Abstract
The phase composition and morphological characteristics of eutectic carbides are key factors affecting the wear resistance and fatigue life of high-speed steel. In this study, a combination of experimental characterization and thermodynamic calculations was used to systematically reveal the dynamic regulation mechanism of cooling rate on eutectic carbides in M2Al high-speed steel. The results indicate that within a cooling rate range of 5 to 225 °C/min, the steel always contains a small amount of face-centered cubic-structured MC-type eutectic carbides and a large number of hexagonal close-packed structured M2C-type eutectic carbides. The three-dimensional morphology of MC-type eutectic carbides is smooth and rod-like, and is insensitive to the cooling rate, while the three-dimensional morphology of M2C-type eutectic carbides evolves from lamellar to dendritic with an increasing cooling rate. The increase in cooling rate significantly reduces the average size of eutectic carbides, increases the total area fraction, and improves the distribution uniformity. Additionally, the increase in cooling rate also promotes the significant refinement of secondary dendrites in M2Al high-speed steel, and the relationship between secondary dendrite arm spacing and cooling rate is . Finally, combining thermodynamic calculations with kinetic analysis, this study found that the formation of eutectic carbides is dominated by the segregation of elements such as V, Mo, and C during the final stage of solidification, while the chemical composition and three-dimensional morphological evolution of M2C-type eutectic carbides are synergistically controlled by the diffusion and competitive growth of elements such as W, Mo, and C in austenite. This study provides a theoretical basis for the solidification process and eutectic carbide control of M2Al high-speed steel.
1. Introduction
M2 high-speed steel (HSS) is a molybdenum-based high-speed tool steel, well-known for its excellent red hardness, wear resistance, and high-temperature strength [1]. M2Al HSS is a novel superhard HSS derived from M2 by increasing its carbon content and incorporating aluminum. Due to its excellent hardness, toughness, and wear resistance, M2Al HSS is widely used in the production of cutting tools, especially for processing high-temperature alloys, ultra-high-strength steel, and other difficult-to-cut materials [2]. However, segregation of tungsten (W), molybdenum (Mo), chromium (Cr), vanadium (V), and carbon (C) is prone to occur during the solidification of HSS, resulting in eutectic carbides that are large-sized, irregularly shaped, and unevenly distributed [3,4,5]. These eutectic carbides significantly influence the mechanical properties of the material, including hardness, wear resistance, toughness, and service life [6,7]. During subsequent heat treatment and hot working, these carbides, even after extensive thermoplastic deformation, are prone to forming large and inhomogeneous carbide bands, which compromise the toughness and isotropic properties of the steel [8]. Moreover, the presence of large-sized eutectic carbides serves as preferential pathways for crack initiation during service [9]. Therefore, controlling the precipitation behavior of these carbides is critical to enhancing the machinability and service performance of M2Al HSS.
To regulate the precipitation of eutectic carbides during solidification, various strategies have been proposed, including alloy composition optimization [10,11], microalloying [12,13,14], semi-solid forging [15,16,17], electromagnetic stirring [18,19], and increasing the cooling rate [20,21,22,23]. For instance, Li et al. [10] investigated the effect of niobium (Nb) on the microstructure, hardness, and wear behavior of HSS. The results showed that the addition of Nb changed the solidification mode of HSS and refined M6C-, M2C-, and Cr7C3-type eutectic carbides. However, the formation of incipient NbC particles reduced the carbon content in austenite, which may weaken the precipitation strengthening of HSS after heat treatment. Zheng et al. [12] studied the influence of rare earth cerium (Ce) on the microstructure, incipient carbides, and inclusions of M35 HSS; the study showed that rare earth Ce changed the type of inclusions in the steel, and a small amount of rare earth Ce reduced the area fraction of carbides by increasing the compositional subcooling and heterogeneous nucleation mechanism of rare earth inclusions; meanwhile, the further increase in the Ce content resulted in the generation of Ce-As-P inclusions in the steel promoted the precipitation of M2C-type carbides. While microalloying offers a promising approach to improving eutectic carbides in HSS, its effectiveness depends on the specific alloy composition and the precise timing of the element addition, making it challenging to generalize. Semi-solid forging leverages the thermal gradient during solidification to forge the material when the surface layer has solidified but the core remains in a mushy state. It has been shown to promote the diffusion of alloying elements in Cr4Mo4V-bearing steel, alleviate liquid segregation, and significantly reduce the volume fraction of eutectic carbides [16]. Nevertheless, the difficulty in controlling process parameters such as forging temperature and pressure limits the application of this technology. Zheng et al. [18] demonstrated that applying a transverse static magnetic field during the directional solidification of M2 HSS could transform layered eutectic carbides into fibrous ones. However, improper control of the magnetic flux density could result in segregation channels or uneven microhardness distribution in the austenitic matrix, thereby degrading HSS properties.
Numerous studies have demonstrated that the characteristic parameters such as the morphology, number, and size of eutectic carbides can be controlled by increasing the solidification cooling rate [20,21,22,23]. In conventional HSS melt-casting processes, the cooling rate can be enhanced through measures such as utilizing high-thermal-conductivity casting molds, adjusting casting techniques, and optimizing the electroslag remelting process. Ji et al. [24] introduced the fusible metal mold-casting method, which reduces the thermal resistance at the interface between M2 HSS and the mold during casting. This method doubles the average cooling rate, resulting in ingots with fine microstructures and uniform carbide distribution. Zhou et al. [4,25] found that replacing sand molds with cast iron molds significantly increases the solidification rate and transforms the micro-morphology of sub-stable M2C-type eutectic carbides in M2 HSS from flaky to fibrous. Fibrous M2C-type eutectic carbides exhibit lower thermal stability at elevated temperatures and are more prone to decomposing into fine, spherical M6C- and MC-type carbides upon heating. Mao et al. [26] investigated the effect of cooling rate on incipient carbides in H13 steel and observed that as the cooling rate increases, the number, size, quantity, and average area of incipient carbides decrease significantly, while their morphology remains largely unaffected by the cooling rate. Conversely, Zhao et al. [27] noted that while the cooling rate has little impact on the morphology of Mo-Cr-rich carbides in H13 steel, it significantly influences the morphology of V-rich carbides.
