A.B.; Mohamed, F.S.; Alnuaimi, N.A.; Abdullah, A.M. An Efficient Green Ionic Liquid for the Corrosion Inhibition of Reinforcement Steel in Neutral and Alkaline Highly Saline Simulated Concrete Pore Solutions. Sci. Rep. 2020, 10, 14565. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Daniyan, A.A.; Umoru, L.E.; Fayomi, O.S.I. Effect of WO3 Nanoparticle Loading on the Microstructural, Mechanical and Corrosion Resistance of Zn Matrix/TiO2–WO3 Nanocomposite Coatings for Marine Application. J. Mar. Sci. Appl. 2017, 16, 8. [Google Scholar] [CrossRef]
- Feng, X.; Qiu, F. Study on the Corrosion Resistance and Application of Nano-Y2O3/Al2O3-Modified Anchor Rod Coatings Based on Electrodeposition Method. Electrochem 2025, 6, 14. [Google Scholar] [CrossRef]
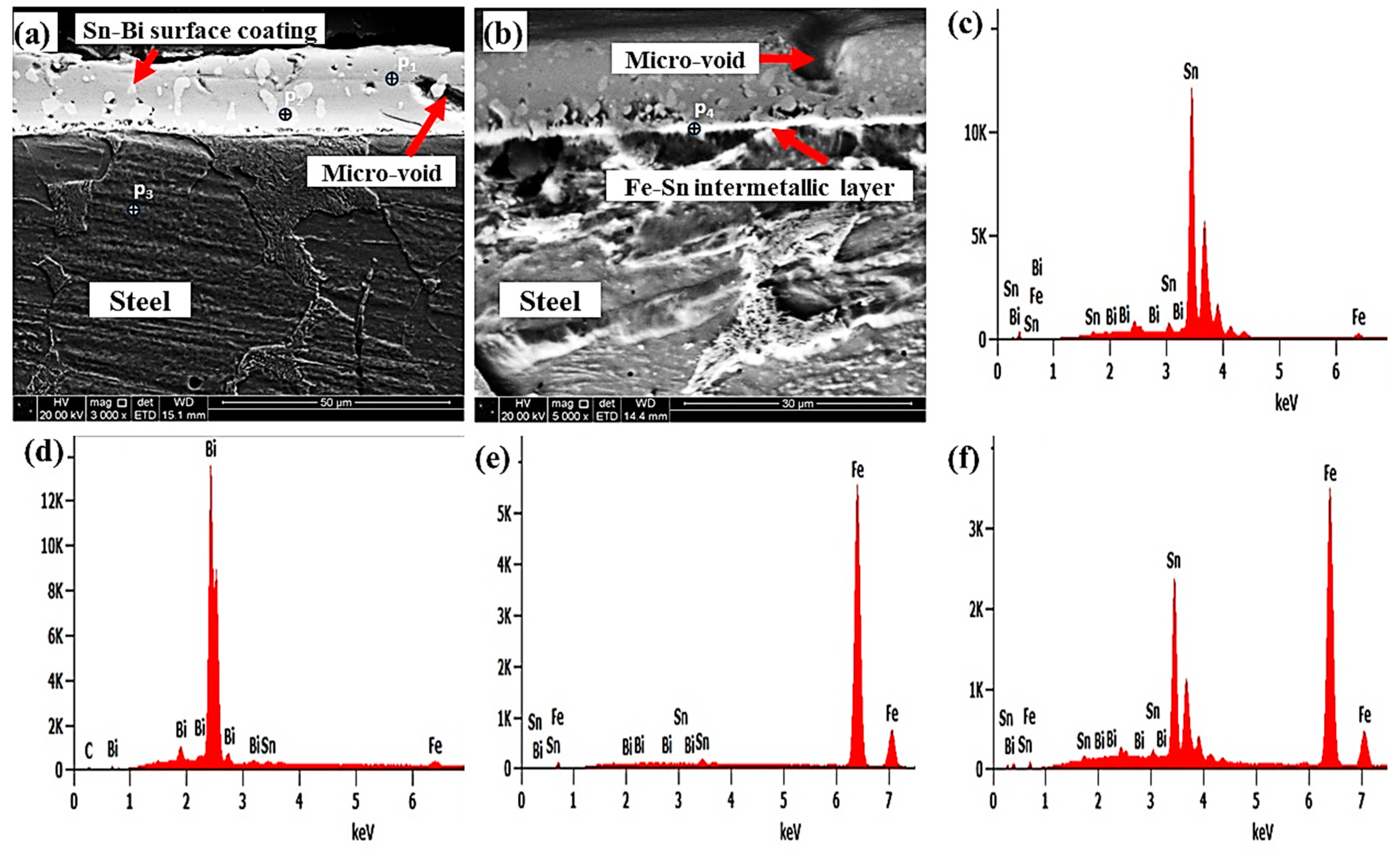
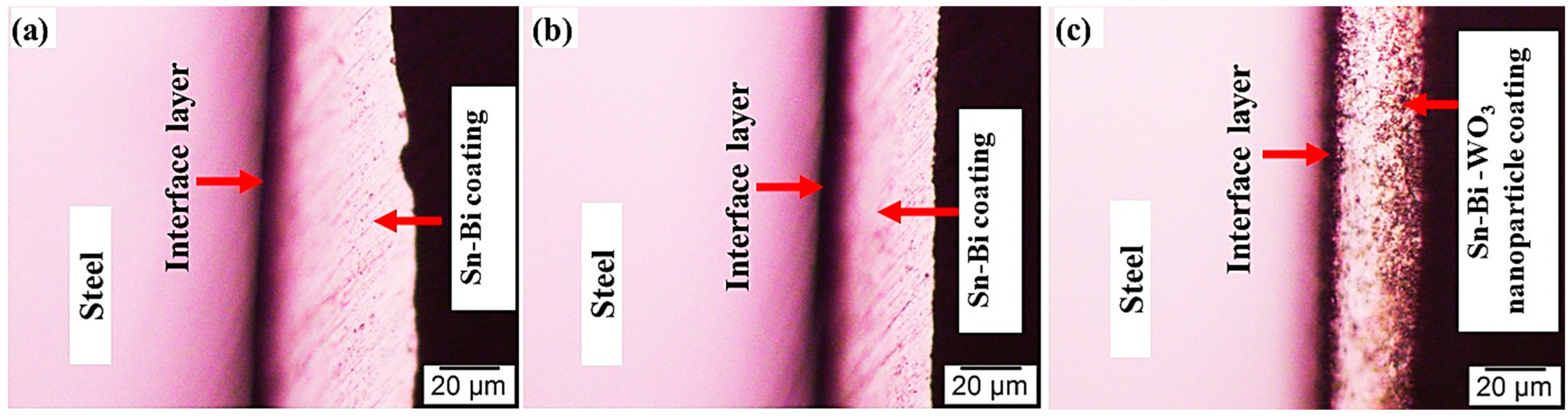



| Elements | C | Si | Mn | Cu | Cr | Ni | Al | Fe |
|---|---|---|---|---|---|---|---|---|
| Low-carbon steel substrate (LCS) | 0.18 | 0.08 | 0.35 | 0.03 | 0.02 | 0.05 | 0.05 | Bal. |
| Sample Number | Coating Powder/Flux P:F | Heating Time, min | Sample Composition |
|---|---|---|---|
| Sample #1 | 0 | 0 | Uncoated (LCS) |
| Sample #2 | 1:8 | 3 | LCS + S20B alloy coating |
| Sample #3 | 1:6 | 3 | LCS + S20B alloy coating |
| Sample #4 | 1:6 | 4 | LCS + S20B alloy coating |
| Sample #5 | 1:6 | 5 | LCS + S20B alloy coating |
