Crystallization of Small Molecules in Microgravity Using Pharmaceutical In-Space Laboratory–Biocrystal Optimization eXperiment (PIL-BOX)
Abstract
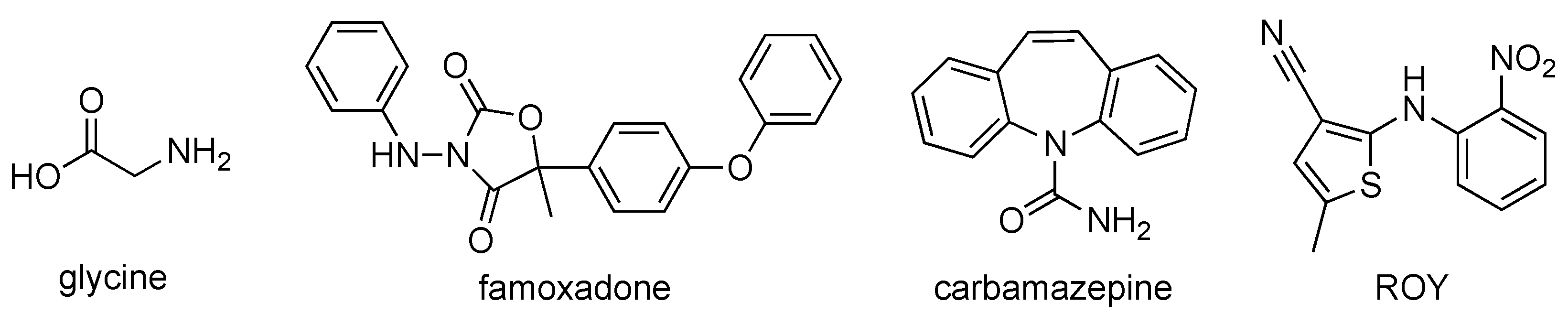
:1. Introduction
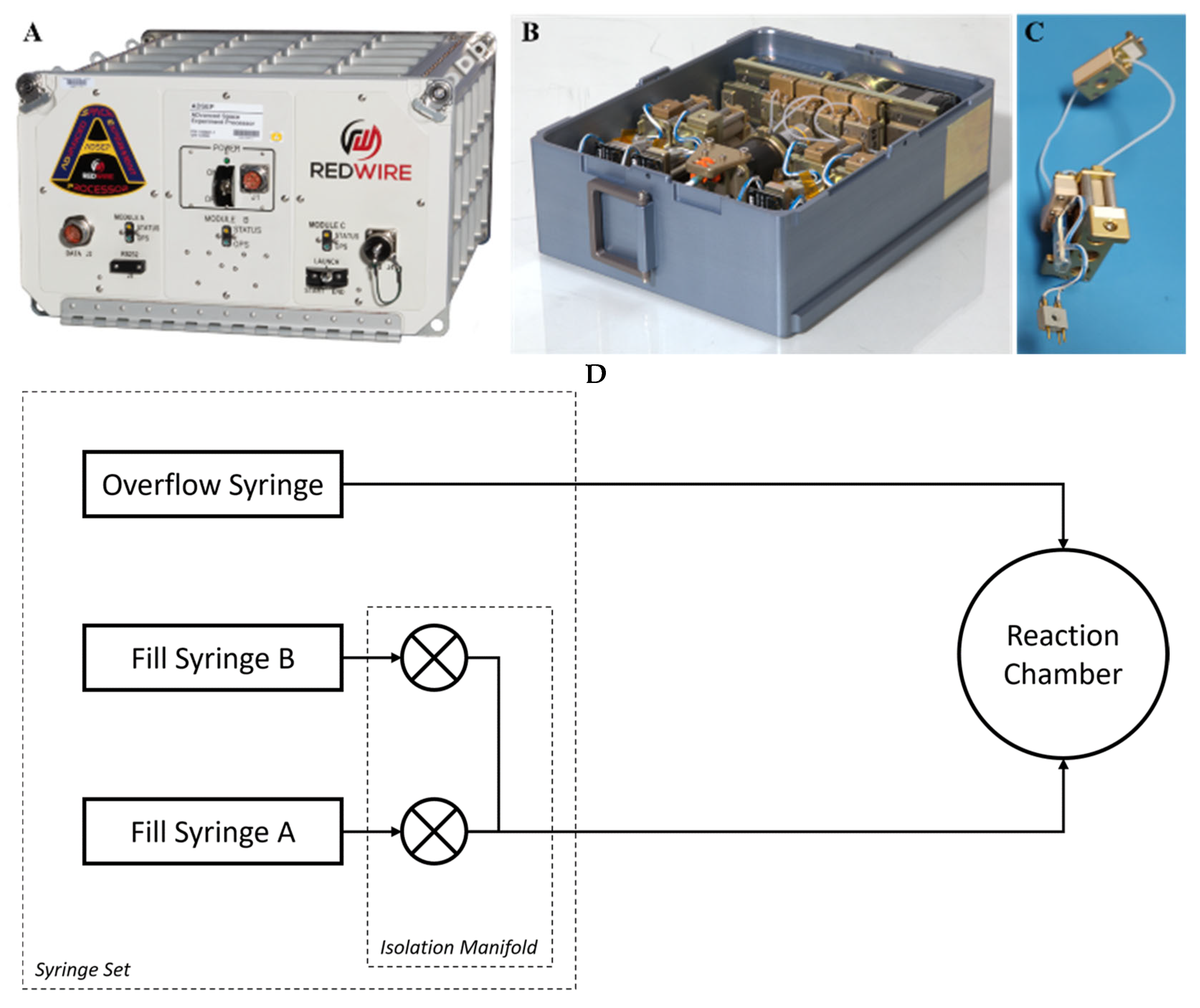
2. Materials and Methods
3. Results
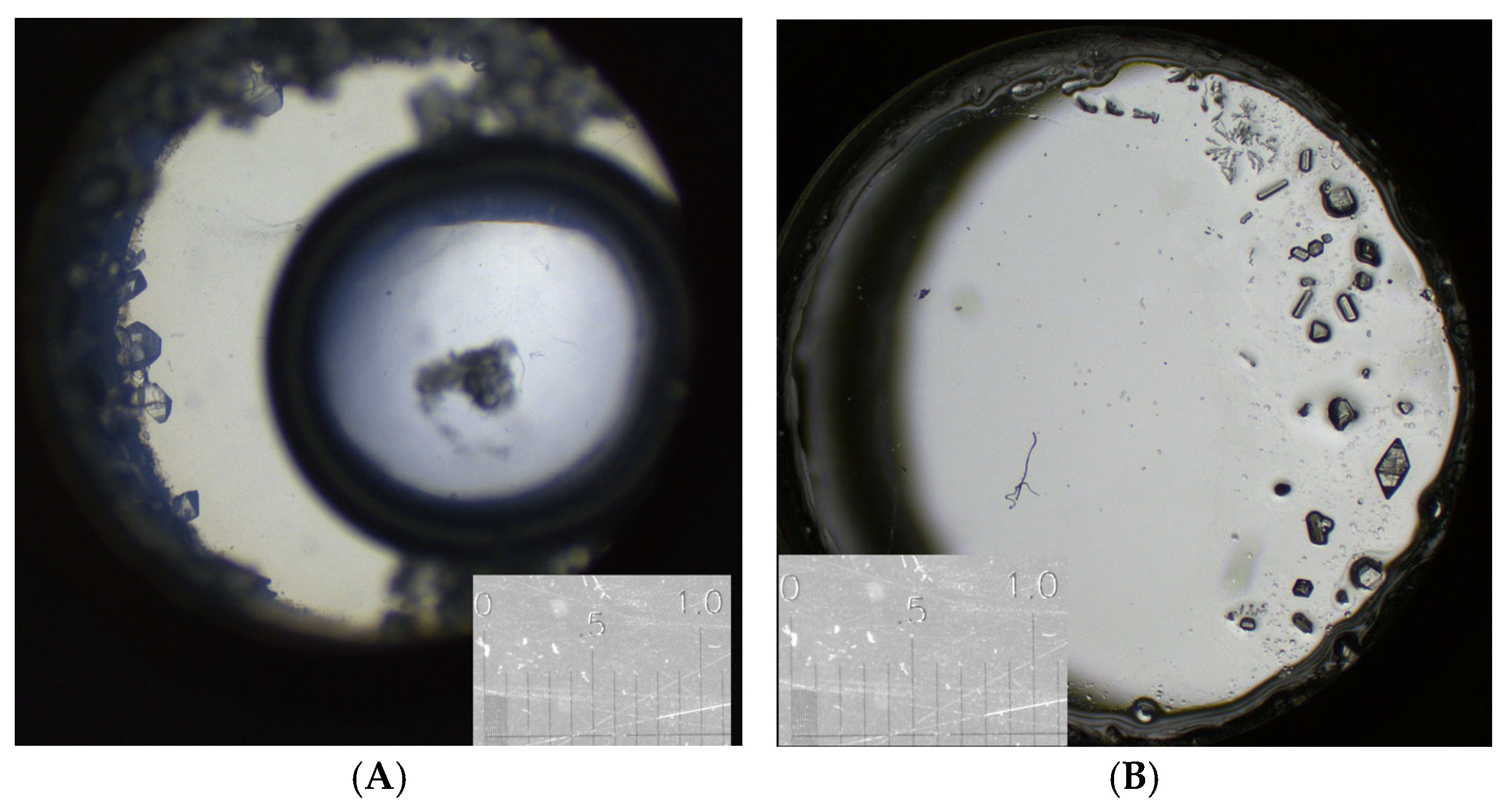
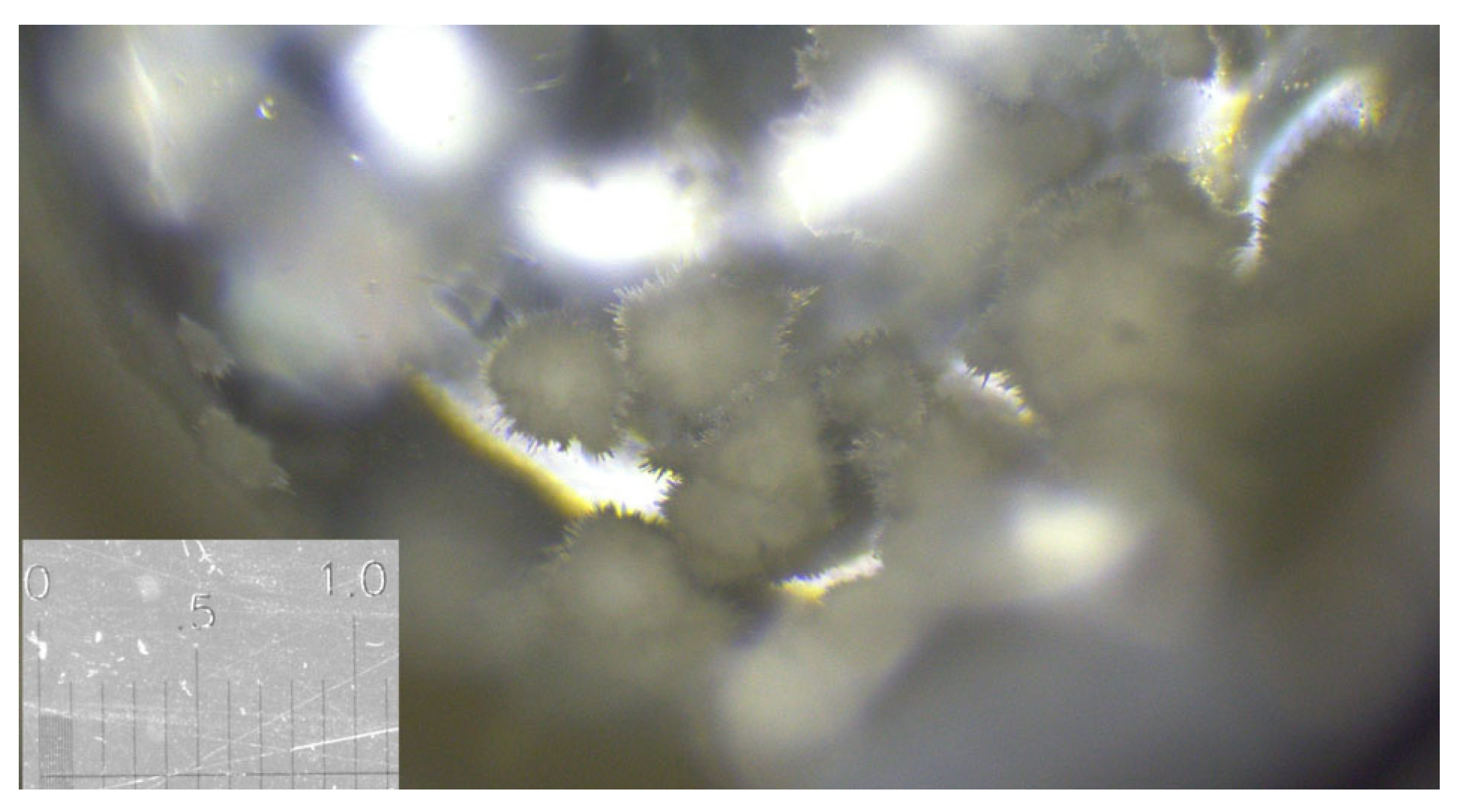
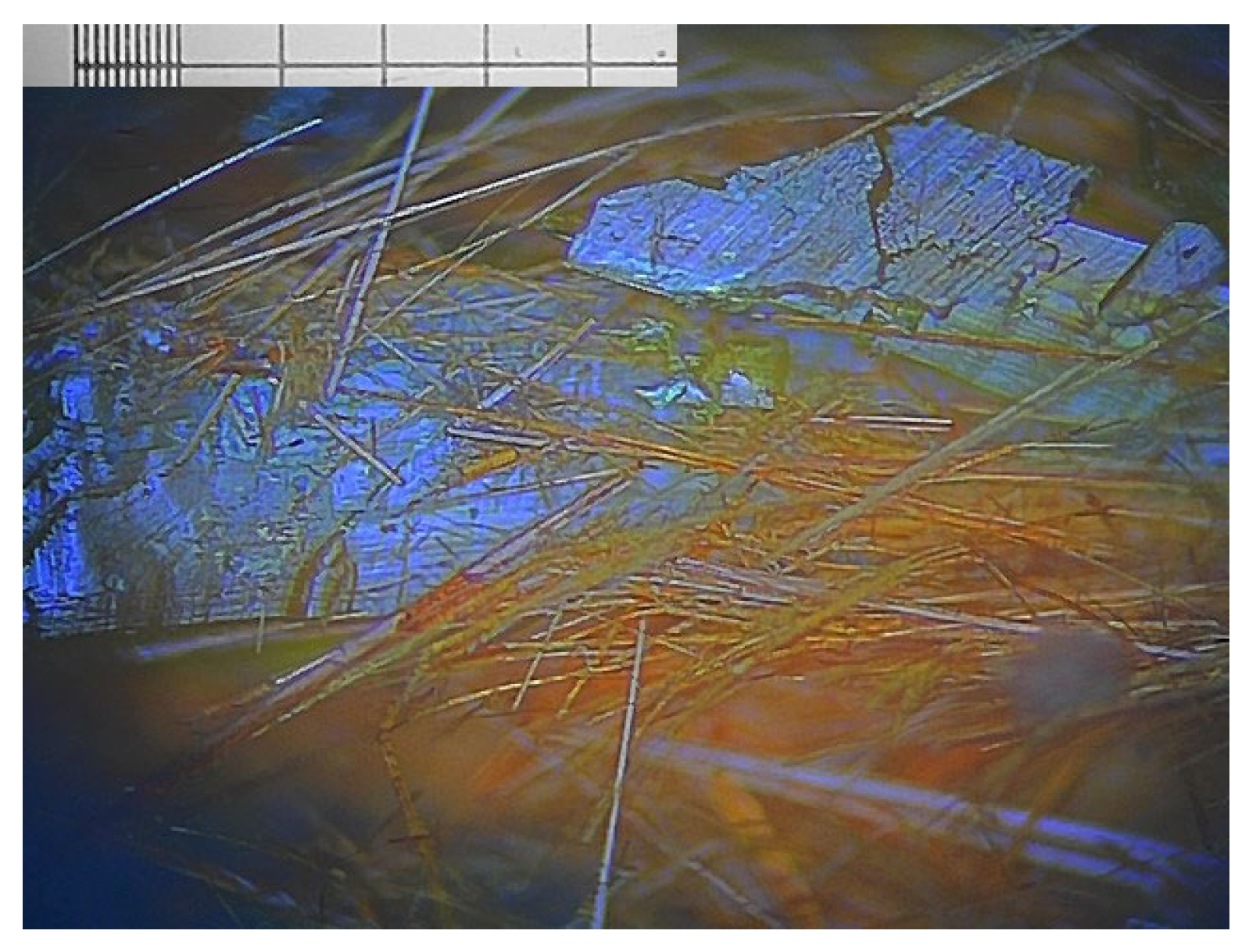
3.1. Glycine
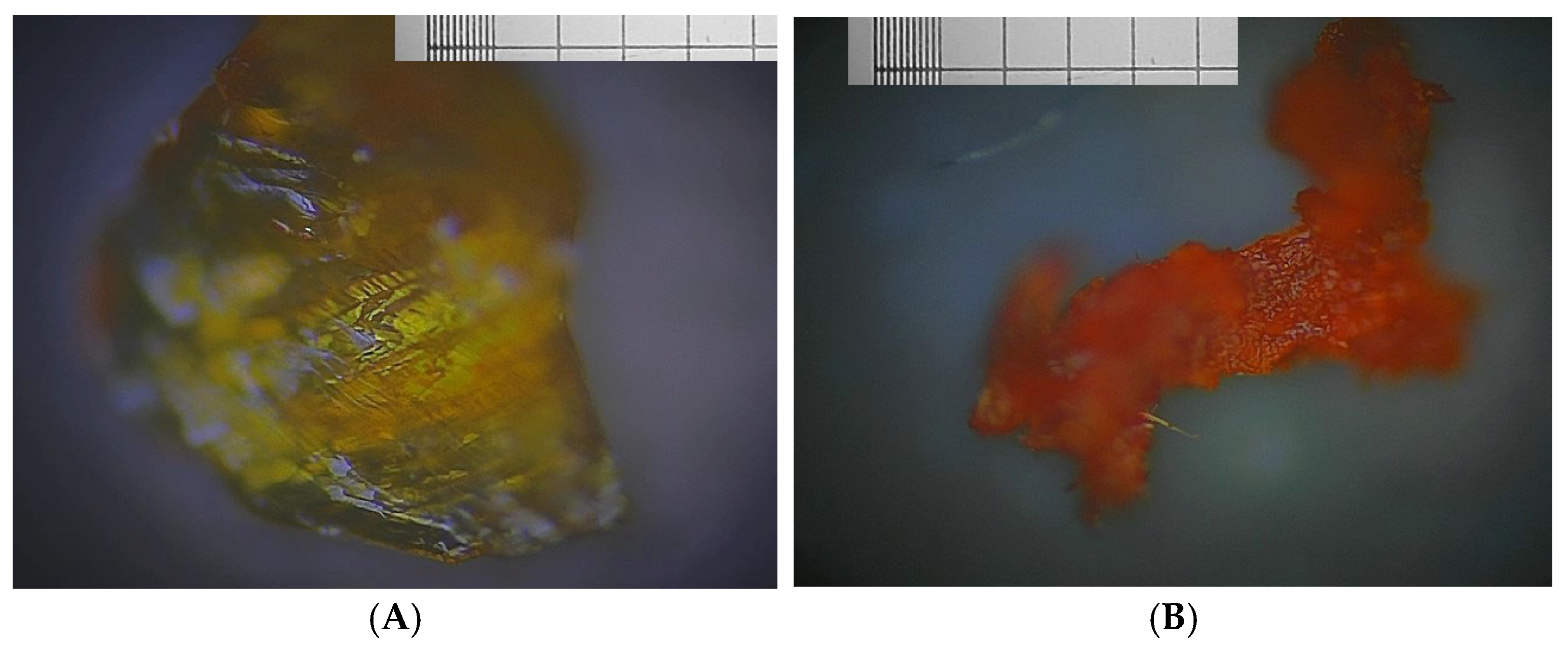
3.2. Famoxadone
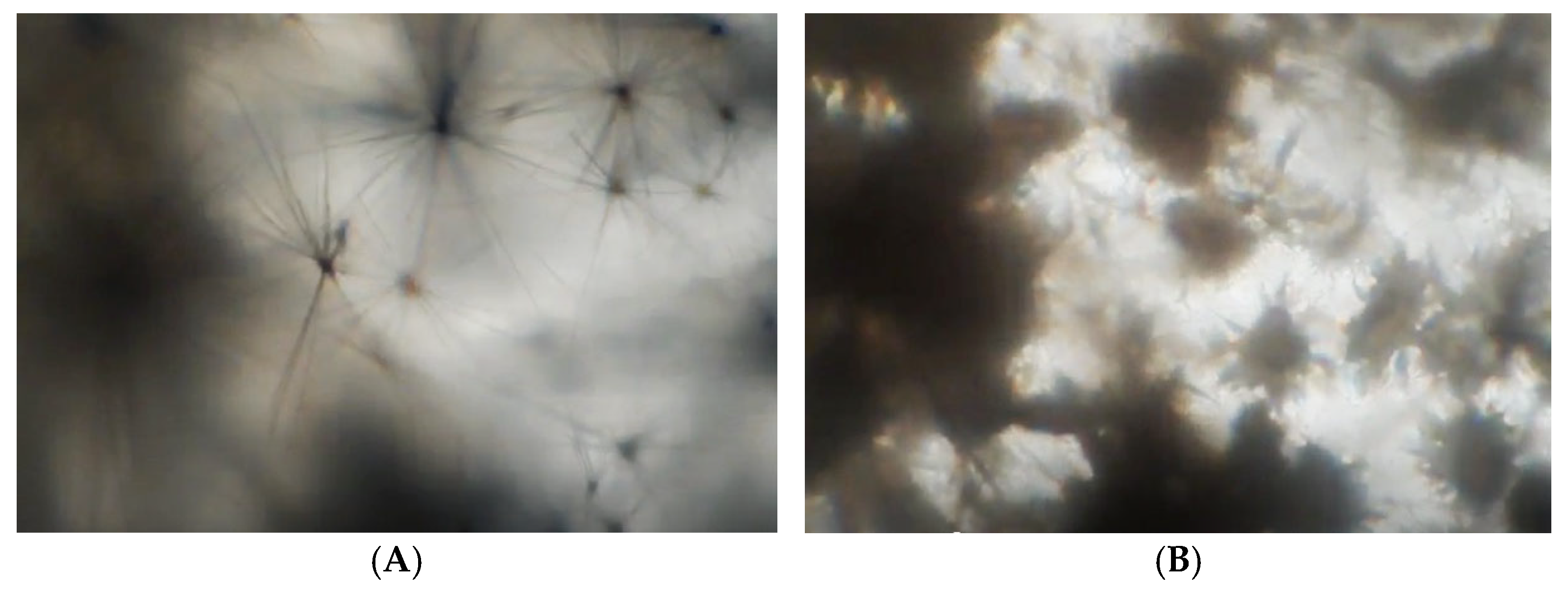
3.3. Carbamazepine
3.4. ROY
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISS | International Space Station |
