Abstract
Pursuing cost-effective non-precious metal electrocatalysts is a key challenge in the field of sustainable energy conversion. Transition metal dichalcogenides, known for their unique electronic structure, demonstrate superior electrocatalytic capabilities for the hydrogen evolution reaction (HER), yet their effectiveness is still lacking. In the present study, a CuS/Co3S4@MWCNT composite was fabricated via single-step hydrothermal synthesis for HER applications. This catalyst exploited the synergistic effects between CuS and Co3S4 to increase edge site functionalities and metallic conductivity, thereby resulting in high catalytical activity within the material. Furthermore, the incorporation of multi-walled carbon nanotubes (MWCNTs) into the composite effectively enhanced electron transfer kinetics throughout the HER process. Notably, thiourea serves a dual function in this synthesis, acting both as a reducing agent and as a sulfur source for the formation of metal sulfides. When evaluated in a 1 M KOH alkaline electrolyte, the synthesized nanocomposite exhibited a minimal overpotential of 300 mV to reach a current density of 10 mA/cm2, and a Tafel slope of merely 76.2 mV/dec, indicative of its good HER catalytic activity. These findings underscore the composite’s potential for application in hydrogen production technologies.
1. Introduction
The increase in population and developments in industry and technology have precipitated a rise in global energy demand, exacerbating the depletion of conventional fossil fuels, inflating fuel prices, and precipitating environmental degradation [1]. Hydrogen energy, considered an important alternative energy source, has attracted much attention owing to its high energy density and environmentally friendly, pollution-free traits. Electrocatalytic water splitting is one of the desirable methods available for hydrogen production due to its cleanliness and recyclability [2,3]. However, the efficiency of electrolysis in practical applications is currently hindered by several factors, including cathodic polarization, solution resistance, and contact resistance [4,5]. To address these issues, the utilization of electrocatalysts that can diminish cathode overpotential and boost the reaction kinetics associated with the HER is essential. In this field of study, platinum or Pt–based materials exhibit the highest activity in the hydrogen evolution reaction; however, their high cost and scarcity preclude widespread application [4]. The identification of a catalyst that exhibits both high performance and low cost remains a significant challenge.
Presently, numerous earth-abundant materials are under development to address these challenges. For instance, metal sulfides [5,6,7,8,9,10], carbides, nitrides, and phosphates [11,12,13] have demonstrated considerable potential, particularly in the realm of electrocatalytic water decomposition, enhancing HER performance through augmentation of the active site and enhancement of electrical conductivity on metal surfaces. The discovery and subsequent development of these materials offer novel methodologies for surmounting challenges associated with the conversion and storage of renewable energy [14,15,16]. The cost-effectiveness and robust stability of these materials render them highly appealing for industrial applications. Amid the array of materials, sulfides stand out as an economical and environmentally benign HER catalyst, attributed to their high specific capacitance, low cost, and superior catalytic efficacy [17,18]. Compared with other metal sulfides, Co3S4 has gained widespread usage owing to its affordability and high stability [19,20]. In particular, the integration of carbon-based materials into sulfides enhances their conductivity and HER activity. Carbon nanotubes (CNTs), graphene (G), and graphene oxide (GO) have recently been demonstrated to be efficacious materials [21,22,23], characterized by their high specific surface area, which in turn enhances the stability and dispersion of the catalytic active sites. Consequently, the enhancement of ion transport dynamics is accompanied by the formation of additional layered porous channels and the provision of enhanced structural mechanical stability. Nonetheless, the literature is sparse regarding supercapacitors constructed through the loading of monometallic sulfides onto carbon nanotubes [24,25,26]. For instance, Wu et al. [24] successfully synthesized NiFe-NGT nanocomposites under a nitrogen atmosphere via a high-temperature annealing method. In this work, a precisely controlled temperature program was employed to facilitate the formation of uniformly dispersed active sites within a nitrogen-doped carbon matrix. As a novel non-precious metal electrocatalyst, it exhibits remarkable HER activity and long-term stability in acidic environments. Nevertheless, this synthesis approach also has notable limitations: firstly, the high-temperature heat treatment process (>700 °C) results in a substantial increase in energy consumption and production costs; secondly, the stringent requirement for an inert atmosphere and the complexity of temperature control procedures escalates operational difficulty, which is not conducive to large-scale production. Moreover, high-temperature conditions may induce the sintering of metal particles and affect the graphitization degree of carbon supports. Therefore, to enhance the catalytic efficiency of the electrocatalyst by integrating elements with similar charge and lattice size, and to elucidate the electronic interactions between metal ions in the alloy sulfide structure, it is crucial to develop a simple, low-temperature, and cost-effective synthesis method. The integration of elements possessing analogous charges and lattice dimensions has the potential to enhance the catalytic efficacy of electrocatalysts. Consequently, a comprehensive understanding of the electronic interactions occurring between metal ions within the alloyed sulfide structures is essential for the realization of superior electrocatalytic performance. Thus, the primary objective of this investigation is to dope bimetallic sulfides with MWCNTs to synthesize an advanced electrocatalyst for the HER. This approach is anticipated to yield a composite material with enhanced catalytic activity, thereby contributing to the advancement of sustainable hydrogen production technologies.
