Epitaxial Growth of BaBiO3 Thin Films on SrTiO3(001) and MgO(001) Substrates Using Molecular Beam Epitaxy: Controlling the Competition Between Crystal Orientations
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiabrera, F.; Yun, S.; Li, Y.; Dahm, R.T.; Zhang, H.; Kirchert, C.K.R.; Christensen, D.V.; Trier, F.; Jespersen, T.S.; Pryds, N.; et al. Freestanding perovskite oxide films: Synthesis, challenges, and properties. Ann. Phys. 2022, 534, 2200084. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Lee, S.A.; Choi, W.S.; Kang, K.T. Complex oxide thin films: A review on pulsed laser epitaxy growth. Curr. Appl. Phys. 2024, 68, 113–130. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Liu, Z.; Zhou, B.; Zhou, L.; Deng, H.; Long, Y. Emergent physical properties of perovskite-type oxides prepared under high pressure. Dalton Trans. 2022, 51, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Scholder, R.; Ganter, K.; Glaser, H.; Merz, G. Alkali and alkali earth bismuthates (V). Z. FüR Anorg. Und Allg. Chem. 1963, 319, 375–377. [Google Scholar] [CrossRef]
- Cox, D.; Sleight, A. Mixed-valent Ba2Bi3+Bi5+O6: Structure and properties vs temperature. Struct. Sci. 1979, 35, 1–10. [Google Scholar] [CrossRef]
- Vesto, R.; Choi, H.; Kim, K. Observation of bandgap closing in SrxBa1-xBiO3 films: Evidence toward topological order in BaBiO3. J. Appl. Phys. 2022, 132, 015102. [Google Scholar] [CrossRef]