| PIL-BOX | Pharmaceutical In-space Laboratory (PIL)–Biocrystal Optimization eXperiment |
| ROY | 5-methyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile |
| ADSEP | ADvanced Space Experiment Processor |
| SMALS | Small Molecule Accelerated Laboratory for Structure |
References
- Lewis, A.E.; Seckler, M.M.; Kramer, H.; Van Rosmalen, G. Industrial Crystallization: Fundamentals and Applications; Cambridge University Press: Padstow Cornwall, UK, 2015. [Google Scholar]
- Ostwald, W. Studien Über Die Bildung Und Umwandlung Fester Körper. 1. Abhandlung: Übersattigung Und Uberkaltung. Z. Phys. Chemie 1897, 22, 289–330. [Google Scholar] [CrossRef]
- Volmer, M.; Weber, A. Keimbildung in Übersattigten Gebilden. Z. Phys. Chemie 1926, 119U, 277–301. [Google Scholar] [CrossRef]
- Beckmann, W. Seeding the Desired Polymorph: Background, Possibilities, Limitations, and Case Studies. Org. Proc. Res. Dev. 2000, 4, 372–383. [Google Scholar] [CrossRef]
- Yu, L. Nucleation of One Polymorph by Another. J. Am. Chem. Soc. 2003, 125, 6380–6381. [Google Scholar] [CrossRef]
- Cardew, P.T. Ostwald Rule of Stages—Myth or Reality? Cryst. Growth Des. 2023, 23, 3958–3969. [Google Scholar] [CrossRef]