In this study, a CuS/Co3S4@MWCNT composite was successfully synthesized via a facile one-pot hydrothermal process. This synthesis strategy leverages the synergistic effects between CuS and Co3S4 to exploit the catalytic performance of the composite. The composite was deposited onto multi-walled carbon nanotubes, which not only increase the number of active sites but also enhance electrical conductivity during the catalytic process. Notably, thiourea functions not only as a reducing agent in the reaction but also serves as a sulfur precursor for the formation of metal sulfides [27]. Under testing conditions in a 1 M KOH alkaline electrolyte solution, the optimized nanocomposites exhibited an overpotential of 300 mV at a current density of 10 mA/cm2, with a Tafel slope of 76.2 mV/dec, indicative of superior HER catalytic activity. The composite material was also found to retain high stability for approximately 12 h in an alkaline medium. The low-cost preparation, outstanding activity, and stability of the CuS/Co3S4@MWCNT composite underscore its immense potential as an inexpensive, efficient, and sustainable catalyst for water electrolysis applications.
2. Materials and Methods
2.1. Materials
Multi-walled carbon nanotubes (≥95%, length: 10–20 μm), cobalt (II) acetate tetrahydrate (Co (CH3COO)2·4H2O, AR), copper (II) nitrate trihydrate (Cu(NO3)2·6H2O, AR), and thiourea (CH4N2S, AR) were supplied by Aladdin Industrial Corporation (Shanghai, China). All precursors and chemicals were used without further purification.
2.2. Synthesis and Preparation
The CuS/Co3S4@MWCNT composite was synthesized through the following steps: Initially, 0.3 g of Co(CH3COO)2·4H2O and 0.3 g Cu(NO3)2·6H2O were dissolved in 40 mL of deionized water, and stirred for 10 min. Subsequently, 0.46 g of thiourea was added to the solution, followed by vigorous stirring for over 20 min to ensure complete dissolution. Thereafter, 0.05 g of multi-walled carbon nanotubes were incorporated into the mixture and stirred vigorously for an additional 40 min.
The resultant suspension was then transferred into a 100 mL high-pressure reactor and subjected to a hydrothermal process at 180 °C for 12 h. After the hydrothermal reaction, the precipitate was collected and subjected to a triple washing process with deionized water and anhydrous ethanol to remove impurities. The purified product was subsequently dried in an oven at 60 °C for 8 h, yielding the composite nanomaterials, hereafter denoted as 1CuS/1Co3S4@MWCNTs.
Through manipulation of the ratios of copper and cobalt salts, an identical synthesis protocol was adapted to produce complex materials with varying metal compositions, which are designated as 1CuS/2Co3S4@MWCNTs and 2CuS/1Co3S4@MWCNTs, respectively, to reflect the distinct metal ratios within the composite structures. The preparation procedure of CuS/Co3S4@MWCNT composite is detailed in Figure 1.
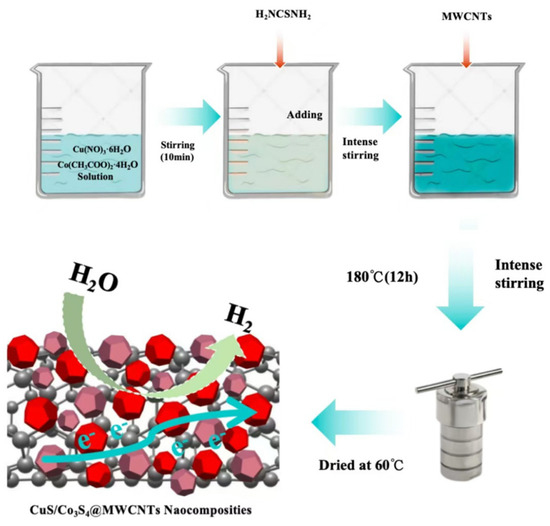
Figure 1.
Schematic diagram for preparation process of CuS/Co3S4@MWCNTs.
2.3. Electrochemical Studies
Electrochemical measurements were conducted using a conventional three-electrode system in conjunction with a GAMRY Electrochemical Workstation (Gamry Instruments, Warminster, PA, USA). All measurements were performed in an electrolyte solution of 50 mL of 1.0 M KOH with a pH of 14, which was prepared using 18 MΩ deionized water that had been purged with N2 gas (99.999%). The as-prepared material was deposited onto a rotating disk electrode (RDE, φ = 5 mm), which served as the working electrode. A platinum wire electrode and a saturated calomel electrode were utilized as the counter electrode and reference electrode, respectively. The electrode potentials were calibrated by a reversible hydrogen electrode (RHE), using the equation ERHE = ESCE + (0.241 + 0.059 pH) V, where ESCE is the potential of the saturated calomel electrode. For the RDE experiment, a GAMRY Reference 600+ type electrode rotator was employed to control the rotation speed.
2.4. Characterization
Scanning electron microscope (SEM) images were acquired using a Philips XL-30 emission scanning electron microscope (Philips Company, Amsterdam, The Netherlands) operated at 3.0 kV, equipped with an energy-dispersive X-ray spectroscopy (EDS) unit (Thermo Fisher Scientific, Waltham, MA, USA) operated at 200 kV. X-ray diffraction (XRD) patterns were obtained using an X-ray D/max-2200vpc diffractometer (Rigaku Corporation, Woodlands, TX, USA) with Cu kα radiation at a wavelength of 1.54056 Å. X-ray photoelectron spectroscopy (XPS) patterns were collected with a Thermo ESCA LAB spectrometer (Thermo Fisher Scientific, USA).
