Boundary Between Amorphously and Molecularly Dispersed Curcumin of Its Amorphous Solid Dispersions Determined by Fluorescence Spectroscopy
Abstract
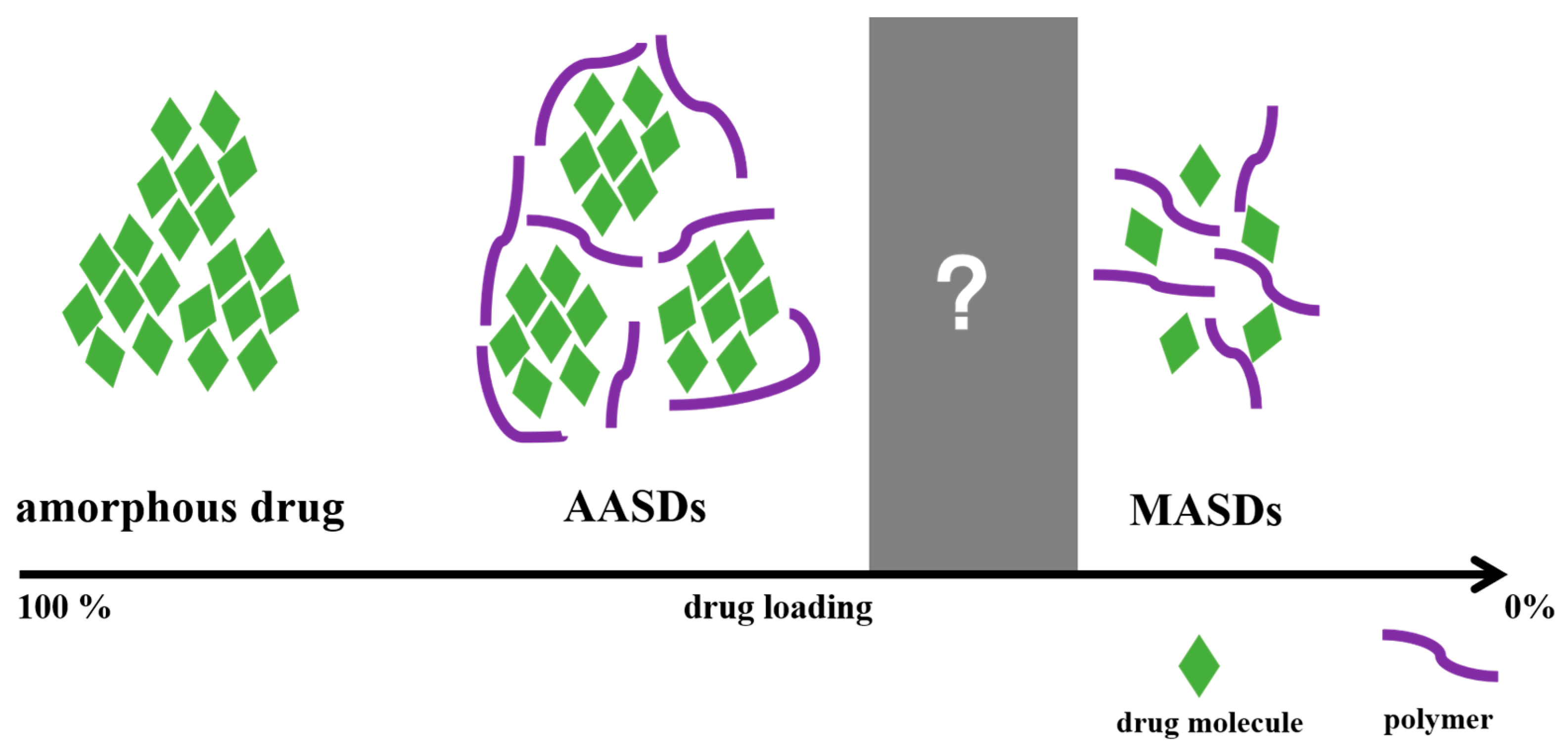
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of Curcumin (CUR) ASDs
2.2.2. Preparation of Amorphous CUR
2.2.3. Preparation of Indomethacin ASDs
2.2.4. Characterization
2.2.5. Working Curve
2.2.6. Dissolution Profiles
3. Results
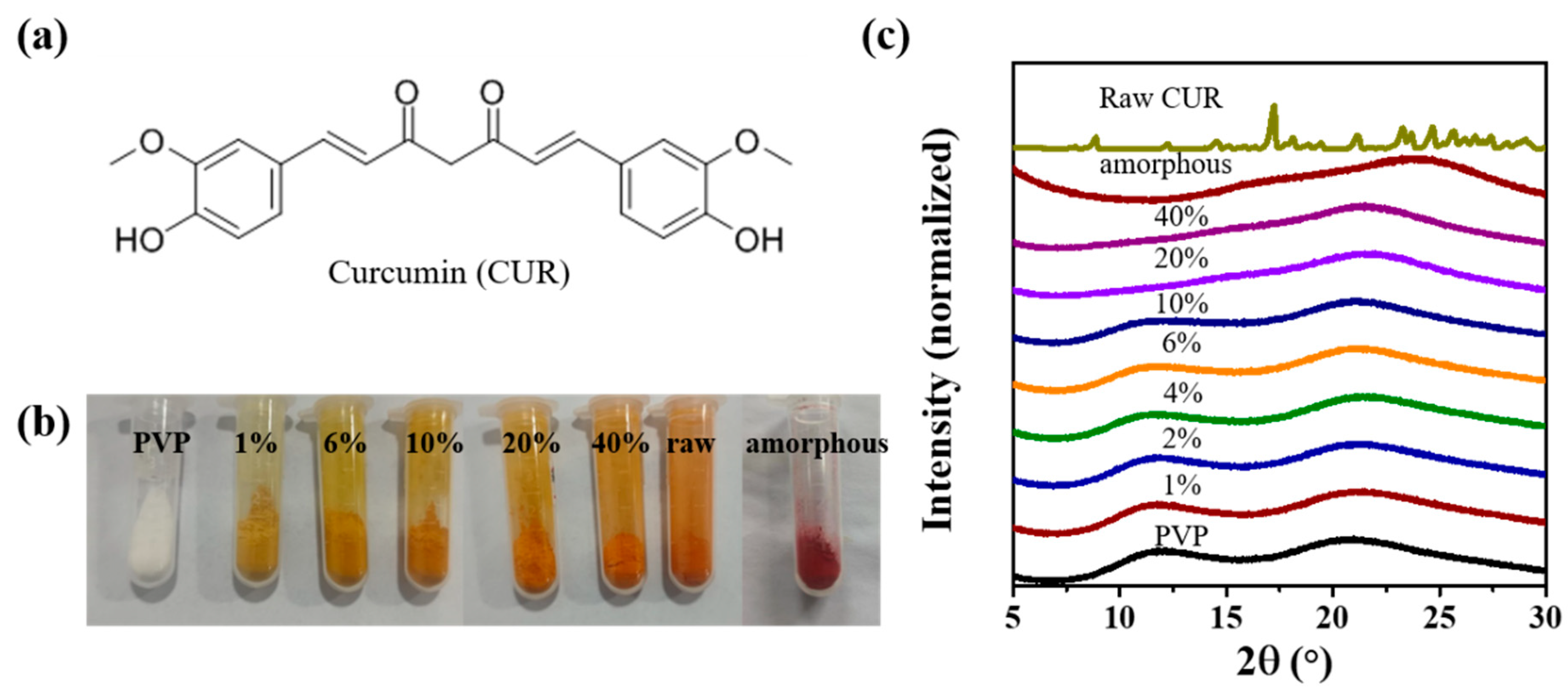
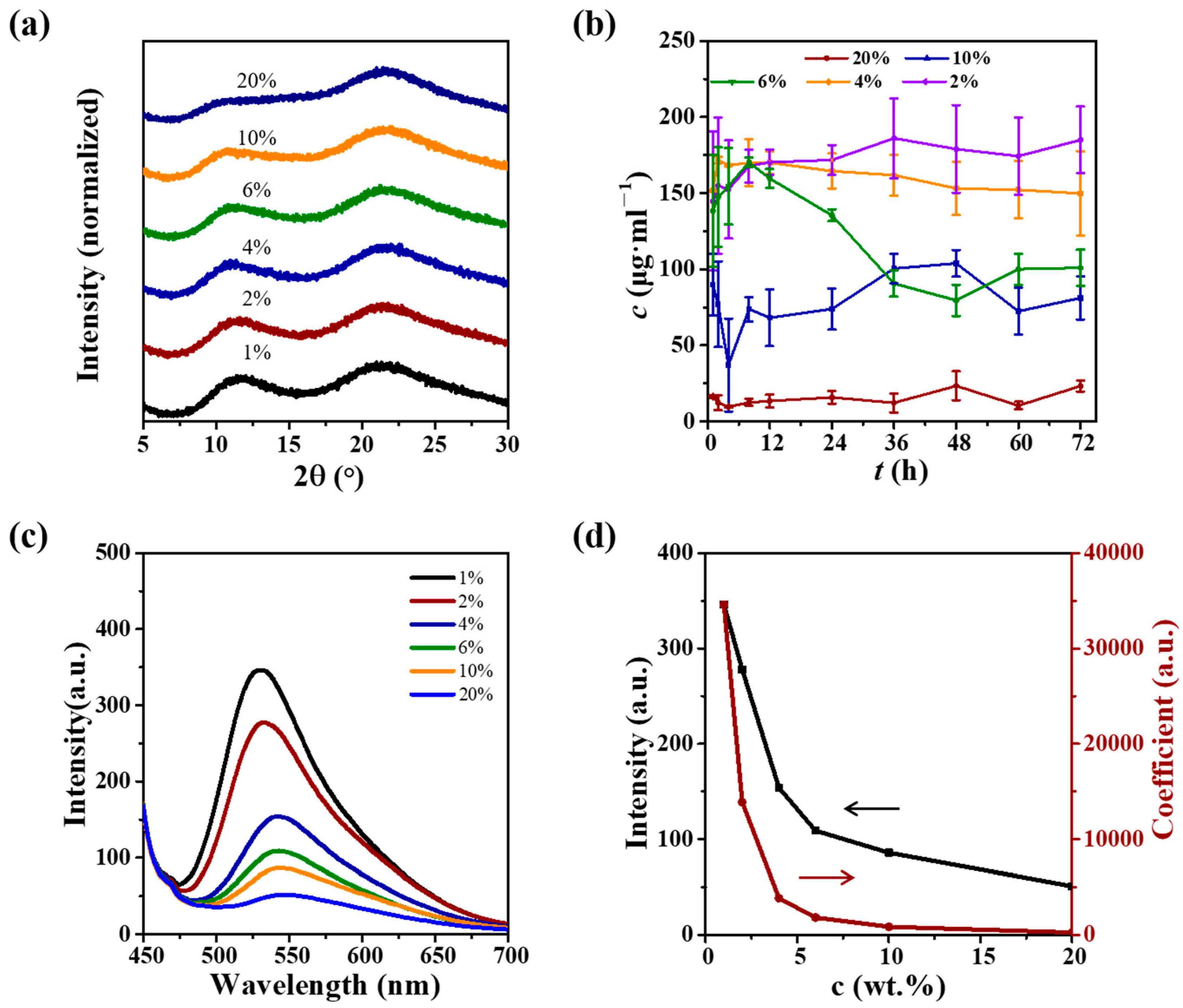
3.1. Preparation and Characterizations of CUR ASDs with PVP
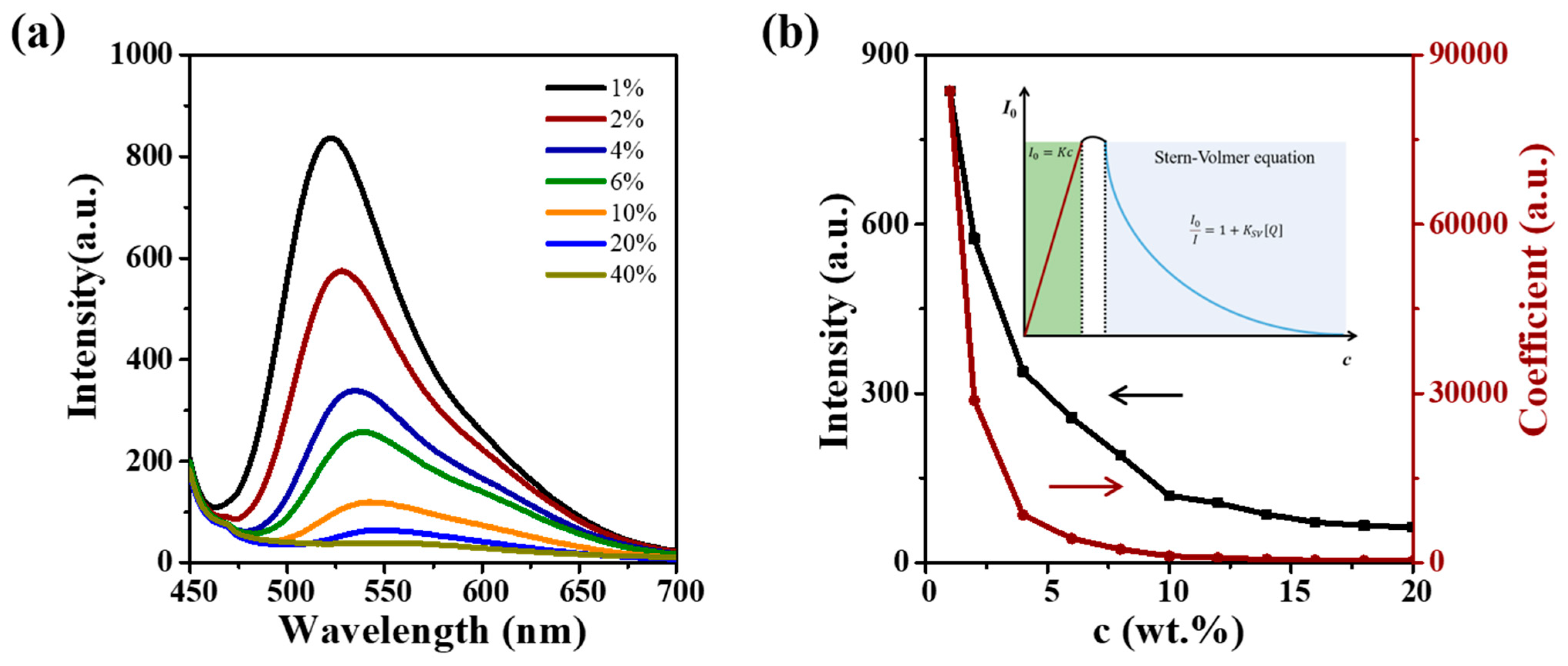
3.2. A–M Boundary Determination for CUR ASDs with PVP
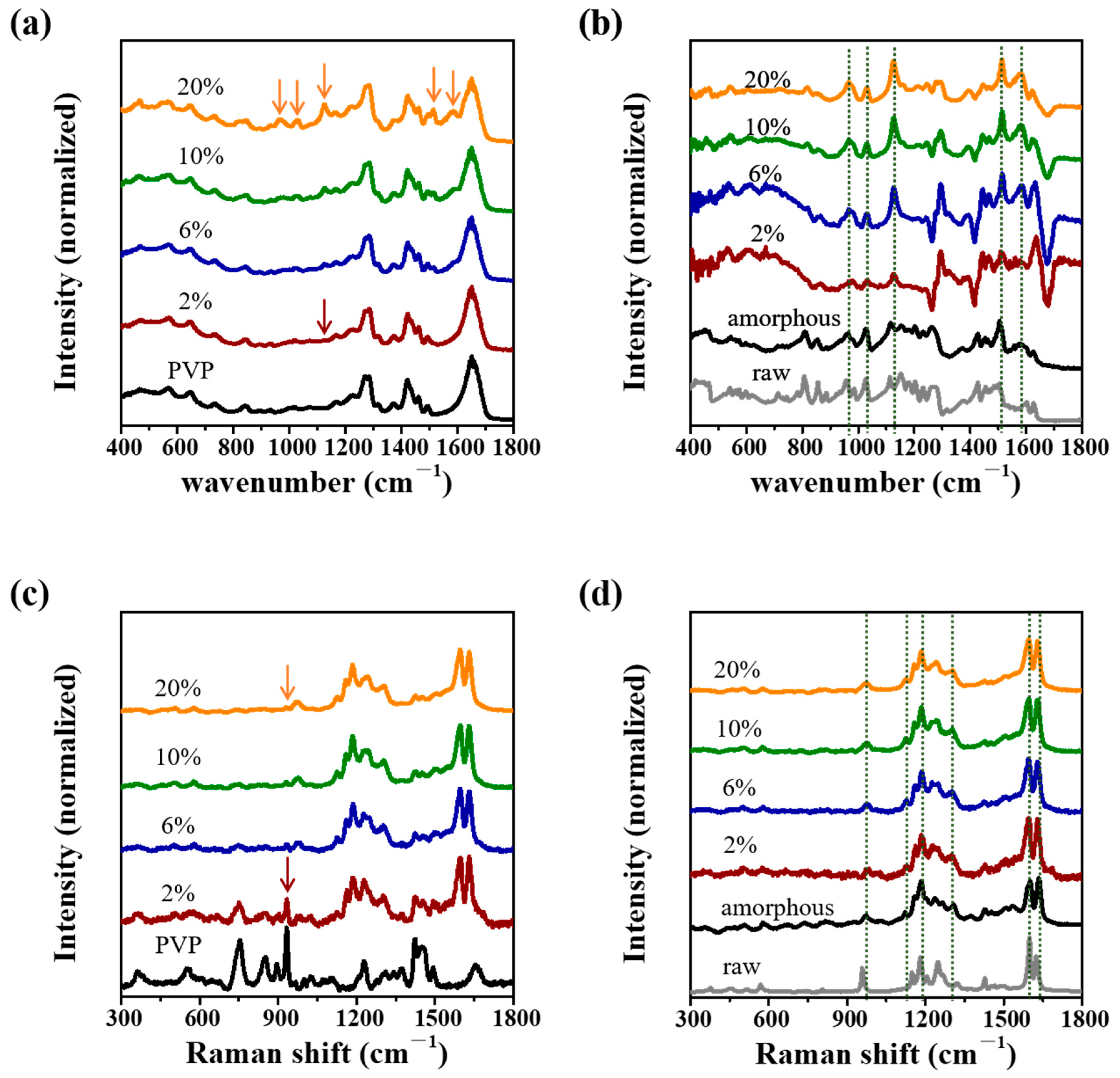
3.3. IR and Raman Spectra of CUR ASDs with PVP
3.4. Dissolution Behavior and Stability of Curcumin ASDs with PVP
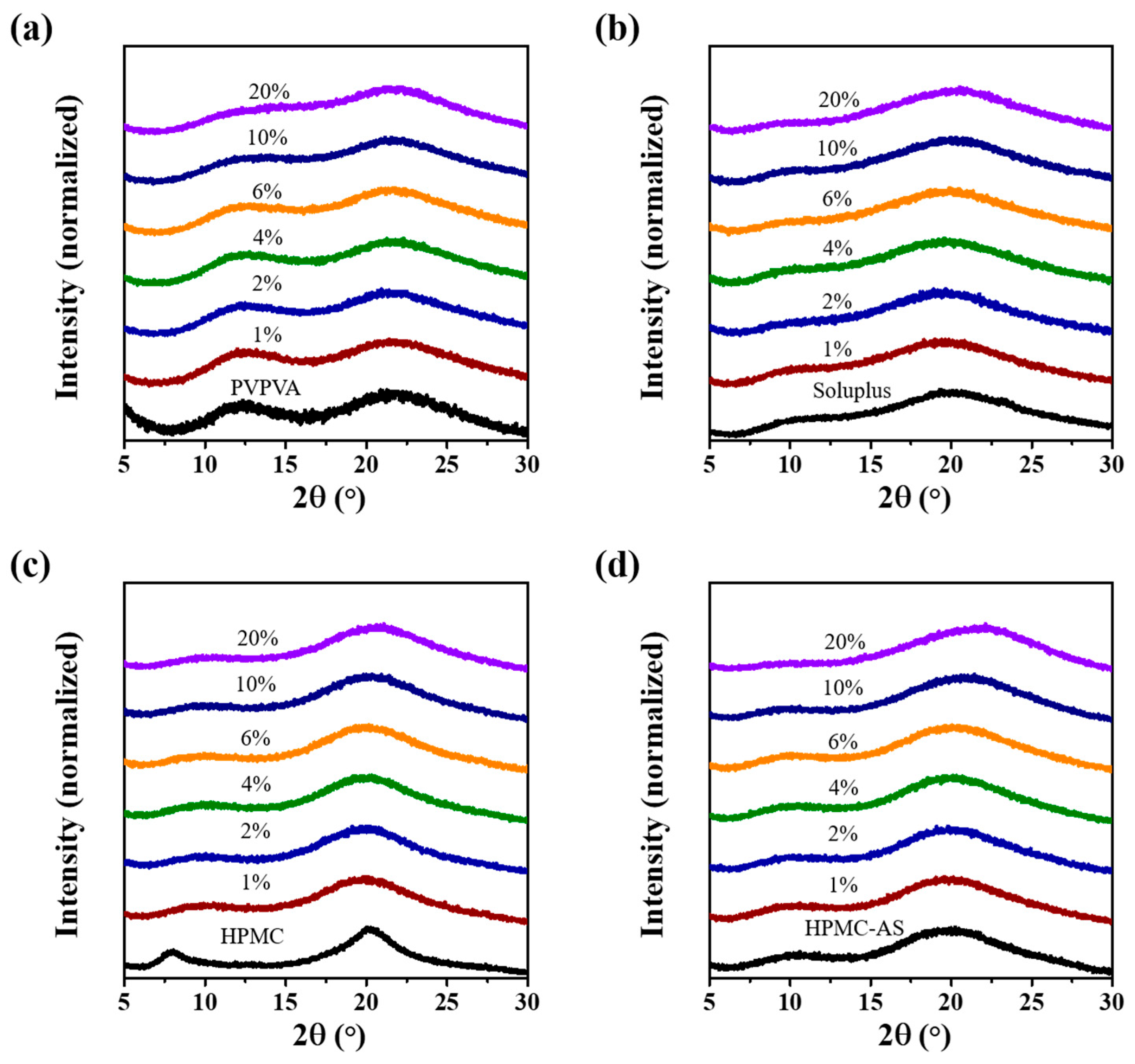
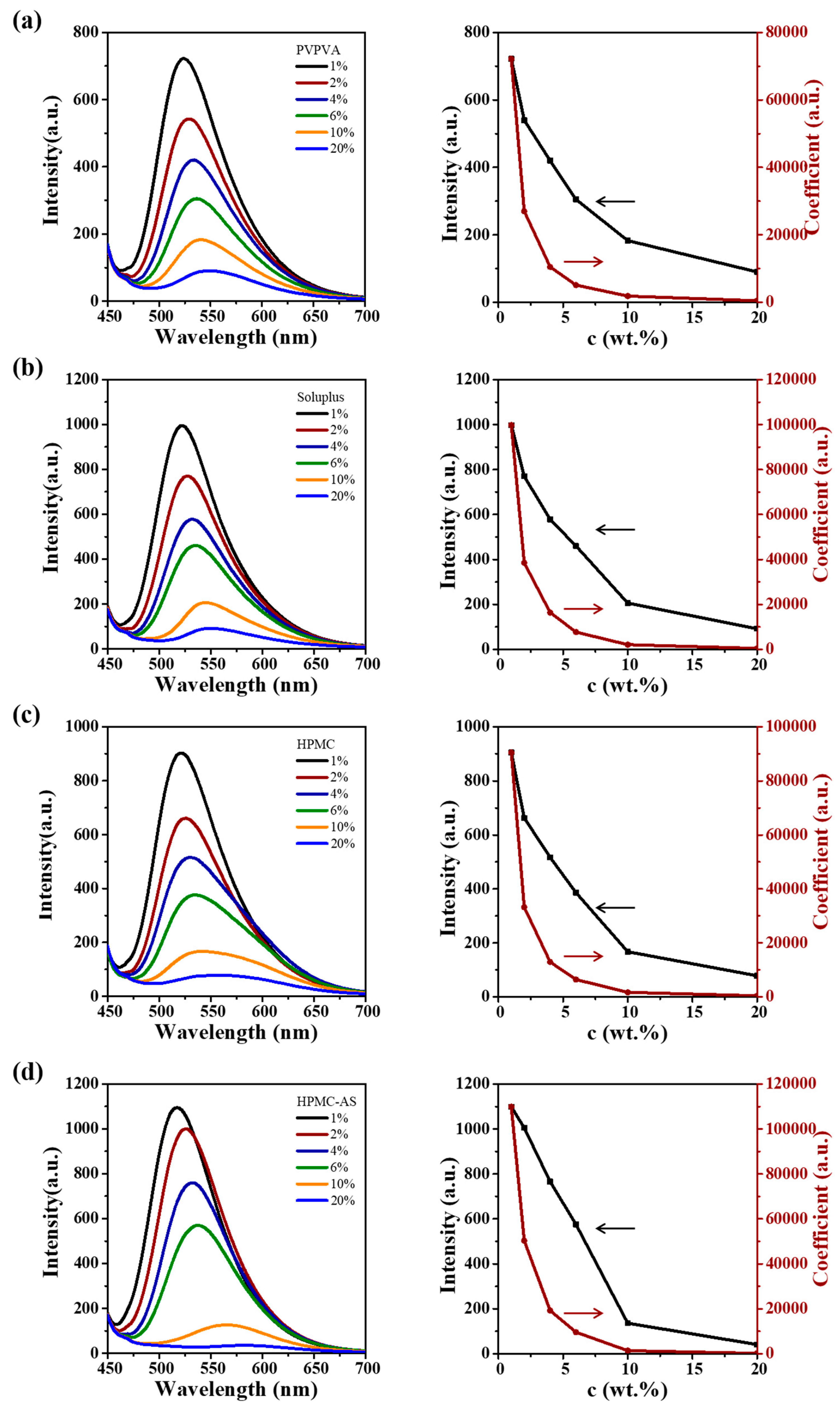
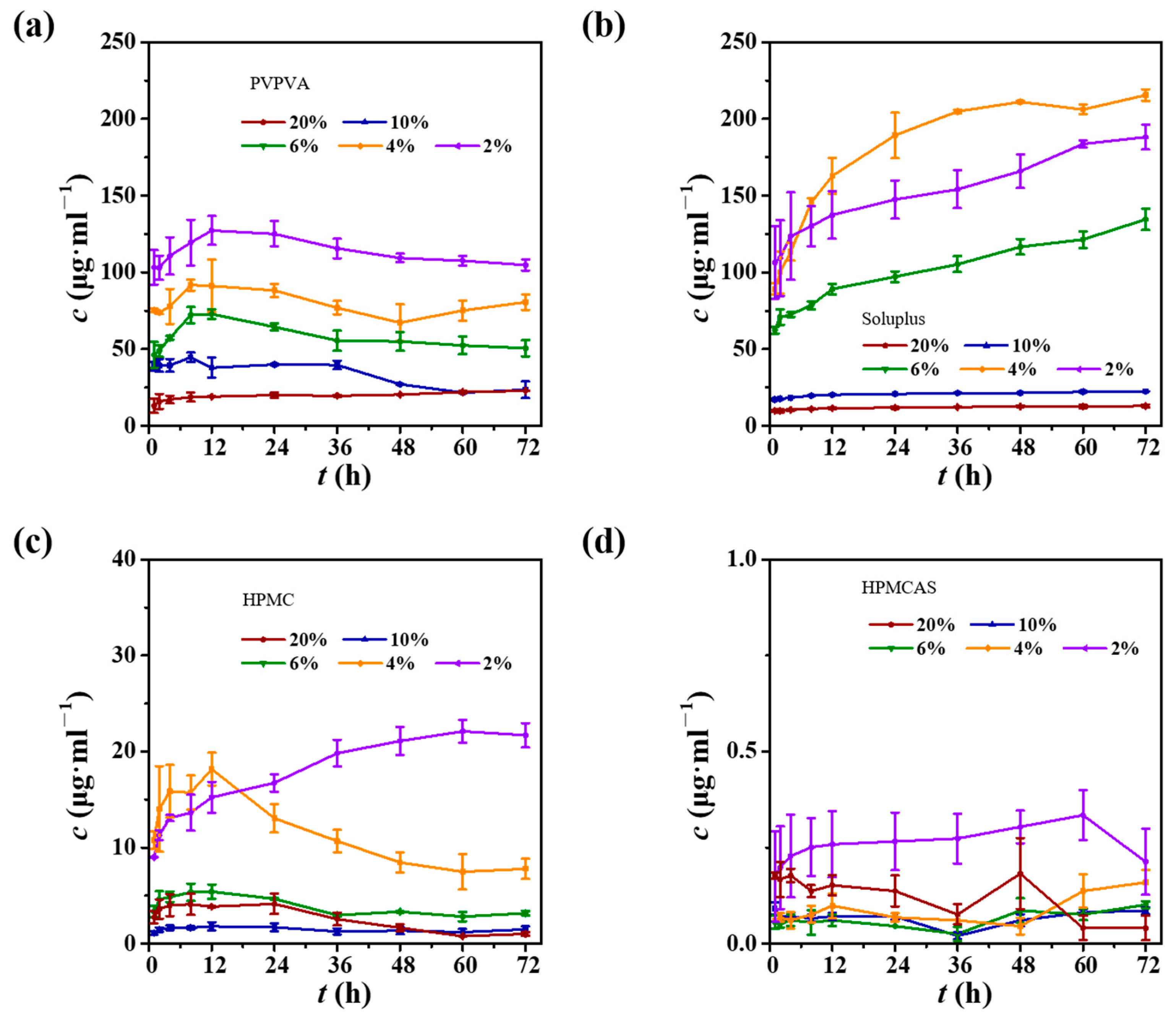
3.5. CUR ASDs with Different Polymers
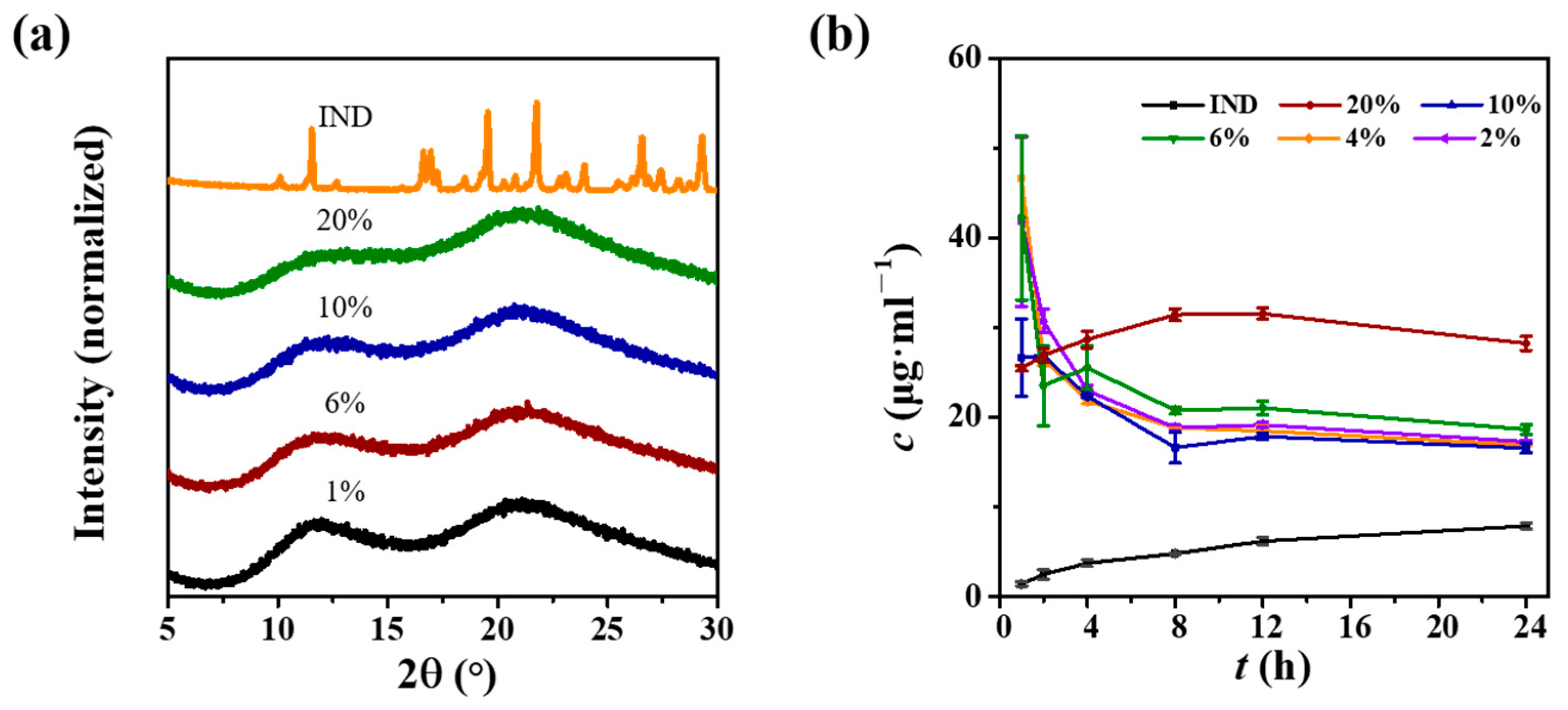
3.6. Indomethacin ASDs with PVP
4. Discussion
4.1. A–M Boundary and M–A Boundary
4.2. A–M Boundary and Stability
4.3. A–M Boundary and Dissolution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASDs | Amorphous Solid Dispersions |
| AASDs | ASDs with Drug Dispersed Amorphously |
| MASDs | ASDs with Drug Dispersed Molecularly |
| A–M Boundary | Boundary between AASDs and MASDs |
| M–A Boundary | Boundary between MASDs and AASDs |
| ACQ | Aggregation-Caused Quenching |
| CUR | Curcumin |
| IND | Indomethacin |
| PVP | Polyvinylpyrrolidone |
| PVPVA | Polyvinylpyrrolidone-Vinylacetate Copolymer |
| HPMC | Hydroxypropyl Methyl Cellulose |
| HPMCAS | Hydroxypropyl Methyl Cellulose Acetate Succinate |
References