- Urquidi, O.; Brazard, J.; LeMessurier, N.; Simine, L.; Adachi, T.B.M. In situ optical spectroscopy of crystallization: One crystal nucleation oat a time. Proc. Natl. Acad. Sci. USA 2022, 119, e2122990119. [Google Scholar] [CrossRef]
- Cotting, G.; Urquidi, O.; Besnard, C.; Adachi, T.B.M. The effect of salt additives on the glycine crystallization pathway revealed by studying one crystal nucleation at a time. Proc. Natl. Acad. Sci. USA 2025, 122, e2419638122. [Google Scholar] [CrossRef]
- Burcham, C.L.; Doherty, M.F.; Peters, B.G.; Price, S.L.; Salvalaglio, M.; Reutzel-Edens, S.M.; Price, L.S.; Reddy Addula, R.K.; Francia, N.; Khanna, V.; et al. Pharmaceutical Digital Design: From Chemical Structure through Crystal Polymorph to Conceptual Crystallization Process. Cryst. Growth Des. 2024, 24, 5417–5438. [Google Scholar] [CrossRef]
- Jackson, K.; Brewer, F.; Wilkinson, A.; Williams, A.; Whiteside, B.; Wright, H.; Harper, L.; Wilson, A.M. Microgravity Crystal Formation. Crystals 2024, 14, 12. [Google Scholar] [CrossRef]
- Yoo, H.-D.; Wilcox, W.R.; Lal, R.; Trolinger, J.D. Modeling the growth of triglycine sulphate crystals in spacelab 3. J. Cryst. Growth 1988, 92, 101–117. [Google Scholar] [CrossRef]
- Lal, R.B.; Batra, A.K.; Trolinger, J.D.; Wilcox, W.R.; Steiner, B. Growth and characteristics of tgs crystals grown aboard first international microgravity laboratory (IML-1). Ferroelectrics 1994, 158, 81–85. [Google Scholar] [CrossRef]
- Aggarwal, M.D.; Batra, A.K.; Lal, R.B.; Penn, B.G.; Frazier, D.O. Growth and Characteristics of Bulk Single Crystals Grown from Solution on Earth and in Microgravity. NASA Technical Reports Server. Available online: https://ntrs.nasa.gov/citations/20110006347 (accessed on 10 September 2024).