Thus, enhancing the solidification cooling rate presents a highly promising approach to optimizing eutectic carbides in HSS. Prior studies on HSS eutectic carbides have predominantly centered on conventional grades like M2, M35, and M42 [12,28,29]. Nevertheless, the precipitation behavior of eutectic carbides in M2Al HSS remains unexplored. Additionally, no reports have analyzed the thermodynamics of eutectic carbide precipitation in high-aluminum M2Al HSS. In this study, we investigated the precipitation and evolution of eutectic carbides in M2Al HSS by varying the solidification cooling rate. Through thermodynamic calculations and in situ solidification observations, we analyzed the precipitation process of eutectic carbides and elucidated the mechanism by which cooling rate influences microstructure and eutectic carbides. This research intends to offer insights into controlling eutectic carbide precipitation in M2Al HSS.
2. Materials and Methods
In accordance with the compositional specifications outlined in the national standard GB/T 9943-2008 [30], approximately 500 g of M2Al HSS ingots were synthesized in a high-temperature energy-saving tube furnace. The base materials used were commercially annealed M2 HSS, high-purity Cr–Fe powder, high-purity graphite powder, and electrolytic Al. Initially, M2 HSS, high-purity Cr–Fe powder, and high-purity graphite powder were loaded into an alumina crucible in proportion to the desired yield. A graphite crucible was placed around the alumina crucible, and remelting was conducted under argon protection following three cycles of evacuation followed by backfilling with high-purity argon (99.9999%). Once the molten steel was fully melted, electrolytic Al was introduced, stirred for 30 s, and held for 15 min to ensure homogenization before solidification. This study employed four distinct cooling rates: furnace cooling at 5 °C/min and 44 °C/min, sand mold cooling (with an average cooling rate from 1600 °C to 1100 °C of approximately 89 °C/min), and air cooling (with an average cooling rate from 1600 °C to 1100 °C of approximately 225 °C/min). Subsequently, four samples were air-cooled at 1100 °C and solidified into small cylindrical ingots with a diameter of 47 mm. The cooling rates for sand mold cooling and air cooling were measured using a specialized multi-functional infrared thermometer (TM-980D, Tekman Electronics Co., Ltd., Shenzhen, China), designed for precise temperature measurement of high-temperature liquid metals (e.g., molten steel, iron, and copper), with an accuracy error within ±1.5%. This instrument uses a metallurgical short-wave infrared sensor (wavelength range: 0.9–1.7 μm), equipped with a laser sighting port and featuring an ultra-long distance ratio of 80:1. During temperature measurement, the laser port of the instrument was aligned vertically with the center of the molten steel surface, at a distance of 0.8 m. The temperature was recorded every 10 s until it decreased to 1100 °C. The molten steel melting setup is depicted in Figure 1. Given the small dimensions of the samples, the entire sample could be considered to undergo isothermal solidification during cooling.
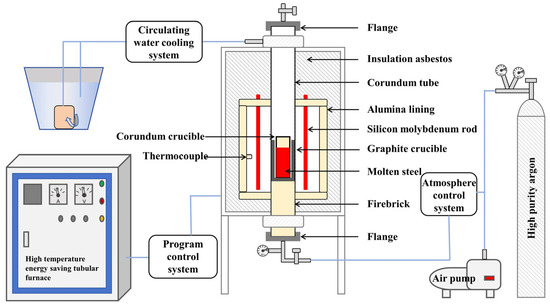
Figure 1.
Schematic diagram of M2Al HSS smelting equipment.
The The chemical composition of M2Al HSS was analyzed using a carbon-sulfur analyzer (CS-320, Yanrui Instruments, Chongqing, China), an oxygen-nitrogen-hydrogen analyzer (ONH-330, Yanrui Instruments, Chongqing, China), a direct reading spectrometer (SPECTRO MAXx07-F, Spectro Analytical Instruments GmbH, Kleve, Germany), and an inductively coupled plasma mass spectrometer (Plasma MS 400, Chengdu, China). The specific mass fractions are listed in Table 1.

Table 1.
Chemical composition of M2Al HSS (wt.%).
Remelting tests on M2Al HSS were conducted using a high-temperature laser confocal scanning microscope (HT-CLSM; VL2000DX-SVF17SP, Yonekura MFG CO., LTD, Tokyo, Japan) for in situ observation of eutectic carbide precipitation. Initially, the steel samples were machined into cylindrical specimens of Φ7 mm × 3 mm to eliminate temperature gradients through their thickness. Each specimen’s surfaces were polished and descaled, with the upper and lower surfaces maintained parallel through mechanical polishing. The specimens were then cleaned with anhydrous ethanol and dried. Prior to experimentation, the instrument’s temperature was calibrated using pure iron. To prevent surface oxidation, the sample chamber was evacuated to 6 × 10−3 Pa and backfilled with high-purity argon (99.9999%) before heating. The specimens were placed in an elliptical, gold-plated heating furnace equipped with a 1.5 kW halogen lamp as the infrared heat source for HT-CLSM analysis. Given the high aluminum and carbon content of M2Al HSS, the argon gas flow was increased during the experiment to prevent lens damage from gas vaporization during melting. Additionally, helium was introduced during high-speed solidification to precisely control the cooling rate. The specimens were heated to 1500 °C at 120 °C/min, held for 5 min to ensure complete melting, and subsequently cooled to room temperature at 120 °C/min.
To comprehensively understand the three-dimensional morphology of eutectic carbides in M2Al HSS, carbides were extracted from the samples via non-aqueous solution electrolysis. During electrolysis, the sample matrix gradually dissolved, exposing the eutectic carbides. The electrolysis setup is depicted in Figure 2, where the M2Al HSS sample is mounted on a platinum plate (anode) and a stainless steel plate serves as the cathode. The electrolyte comprises 1% tetramethylammonium chloride, 10% acetylacetone, and 89% methanol. Electrolysis was conducted at a constant voltage of 10 V and a temperature of 0~5 °C. After 30 min of electrolysis, the anode sample was removed and ultrasonically cleaned in anhydrous methanol. The sample was then reground for further cleaning and cyclic electrolysis until sufficient eutectic carbides were collected. The electrolyte and cleaning solution were filtered using a 0.22 μm organic filter membrane. The collected carbides were dried under vacuum for 12 h. Subsequently, the dried powder was stirred in a 20% hydrochloric acid ethanol solution at 70 °C for 4 h to remove iron and other impurities. Finally, pure carbides were obtained through another cycle of cleaning, filtration, and drying with anhydrous ethanol.