- Ahmed, I.; Kortov, M.; Sergeant, S.; Nuytten, T.; Conrad, T.; De Gendt, S.; Merckling, C. Influence of thickness scaling on the electronic structure and optical properties of oxygen-deficient BaBiO3-δ thin films grown on SrTiO3-buffered Si (001) substrate. APL Mater. 2024, 12, 031105. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient photocatalysis on BaBiO3 driven by visible light. J. Phys. Chem. C 2007, 111, 12779–12785. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Athresh, E.; Ranjan, R.; Raghavan, S.; Avasthi, S. BaBiO3: A potential absorber for all-oxide photovoltaics. Mater. Lett. 2018, 210, 218–222. [Google Scholar] [CrossRef]
- Huerta-Flores, M.A.; Sánchez-Martínez, D.; Fernández-Romo, M.R.; Zarazúa-Morín, M.E.; Torres-Martínez, L.M. Visible-light-driven BaBiO3 perovskite photocatalyst: Effect of physicochemical properties on the photoactivity towards water splitting and the removal of rhodamine B from aqueous systems. J. Photochem. Photobiol. A Chem. 2019, 368, 70–77. [Google Scholar] [CrossRef]
- Khraisheh, M.M.; Khzandar, A.; Al-Ghouti, M.A. Visible light-driven metal-oxide photocatalytic CO2 conversion. Int. J. Energy Res. 2015, 39, 1142–1152. [Google Scholar] [CrossRef]
- Acero, G.; Moreno, H.; Ortega, P.; Ramirez, M.; Montes, M.; Moura, F.; Simões, A. Unveiling the polar properties on barium bismuthate perovskite thin films with distinct Ba/Bi ratios. J. Alloys Compd. 2022, 974, 172871. [Google Scholar] [CrossRef]
- Silhue, K.V.; Sahoo, S.C.; Thomas, K.J. Novel ferromagnetism and negative magnetoresistance in BaBiO3 nanoparticles. Appl. Mater. Today 2022, 27, 101427. [Google Scholar]
