Delivery Systems for Curcumin Derivatives Based on Calcium Carbonate Structures for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hybrid Materials AP1–AP5
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niu, Y.Q.; Liu, J.H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883. [Google Scholar] [CrossRef] [PubMed]
- Comes, J.; Islamovic, E.; Lizandara-Pueyo, C.; Seto, J. Improvements in the utilization of calcium carbonate in promoting sustainability and environmental health. Front. Chem. 2024, 12, 1472284. [Google Scholar] [CrossRef] [PubMed]
- Azarian, M.H.; Sutapun, W. Biogenic calcium carbonate derived from waste shells for advanced material applications: A review. Front. Mater. 2022, 9, 1024977. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Q.; Lei, Z.; Zhang, K.; Zhen, S.; Yao, H.; Zu, Y. Calcium Carbonate-Based Nanoplatforms for Cancer Therapeutics: Current State of Art and Future Breakthroughs. ACS Omega 2024, 9, 12539–12552. [Google Scholar] [CrossRef]
- Liendo, F.; Arduino, M.; Deorsola, F.A.; Bensaid, S. Factors controlling and influencing polymorphism, morphology and size of calcium carbonate synthesized through the carbonation route: A review. Powder Technol. 2022, 398, 117050. [Google Scholar] [CrossRef]
- Tobler, D.J.; Blanco, J.D.R.; Sørensen, H.O.; Stipp, S.L.S.; Dideriksen, K. Effect of pH on Amorphous Calcium Carbonate Structure and Transformation. Cryst. Growth Des. 2016, 16, 4500–4508. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.S.; Volodkin, D. CaCO3 crystals as versatile carriers for controlled delivery of antimicrobials. J. Contr. Release 2020, 328, 470–489. [Google Scholar] [CrossRef]
- Merchant, J.; Müllertz, A.; Rades, T.; Bannow, J. Functionalized calcium carbonate (FCC) as a novel carrier to solidify supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). Eur. J. Pharm. Sci. 2023, 193, 198–207. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Wang, K.; Dai, M.; Duan, Y.; Wan, C.M.M. Biomimetic mineralization: Construction and biomedical applications of biohybrid materials. Mater. Chem. Front. 2024, 8, 3383. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef]
- Kabacinska, Z.; Krzyminiewski, R.; Dobosz, B. EPR investigation of UV light effecton calcium carbonate powders with different grain sizes. Radiat. Prot. Dosim. 2014, 159, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.M.; Hazoor, S.; Vuong, T.; Kydd, L.; Shortt, I.; Foss, F.W.; LaPlante, E. Synthesis of Metastable Calcium Carbonate Using Long-Chain Bisphosphonate Molecules. ACS Appl. Mater. Interfaces 2024, 16, 30567–30579. [Google Scholar] [CrossRef] [PubMed]
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium carbonate nano and microparticles: Synthesis methods and biological applications. Biotech 2021, 11, 457. [Google Scholar] [CrossRef]
- Trushina, D.B.; Borodina, T.N.; Belyakov, S.; Antipina, M.N. Calcium carbonate vaterite particles for drug delivery: Advances and challenges. Mater. Today Adv. 2022, 14, 100214. [Google Scholar] [CrossRef]
- Li, S.; Lian, B. Application of Calcium Carbonate as a Controlled Release Carrier for Therapeutic Drugs. Minerals 2023, 13, 1136. [Google Scholar] [CrossRef]
- Ambrogi, V. A New Challenge for the Old Excipient Calcium Carbonate: To Improve the Dissolution Rate of Poorly Soluble Drugs. Pharmaceutics 2023, 15, 300. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, P.; Gaikwad, K.K. UV-shielding and antioxidant properties of chitosan film impregnated with Acacia catechu modified with calcium carbonate for food packaging. Int. J. Biol. Macromol. 2024, 257, 128790. [Google Scholar] [CrossRef] [PubMed]
- Biny, L.; Gerasimovich, E.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Functionalized Calcium Carbonate-Based Microparticles as a Versatile Tool for Targeted Drug Delivery and Cancer Treatment. Pharmaceutics 2024, 16, 653. [Google Scholar] [CrossRef]
- Xing, J.; Cai, Y.; Wang, Y.; Zheng, H.; Liu, Y. Synthesis of Polymer Assembled Mesoporous CaCO3 Nanoparticles for Molecular Targeting and pH-Responsive Controlled Drug Release. Adv. Polym. Technol. 2020, 2020, 8749238. [Google Scholar] [CrossRef]