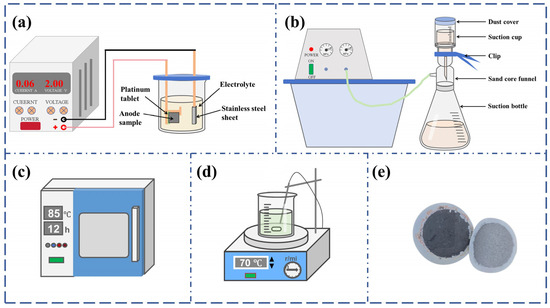
Figure 2.
Schematic diagram of electrolysis and extraction device and physical drawing of extracted eutectic carbides of M2Al HSS: (a) electrolytic device; (b) suction filter device; (c) drying oven; (d) battery mixing device; (e) eutectic carbide.
To analyze the carbides in M2Al HSS, cubic metallographic specimens with a side length of 10 mm were cut from the ingot at half its radius and subjected to grinding and mechanical polishing. A field emission scanning electron microscope (FE-SEM; ZEISS SIGMA 300, Carl Zeiss AG, Oberkochen, Germany), equipped with an energy spectrometer, was employed to observe the two-dimensional and three-dimensional morphology, distribution, and elemental composition of different carbide forms. The network spacing of eutectic carbides was quantified using Nano measurer software (1.2.5), while Image-J software (v1.8.0.112) was used to measure the phase area of carbides in M2Al HSS ingots with varying cooling rates. X-ray diffraction (XRD; XRDynamic 500, Anton Paar GmbH, Graz, Austria) was utilized to identify the carbide types present in the samples, with a scanning range of 30°~90° and a scanning speed of 2°/min.
3. Results and Discussion
3.1. Effect of Cooling Rate on the Organization of M2Al HSS in the As-Cast State
Figure 3 presents SEM-BSE images of the eutectic carbide network structure in M2Al HSS cast samples subjected to different cooling rates. As shown, the eutectic carbides become progressively finer and their distribution more uniform as the cooling rate increases, with a marked reduction in carbide network spacing. This variation in eutectic carbide spacing and distribution with cooling rate is attributed to the enhanced subcooling during solidification at higher cooling rates. According to classical nucleation theory, increased subcooling reduces the critical nucleation radius, thereby decreasing average carbide spacing with rising cooling rates [22]. Additionally, as the solidification time shortens with the increasing cooling rate, the diffusion of alloying elements in the liquid phase becomes incomplete, and crystal growth is restricted [26,31]. In terms of microstructure, the average secondary dendrite arm spacing (SDAS) of the specimens decreases significantly with increasing cooling rate, and carbide distribution becomes more uniform.
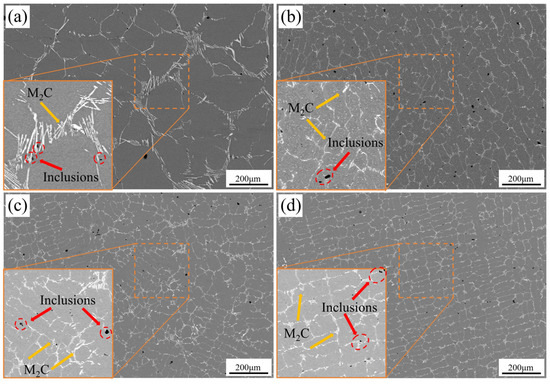
Figure 3.
Eutectic carbide network of M2Al high-speed steel under different cooling rate conditions: (a) 5 °C/min; (b) 44 °C/min; (c) 89 °C/min; (d) 225 °C/min.
To further investigate the relationship between cooling rate and average SDAS, Nano measurer software quantitatively analyzed 200 randomly selected secondary dendrite arms via the intercept method to determine SDAS as the average value. At solidification cooling rates of 5, 44, 89, and 225 °C/min, the SDAS values of M2Al HSS ingots were 83.44, 33.38, 23.35, and 21.79 μm, respectively. The results show that under the current solidification conditions, increasing the cooling rate below 89 °C/min significantly reduces SDAS, whereas further cooling rate increases have minimal impact on SDAS. This is attributed to the solidification process deviating from equilibrium at higher cooling rates, altering the solidified structure morphology and making further changes in crystal spacing difficult [22]. The relationship between SDAS (λ, μm) and local cooling rate (CR, °C/min) can be expressed by Equation (1) [26]:
where a and b are constants of the alloy.
Power function fitting was performed on the measured SDAS and CR, yielding the relationship shown in Figure 4. For M2Al HSS, the fitted alloy constants a and b are 149.42 and 0.39, respectively, with a high fitting correlation coefficient R2 of 0.98, indicating a robust functional relationship between cooling rate and carbide network spacing. Determining the local cooling rate of ingots in commercial production is typically challenging due to the complex solidification system and varying local cooling rates. However, SDAS measurement is relatively straightforward. Using the fitted relationship, the local cooling rate of commercial-scale M2Al HSS ingots can be easily determined by measuring SDAS.
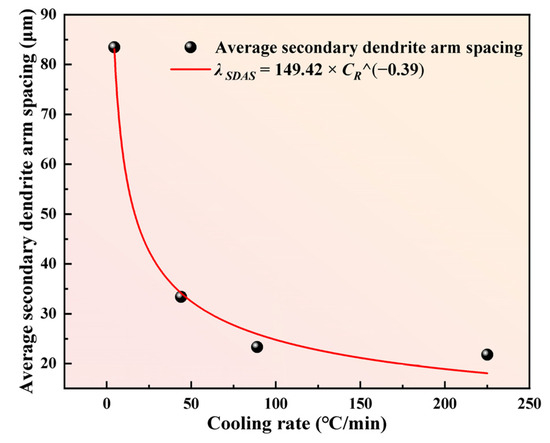
Figure 4.
The relationship between cooling rate CR and average SDAS of M2Al HSS.