- Li, J.; Wang, X.; Yu, D.; Zhoujin, Y.; Wang, K. Molecular complexes of drug combinations: A review of cocrystals, salts, coamorphous systems and amorphous solid dispersions. Int. J. Pharm. 2023, 648, 123555. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, H.; Palekar, S.; Patel, H.; Nukala, P.K.; Patel, K. Formulation strategies for the development of high drug-loaded amorphous solid dispersions. Drug Discovery Today 2023, 28, 103806. [Google Scholar] [CrossRef] [PubMed]
- Azari Torbat, N.; Akbarzadeh, I.; Rezaei, N.; Salehi Moghaddam, Z.; Bazzazan, S.; Mostafavi, E. Curcumin-incorporated biomaterials: In silico and in vitro evaluation of biological potentials. Coord. Chem. Rev. 2023, 492, 215233. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G. Curcumin, a biological wonder molecule: A crystal engineering point of view. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Samsoen, S.; Dudognon, É.; Le Fer, G.; Fournier, D.; Woisel, P.; Affouard, F. Impact of the polymer dispersity on the properties of curcumin/polyvinylpyrrolidone amorphous solid dispersions. Int. J. Pharm. 2024, 653, 123895. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, C.; Yuan, H.; Wu, W. Mapping multiple phases in curcumin binary solid dispersions by fluorescence contrasting. Chin. Chem. Lett. 2023, 35, 109324. [Google Scholar] [CrossRef]
- Al Fatease, A.; Abourehab, M.A.S.; Alqahtani, A.M.; Chidambaram, K.; Qureshi, A.A.; Venkatesan, K.; Alshahrani, S.M.; Abdelkader, H. Polymeric/dextran wafer dressings as promising long-acting delivery systems for curcumin topical delivery and enhancing wound healing in male wistar albino rats. Pharmaceuticals 2023, 16, 38. [Google Scholar] [CrossRef]
- Pan-On, S.; Tiyaboonchai, W. Development, characterization and Caco-2 cells absorption of curcumin solid dispersion for oral administration. J. Drug Deliv. Sci. Technol. 2023, 86, 104574. [Google Scholar] [CrossRef]