- Liu, H.; Wen, Z.; Liu, Z.; Yang, Y.; Wang, H.; Xia, X.; Ye, J.; Liu, Y. Unlocking the potential of amorphous calcium carbonate: A star ascending in the realm of biomedical application. Acta Pharm. Sin. B 2024, 14, 602–622. [Google Scholar] [CrossRef]
- Tan, C.; Dima, C.; Huang, M.; Assadpour, E.; Wang, J.; Sun, B.; Kharazmi, M.S.; Jafari, S.M. Advanced CaCO3-derived delivery systems for bioactive compounds. Adv. Colloid Interface Sci. 2022, 309, 102791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Tian, Y.; You, J.; Hu, X.; Liu, Y. Recent Advances of Calcium Carbonate Nanoparticles for Biomedical Applications. Bioengineering 2022, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Feng, Z.; Zhang, X.; Li, T.; Yang, T.; Yu, L. Research progress of calcium carbonate nanomaterials in cancer therapy: Challenge and opportunity. Front. Bioeng. Biotechnol. 2023, 11, 1266888. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.Q.; Thao, D.T.T. Enhancing the Solubility of Curcumin Metal Complexes and Investigating Some of Their Biological Activities. J. Chem. 2019, 2019, 8082195. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V. Curcumin: Modern Applications for a Versatile Additive. Coatings 2021, 11, 519. [Google Scholar] [CrossRef]
- Heng, M.C.Y. Topical Curcumin: A Review of Mechanisms and uses in Dermatology. Int. J. Dermatol. Clin. Res. 2017, 3, 010–017. [Google Scholar] [CrossRef]
- Mo, Z.; Yuan, J.; Guan, X.; Peng, J. Advancements in Dermatological Applications of Curcumin: Clinical Efficacy and Mechanistic Insights in the Management of Skin Disorders. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1083–1092. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Sathee, D.; Krishnan, L.K. Aqueous Solubility and Bioavailability of Albumin Conjugated Curcumin in Endothelial Cells Allege Potential of the Formulation for Anti-Inflammation Therapy. Cardiol. Cardiovasc. Med. 2021, 5, 86–105. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H. Curcumin and Its Derivatives: A Review of Their Biological Activities. Sys. Rev. Pharm. 2020, 11, 472–481. [Google Scholar]
- Kuzminska, J.; Szyk, P.; Mlynarczyk, D.T.; Bakun, P.; Muszalska-Kolos, I.; Dettlaff, K.; Sobczak, A.; Goslinski, T.; Jelinska, A. Curcumin Derivatives in Medicinal Chemistry: Potential Applications in Cancer Treatment. Molecules 2024, 29, 5321. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Lo, C.; Han, J.; Yang, F.; Leung, A.W.; Xu, C. Design, Synthesis and Characterization of Novel Curcumin Derivatives. Nat. Prod. Chem. Res. 2020, 8, 367. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative Delivery Systems for Curcumin: Exploring Nanosized and Conventional Formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, F.; Chen, J.; Wu, Q.; Zou, Z. Research progress on natural bio-based encapsulation system of curcumin and its stabilization mechanism. Food Sci. Technol. Camp. 2022, 42, e78422. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, C.; McClements, D.J.; Qin, Y.; Long, J.; Jiao, A.; Li, X.; Wang, J.; Jin, Z. Encapsulation, protection, and delivery of curcumin using succinylated-cyclodextrin systems with strong resistance to environmental and physiological stimuli. Food Chem. 2022, 376, 131869. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Y. Research progress on drug delivery systems for curcumin in the treatment of gastrointestinal tumors. World J. Gastrointest. Oncol. 2023, 15, 1342–1348. [Google Scholar] [CrossRef]
- Sable, A.A.; Kunwar, A.; Barik, A. Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin. Pharmaceutics 2024, 16, 423. [Google Scholar] [CrossRef]
- Raduly, M.F.; Raditoiu, V.; Raditoiu, A.; Wagner, L.A.; Amariutei, V.; Ailiesei, G.L. Facile synthesis of curcumin and curcuminoid like derivatives at microwaves. Rev. Chim. 2018, 69, 1327–1331. [Google Scholar] [CrossRef]
- Altundag, E.M.; Özbilenler, C.; Ustürk, S.; Kerküklü, N.R.; Afshani, M.; Yilmaz, E. Metal-based curcumin and quercetin complexes: Cell viability, ROS production and antioxidant activity. J. Mol. Struct. 2021, 1245, 131107. [Google Scholar] [CrossRef]
- ISO 24444:2019; Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). ISO: Geneva, Switzerland, 2019.