3. Results
3.1. Research on Crystal Properties
X-ray diffraction (XRD) analysis is used to ascertain the crystalline structure and phase composition of the synthesized materials as depicted in Figure 2. All the XRD patterns of composites with different metal ratios reveal characteristic reflections at 2θ = 27.78°, 31.27°, and 46.94°, corresponding to the (101), (103), and (107) planes, respectively, which are evident in the CuS diffraction pattern, confirming the formation of hexagonal CuS nanocrystals (JCPDS No.85-0620) [28]. In particular, (101) facets can provide more active sites in the electrocatalytic reaction, thus increasing catalytic activity. On the other hand, Co3S4 displays diffraction peaks at 2θ = 26.54°, 31.27°, 37.95°, 50.00°, and 54.81°, corresponding to the (220), (331), (400), (511), and (440) planes, respectively, and demonstrates more pronounced crystallinity than CuS (JCPDS No. 74-0138). The XRD pattern of the CuS/Co3S4@MWCNT composite is sharp enough, indicating its good crystallinity, and shows the presence of CuS and Co3S4 phases. Obviously, there are no additional peaks observed, which indicates that the prepared products are pure. Furthermore, the diffraction peak intensity of CuS in the 2CuS/Co3S4@MWCNT sample appears weaker than that of Co3S4, which may be due to the small usage of copper salt in the preparation process or the low crystallinity of CuS during formation. Finally, it should be pointed out the MWCNT–related diffraction peaks are difficult to identify. These phenomena might be explained by the fact that the main peak of MWCNTs, which is at 26°, overlaps with the main peak of Co3S4, which is at 26.54°. This overlap may be the cause, and the peaks of MWCNTs are shielded by those of Co3S4 because the crystalline extent of MWCNTs is significantly lower than that of Co3S4 [29].
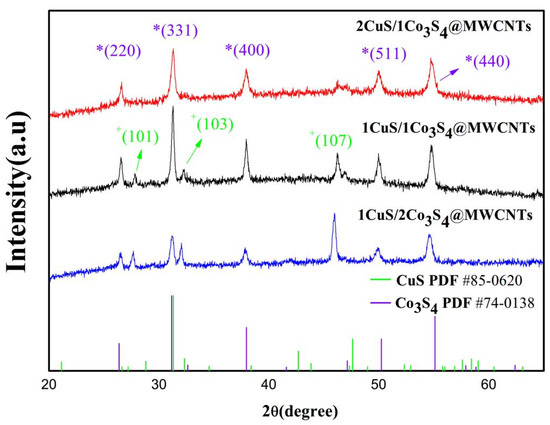
Figure 2.
XRD patterns of 1CuS/1Co3S4@MWCNTs, 1CuS/2Co3S4@MWCNTs, and 2CuS/1Co3S4@MWCNTs (Purple * represents Co3S4 crystal plane, green * represents CuS crystal plane).
3.2. Morphological Identification
The structural and morphological characteristics of the materials were investigated using scanning electron microscopy (SEM). SEM images (Figure 3A) revealed that the 2CuS/1Co3S4@MWCNT composite, as well as individual Co3S4 and CuS samples, exhibited an interconnected particles/spheres morphology. Figure 3B displays the morphology of the 2CuS/1Co3S4@MWCNT composite, where nanoparticles, spheres, and hexagons with sizes ranging from about 100 nm to 200 nm were observed. These particles are deeply embedded within the carbon nanotubes, indicating strong integration of the metal sulfides with the MWCNT matrix. This uniform distribution contributes to an increased specific surface area of the material, which in turn provides a greater number of active sites. Additionally, it enhances the contact area between the catalyst and the electrolyte, thereby facilitating the electrochemical reaction. Figure 3D–H present color elemental mapping of the 2CuS/1Co3S4@MWCNT composite, confirming the distribution of elements Co, Cu, C, O, and S throughout the hybrid material. The corresponding energy-dispersive X-ray spectroscopy (EDS) continuum of the composite is also shown in Figure 3I. This spectrum further substantiates the effective dispersion of the metal sulfides within the composite, which is essential for the catalyst’s performance. The mass percentages of Cu, Co, and S are 15.83%, 5.54%, and 7.33%, respectively, confirming the successful preparation of the 2CuS/1Co3S4@MWCNT composite. Such distribution ensures that the active sites of the catalyst are optimally exposed, thereby enhancing their participation in the catalytic reaction.