- Nogami, S.; Minoura, K.; Kiminami, N.; Kitaura, Y.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Stabilizing effect of the cyclodextrins additive to spray-dried particles of curcumin/polyvinylpyrrolidone on the supersaturated state of curcumin. Adv. Powder Technol. 2021, 32, 1750–1756. [Google Scholar] [CrossRef]
- Meng, F.; Paul, S.K.; Borde, S.; Chauhan, H. Investigating crystallization tendency, miscibility, and molecular interactions of drug–polymer systems for the development of amorphous solid dispersions. Drug Dev. Ind. Pharm. 2021, 47, 579–608. [Google Scholar] [CrossRef] [PubMed]
- Sekitoh, T.; Okamoto, T.; Fujioka, A.; Tramis, O.; Takeda, K.; Matsuura, T.; Imanaka, H.; Ishida, N.; Imamura, K. Sole-amorphous-sugar-based solid dispersion of curcumin and the influence of formulation composition and heat treatment on the dissolution of curcumin. Dry. Technol. 2021, 39, 2065–2074. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-core/PHBV-shell fibers to eliminate tailing off for an improved sustained release of curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Jamal Moideen, M.M.; Alqahtani, A.; Venkatesan, K.; Ahmad, F.; Krisharaju, K.; Gayasuddin, M.; Shaik, R.A.; Ibraheem, K.M.M.; Salama, M.E.-d.M.; Abed, S.Y. Application of the Box–Behnken design for the production of soluble curcumin: Skimmed milk powder inclusion complex for improving the treatment of colorectal cancer. Food Sci. Nutr. 2020, 8, 6643–6659. [Google Scholar] [CrossRef]