- Bouwmeester, R.L.; de Hond, K.; Gauquelin, N.; Verbeeck, J.; Koster, G.; Brinkman, A. Stabilization of the perovskite phase in the Y–Bi–O system by using a BaBiO3 buffer layer. Phys. Status Solidi (RRL) Rapid Res. Lett. 2019, 13, 1800679. [Google Scholar] [CrossRef]
- Chambers, S.A. Epitaxial growth and properties of doped transition metal and complex oxide films. Adv. Mater. 2010, 22, 219–248. [Google Scholar] [CrossRef]
- Harris, D.T.; Campbell, N.; Uecker, R.; Brützam, M.; Schlom, D.G.; Levchenko, A.; Rzchowski, M.S.; Eom, C.B. Superconductivity-localization interplay and fluctuation magnetoresistance in epitaxial BaPb1-xBixO3 thin films. Phys. Rev. Mater. 2018, 2, 041801. [Google Scholar] [CrossRef]
- Griffitt, S.; Spaić, M.; Joe, J.; Anderson, Z.W.; Zhai, D.; Krogstad, M.J.; Osborn, R.; Pelc, D.; Greven, M. Local inversion-symmetry breaking in a bismuthate high-Tc superconductor. Nat. Commun. 2023, 14, 845. [Google Scholar] [CrossRef]
- Yan, B.; Jansen, M.; Felser, C. A large-energy-gap oxide topological insulator based on the superconductor BaBiO3. Nat. Phys. 2013, 9, 709–711. [Google Scholar] [CrossRef]
- Tian, W.; Yu, W.; Shi, J.; Wang, Y. The property, preparation and application of topological insulators: A review. Mater. Today 2017, 10, 814. [Google Scholar] [CrossRef]
- Xu, N.; Xu, Y.; Zhu, J. Topological insulators for thermoelectrics. NPJ Quantum Mater. 2017, 2, 51. [Google Scholar] [CrossRef]