| Sample #6 | 1:6 | 4 | LCS + S20B + 0.25% WO3 nanoparticle alloy coating |
| Sample #7 | 1:6 | 5 | LCS + S20B + 0.25% WO3 nanoparticle alloy coating |
| Sample | βc (mV/dec) | ECorr (mV) | βa (mV/dec) | jCorr (μA/cm2) | EPit (mV) | EProt (mV) | RP (Ω cm2) | RCorr (mmy−1) | PE (%) |
|---|---|---|---|---|---|---|---|---|---|
| Sample #1 | 110 | −625 | 100 | 36 | −360 | −495 | 633 | 0.424 | -- |
| Sample #2 | 105 | −695 | 95 | 18 | −540 | −600 | 1205 | 0.212 | 50 |
| Sample #3 | 100 | −655 | 90 | 17 | −260 | −455 | 1211 | 0.200 | 53 |
| Sample #5 | 80 | −690 | 90 | 12 | −540 | −655 | 1535 | 0.141 | 67 |
| Sample #7 | 75 | −665 | 95 | 10 | −420 | −225 | 1822 | 0.118 | 72 |
| Sample | Impedance Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RS/ Ω cm2 | Q1 | RP1/ Ω cm2 | Q2 | RP2/ Ω cm2 | RPT Ω cm2 | PE % | |||
| YQ1/µF cm−2 | n | YQ2/F cm−2 | n | ||||||
| Sample #1 | 9.81 | 0.0072 | 1.0 | 11.1 | 0.0016 | 0.80 | 682 | 693.1 | -- |
| Sample #2 | 10.98 | 0.0695 | 1.0 | 13.73 | 0.00318 | 0.71 | 730 | 743.73 | 6.8 |
| Sample #3 | 11.34 | 0.0359 | 0.8 | 17.57 | 0.00598 | 0.76 | 785 | 802.57 | 13.6 |
| Sample #5 | 13.32 | 0.0062 | 1.0 | 18.98 | 0.0012 | 0.89 | 1400 | 1418.98 | 51.2 |