- Fan, N.; Lu, T.; Li, J. Surface tracking of curcumin amorphous solid dispersions formulated by binary polymers. J. Pharm. Sci. 2020, 109, 1068–1078. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.; Bian, W.; Liu, Y.; Liu, X.; Ma, S.; Zheng, X.; Du, Z.; Zhang, K.; Ouyang, D. Molecular interactions for the curcumin-polymer complex with enhanced anti-inflammatory effects. Pharmaceutics 2019, 11, 442. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, G.; Luan, X.; Zou, M.; Piao, H.; Cheng, G. Improved oral absorption of poorly soluble curcumin via the concomitant use of borneol. AAPS PharmSciTech 2019, 20, 150. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Prosapio, V.; McConville, C.; Leeke, G.; Ingram, A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J. CO2 Util. 2019, 30, 48–62. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, Y.-R.; Deng, Y.-Y. Effect of HPMCAS/curcumin amorphous solid dispersion in enhancing dissolution and chemical stability of curcumin. China J. Chin. Mater. Med. 2019, 44, 3305–3311. [Google Scholar]
- de Almeida, M.; da Rocha, B.A.; Francisco, C.R.L.; Miranda, C.G.; Santos, P.D.d.F.; de Araújo, P.H.H.; Sayer, C.; Leimann, F.V.; Gonçalves, O.H.; Bersani-Amado, C.A. Evaluation of the in vivo acute antiinflammatory response of curcumin-loaded nanoparticles. Food Funct. 2018, 9, 440–449. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Li, C.; Fan, N.; Wang, J.; He, Z.; Sun, J. Viewing molecular and interface interactions of curcumin amorphous solid dispersions for comprehending dissolution mechanisms. Mol. Pharm. 2017, 14, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. 2017, 73, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Tsekova, P.; Spasova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Georgieva, A.; Toshkova, R. Electrospun cellulose acetate membranes decorated with curcumin-PVP particles: Preparation, antibacterial and antitumor activities. J. Mater. Sci. Mater. Med. 2017, 29, 9. [Google Scholar] [CrossRef]