- Mitra, A.K. Chemistry and Biomedical Applications of Cumin and Turmeric: A Review, Challenge and Perspective. Chem. Afr. 2022, 5, 1191–1213. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Vazifehdoost, M.; Alhashemi, S.H.; Davoudi, H.; Zarrabi, A.; Dehshahri, A.; Fekri, H.S.; Mohammadinejad, R.; Thakur, V.K. Next-Generation Hydrogels as Biomaterials for Biomedical Applications: Exploring the Role of Curcumin. ACS Omega 2023, 8, 8960–8976. [Google Scholar] [CrossRef]
- Tkachenko, Y.; Niedzielski, P. FTIR as a Method for Qualitative Assessment of Solid Samples in Geochemical Research: A Review. Molecules 2022, 27, 8846. [Google Scholar] [CrossRef]
- Toffolo, M.B.; Regev, L.; Dubernet, S.; Lefrais, Y.; Boaretto, E. FTIR-Based Crystallinity Assessment of Aragonite–Calcite Mixtures in Archaeological Lime Binders Altered by Diagenesis. Minerals 2019, 9, 121. [Google Scholar] [CrossRef]
- Xiao, J.; Song, Y.; Li, Y. Comparison of Quantitative X-ray Diffraction Mineral Analysis Methods. Minerals 2023, 13, 566. [Google Scholar] [CrossRef]
- Liu, M.; Yang, M.; Wan, X.; Tang, Z.; Jiang, L.; Wang, S. From Nanoscopic to Macroscopic Materials by Stimuli-Responsive Nanoparticle Aggregation. Adv. Mater. 2023, 35, 2208995. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikandan, E.; Jayachandran, M. Enhanced Polymer Induced Precipitation of Polymorphous in Calcium Carbonate: Calcite Aragonite Vaterite Phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Deng, H.; Wan, M.; Li, H.; Chen, Q.; Li, R.; Liang, B.; Zhu, H. Curcumin protection against ultraviolet-induced photo-damage in Hacat cells by regulating nuclear factor erythroid 2-related factor 2. Bioengineered 2021, 12, 9993–10006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, W.; Deng, Y.; Chu, Y.; Zhong, Y.; Wang, G.; Xiong, Y.; Liu, X.; Chen, L.; Li, H. Curcumin-based waterborne polyurethane-gelatin composite bioactive films for effective UV shielding and inhibition of oil oxidation. Food Control 2022, 141, 109199. [Google Scholar] [CrossRef]