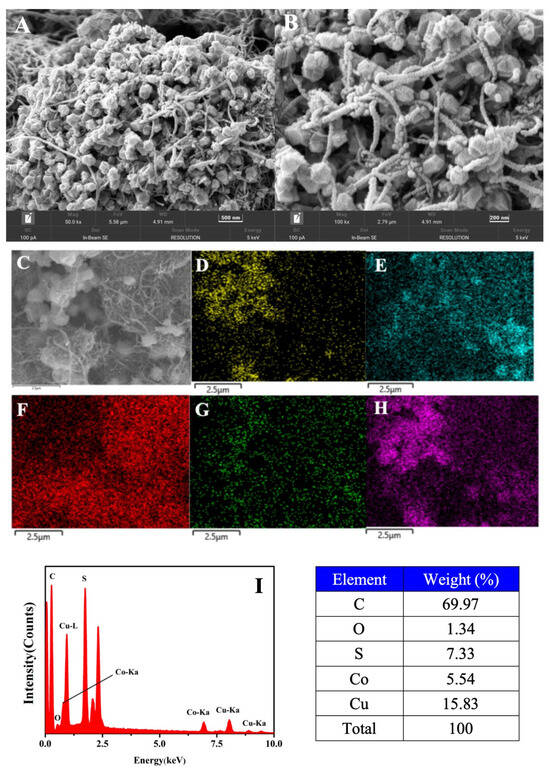
Figure 3.
(A) SEM images of the 2CuS/1Co3S4@MWCNT composite; (B) broad-field SEM image of (A); (C–H) the corresponding EDS elemental mappings of the composite; (D) Co (yellow), (E) Cu (blue), (F) C (red), (G) O (green), and (H) S (pink); (I) EDAX spectrum of the composite and the distribution of elemental content.
3.3. Investigation of X-Ray Photoelectron Spectroscopy (XPS) Characteristics of Crystals
Figure 4 shows X-ray photoelectron spectroscopy (XPS) data, which reveals the elemental composition and electronic state of the various components within the 2CuS/1Co3S4@MWCNT composite. The presence of the individual elements Co, Cu, S, C, and O in the composite is verified by the full-scan survey spectra presented in Figure 4A. As shown in Figure 4B, the high-resolution Co 2p XPS spectrum is deconvolved into two spin orbits. The first state is attributed to Co3+ at 778.9 eV (Co 2p3/2) and 794.2 eV (Co 2p1/2), and the second state is allocated to Co2+ at 781.2 eV (Co 2p3/2) and 797.6 eV (Co 2p1/2), indicating the coexistence of Co2+ and Co3+. In addition, the wide peaks at 786.6 eV and 802.1 eV belong to satellite peaks. It is widely known that metal ions in a high oxidation state suggest a specific electronic structure that can influence a material’s properties, such as its catalytic activity and electronic conductivity. The predominance of Co3+ ions might contribute to higher catalytic activity in the HER [30]. Figure 4C displays the high-resolution XPS spectrum of the Cu 2p region, which exhibits two distinct peaks at binding energies of 931.2 eV and 951.2 eV. The 20 eV binding energy separation observed in the XPS spectrum is a characteristic feature of the Cu–S bond in CuS. These peaks correspond to the Cu 2p3/2 and Cu 2p1/2 orbitals, respectively, and indicate the presence of Cu+/Cu2+ oxidation states in the hybrid material as supported by references [31,32]. The attribution of these peaks to the Cu 2p3/2 and Cu 2p1/2 orbitals suggests the coexistence of copper in different oxidation states within the composite, which is significant for understanding its electronic properties and potential catalytic behavior. Additionally, three smaller satellite peaks are observed at 933.1 eV, 941.9 eV, and 953.9 eV. Figure 4D presents the high-resolution XPS spectrum of the S 2p region with two main peaks at binding energies of 161.5 eV and 160.2 eV, corresponding to S 2p1/2 and S 2p3/2, respectively, which are attributed to M–S bonding and confirm the presence of metal–sulfur bonds in the compound. It is worth noting that the broad peak near 166.9 eV is attributed to sulfur in a higher oxidation state, which might act as active sites for the formation of heterostructures. The XPS analysis provided herein offers a thorough illustration of the successful formation of the 2CuS/1Co3S4@MWCNT composite. In Figure 4E, the C1s in 2CuS/1Co3S4@MWCNTs can be deconvolved into carbon (284.6 eV) with a hybridization mode of sp2, which promotes charge transport due to its high electron mobility. As shown in Figure 4F, the characteristic peak at 530.2 eV indicates the existence of Co–O bonding, which might be caused by incomplete vulcanization in part during the preparation process. The peak at 531.8 eV corresponds to the surface hydroxyl group, which might originate from air exposure.
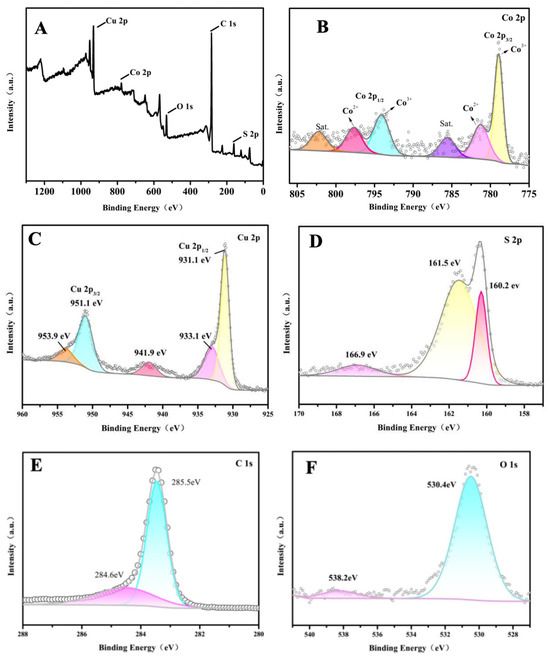
Figure 4.