- Adami, R.; Di Capua, A.; Reverchon, E. Supercritical Assisted Atomization for the production of curcumin-biopolymer microspheres. Powder Technol. 2017, 305, 455–461. [Google Scholar] [CrossRef]
- Sadeghi, F.; Ashofteh, M.; Homayouni, A.; Abbaspour, M.; Nokhodchi, A.; Garekani, H.A. Antisolvent precipitation technique: A very promising approach to crystallize curcumin in presence of polyvinyl pyrrolidon for solubility and dissolution enhancement. Colloids Surf. B 2016, 147, 258–264. [Google Scholar] [CrossRef]
- Rahma, A.; Munir, M.M.; Khairurrijal; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular interactions and the release pattern of electrospun curcumin-polyvinyl (pyrrolidone) fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef]
- Wegiel, L.A.; Zhao, Y.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2014, 19, 976–986. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Tan, Y.W.; Li, L. Ternary dispersions to enhance solubility of poorly water soluble antioxidants. Colloids Surf. A 2013, 433, 111–121. [Google Scholar] [CrossRef]
- Munjal, B.; Pawar, Y.B.; Patel, S.B.; Bansal, A.K. Comparative oral bioavailability advantage from curcumin formulations. Drug Deliv. Transl. Res. 2011, 1, 322–331. [Google Scholar] [CrossRef]
- Paradkar, A.; Ambike, A.A.; Jadhav, B.K.; Mahadik, K.R. Characterization of curcumin–PVP solid dispersion obtained by spray drying. Int. J. Pharm. 2004, 271, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Tomar, Y.; Maheshwari, S.; Gorantla, S.; Singhvi, G. Curcumin loaded liquid crystalline nanoparticles for enhanced topical application: Design, characterization, ex vivo and dermatokinetic evaluation. J. Drug Deliv. Sci. Technol. 2024, 92, 105391. [Google Scholar] [CrossRef]