- Fu, Q.; Qin, Y.; Zhang, X.; Sun, L.; Chang, J. Seeking materials from nature for interrupting eye damage: Ultraviolet to blue light blocking clear cellulose films enabled by curcumin. Int. J. Biol. Macromol. 2024, 279, 135325. [Google Scholar] [CrossRef] [PubMed]
- Shabrina, A.M.; Azzahra, R.S.S.; Permata, I.N.; Dewi, H.P.; Safitri, R.A.; Maya, I.; Aulia, R.N.; Sriwidodo, S.; Mita, S.R.; Amalia, E.; et al. Potential of Natural-Based Sun Protection Factor (SPF): A Systematic Review of Curcumin as Sunscreen. Cosmetics 2025, 12, 10. [Google Scholar] [CrossRef]
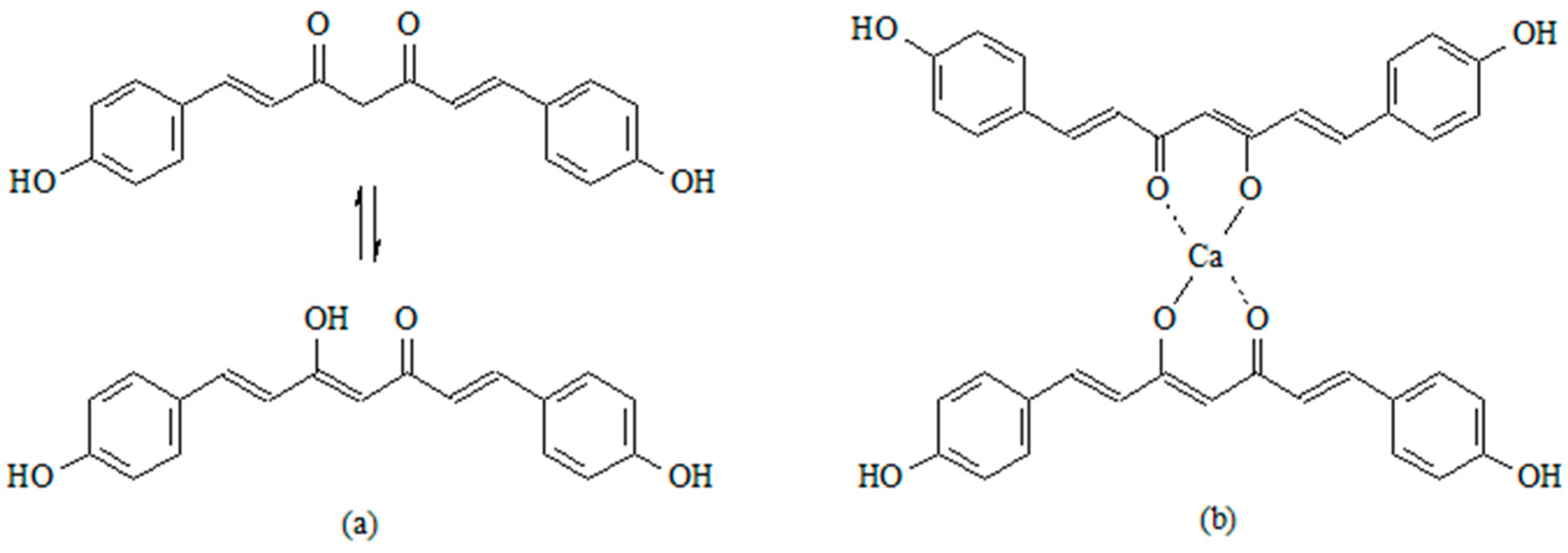
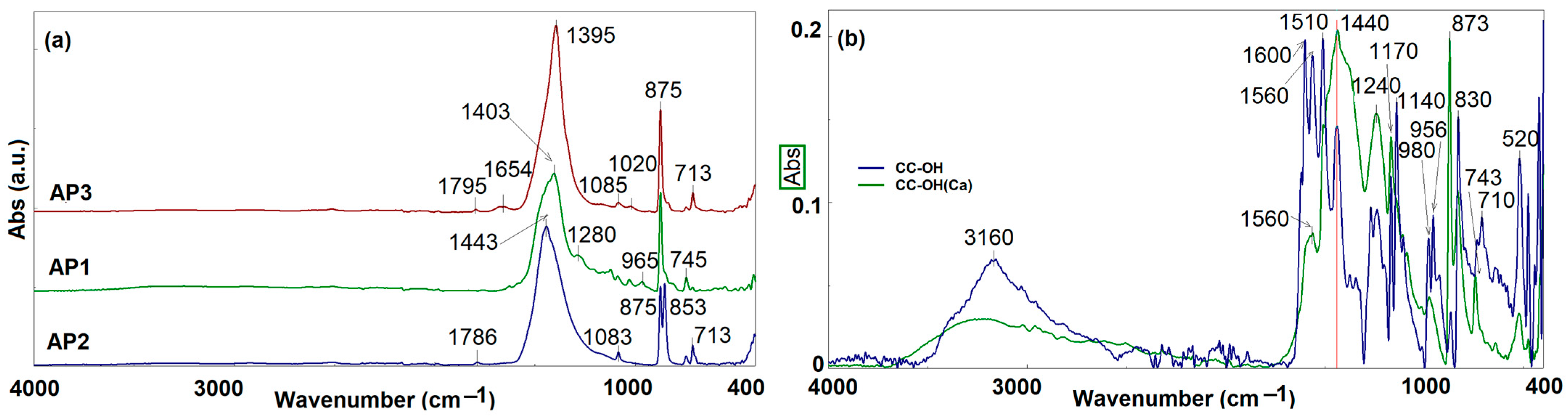
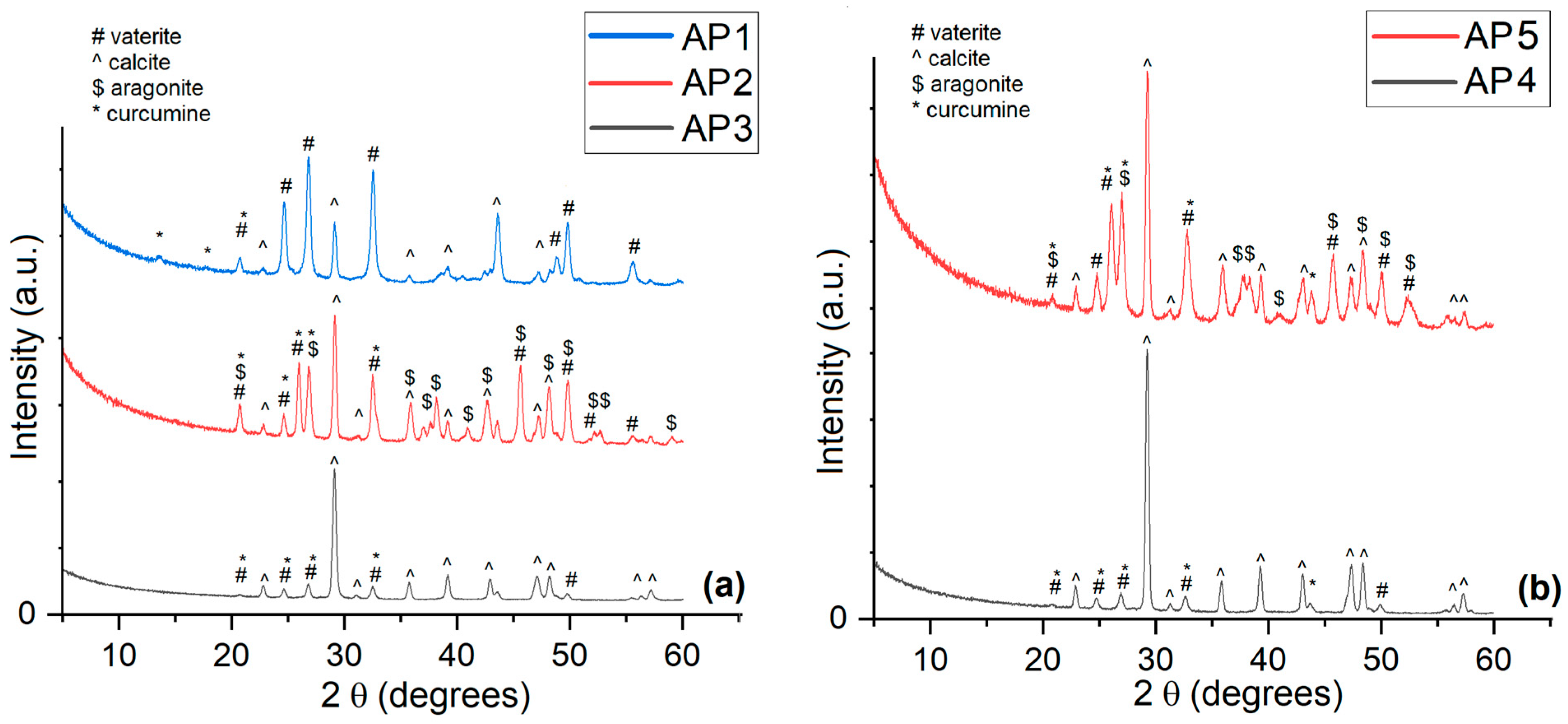
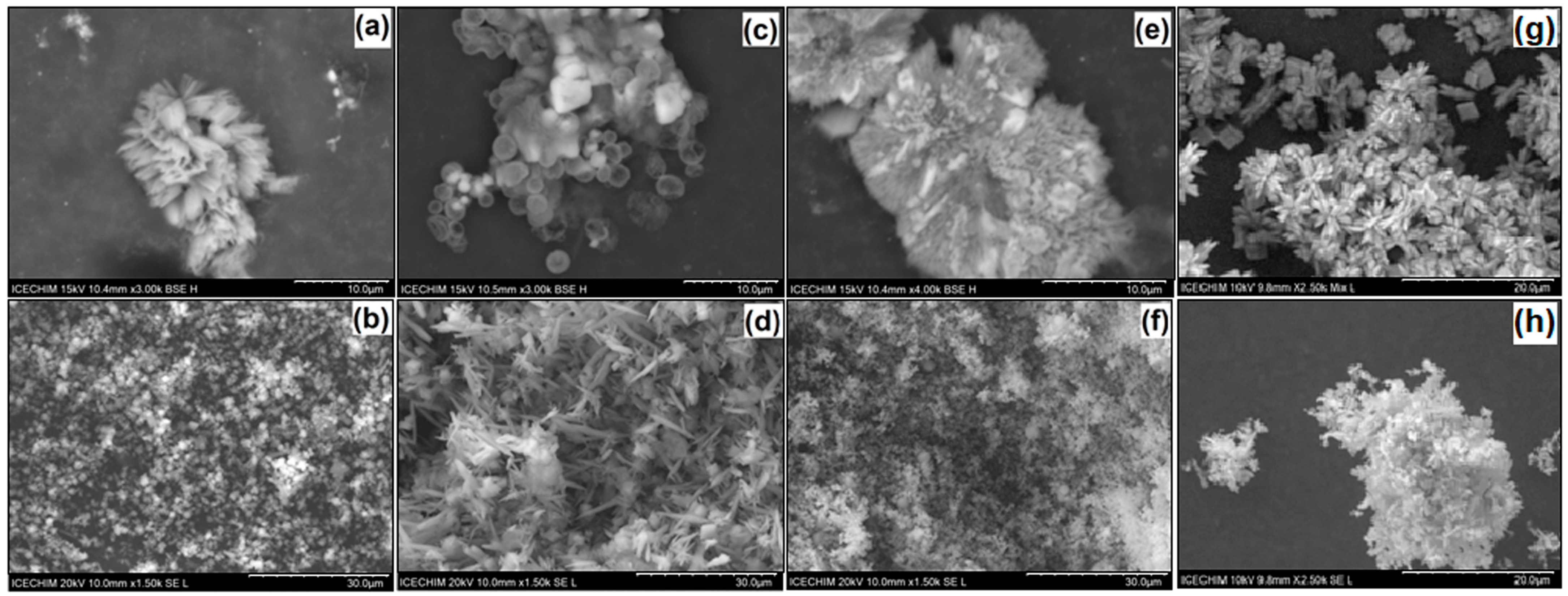
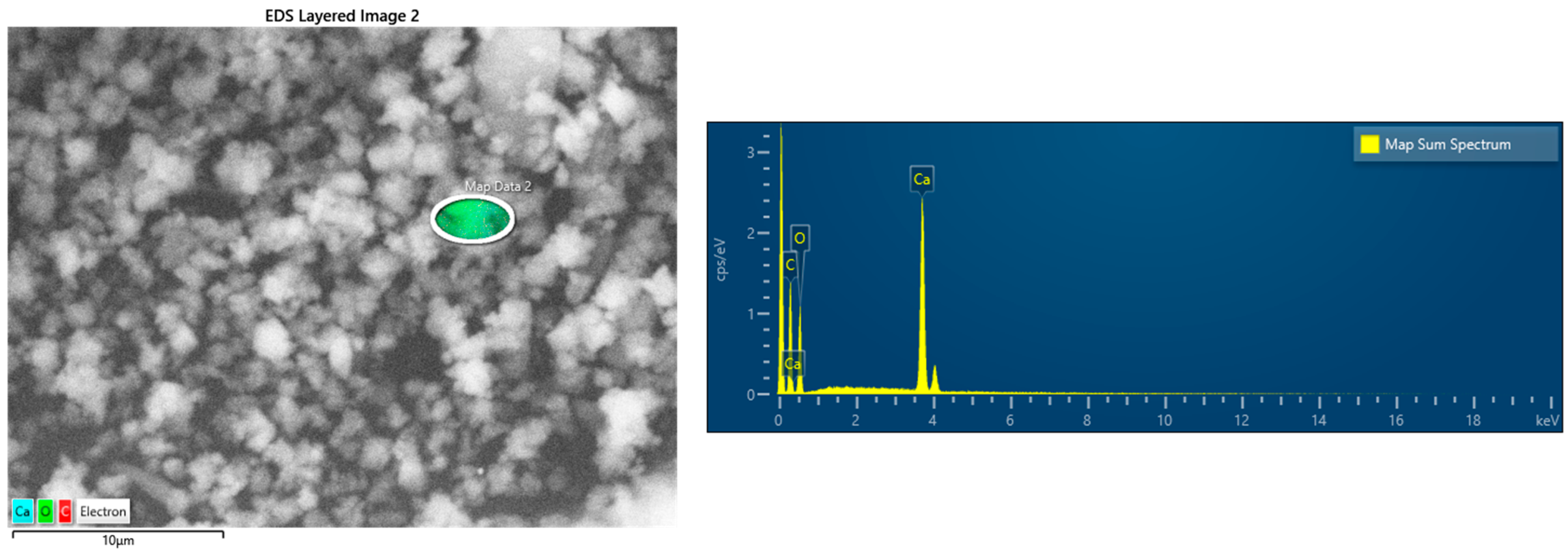