3.2. Effect of Cooling Rate on Eutectic Carbide Type in M2Al HSS
To investigate the effect of cooling rate on the carbide type of M2Al HSS, XRD was used to analyze the physical phase of the cast steel. The XRD pattern in Figure 5a shows that under the current cooling rate conditions, M2Al HSS consists of an α-Fe matrix with a body-centered cubic structure, M2C-type carbides with a densely packed hexagonal structure, and MC-type carbides with a face-centered cubic structure. The (222) and (400) peak intensities of MC carbides increase slightly with the rising cooling rate, indicating that the relative content of MC carbides in M2Al alloys rises with the cooling rate, aligning with the findings of Yin et al. [32] and Luan et al. [21]. The broadening of α-Fe diffraction peaks with the increasing cooling rate correlates with α-Fe grain refinement, consistent with Figure 5. However, the current cooling rate range does not alter the carbide types in M2Al HSS, a conclusion supported by multiple authors [33].
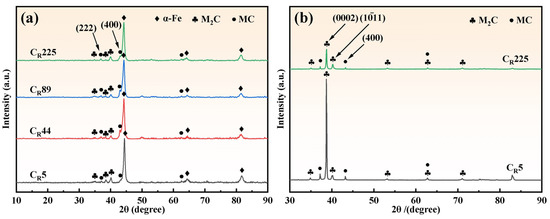
Figure 5.
XRD analysis results of specimens with different cooling rates: (a) XRD patterns of as-cast organization; (b) XRD patterns of carbides.
To reduce the matrix’s influence on the carbide peak, eutectic carbides were extracted from M2Al HSS via non-aqueous electrolysis at cooling rates of 5 and 225 °C/min for physical phase analysis, as shown in Figure 5b. The results reveal a significant decrease in the diffraction intensity of M2C-type eutectic carbides on the grain surface as the cooling rate increases. This change in diffraction peak intensity is attributed to alterations in carbide morphology, with the surface of lamellar M2C carbides being parallel to the (0002) crystal plane [34].
3.3. Effect of Cooling Rate on Two-Dimensional Morphology and Composition of Eutectic Carbides in M2Al HSS
Figure 6 illustrates the two-dimensional morphology of two primary carbides in the as-cast specimen of M2Al HSS. Under SEM-BSE imaging, two carbides with distinct linings are present. The carbides formed by high atomic number elements W and Mo appear bright white and, when combined with XRD data, are identified as M2C-type carbides. In contrast, the chrysanthemum or granular light gray carbides, rich in V elements, are classified as MC-type carbides [35].
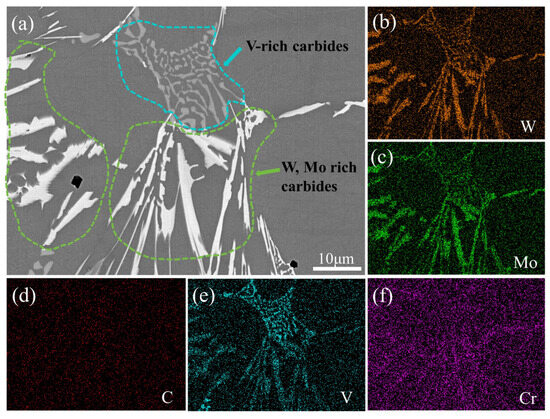
Figure 6.
Typical morphology and elemental distribution of M2C-type and MC-type carbides in M2Al HSS: (a) carbide morphology; (b) distribution of W elements; (c) distribution of Mo elements; (d) distribution of C elements; (e) distribution of V elements; (f) distribution of Cr elements.
Figure 7 illustrates the typical two-dimensional morphology of carbides in M2Al HSS cast specimens under varying cooling rates. At a cooling rate of 5 °C/min, the HSS contains numerous bright white acicular M2C-type carbides and light gray MC-type carbides (Figure 7a). The M2C-type carbides can reach approximately 3 μm in width and tens of microns in length, exhibiting varying sizes and cross-linking or parallel arrangements with local surface defects, consistent with the findings of Liang et al. [5]. The smaller, light gray MC-type carbides form independently or attach to the larger M2C-type carbides. Spherical secondary carbides are dispersed within the grains, and a few black inclusions are observed at the edges of the primary carbides. At 44 °C/min, some M2C-type eutectic carbides in M2Al HSS become narrower and shorter, even forming lumps, while acicular secondary carbides precipitate within the grains (Figure 7b). Further increasing the cooling rate to 89 °C/min refines the eutectic carbides, reducing the width of M2C-type carbides and causing carbide fractures, with almost no secondary carbides in the grain centers (Figure 7c). At 225 °C/min, the morphology of some acicular M2C-type eutectic carbides evolves into curved rods or meshes, with significantly reduced thickness and length of carbide lamellae (Figure 7d).
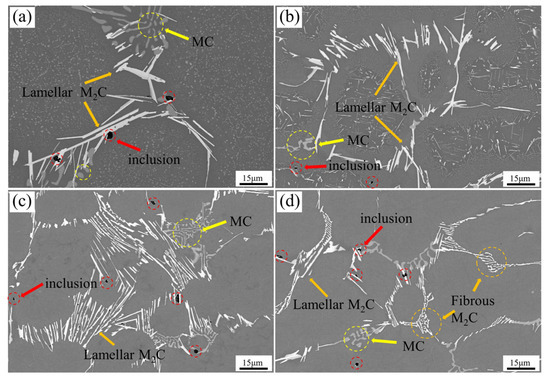
Figure 7.
Two-dimensional morphology of carbides in the as-cast organization of M2Al HSS at different cooling rate conditions: (a) 5 °C/min; (b) 44 °C/min; (c) 89 °C/min; (d) 225 °C/min.
To explore potential elemental differences in the morphological variation in M2C-type carbides, EDS was used for the semi-quantitative analysis of carbides in M2Al HSS ingots, with the results presented in Figure 8. The analysis indicated that the mass fraction of W and Mo in acicular M2C-type carbides is roughly one-third higher than in bent rod-like M2C-type carbides, which show significant Fe enrichment. This aligns with the findings of Zhou et al. [36] and Liu et al. [37]. It is suggested that increased cooling rates restrict the diffusion of W and Mo, thereby decreasing their proportion in fibrous M2C-type eutectic carbides. Moreover, light gray MC-type carbides are V-rich with lesser amounts of W and Mo.