- Dai, Y.; Terskikh, V.; Brinmkmann, A.; Wu, G. Solid-state 1H, 13C, and 17O NMR characterization of the two uncommon polymorphs of curcumin. Cryst. Growth Des. 2020, 20, 7484–7491. [Google Scholar] [CrossRef]
- Han, J.; Wei, Y.; Li, L.; Song, Y.; Pang, Z.; Qian, S.; Zhang, J.; Gao, Y.; Heng, W. Gelation elimination and crystallization Inhibition by co-amorphous strategy for amorphous curcumin. J. Pharm. Sci. 2023, 112, 182–194. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C 2020, 42, 100338. [Google Scholar] [CrossRef]
- Puglisi, A.; Giovannini, T.; Antonov, L.; Cappelli, C. Interplay between conformational and solvent effects in UV-visible absorption spectra: Curcumin tautomers as a case study. Phys. Chem. Chem. Phys. 2019, 21, 15504–15514. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo*. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Cornago, P.; Claramunt, R.M.; Bouissane, L.; Alkorta, I.; Elguero, J. A study of the tautomerism of β-dicarbonyl compounds with special emphasis on curcuminoids. Tetrahedron 2008, 64, 8089–8094. [Google Scholar] [CrossRef]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Theoretical study on physicochemical properties of curcumin. Spectrochim. Acta Part A 2007, 67, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, W.-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. B 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Newman, A.; Engers, D.; Bates, S.; Ivanisevic, I.; Kelly, R.C.; Zografi, G. Characterization of amorphous API: Polymer mixtures using X-ray powder diffraction. J. Pharm. Sci. 2008, 97, 4840–4856. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. Commentary: Considerations in the measurement of glass transition temperatures of pharmaceutical amorphous solids. AAPS PharmSciTech 2019, 21, 26. [Google Scholar] [CrossRef]