- Ni, X.; Yves, S.; Krasnok, A.; Alu, A. Topological metamaterials. Chem. Rev. 2023, 123, 7585–7654. [Google Scholar] [CrossRef]
- Bhatia, A.A.; Hautier, G.; Nilgianskul, T.; Miglio, A.; Sun, J.; Kim, H.J.; Kim, K.H.; Chen, S.; Rignanese, G.M.; Gonze, X.; et al. High-mobility bismuth-based transparent p-type oxide from high-throughput material screening. Chem. Mater. 2016, 28, 30–34. [Google Scholar] [CrossRef]
- Pan, Z.; Hu, Y.; Chen, J.; Wang, F.; Jeong, Y.; Pham, D.P.; Yi, J. Approaches to improve mobility and stability of IGZO TFTs: A brief review. Trans. Electr. Electron. Mater. 2024, 25, 371–379. [Google Scholar] [CrossRef]
- Nunn, K.; Kruitman, T.; Jalan, B. A review of molecular-beam epitaxy of wide bandgap complex oxide semiconductors. J. Mater. Res. 2021, 36, 1–19. [Google Scholar] [CrossRef]
- Brahlek, M.; Gupta, A.S.; Lapano, J.; Roth, J.; Zhang, H.T.; Zhang, L.; Haislmaier, R.; Engel-Herbert, R. Frontiers in the growth of complex oxide thin films: Past, present, and future of hybrid MBE. Adv. Funct. Mater. 2018, 28, 1702772. [Google Scholar] [CrossRef]
- Ahmed, I.; De Gendt, S.; Merckling, C. Self-regulating plasma-assisted growth of epitaxial BaBiO3 thin film on SrTiO3-buffered Si (001) substrate. J. Appl. Phys. 2022, 132, 225304. [Google Scholar] [CrossRef]
- Hellman, E.; Hartford, E.; Fleming, R. Molecular beam epitaxy of superconducting (Rb, Ba)BiO3. Appl. Phys. Lett. 1989, 55, 2120–2122. [Google Scholar] [CrossRef]