XPS spectra of 2CuS/1Co3S4@MWCNT composite: (A) survey; (B) high-resolution XPS spectra of Co 2p3; (C) Cu 2p; (D) S 2p; (E) C 1s; and (F) O 1s.
3.4. The N2 Adsorption-Desorption Isotherm of the Material
Figure 5A indeed illustrates the nitrogen adsorption-desorption isotherm curve at 77K for the CuS/Co3S4@MWCNT composite, which is standard procedure for characterizing surface area and porosity using the Brunauer–Emmett–Teller (BET) method. Calculations reveal that the material possesses an average specific surface area of 12.8585 m2·g−1. The isothermal curve, ranging from 0.0 to 1.0 (P/P0), exhibits an H4-type hysteresis loop. This isotherm belongs to V-type, indicating that the material exhibits mesoporous adsorption characteristics. Further, the pore size distribution was calculated using the Barret–Jeyner–Halenda (BJH) curve, and the resulting curve is illustrated in Figure 5B. The curve reveals a distinct distribution that is predominantly centered around a pore diameter of 32.74 nm, with a pore volume of approximately 0.079 cc/g (as shown in Table 1). This pore size distribution is characteristic of mesoporous materials, which is consistent with observations from the adsorption-desorption isotherm. The mesoporous structure enhances the permeability of the electrode material to the electrolyte, and facilitates full utilization of the active ingredients. The distinctive architecture of the composite provides ample channels for efficient ion transport between the electrolyte and the electrode surface. This feature facilitates accelerated ion diffusion and electron transfer, which in turn minimizes volume expansion and contraction associated with the charging and discharging processes. Consequently, the composite’s structural integrity is maintained, leading to an enhanced cycling stability, which is a critical attribute for the long-term performance of electrocatalytic materials in energy storage and conversion applications.
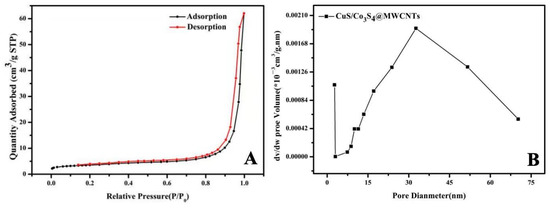
Figure 5.
(A) Nitrogen adsorption-desorption isotherm curve for the CuS/Co3S4@MWCNT composite; (B) pore size distribution curve of the CuS/Co3S4@MWCNT composite.

Table 1.
Analysis of void characteristics of materials.
3.5. Electrochemical Characterization of CuS/Co3S4@MWCNTs
In this study, we assessed the hydrogen evolution performance of CuS/Co3S4@MWCNTs catalysts with varying metal ratios in the alkaline conditions of a 1.0 M KOH solution. We note that all polarization curves are corrected for iR loss, and linear sweep voltammetry (LSV) was employed to evaluate the catalytic activity of these materials. A typical polarization curve (I–V plot) demonstrates that the 2CuS/1Co3S4@MWCNT electrode presents a low onset overpotential (η) of ~500 mV versus RHE for taking off HER activity (Figure 6A). This phenomenon can be attributed to enhanced electrical conductivity, which significantly improves the HER performance, particularly with respect to current density. As a typical reference metric for electrochemical catalytic performance, the potential value for 10 mA/cm2 current density is frequently employed; the 2CuS/1Co3S4@MWCNTs catalysts only require 423 mV to achieve 10 mA/cm2, which is better than the other two.
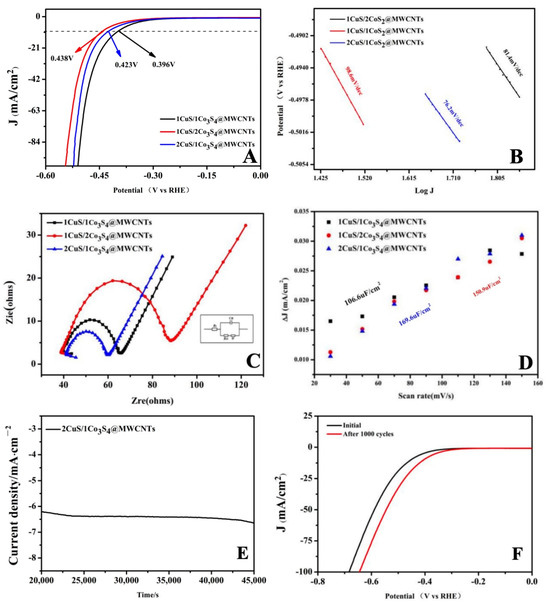
Figure 6.
(A) Polarization curves of the CuS/Co3S4@MWCNT composite with different metal ratios in 1.0 M N2–purged KOH solution; (B) Tafel slopes of different catalysts derived from the polarization curves; (C) ESI of catalysts in a 0.5 mM impedance solution; (D) linear fitting for the capacitive currents of the catalyst vs scan rates; (E) i−t curve obtained under a static overpotential of −1.3 V; (F) HER polarization curves before and after 1000 cycles for the 2CuS/1Co3S4@MWCNT composite modified.