| Sample #7 | 14.29 | 0.0055 | 1.0 | 23.44 | 0.0014 | 0.78 | 4363 | 4386.44 | 84.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, N.; Ramadan, M.; Al-Shomar, S.M.; Hafez, K.M.; Sherif, E.-S.M.; Alshammari, A.H.; Abdel Halim, K.S. The Effect of Powder-to-Flux Ratio and Heating Duration on the Microstructure and Corrosion Resistance of WO3 Nanoparticle-Reinforced Sn–20Bi Coatings on Low-Carbon Steel. Crystals 2025, 15, 551. https://doi.org/10.3390/cryst15060551
Fathy N, Ramadan M, Al-Shomar SM, Hafez KM, Sherif E-SM, Alshammari AH, Abdel Halim KS. The Effect of Powder-to-Flux Ratio and Heating Duration on the Microstructure and Corrosion Resistance of WO3 Nanoparticle-Reinforced Sn–20Bi Coatings on Low-Carbon Steel. Crystals. 2025; 15(6):551. https://doi.org/10.3390/cryst15060551
Chicago/Turabian StyleFathy, Naglaa, Mohamed Ramadan, Shereen Mohammad Al-Shomar, Khalid M. Hafez, El-Sayed M. Sherif, Alhulw H. Alshammari, and K. S. Abdel Halim. 2025. "The Effect of Powder-to-Flux Ratio and Heating Duration on the Microstructure and Corrosion Resistance of WO3 Nanoparticle-Reinforced Sn–20Bi Coatings on Low-Carbon Steel" Crystals 15, no. 6: 551. https://doi.org/10.3390/cryst15060551
APA StyleFathy, N., Ramadan, M., Al-Shomar, S. M., Hafez, K. M., Sherif, E.-S. M., Alshammari, A. H., & Abdel Halim, K. S. (2025). The Effect of Powder-to-Flux Ratio and Heating Duration on the Microstructure and Corrosion Resistance of WO3 Nanoparticle-Reinforced Sn–20Bi Coatings on Low-Carbon Steel. Crystals, 15(6), 551. https://doi.org/10.3390/cryst15060551