- Iyori, M.; Suzuki, S.; Yamano, K.; Suzuki, H.; Takahashi, K.; Yoshisada, Y. Preparation of BaBiO3 thin films using an oxygen radical beam source. J. Cryst. Growth 1995, 150, 1086–1089. [Google Scholar] [CrossRef]
- Makita, T.T.; Abe, H.A. Control of crystal orientation for BaBiO3 thin film on SrTiO3 (100) substrate using BaO buffer layer. Jpn. J. Appl. Phys. 1997, 36, L96. [Google Scholar] [CrossRef]
- Mijatovic, D.; Rijnders, G.; Hilgenkamp, H.; Blank, D.H.; MacManus-Driscoll, J. Growth studies of Ba1-xKxBiO3 thin films using pulsed-laser deposition. Phys. C Supercond. 2002, 372, 594–598. [Google Scholar]
- Muta, S.; Nishikawa, S.; Ichinose, A.; Sato, Y.; Arita, M.; Shimakawa, Y.; Mukaida, H. Growth and photo-response of c-axis-oriented BaBiO3 films on SrTiO3 (001) substrates. Thin Solid Films 2022, 749, 139167. [Google Scholar] [CrossRef]
- Tellekamp, M.B.; Shank, J.C.; Goorsky, M.S.; Doering, W.A. Molecular beam epitaxy growth of high crystalline-quality LiNbO3. J. Electron. Mater. 2016, 45, 6292–6299. [Google Scholar] [CrossRef]
- Springell, R.; Bright, E.L.; Chaney, D.A.; Harding, L.M.; Bell, C.; Ward, R.C.C.; Lander, G.H. A review of uranium-based thin films. Adv. Phys. 2022, 71, 87–165. [Google Scholar] [CrossRef]
- Dahiya, A.; Chuhadiya, S.; Nehra, S.P.; Dhaka, M.S.; Himanshu; Suthar, D.; Nehra, S.P.; Dhaka, M.S. Achieving phase stability in ZnSe thin films by thickness and annealing recipes for optical window applications. J. Mater. Sci. Mater. Electron. 2023, 34, 410. [Google Scholar] [CrossRef]