To understand the detailed underlying mechanism of HER activity, Tafel plots based on polarization curves are acquired as shown in Figure 6B. The linear regions of Tafel plots were fit to the Tafel equation (η = b log j + a, where j is the current density, and b is the Tafel slope) to obtain slope b. Tafel slopes of 76.2, 81.4, and 98.6 mV/dec are obtained for 2CuS/1Co3S4@MWCNTs, 1CuS/2Co3S4@MWCNTs, and CuS/Co3S4@MWCNTs, respectively. The small Tafel slope of the 2CuS/1Co3S4@MWCNT catalyst is advantageous for practical catalytic application since it leads to a rapid increase in the HER rate with overpotential. This is attributed to strong electronic coupling between the nanoscale thin metal sulfide catalyst and MWCNT surface, which optimizes the electronic structure and accelerates gaseous hydrogen release from catalytically active sites. While both CuS and Co3S4 contribute catalytical active sites, the metal ratio significantly influences the catalyst’s performance. Therefore, optimizing the metal ratio plays a crucial role in enhancing catalytic activity. Notably, prior studies [33] report that the Tafel slope of MWCNTs in electrocatalytic hydrogen precipitation is about 101 mV/dec. This value is larger than the Tafel slope demonstrated by our prepared catalysts, therefore, MWCNTs are mainly used as a structural support material and conductive carrier in composite catalyst systems. Crucially, the catalytic performance of these composites is governed by the composition and stoichiometry of the incorporated metal sulfides, which directly modulate the density of active sites and kinetics of hydrogen adsorption/desorption processes. Thereby, the composition, as well as the ratio of the metal sulfide, is a key factor in determining the performance of the catalysts.
Fluent charge transport at the electrode interface could also be characterized by electrochemical impedance spectroscopy (EIS) as shown in Figure 6C. The charge transfer resistance (Rct) of the material is obtained by fitting the semi-circle in the high-frequency region of the Nyquist diagram. As illustrated in Figure 6C, the impedances vary with the metal proportions in the materials. The order of impedance magnitude is 1CuS/2Co3S4@MWCNTs > CuS/Co3S4@MWCNTs > 2CuS/1Co3S4@MWCNTs. This data indicate that although all materials possess some level of conductivity, a comparative analysis reveals that the 2CuS/1Co3S4@MWCNT composite has the lowest internal resistance among the three, conferring it with the highest conductivity. In addition, the vertical slope of the linear part in the low-frequency region further indicates the low diffusion resistance of ion and electron transfer for the 2CuS/1Co3S4@MWCNT nanocomposite. The apparently enhanced electrocatalytic activity benefits from the low-resistance Ohmic contact between metal sulfide nanostructures and the highly conductive MWCNT networks, largely facilitating fast ion penetration and electron transport.
Effective electrochemical surface area (ECSA) is an important parameter for assessing the performance of electrocatalysts as it provides an indication of the number of active sites available for catalysis. The ECSA of the composites during the HER process is further estimated by the double-layer capacitances (Cdl) in the CV curves, which are a measure of the charge stored in the electrical double layer at the electrode–electrolyte interface. Figure 6D shows the Cdl value of the catalysts is extracted by plotting the Δj at a given potential of 0–0.1 V (vs RHE) at varying scan rates of 30, 50, 70, 90, 110, 130, and 150 mV·s−1 in the non-Faraday range. Upon testing these catalysts, the Cdl values of 1CuS/1Co3S4@MWCNTs, 1CuS/2Co3S4@MWCNTs, and 2CuS/1Co3S4@MWCNTs were found to be 106.6 μF·cm−2, 150.9 μF·cm−2, and 169.6 μF·cm−2, respectively. Obviously, the 2CuS/1Co3S4@MWCNT catalyst exhibits the highest double-layer capacitance value, further demonstrating that it possesses the largest ECSA.
The HER not only necessitates high electrocatalytic activity but also demands long-term stability to improve energy transfer and conversion efficiency. At a fixed overpotential of −1.3007 V, an i–t curve was obtained using the chronoamperometry method. The stability of 2CuS/1Co3S4@MWCNTs in a 1.0 M KOH solution was evaluated over a duration of 50,000 s as shown in Figure 6E. The figure clearly illustrates that the catalyst maintains a stable current response throughout the test period. Furthermore, the catalytic stability of our 2CuS/1Co3S4@MWCNT catalyst is also characterized by continuous cyclic voltammetry performed between −1.0 and 0.2 V vs RHE at a 100 m/s scan rate (Figure 6F). A comparison of the linear sweep voltammetry (LSV) curve before and after 1000 CV cycles reveals that a minor change in cathodic current density is minimal. These results further confirm that the 2CuS/1Co3S4@MWCNT catalyst exhibits excellent electrocatalytic stability for the HER. In general, the durability of the supported catalyst is known to strongly depend on the catalyst support chemical and electronic coupling interaction. Previous simulation and experimental results have established that strong interaction of the d-orbital of transition metal with the p-orbital of MWCNTs may give rise to robust bonding. Comparative analysis of catalytic properties in Table 2 reveals that the materials synthesized in this study exhibit superior Tafel characteristics (slope range: 76.2 mV/dec−1) and robust catalytic activity, achieved through a facile one-pot hydrothermal method.

Table 2.
A comparison of the CuS/Co3S4@MWCNT electrocatalyst in this work with previously reported sulfide electrocatalysts for the HER.