| CUR% | Preparation Method | Characterizations | Dispersion State | Ref |
|---|---|---|---|---|
| 5–90 | co-milling or solvent evaporation | PXRD | amorphous | [6] |
| 5–50 | solvent co-precipitation | PXRD, DSC | amorphous | [7] |
| - | solvent casting | PXRD, DSC, IR | amorphous or molecular | [8] |
| 10–25 | melting solvent | PXRD | amorphous | [9] |
| 10 | solvent evaporation | PXRD, IR | amorphous | [10] |
| 30–70 | solvent evaporation | PXRD, DSC, IR | amorphous | [11] |
| 1–10 | vacuum foam drying | - | - | [12] |
| 10 | electrospinning | PXRD | amorphous | [13] |
| 12.5–25 | solvent evaporation | PXRD, IR | amorphous | [14] |
| 20 | solvent evaporation | PXRD, IR, Raman | amorphous | [15] |
| 10–50 | solvent evaporation | PXRD, DSC | amorphous or molecular | [16] |
| 25–50 | solvent evaporation | PXRD, DSC, IR | amorphous | [17] |
| 10–25 | supercritical antisolvent | PXRD, DSC, IR | amorphous | [18] |
| 10–25 | solvent evaporation | DSC | amorphous | [19] |
| 14 | solvent evaporation | IR | amorphous | [20] |
| 25–50 | rotary evaporation | IR, Raman | amorphous | [21] |
| 10 | electrospinning | PXRD, DSC | amorphous | [22] |
| 3–5 | electrospinning | PXRD, IR | amorphous | [23] |
| 10–30 | supercritical assisted atomization | PXRD, DSC, IR | amorphous | [24] |
| 33–100 | antisolvent crystallization | PXRD, DSC, IR | amorphous | [25] |
| 9 | electrospinning | IR, Raman | amorphous | [26] |
| 70 | solvent evaporation | PXRD, DSC, IR, Raman | amorphous | [27] |
| 25 | rapid solvent evaporation | PXRD, DSC, IR | amorphous | [28] |
| - | solvent evaporation | PXRD, DSC, IR, Raman | amorphous or molecular | [29] |
| 50 | solvent evaporation | PXRD, DSC | molecular | [30] |