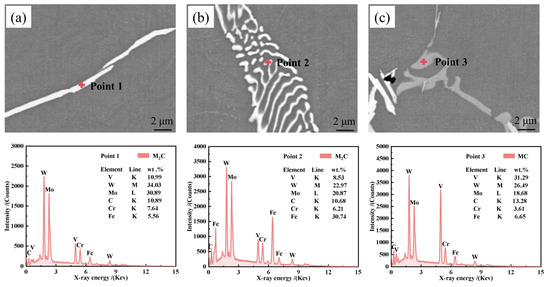
Figure 8.
Presents the SEM-EDS analyses of M2C and MC-type carbides: (a) needle-like M2C-type carbides; (b) curved rod-like M2C-type carbides; (c) granular or rod-like MC-type carbides.
3.4. Effect of Cooling Rate on the Three-Dimensional Morphology of Eutectic Carbides in M2Al HSS
To investigate the three-dimensional (3D) morphology of carbides in as-cast M2Al HSS under different cooling rates, carbides were extracted via non-aqueous electrolysis from specimens cooled at 5 and 225 °C/min, as shown in Figure 9. The 3D morphology of eutectic M2C-type carbides differs significantly from their 2D appearance, and the cooling rate substantially affects the carbide morphology. At 5 °C/min, individual, unconnected lamellar carbides form at the grain boundaries, exhibiting varying widths and growth directions (Figure 9a). At 225 °C/min, grain boundary carbides develop into smooth dendrites with a trunk and multiple curved branches (Figure 9b). Figure 9c,d show the electrolytically extracted lamellar and dendritic eutectic carbides, respectively. The lamellar M2C-type eutectic carbides, grown with sufficient time, display a selective growth direction and distinct growth steps (Figure 9c). Increasing the cooling rate transforms the carbide surface from growth steps to smooth rods, reducing growth anisotropy (Figure 9d).
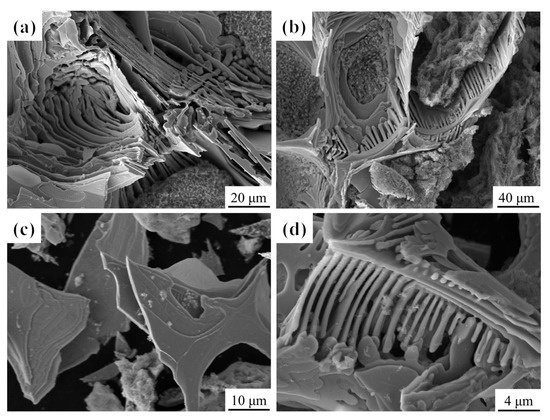
Figure 9.
Three-dimensional morphology of M2C-type carbides in M2Al HSS: (a,c) lamellar M2C carbides; (b,d) fibrous M2C carbides.
During solidification, crystal morphology is determined by the liquid–solid interface structure, which can be classified as polyhedral or non-polyhedral. The polyhedral phase exhibits anisotropic growth, with crystal faces of higher refractive indices growing faster, leaving densely arranged faces as crystal facets on the surface. The presence of growth steps on the lamellar M2C-type eutectic carbides is a key feature of the polyhedral phase. Zhou et al. [25] demonstrated that the surface of lamellar M2C-type eutectic carbides is parallel to densely arranged crystal planes, providing a theoretical basis for their formation. Notably, at higher cooling rates, the polyhedral phase disappears, and M2C-type eutectic carbides transform into smooth curved rods. This transformation is attributed to local thermal and phase transition stresses, where the fiber-like structure reduces interfacial energy in M2C-type carbides, favoring their growth.
3.5. Effect of Cooling Rate on the Area Fraction of Eutectic Carbides in M2Al HSS
To further clarify the effect of cooling rate on carbide number in M2Al HSS, four random SEM-BSE images at 200× magnification were analyzed using Image-J software for carbide area measurement via binary processing. Figure 10 shows the relationship between the carbide area fraction and cooling rate in as-cast M2Al HSS. At 5 °C/min, the carbide area fraction is 7.85%, increasing to 9.42% at 44 °C/min, 10.84% at 89 °C/min, and 11.81% at 225 °C/min. The growth rate of the carbide area fraction diminishes beyond 89 °C/min. This indicates that increasing the cooling rate enhances carbide precipitation, particularly below 89 °C/min. The higher cooling rate increases the subcooling degree of liquid steel, boosting carbide nucleation rate and density in the residual liquid phase, thereby increasing carbide area fraction.
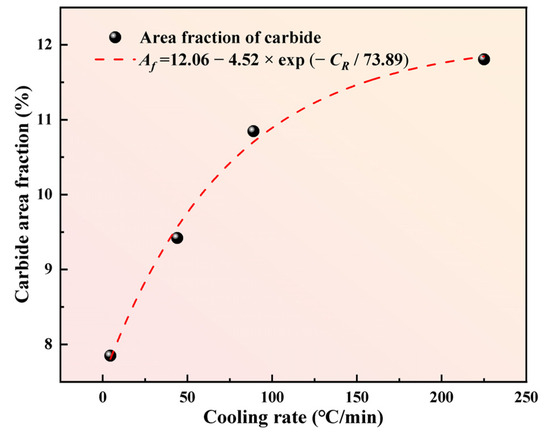
Figure 10.
The relationship between carbide area fraction and cooling rate in M2Al HSS.
3.6. Thermodynamic Analysis of Eutectic Carbide Precipitation in M2Al HSS
To analyze the precipitation behavior of eutectic carbides during the solidification process of M2Al HSS, thermodynamic calculations were performed to investigate the formation of eutectic carbides. The EDS analysis results in Figure 6 and Figure 8 reveal that the M2C-type eutectic carbides are rich in Mo, while V accounts for the largest atomic proportion of the total metal elements in MC-type carbides. To simplify the calculation process, M2C-type and MC-type eutectic carbides were simplified as Mo2C and VC, respectively, in the thermodynamic calculations. The reaction equations and Gibbs free energies for the formation of Mo2C and VC in molten steel are as follows [26]:
where is the standard Gibbs free energy change; is the reaction temperature; is the equilibrium constant; , , and are the activity, activity coefficient, and mass percent of element i, respectively. The activity coefficient can be calculated using the Wagner equation provided below:
The Wagner primary interaction coefficients for the elements Mo, V, and C were systematically compiled from the literature and are presented in Table 2 [12,38].