- Kroes, R.L.; Reiss, D.A.; Lehoczky, S.L. Nucleation of Crystals from Solution in Microgravity-USML-1 Glovebox (GBX) Investigation; Joint Launch + One Year Science Review of USML-1 and USMP-1 with the Microgravity Measurement Group: Huntsville, AL, USA, 1994; Volume 2. [Google Scholar]
- Nielsen, K.F.; Lind, M.D. Results of the TTF-TCNQ- and the Calcium Carbonate-Crystallization on the Long Duration Exposure Facility; First LDEF Post-Retrieval Symposium Abstracts; NASA, Langley Research Center: Kissimmee, FL, USA, 1991. [Google Scholar]
- Bauser, H.C.; Smith, P.A.; Parent, S.D.; Chan, L.R.; Bhavsar, A.S.; Condon, K.H.; McCalip, A.; Croom, J.M.; Purcell, D.K.; Bogdanowich-Knipp, S.J.; et al. Return of Ritanovir: A Study on the Stability of Pharmaceuticals Processed in Orbit and Returned to Earth. ChemRxiv 2024, 3. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/65faecbee9ebbb4db91b4ac1 (accessed on 10 September 2024).
- DeLucas, L.J.; Smith, C.D.; Smith, H.W.; Vijay-Kumar, S.; Senadhi, S.E.; Ealick, S.E.; Carter, D.C.; Salemme, F.R.; Ohlendorf, D.H.; Einspahr, H.M.; et al. Protein Crystal Growth in Microgravity. Science 1989, 246, 651–654. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.; DeLucas, L.J. Microgravity protein crystallization. NPJ Microgravity 2015, 1, 15010. [Google Scholar] [CrossRef]
- Maes, D.; Decanniere, K.; Zegers, I.; Vanhee, C.; Sleutel, M.; Willaert, R.; Weerdt, C.; Martial, J.; Declercq, J.; Evrard, C.; et al. Protein crystallization under microgravity conditions: What did we learn on TIM crystallization from the Soyuz missions? Microgravity Sci. Technol. 2007, XIX, 90–94. [Google Scholar]
- Nicoud, N.; Licordari, F.; Myerson, A.S. Estimation of the Solubility of Metastable Polymorphs: A Critical Review. Cryst. Growth Des. 2018, 18, 7228–7237. [Google Scholar] [CrossRef]
- Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef]
- Surovtev, N.V.; Adichtchev, S.V.; Malinovsky, V.K.; Ogienko, A.G.; Drebushchak, V.A.; Manakov, M.Y.; Ancharov, A.I.; Yunoshev, A.S.; Boldyreva, E.V. Glycine phases formed from frozen aqueous solutions: Revisited. J. Chem. Phys. 2012, 137, 065103. [Google Scholar] [CrossRef]
- Du, D.; Ren, G.-B.; Qi, M.-H.; Li, Z.; Xu, X.-Y. Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems. Crystals 2019, 9, 161. [Google Scholar] [CrossRef]
- Fateixa, S.; Nogueira, H.I.S.; Trinadade, T. Carbamazepine polymorphism: A re-visitation using Raman imaging. Int. J. Pharm. 2022, 617, 121632. [Google Scholar] [CrossRef]
- Gnutzmann, T.; Thi, Y.N.; Rademann, K.; Emmerling, F. Solvent-Triggered Crystallization of Polymorphs Studied in Situ. Cryst. Growth Des. 2014, 14, 6445–6450. [Google Scholar] [CrossRef]
- Lévesque, A.; Maris, T.; Wuest, J.D. ROY Reclaims Its Crown: New Ways to Increase Polymorphic Diversity. J. Am. Chem. Soc. 2020, 142, 11873–11883. [Google Scholar] [CrossRef] [PubMed]
- Weatherston, J.; Probert, M.R.; Hall, M.J. Polymorphic ROYalty: The 14th ROY Polymorph Discovered via High-Throughput Crystallization. J. Am. Chem. Soc. 2025, 147, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G.L.; Hansen, L.K.; Bauer-Brandl, A. The polymorphism of glycine: Thermochemical and structural aspects. J. Therm. Anal. Calorim. 2001, 66, 699–715. [Google Scholar] [CrossRef]
- Xavier, N.F., Jr.; da Silva, A.M., Jr.; Bauerfeldt, G.F. What Rules the Relative stability of a-, b-, and g-Glycine Polymorphs? Cryst. Growth Des. 2020, 20, 4695–4706. [Google Scholar] [CrossRef]
- Sondermann, E.; Voigtmann, T.; Meyer, A. Influence of Gravity on Atomic Mobility in a Liquid. Microgravity Sci. Technol. 2022, 34, 93. [Google Scholar] [CrossRef]