| 9–50 | spray drying | PXRD, DSC, IR | amorphous | [31] |
| Spectra | Raw | Amorphous | 20% | 10% | 6% | 2% |
|---|---|---|---|---|---|---|
| IR | 1598 | 1584 | 1585 | 1589 | 1582 | |
| 1498 | 1510 | 1514 | 1514 | 1515 | 1513 | |
| 1113 | 1115 | 1124 | 1126 | 1126 | 1126 | |
| 1026 | 1026 | 1030 | 1028 | 1030 | 1031 | |
| 958 | 962 | 966 | 966 | 966 | ||
| Raman | 1626 | 1634 | 1631 | 1632 | 1632 | 1630 |
| 1600 | 1601 | 1598 | 1598 | 1597 | 1597 | |
| 1321 | 1308 | 1305 | 1305 | 1305 | ||
| 1183 | 1183 | 1186 | 1186 | 1187 | 1186 | |
| 1151 | 1122 | 1126 | 1127 | 1128 | ||
| 960 | 972 | 974 | 976 | 974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Zheng, W.; Ren, S.; Xiao, W.; Chen, F.; Xue, R. Boundary Between Amorphously and Molecularly Dispersed Curcumin of Its Amorphous Solid Dispersions Determined by Fluorescence Spectroscopy. Crystals 2025, 15, 512. https://doi.org/10.3390/cryst15060512
Fan S, Zheng W, Ren S, Xiao W, Chen F, Xue R. Boundary Between Amorphously and Molecularly Dispersed Curcumin of Its Amorphous Solid Dispersions Determined by Fluorescence Spectroscopy. Crystals. 2025; 15(6):512. https://doi.org/10.3390/cryst15060512
Chicago/Turabian StyleFan, Shixin, Wenling Zheng, Shizhao Ren, Wangchuan Xiao, Fenghua Chen, and Rongrong Xue. 2025. "Boundary Between Amorphously and Molecularly Dispersed Curcumin of Its Amorphous Solid Dispersions Determined by Fluorescence Spectroscopy" Crystals 15, no. 6: 512. https://doi.org/10.3390/cryst15060512
APA StyleFan, S., Zheng, W., Ren, S., Xiao, W., Chen, F., & Xue, R. (2025). Boundary Between Amorphously and Molecularly Dispersed Curcumin of Its Amorphous Solid Dispersions Determined by Fluorescence Spectroscopy. Crystals, 15(6), 512. https://doi.org/10.3390/cryst15060512