Table 2.
First-order interaction parameters used in the present study (×102).
The activity coefficients of Mo, V, and C in M2Al HSS were calculated using Equation (6), yielding values of , , and , respectively. Figure 11 demonstrates the calculated logarithmic solubility products of Mo2C and VC carbides in the liquid steel as a function of temperature. The results indicate that the equilibrium solubility products of both Mo2C and VC are higher than their actual solubility products, suggesting that Mo2C and VC cannot precipitate above the solidus temperature of the steel. It is important to note that carbides precipitating below the solidus temperature are secondary carbides, which typically exhibit nanoscale dimensions. This observation is inconsistent with the eutectic carbide sizes observed via scanning electron microscopy (SEM). Therefore, the carbides identified by SEM must have precipitated during the solidification process. Consequently, the accumulation of carbide-forming elements in the residual liquid steel leads to elemental microsegregation, which promotes carbide precipitation [39].
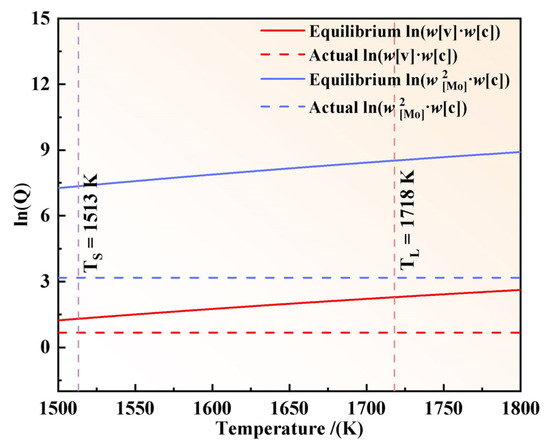
Figure 11.
Logarithm of the solubility product of Mo2C and VC as a function of temperature.
Considering that M2Al HSS is highly susceptible to elemental segregation during the actual solidification process, numerous microscopic segregation models have been developed to predict the solute element content in the residual liquid steel between dendrites. In this study, the Scheil–Gulliver model [40] was employed for the thermodynamic calculations of carbide precipitation in the liquid–solid two-phase region. This model assumes the absence of solid-phase diffusion during rapid solidification. The variation in the content of alloying elements M and C with solid-phase fraction can be expressed by Equations (7) and (8), respectively, while the solid-phase fraction of the system is described by Equation (9).
In the formula, and represent the mass fractions of metal M and carbon element C at the onset of solidification of M2Al HSS, in percent. and denote the equilibrium distribution coefficients of metal M and carbon, respectively. According to Reference [32], the equilibrium distribution coefficients of Mo, V, and C between austenite and the solid phase are 0.585, 0.63, and 0.34, respectively; is the melting point of pure iron in Kelvin. and represent the liquidus and solidus temperatures of M2Al HSS, respectively, in Kelvin.
The logarithm of the solubility product of Mo2C with VC-type eutectic carbides in the liquid–solid two-phase region was calculated as a function of the solid-phase fraction, as shown in Figure 12. It was found that the equilibrium solubility products of both Mo2C and VC in the two-phase region decrease with the increasing solid-phase fraction, whereas the actual solubility products increase with the increasing solid-phase fraction. MC and M2C-type carbides precipitate in the liquid phase when the solid-phase fraction reaches 0.75 and 0.97, respectively. Experimental results indicate that in M2Al HSS ingots, the precipitation of MC-type eutectic carbides precedes that of M2C-type primary carbides.
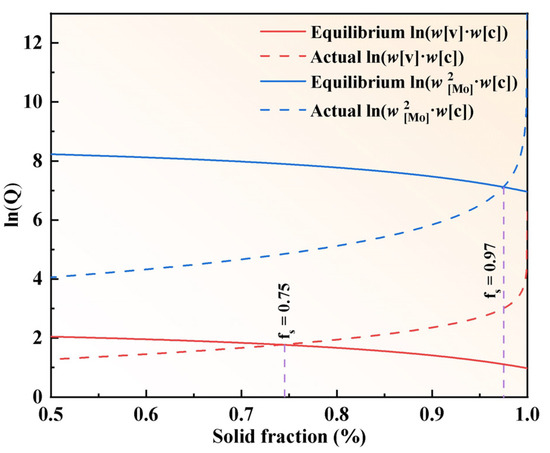
Figure 12.
Logarithm of the solubility product of Mo2C and VC in M2Al ingots as a function of the solid phase fraction.
3.7. In Situ Observation of Melting and Solidification of M2Al HSS
Figure 13 illustrates the microstructural evolution of M2Al HSS during heating. By mechanically polishing the sample to a mirror finish prior to the in situ observation, the eutectic carbides (indicated by orange circles) and a small number of inclusions (indicated by gold circles) can be clearly visualized, as shown in Figure 13a. It should be noted that M2C and MC carbides are indistinct under HT-CLSM. As depicted in Figure 13b, the material undergoes a solid-phase transformation at approximately 976 °C, evidenced by a pronounced relief on the grain surface, which is attributed to the α-Fe to γ-Fe transformation. Concurrently, the eutectic carbides exhibit a distinct dark gray color due to minor volume changes associated with the solid-phase transformation. As the temperature increases further, the specimen oxidizes and darkens. At 1168 °C, the eutectic carbides, which appear white at the grain boundaries, partially melt, as shown in Figure 13c. The liquid phase initially forms at the intersections of polycrystalline boundaries, which are regions of carbide aggregation, consistent with the findings of Ren et al. [41]. The thinning of eutectic carbide lamellae and fracture spheroidization occur concurrently, with the liquid phase advancing toward carbide weaknesses and into the grain interior, as illustrated in Figure 13d. Once the eutectic carbides and matrix are completely melted, the inclusions in the liquid steel continue to float and gather, as shown in Figure 13e. The temperature is then continuously increased and held for a period until the inclusions are essentially removed from the field of view, as depicted in Figure 13f. It is important to note that the temperature gradient induced by the sample thickness is non-negligible in the in situ melting test of M2Al HSS, and the actual temperature at the sample surface is likely higher than that measured by the thermocouple [42].