- Hao, M.H.; Van Thourhout, D.; Pantouvaki, M.; Meersschaut, J.; Conard, T.; Richard, O.; Bender, H.; Favia, P.; Vila, M.; Cid, R.; et al. Controlled orientation of molecular-beam-epitaxial BaTiO3 on Si (001) using thickness engineering of BaTiO3 and SrTiO3 buffer layers. Appl. Phys. Express 2017, 10, 065501. [Google Scholar]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Zapf, M.; Stübinger, M.; Jin, L.; Kamp, M.; Pfaff, F.; Lubk, A.; Büchner, B.; Sing, M.; Claessen, R. Domain matching epitaxy of BaBiO3 on SrTiO3 with structurally modified interfaces. Appl. Phys. Lett. 2018, 112, 141601. [Google Scholar] [CrossRef]
- Jin, L.; Zapf, M.; Stübinger, M.; Kamp, M.; Sing, M.; Claessen, R.; Jia, C.L. Atomic-scale interface structure in domain matching epitaxial BaBiO3 thin films grown on SrTiO3 substrates. Phys. Status Solidi (RRL) Rapid Res. Lett. 2020, 14, 2000054. [Google Scholar] [CrossRef]
- Egitis, R.I.; Jia, R. Review of systematic tendencies in (001),(011) and (111) surfaces using B3PW and B3LYP computations of BaTiO3, CaTiO3, PbTiO3, SrTiO3, BaZrO3, CaZrO3, PbZrO3 and SrZrO3 perovskites. Materials 2023, 16, 7623. [Google Scholar] [CrossRef]
- Zhong, M.; Zeng, W.; Liu, F.S.; Tang, B.; Liu, Q.J. First-principles study of the atomic structures, electronic properties, and surface stability of BaTiO3 (001) and (011) surfaces. Surf. Interface Anal. 2019, 51, 1021–1032. [Google Scholar] [CrossRef]
- Ye, J.; Mou, S.; Zhu, R.; Liu, L.; Li, Y. Orientation competition growth and mechanism of SrTiO3 film on CeO2 layer. Vacuum 2021, 194, 110626. [Google Scholar] [CrossRef]