4. Conclusions
In this study, we report the synthesis of CuS and Co3S4 co-loaded heterogeneous structures on multi-walled carbon nanotubes via a one-step hydrothermal method. In this process, thiourea serves a dual role as not only a reducing agent but also a sulfur source for the metals, streamlining the synthesis procedure and enhancing its operational ease. The resulting three-dimensional architecture, composed of nanospheres and carbon nanotubes, boasts a substantial specific surface area for reaction and a high density of catalytic active sites, which collectively endow it with superior catalytic performance for the HER. As evidenced by the linear sweep voltammetry test, the catalyst exhibits a Tafel slope as low as 76.2 mV/dec. In summary, the materials synthesized herein provide a valuable prototype for the development of simple synthesis strategies for transition metal sulfides loaded onto carbon nanotube substrates.
Author Contributions
X.Z.: conceptualization, methodology, formal analysis, investigation, and writing—original draft of the manuscript. M.S. (Meng Sun): formal analysis, writing—original draft, and methodology. H.G.: formal analysis and investigation. M.S. (Ming Su): writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Hebei Province (B2023201032), Hebei Province Innovation Capacity Enhancement Program (225A4802D), Hebei University Innovation Team Program (IT2023A03), and the Science Research Project of Hebei Education Department (JZX2024019).
Data Availability Statement
All data are included in the text.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Li, J.; He, X.; Zheng, Y.; Sun, S.; Fang, X.; Zheng, D.; Luo, Y.; Wang, Y.; et al. Highefficiency overall alkaline seawater splitting: Using a nickel-iron sulfide nanosheet array as a bifunctional electrocatalyst. J. Mater. Chem. A 2023, 11, 1116–1122. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Q.; Sun, W.; Sun, K.; Shen, Y.; An, W.; Zhang, L.; Chen, H.; Zou, X. Nanostructured intermetallics: From rational synthesis to energy electrocatalysis. Chem. Synth. 2023, 3, 28. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Sun, C.; Xi, P.; Peng, S.; Gao, D.; Xue, D. Accelerated hydrogen evolution reaction in CoS2 by transition-metal doping. ACS Energy Lett. 2018, 3, 779–786. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Bose, R.; Jothi, V.R.; Vikraman, D.; Jeong, Y.-T.; Arunkumar, P.; Velusamy, D.B.; Maiyalagan, T.; Alfantazi, A.; Kim, H.-S. High performance, 3D-hierarchical CoS2/CoSe@C nanohybrid as an efficient electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2020, 838, 155537. [Google Scholar] [CrossRef]
- Aftab, U.; Tahira, A.; Samo, A.H.; Abro, M.I.; Baloch, M.M.; Kumar, M.; Ibupoto, Z.H. Mixed CoS2@Co3O4 composite material: An efficient nonprecious electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 13805–13813. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, M.; Wang, C.; Zhu, Y.; Li, N.; Pu, X.; Yu, A.; Zhai, J. Vertically aligned NiS2/CoS2/MoS2 nanosheet array as an efficient and low-cost electrocatalyst for hydrogen evolution reaction in alkaline media. Sci. Bull. 2020, 65, 359–366. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent Advances in Transition-Metal-Sulfide-Based Bifunctional Electrocatalysts for Overall Water Splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Xu, K.; Yu, T.; Yao, S.; Peng, Q.; Yuan, C. Crystal plane dependent electrocatalytic performance of NiS2 nanocrystals for hydrogen evolution reaction. J. Catal. 2020, 381, 63–69. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Zhang, D.; Pi, M.; Feng, J.; Chen, S. Mn-doped NiP2 nanosheets as an efficient electrocatalyst for enhanced hydrogen evolution reaction at all pH values. J. Power Sources 2018, 387, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Shang, J.; Su, C.; Zhang, S.; Wu, M.; Guo, Y. Preparation of amorphous NiP-based catalysts for hydrogen evolution reactions. J. Fuel Chem. Technol. 2018, 46, 473–478. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, H.; Du, Y.; Yang, Y.; Liu, Y.; Wang, L. RuP4 decorated CoP acacia-like array: An efficiently electrocatalyst for hydrogen evolution reaction at acidic and alkaline condition. Appl. Surf. Sci. 2020, 534, 147626. [Google Scholar] [CrossRef]
- Li, Y.W.; Lu, M.T.; Wu, Y.H.; Ji, Q.H.; Xu, H.; Gao, J.K.; Qian, G.D.; Zhang, Q.C. Morphology regulation of metal-organic framework-derived nanostructures for efficient oxygen evolution electrocatalysis. J. Mater. Chem. A 2020, 8, 18215–18219. [Google Scholar] [CrossRef]