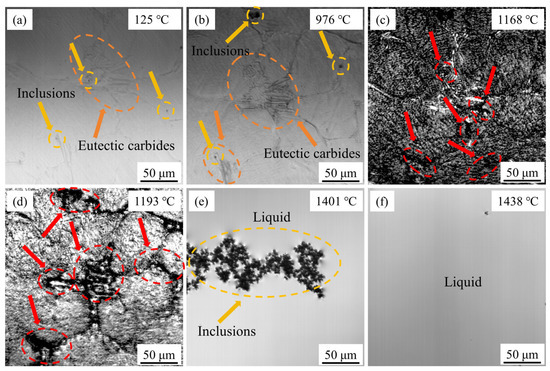
Figure 13.
Observation of in situ melting of M2Al HSS ingots: (a) 125 °C; (b) 976 °C; (c) 1168 °C; (d) 1193 °C; (e) 1401 °C; (f) 1438 °C.
The in situ solidification process of M2Al HSS at a cooling rate of 120 °C/min is depicted in Figure 14. When the temperature is reduced to 1304 °C, the initial precipitation of high-temperature δ-Fe, accompanied by the nucleation and aggregation of inclusions, can be observed within the yellow-circled region. Upon further cooling to 1250 °C, a substantial dendritic δ-Fe phase begins to precipitate throughout the field of view. As the temperature decreases to 1215 °C, continuous precipitation of new δ-Fe is noted, while pre-existing δ-Fe has already undergone significant growth. Concurrently, a distinct black interface emerges at the boundary between δ-Fe and the liquid phase, signifying the formation of γ-Fe. This interface is attributed to solidification shrinkage of the liquid steel surface. Unlike the direct precipitation of δ-Fe from the liquid steel, the inclusion-induced crystallization reaction results in substantial volumetric contraction in the thickness direction, manifesting as a black interface under confocal microscopy [43].
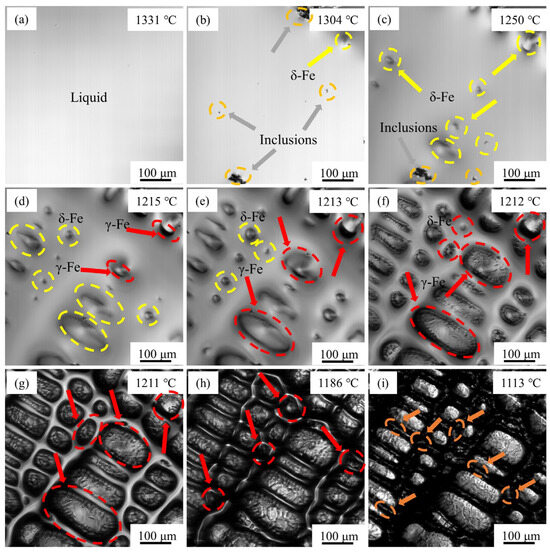
Figure 14.
In situ observation of rapid solidification of M2Al HSS: (a) 1331 °C; (b) 1304 °C; (c) 1250 °C; (d) 1215 °C; (e) 1213 °C; (f) 1212 °C; (g) 1211 °C; (h) 1186 °C; (i) 1113 °C.
As the temperature continues to drop to 1213 °C, most δ-Fe is enveloped by γ-Fe. When the temperature is further reduced to 1212 °C and 1211 °C, the smooth δ-Fe undergoes an encapsulation transition to γ-Fe, consuming a significant portion of the liquid phase. Under the current cooling rate conditions, the pack-crystal transformation in M2Al HSS is rapid and follows a block transformation mechanism [44]. Upon further cooling to 1186 °C, fine γ-Fe precipitates in the residual liquid phase, with existing γ-Fe exhibiting minimal growth. When the temperature is lowered to 1113 °C, complete solidification of M2Al HSS is achieved, accompanied by the formation of extensive white reticulated eutectic carbides via interdendritic segregation.
3.8. Kinetic Precipitation Mechanism of Eutectic Carbides
Based on the XRD phase analysis in Figure 5 and the SEM morphology analysis in Figure 7 and Figure 9, a large number of eutectic carbides were observed in M2Al HSS ingots within the current cooling rate range. The type of eutectic carbide remained unchanged, but its three-dimensional morphology evolved from lamellar to fibrous. The size of these carbides, smaller than ferrite grains, exhibited a gradual reduction, while their area fraction increased. The distribution of eutectic carbides became more uniform, primarily localized in interdendritic regions. Notably, the precipitation of eutectic carbides between dendrites occurred randomly, with the lamellar growth direction of the carbides exhibiting no specific orientation.
Given the high solute content of eutectic carbides, it is reasonable to posit that nucleation of these carbides from the supercooled melt occurs predominantly during the mid-to-late stages of solidification. This happens when the residual liquid phase experiences severe microsegregation, attaining a eutectic composition. The Scheil model solidification calculation in Figure 12 and the in situ solidification experiment in Figure 14 both support this conclusion. The growth of eutectic carbides is predominantly governed by the diffusion of alloying elements, as evidenced by the significant variations in the morphology, size, and alloying content of eutectic carbides under different cooling conditions.
According to the Scheil model solidification calculation results in Figure 12 and the in situ solidification characterization in Figure 14, the precipitation and growth process of eutectic carbides can be summarized as follows. Initially, δ-Fe nucleates and grows in the supercooled liquid phase, followed by inclusion-induced crystallization and phase transformation. Subsequently, γ-Fe slowly grows and expels alloying elements such as V, W, Mo, and C into the residual liquid phase. As the solid-phase fraction increases, these alloying elements continuously enrich in the residual liquid phase. Under the combined effects of local compositional and thermal fluctuations, when the critical nucleation size for eutectic carbide is achieved, nucleation and precipitation occur in the interdendritic regions. The growth of eutectic carbides consumes significant amounts of V, W, Mo, and C from the residual liquid phase and is predominantly governed by the diffusion of these elements. During solidification, γ-Fe continuously expels alloying elements such as V, W, Mo, and C, thereby promoting the growth of γ-Fe and eutectic carbides.