- Praizey, J.P.; Garandet, J.P.; Frohberg, G.; Griesche, A.; Kraatz, K.H. Diffusion experiments in liquid metals preliminary results (Agat-module on Foton12). In First International Symposium on Microgravity Research & Applications in Physical Sciences and Biotechnology; Vols. I and II, Proceedings; Schurmann, B., Ed.; ESA Special Publications: Paris, France, 2001; Volume 454, pp. 481–490. [Google Scholar]
- Chen, S.; Xi, H.; Yu, L. Cross-Nucleation between ROY Polymorphs. J. Am. Chem. Soc. 2005, 127, 17439–17444. [Google Scholar] [CrossRef]
- Debushchak, T.N.; Boldyreva, E.V.; Seryotkin, Y.V.; Shutova, E.S. Crystal Structure Study of the Metastable b-Modification of Glycine and Its Tranformation into the a-Modification. J. Struct. Chem. 2002, 43, 835–842. [Google Scholar] [CrossRef]
- Vesga, M.J.; McKechnie, D.; Mulheran, P.A.; Johnston, K.; Sefcik, J. Conundrum of g glycine nucleation revisited: To stir or not to stir? Cryst. Eng. Comm. 2019, 21, 2234–2243. [Google Scholar] [CrossRef]
- Srinivasan, K. Crystal growth of a and g glycine polymorphs and their polymorphic phase transformations. J. Cryst. Growth 2008, 311, 156–162. [Google Scholar] [CrossRef]
- Duff, N.; Peters, B. Polymorph specific RMSD local order parameters for molecular crystals and nuclei: A-, b-, and g-glycine. J. Chem. Phys. 2011, 134, 134101. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, E. Principles of Crystal Chemistry; The Royal Institute of Chemistry: Letchworth, UK, 1971; p. 16. [Google Scholar]
- Grzesiak, A.L.; Lang, M.; Kim, K.; Matzger, A.J. Comparison of the Four Anhydrous Polymorphs of Carbamazepine and the Crystal Structure of Form I. J. Pharm. Sci. 2003, 92, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Czernicki, W.; Baranska, M. Carbamazepine polymorphs: Theoretical and experimental vibrational spectroscopy studies. Vib. Spec. 2013, 65, 12–23. [Google Scholar] [CrossRef]
- Yu, L.; Stephenson, G.A.; Mitchell, C.A.; Bunnell, C.A.; Snorek, S.V.; Bowyer, J.J.; Borchardt, T.B.; Stowell, J.G.; Byrn, S.R. Thermochemistry and Conformational Polymorphism of a Hexamorphic Crystal System. J. Am. Chem. Soc. 2000, 122, 585–591. [Google Scholar] [CrossRef]







| Substrate (Amount) | Solvent (Amount) | Antisolvent |
|---|---|---|
| Glycine (198.7 mg) | Water (2 mL) | Ethanol |
| Famoxadone (48.3 mg) | Acetone (4.5 mL) | Water–acetone (4:1) |
| Carbamazepine (117.4 mg) | Acetonitrile–water (2:1, 9 mL) | Water |
| ROY (94.6 mg) | Acetone (9 mL) | Water–acetone (4:1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, L.; Mulligan, M.K.; Savin, K.A.; Tuma, S.; Wilson, A.M. Crystallization of Small Molecules in Microgravity Using Pharmaceutical In-Space Laboratory–Biocrystal Optimization eXperiment (PIL-BOX). Crystals 2025, 15, 527. https://doi.org/10.3390/cryst15060527
Miller L, Mulligan MK, Savin KA, Tuma S, Wilson AM. Crystallization of Small Molecules in Microgravity Using Pharmaceutical In-Space Laboratory–Biocrystal Optimization eXperiment (PIL-BOX). Crystals. 2025; 15(6):527. https://doi.org/10.3390/cryst15060527
Chicago/Turabian StyleMiller, Lillian, Molly K. Mulligan, Kenneth A. Savin, Stephen Tuma, and Anne M. Wilson. 2025. "Crystallization of Small Molecules in Microgravity Using Pharmaceutical In-Space Laboratory–Biocrystal Optimization eXperiment (PIL-BOX)" Crystals 15, no. 6: 527. https://doi.org/10.3390/cryst15060527
APA StyleMiller, L., Mulligan, M. K., Savin, K. A., Tuma, S., & Wilson, A. M. (2025). Crystallization of Small Molecules in Microgravity Using Pharmaceutical In-Space Laboratory–Biocrystal Optimization eXperiment (PIL-BOX). Crystals, 15(6), 527. https://doi.org/10.3390/cryst15060527