- Queralt, O.M.; De La Mata, M.; Arbiol, J.; Obradors, X.; Puig, T. Disentangling epitaxial growth mechanisms of solution derived functional oxide thin film. Adv. Funct. Mater. Interfaces 2016, 3, 1600392. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, Q.; Walker, B.; Gonder, C.A.; Romain, G.C.; Mundell, R.; Bohura, M.; Pradhan, A. Competition between (001) and (111) MgO thin film growth on Al-doped ZnO by oxygen plasma assisted pulsed laser deposition. J. Appl. Phys. 2013, 113, 214102. [Google Scholar] [CrossRef]
- Kamran, M.A.; Siddique, S.; Ullah, S.; Alharbi, T.; Raza, M.; Usama, M.; Zou, B. Bifunctional strontium-doped barium oxide nanorods as promising photocatalysts and electrodes for energy storage applications. J. Energy Storage 2023, 67, 107598. [Google Scholar] [CrossRef]
- Hellman, E.; Hartford, E. Adsorption controlled molecular beam epitaxy of rubidium barium bismuth oxide. J. Vac. Sci. Technol. B 1990, 8, 332–335. [Google Scholar] [CrossRef]
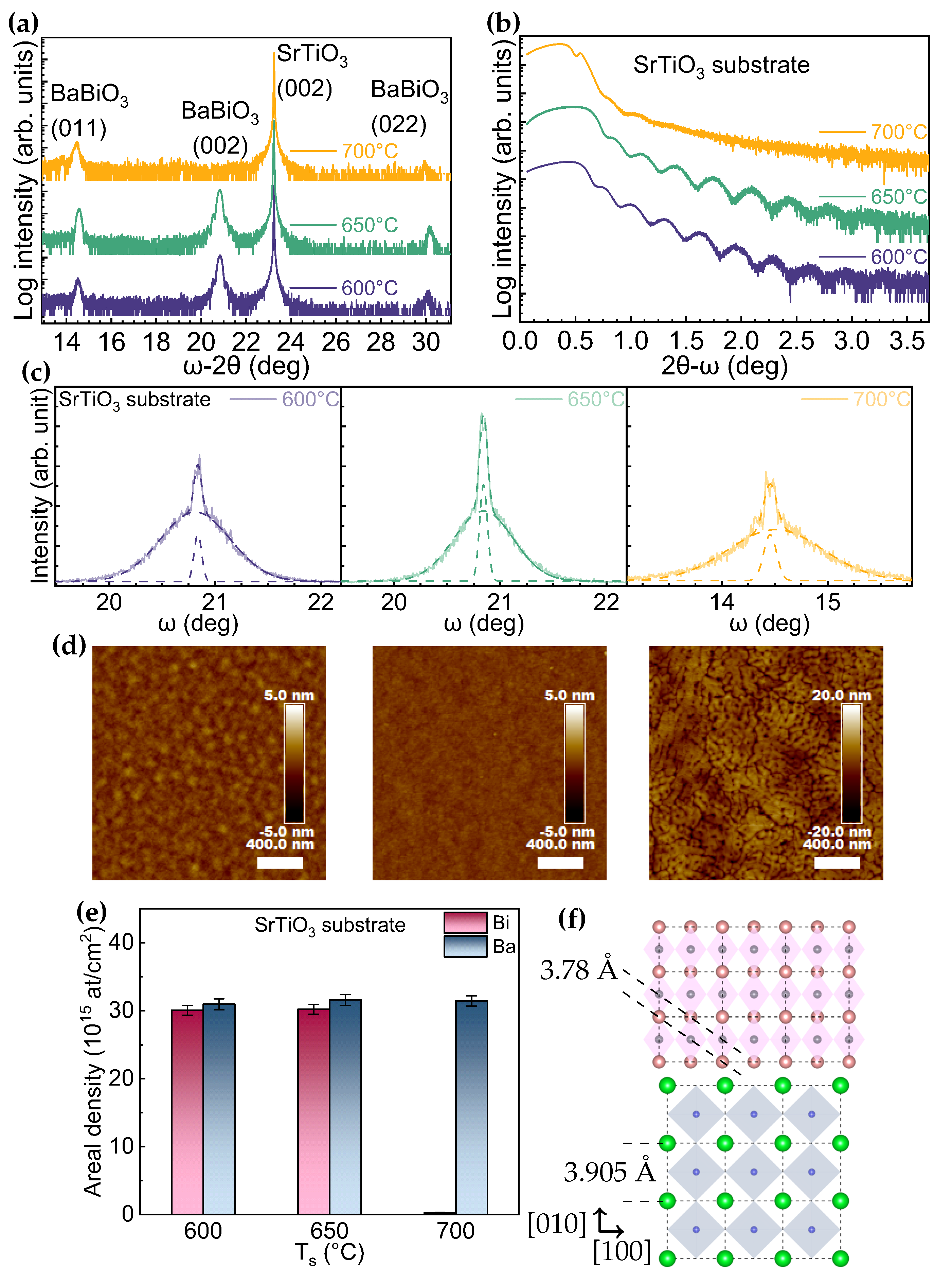
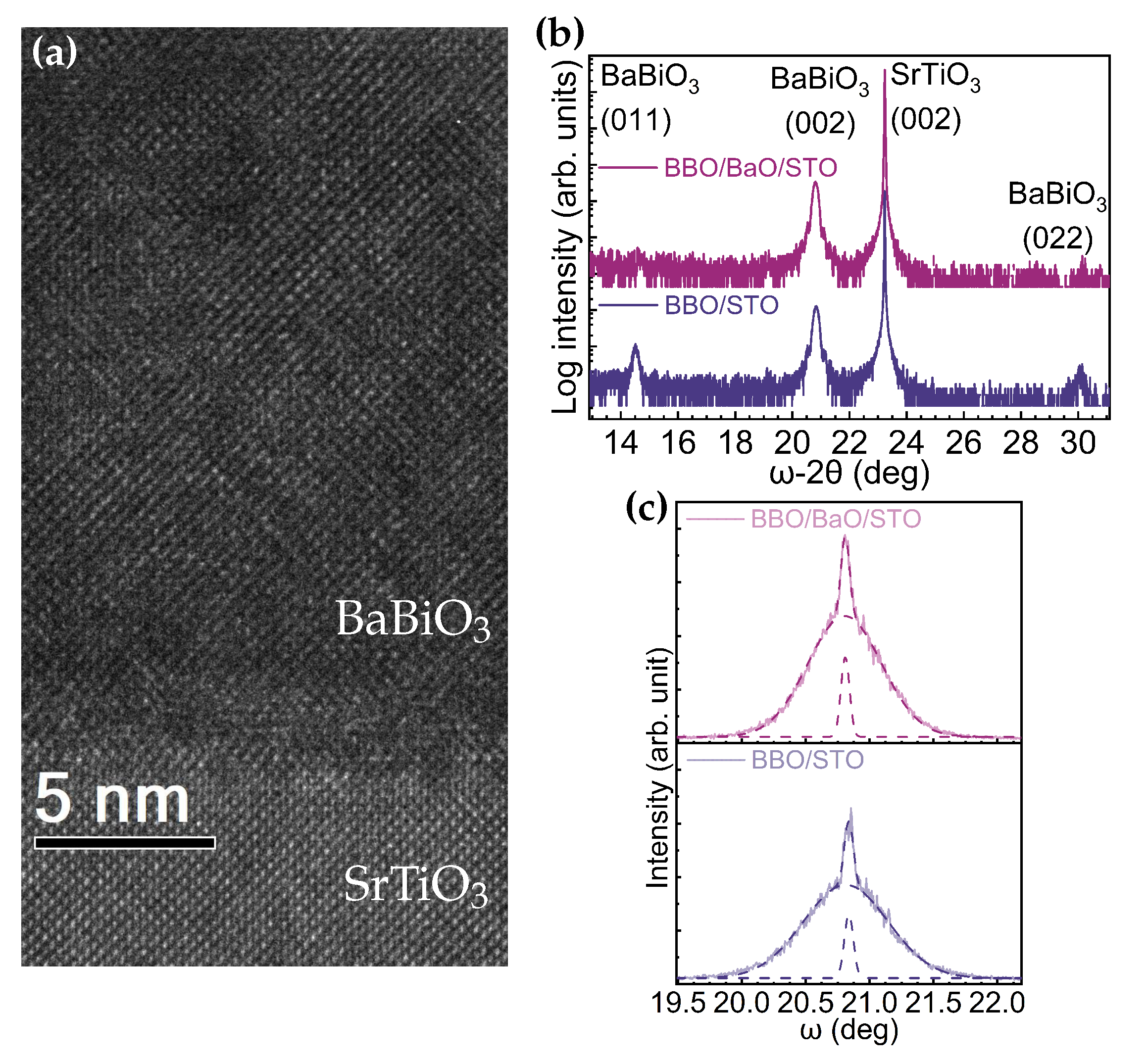
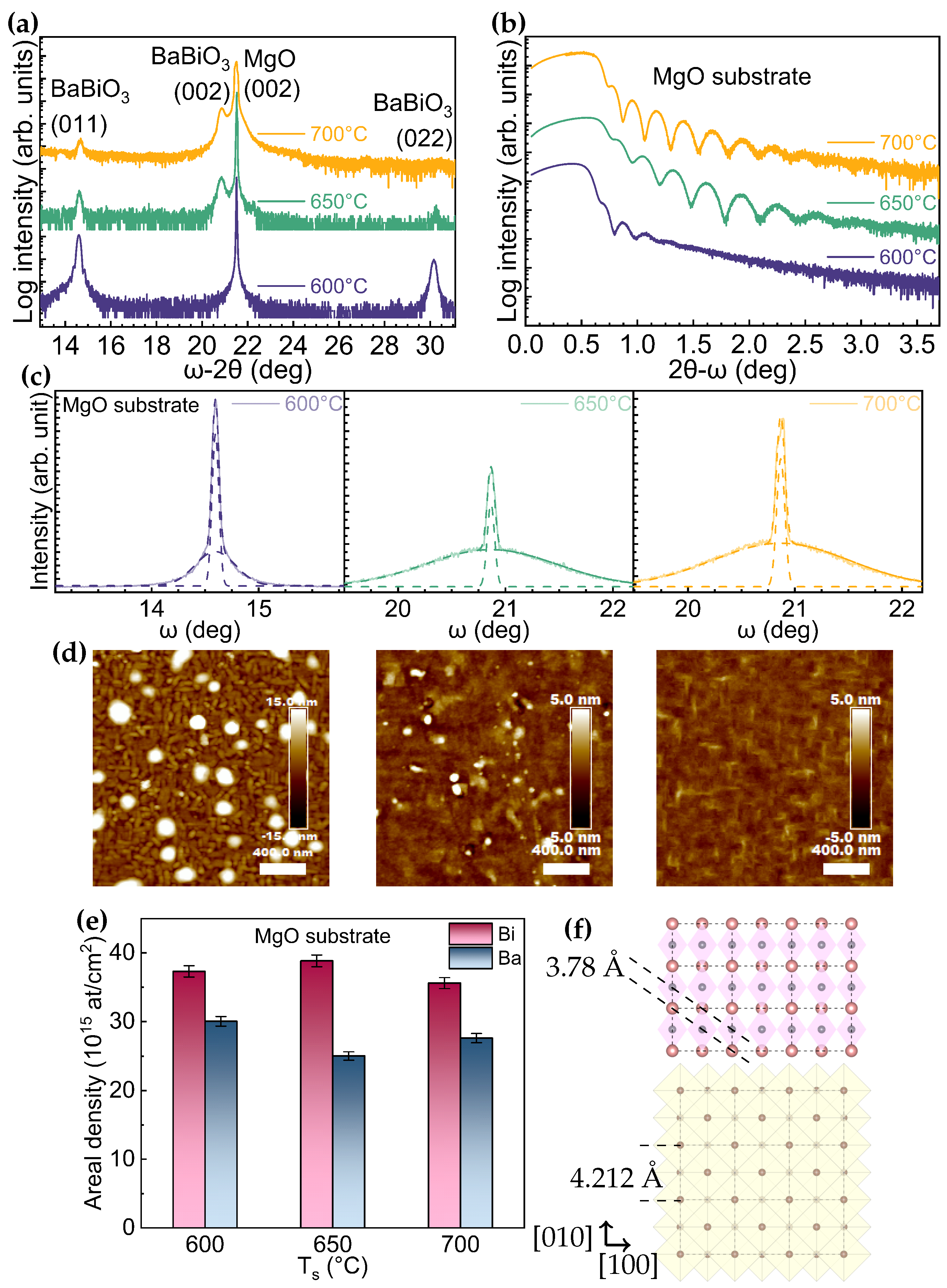
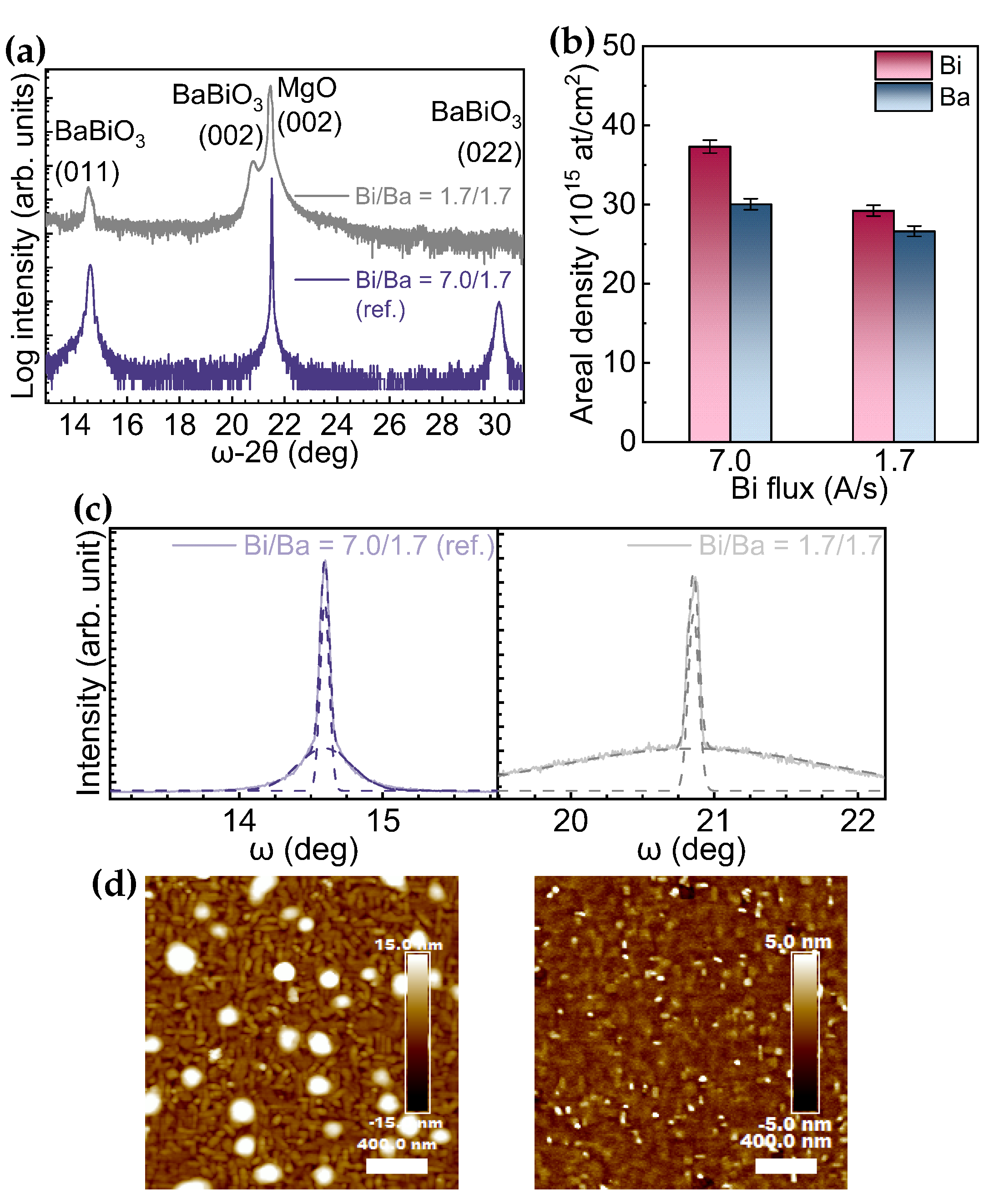
| Growth Condition | (°) | (011)/(001)+(011) | t (nm) | Rq (nm) | Bi/Ba (RBS Data) | ||
|---|---|---|---|---|---|---|---|
| STO substrate | 600 °C | 14.41 | 20.81 | 7.56% | 30 | 0.4 | 0.97 ± 0.03 |
| 650 °C | 14.56 | 20.80 | 11.81% | 27 | 0.2 | 0.96 ± 0.03 | |
| 700 °C | 14.45 | – | – | 35 | 3.5 | 0.008 ± 0.002 | |