| Sample | Calcium Source for Host Matrix | Curcumin Derivative—Guest | Molar Ratio (CaCl2–CC) | Temp. [°C] | pH | Active Compound Entrapment (%) | Active Compound Content (% w/w) |
|---|---|---|---|---|---|---|---|
| AP1 | CaCl2 | CCOH | 1.5:1 | 70 | 11–12 | 20.8 | 14.1 |
| AP2 | CaCl2 | CCOH | 3:1 | 70 | 10 | 20.1 | 11.8 |
| AP3 | CaCl2 | Ca(CCOH)2 | 5:1 | 70 | 11–12 | 54.7 | 25.8 |
| AP4 | CaCl2 | Ca(CCOH)2 | 2.5:1 | 90 | 10 | 75.3 | 19.3 |
| AP5 | CaCl2 | CCOH | 5:1 | 90 | 10 | 97.2 | 11.5 |
| Element | Line Type | AP1 Weight % | AP2 Weight % | AP3 Weight % | AP4 Weight % | AP5 Weight % |
|---|---|---|---|---|---|---|
| C | K series | 42.9 ± 0.6 | 37.2 ± 0.7 | 45.8 ± 1.1 | 43.1 ± 0.7 | 32.6 ± 1.0 |
| O | K series | 41.8 ± 0.6 | 38.0 ± 0.7 | 38.2 ± 1.1 | 39.9 ± 0.6 | 25.0 ± 1.1 |
| Ca | K series | 15.3 ± 0.2 | 24.1 ± 0.1 | 16.0 ± 0.7 | 15.9 ± 0.4 | 42.4 ± 1.0 |
| Sample | L* | a* | b* | K/S (λ = 420) | Fluoresc. Int. (a.u.) λ = 420 nm |
|---|---|---|---|---|---|
| AP1 | 63.53 | 22.99 | 35.38 | 3.0641 | 175 |