- Weng, B.C.; Grice, C.R.; Meng, W.W.; Guan, L.; Xu, F.H.; Yu, Y.; Wang, C.L.; Zhao, D.W.; Yan, Y.F. Metal-organic framework-Derived CoWP@C Composite Nanowire electrocatalyst for efficient water splitting. ACS Energy Lett. 2018, 3, 1434–1442. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhao, T.; Lu, M.T.; Wu, Y.H.; Xie, Y.B.; Xu, H.H.; Gao, J.K.; Yao, J.M.; Qian, G.D.; Zhang, Q.C. Enhancing oxygen evolution reaction through modulating electronic structure of trimetallic electrocatalysts derived from Metal-organic frameworks. Small 2019, 15, 1901940. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.; Zhang, S.; Mao, C.; Song, J.; Niu, H.; Jin, B.; Tian, Y. Controlled synthesis of nickel sulfide/graphene oxide nanocomposite for high-performance supercapacitor. Appl. Surf. Sci. 2013, 282, 704–708. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, B.; Bonakdarpour, A.; Sun, A.; Wilkinson, D.P.; Wang, D. WS2 nanosheets as a highly efficient electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2012, 125, 59–66. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, W.; Xi, P.; Xi, S.; Du, Y.; Gao, D.; Ding, J. Activating and optimizing activity of CoS2 for hydrogen evolution reaction through the synergic effect of N dopants and S vacancies. ACS Energy Lett. 2017, 2, 1022–1028. [Google Scholar] [CrossRef]
- Masar, M.; Urbanek, M.; Urbanek, P.; Machovska, Z.; Maslik, J.; Yadav, R.S.; Skoda, D.; Machovsky, M.; Kuritka, I. Synthesis, characterization and examination of photocatalytic performance of hexagonal covellite CuS nanoplates. Mater. Chem. Phys. 2019, 237, 121823. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Wu, S.; Shen, X.; Zhu, G.; Zhou, H.; Ji, Z.; Ma, L.; Xu, K.; Yang, J.; Yuan, A. Metal organic framework derived NiFe@N-doped graphene microtube composites for hydrogen evolution catalyst. Carbon 2017, 116, 68–76. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef]
- Wang, D.Y.; Gong, M.; Chou, H.L.; Pan, C.J.; Chen, H.A.; Wu, Y.; Lin, M.C.; Guan, M.; Yang, J.; Chen, C.W. Highly Active and Stable Hybrid Catalyst of Cobalt-Doped FeS2Nanosheets–Carbon Nanotubes for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Duraisamy, S.; Ganguly, A.; Sharma, P.K.; Benson, J.; Davis, J.; Papakonstantinou, P. One-Step Hydrothermal Synthesis of Phase-Engineered MoS2/MoO3 Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2021, 4, 2642–2656. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Sekar, S.; Devi, S.B.; Maruthasalamoorthy, S.; Maiyalagan, T.; Kim, D.Y.; Lee, S.; Navamathavan, R. One-step facile hydrothermal synthesis of rGO-CoS2 nanocomposites for high performance HER electrocatalyst. Int. J. Hydrogen Energy 2022, 47, 40359–40367. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, Y.; Dai, K.; Wang, J.; Zhang, B.; Shen, X. NiCoP Nanowire@NiCo-Layered Double Hydroxides Nanosheet Heterostructure for Flexible Asymmetric Supercapacitors. Chem. Eng. J. 2020, 384, 123373. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Kollu, P.; Jeong, S.K.; Grace, A.N. A template-free facile approach for the synthesis of CuS-rGO nanocomposites towards enhanced photocatalytic reduction of organic contaminants and textile effluents. RSC Adv. 2015, 5, 15831–15840. [Google Scholar] [CrossRef]
- Shu, Q.W.; Li, C.M.; Gao, P.F.; Gao, M.X.; Huang, C.Z. Porous hollow CuS nanospheres with prominent peroxidase-like activity prepared in large scale by a one-pot controllable hydrothermal step. RSC Adv. 2015, 5, 17458–17465. [Google Scholar] [CrossRef]
- Xu, Y.C.; Yang, C.H.; Deng, Q.H.; Zhou, Y.M.; Mao, C.F.; Song, Y.C.; Zhu, M.; Zhang, Y.W. Bi-Porphyrins MOF with confinement and ion-attracting effects in concert with RuO2-doped CNT as efficient electrocatalysts for HER in acidic and alkaline media. Appl. Surf. Sci. 2023, 612, 155870. [Google Scholar] [CrossRef]
- Sun, X.; Huo, J.; Yang, Y.; Xu, L.; Wang, S.Y. The Co3O4 nanosheet array as support for MoS2 as highly efficient electrocatalysts for hydrogen evolution reaction. JEC 2017, 6, 1136–1139. [Google Scholar] [CrossRef]
- Yao, Y.; He, J.; Yang, X.; Peng, L.; Zhu, X.; Li, K.; Qu, M. Superhydrophilic/underwater superaerophobic self-supporting CuS/Cu foam electrode for efficient oxygen evolution reaction. Colloids Surf. A 2022, 634, 127934. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Wu, L.; Li, G.; Tao, K.; Han, L. Zeolitic Imidazolate Framework-Derived Co3S4@NiFe-LDH Core-hell Heterostructure as Efficient Bifunctional Electrocatalyst for Water Splitting. ACS Appl. Mater. 2024, 16, 8751–8762. [Google Scholar] [CrossRef]
- Fan, Y.J.; Ai, T.T.; Bao, W.W.; Han, J.; Jiang, P.; Deng, Z.F.; Wei, X.L.; Zou, X.Y. Ru doping Co3S4 induced electron and morphology double regulation to promote the kinetics of the bifunctional catalytic reaction. Appl. Surf. Sci. 2025, 689, 162361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).