| 600 °C (with BaO buffer layer) | 14.41 | 20.81 | 1.62% | 30 | |||
| MgO substrate | 600 °C | 14.60 | – | 100% | 33 ± 4 | 4.5 | 1.24 ± 0.04 |
| 650 °C | 14.60 | 20.86 | 6.44% | 32 ± 3 | 0.9 | 1.55 ± 0.05 | |
| 700 °C | 14.68 | 20.87 | 2.37% | 38 ± 5 | 0.5 | 1.29 ± 0.04 | |
| 600 °C (JBi/JBa = 1.7/1.7) | 14.57 | 20.81 | 54.49% | 25 | 1 | 1.09 ± 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.; De Gendt, S.; Merckling, C. Epitaxial Growth of BaBiO3 Thin Films on SrTiO3(001) and MgO(001) Substrates Using Molecular Beam Epitaxy: Controlling the Competition Between Crystal Orientations. Crystals 2025, 15, 534. https://doi.org/10.3390/cryst15060534
Ahmed I, De Gendt S, Merckling C. Epitaxial Growth of BaBiO3 Thin Films on SrTiO3(001) and MgO(001) Substrates Using Molecular Beam Epitaxy: Controlling the Competition Between Crystal Orientations. Crystals. 2025; 15(6):534. https://doi.org/10.3390/cryst15060534
Chicago/Turabian StyleAhmed, Islam, Stefan De Gendt, and Clement Merckling. 2025. "Epitaxial Growth of BaBiO3 Thin Films on SrTiO3(001) and MgO(001) Substrates Using Molecular Beam Epitaxy: Controlling the Competition Between Crystal Orientations" Crystals 15, no. 6: 534. https://doi.org/10.3390/cryst15060534
APA StyleAhmed, I., De Gendt, S., & Merckling, C. (2025). Epitaxial Growth of BaBiO3 Thin Films on SrTiO3(001) and MgO(001) Substrates Using Molecular Beam Epitaxy: Controlling the Competition Between Crystal Orientations. Crystals, 15(6), 534. https://doi.org/10.3390/cryst15060534