| AP2 | 90.10 | 3.24 | 20.89 | 0.2589 | 185 |
| AP3 | 97.46 | 0.27 | 1.85 | 0.0048 | 305 |
| AP4 | 77.25 | 4.67 | 23.71 | 0.9316 | 250 |
| AP5 | 96.93 | 23.24 | 37.11 | 0.6448 | - |
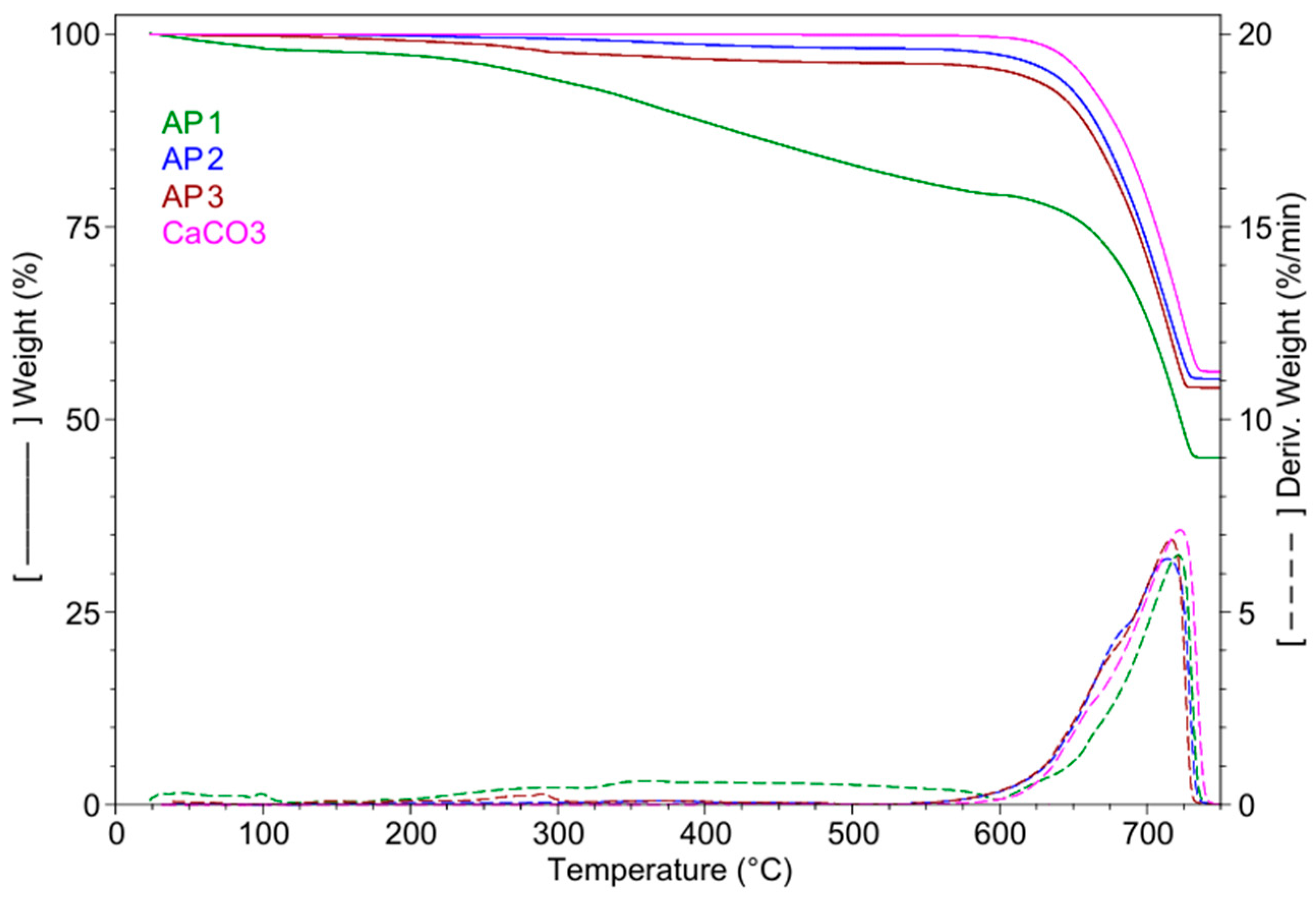
| Sample | RT-155 °C Wt.Loss | 155–600 °C Wt.LossTmax | 600–750 °C Wt.LossTmax | Residue at | SBET | Vtot | Dpor | ||
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (°C) | (%) | (°C) | 750 °C | [m2/g] | [cm3/g]∙10−3 | [nm] | |
| AP 1 | 2.07 | 18.81 | 360.0 | 34.13 | 721.0 | 45.00 | 3.63 (±0.2) | 4.08 (±0.001) | 4.5 (±0.04) |
| AP 2 | 0.06 | 2.61 | 370.7 | 42.04 | 714.1 | 55.26 | 2.72 (±0.3) | 3.48 (±0.001) | 5.1 (±0.03) |
| AP 3 | 0.26 | 4.14 | 373.2 | 41.26 | 716.2 | 54.09 | 3.69 (±0.3) | 3.86 (±0.002) | 4.2 (±0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raditoiu, A.; Raditoiu, V.; Grapin, M.; Fierascu, R.C.; Nicolae, C.A.; Raduly, M.F. Delivery Systems for Curcumin Derivatives Based on Calcium Carbonate Structures for Biomedical Applications. Crystals 2025, 15, 508. https://doi.org/10.3390/cryst15060508
Raditoiu A, Raditoiu V, Grapin M, Fierascu RC, Nicolae CA, Raduly MF. Delivery Systems for Curcumin Derivatives Based on Calcium Carbonate Structures for Biomedical Applications. Crystals. 2025; 15(6):508. https://doi.org/10.3390/cryst15060508
Chicago/Turabian StyleRaditoiu, Alina, Valentin Raditoiu, Maria Grapin, Radu Claudiu Fierascu, Cristian Andi Nicolae, and Monica Florentina Raduly. 2025. "Delivery Systems for Curcumin Derivatives Based on Calcium Carbonate Structures for Biomedical Applications" Crystals 15, no. 6: 508. https://doi.org/10.3390/cryst15060508
APA StyleRaditoiu, A., Raditoiu, V., Grapin, M., Fierascu, R. C., Nicolae, C. A., & Raduly, M. F. (2025). Delivery Systems for Curcumin Derivatives Based on Calcium Carbonate Structures for Biomedical Applications. Crystals, 15(6), 508. https://doi.org/10.3390/cryst15060508