According to classical nucleation theory, higher cooling rates lead to greater degrees of undercooling, enhancing the phase transformation driving force and increasing the nucleation rates of γ-Fe and eutectic carbides. Consequently, higher cooling rates result in a larger number of dendrites and eutectic carbides, as shown in Figure 3. However, the growth of eutectic carbides relies on the diffusion of alloying elements such as V, W, Mo, and C from γ-Fe. As the cooling rate increases, atoms in the γ-Fe matrix have insufficient time to diffuse, failing to meet the compositional requirements for sustained eutectic carbide growth. Thus, while higher cooling rates increase the number of M2C-type eutectic carbides, they simultaneously reduce the W and Mo alloying element content and the size of the carbides, as shown in Figure 7 and Figure 8.
The growth of M2C-type eutectic carbides is closely tied to the growth rate of local γ-Fe, with the relationship between these two rates significantly influencing the three-dimensional morphology of eutectic carbide precipitation. At slower cooling rates, γ-Fe grows faster than or at a rate comparable to that of M2C-type eutectic carbides, resulting in a lamellar three-dimensional morphology of the M2C-type eutectic carbides, as shown in Figure 9. This occurs because, at lower cooling rates, alloying elements such as Mo and C within γ-Fe have sufficient time to diffuse and satisfy the thermodynamic conditions necessary for the continued growth of eutectic carbides. Additionally, the slower cooling rate allows M2C and γ-Fe to maintain lamellar eutectic growth through lateral solute diffusion, adhering to the eutectic synergistic growth model [45]. Conversely, under rapid cooling conditions, the diffusion distance of solutes decreases significantly. Solute enrichment at the solid–liquid interface leads to compositional supercooling, disrupting the eutectic synergistic growth conditions. According to the Kurz–Fisher interfacial stability criterion [46], when the actual temperature gradient is smaller than the critical temperature gradient, interfacial destabilization causes lamellar splitting, prompting M2C to shift toward dendritic growth to minimize the system’s free energy.
4. Conclusions
This study investigates the precipitation and evolution characteristics of eutectic carbides in M2Al HSS under cooling rates ranging from 5 to 225 °C, from both thermodynamic and kinetic perspectives. The key findings are summarized as follows:
- 1.
- The as-cast microstructure of M2Al HSS comprises ferrite dendrites, reticulated eutectic carbides between the dendrites, and fine spherical secondary carbides within the dendrites. As the solidification cooling rate increases, the solidification microstructure of M2Al HSS becomes more refined. The relationship between the secondary dendrite arm spacing and the cooling rate is quantified by the equation .
- 2.
- The as-cast microstructure of M2Al HSS in the interdendritic regions exhibits significant segregation of Mo, W, V, C, and Cr, along with the precipitation of V-rich MC-type and Mo-rich M2C-type eutectic carbides. As the cooling rate increases, the average size of eutectic carbides decreases markedly, while their total area fraction increases and distribution uniformity improves. The three-dimensional morphology of MC-type eutectic carbides is smooth and rod-like, exhibiting low sensitivity to the cooling rate. In contrast, the three-dimensional morphology of M2C-type eutectic carbides transitions from lamellar to dendritic with increasing cooling rate.
- 3.
- Thermodynamic calculations and kinetic analyses indicate that the formation of eutectic carbides is primarily governed by the segregation of elements such as V, Mo, and C during the final stages of solidification. In contrast, the chemical composition and three-dimensional morphology of M2C-type eutectic carbides are synergistically governed by the diffusion of elements like W, Mo, and C in austenite, as well as by competitive growth dynamics.
Author Contributions
Conceptualization, J.X. and C.Z.; methodology, C.Z.; software, J.X.; validation, J.X. and C.Z.; formal analysis, J.X.; investigation, J.X.; resources, C.Z. and H.Y.; data curation, J.X.; writing—original draft preparation, J.X.; writing—review and editing, C.Z.; visualization, J.X.; supervision, C.Z.; project administration, C.Z.; funding acquisition, C.Z. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science foundation of China (Grant No. 52164032, Grant No. 52464038), Science and Technology Program of the Guizhou Province (Grant No. ZK [2023] 071).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study was supported by the National Natural Science Foundation of China and the Guizhou Provincial Science and Technology Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaus, A.S.; Bracik, M.; Sahulb, M.; Domankova, M. Microstructure and properties of M2 high-speed steel cast by the gravity and vacuum investment casting. Vacuum 2019, 162, 183–198. [Google Scholar] [CrossRef]
- Shi, G.; Zhou, S.; Ding, P. Investigation of nonmetallic inclusions in high-speed steels. Mater. Charact. 1997, 38, 19–23. [Google Scholar] [CrossRef]
- Zheng, L.; Yan, B.; Lou, J.; Li, H.; Jiang, Z. Formation Behavior of M2C and M6C Eutectic Carbides in M42 High-Speed Steel. ISIJ Int. 2023, 63, 294–302. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, F.; Li, G.; Jiang, J. Morphology and Properties of M2C Eutectic Carbides in AISI M2 Steel. ISIJ Int. 2010, 50, 1151–1157. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; Zheng, D.; Chai, J. Distribution characteristic and precipitation behavior of primary carbides in industrial grade AISI M35 high-speed steel produced by electroslag remelting. J. Mater. Res. Technol. 2025, 35, 3582–3592. [Google Scholar] [CrossRef]
- Xu, L.; Wei, S.; Xiao, F.; Zhou, H.; Zhang, G.; Li, J. Effects of carbides on abrasive wear properties and failure behaviours of high speed steels with different alloy element content. Wear 2017, 376, 968–974. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.; Xiong, X.; Luan, H.; Hao, Y.; Yang, B.; Chen, J. Microstructure and mechanical properties of high carbon M2 powder metallurgy high-speed steel prepared by the carbide addition. Powder Metall. 2022, 65, 403–412. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Z.; Zhang, W.; Fang, F.; Tu, Y.; Jiang, J. Deformation-Induced Carbide Transformation in M2 High-Speed Steel. Metall. Mater. Trans. A 2020, 51, 568–573. [Google Scholar] [CrossRef]
- Chen, D.; Xu, X.; Zhao, Y.; Fu, X.; Wei, L.; Zhou, Y.; Wu, Z. Superior mechanical properties of M35 high-speed steel obtained by controlling carbide precipitation and distribution via electropulsing treatment. Mat. Sci. Eng. A-Struct. 2023, 888, 145691. [Google Scholar] [CrossRef]
- Li, H.; Han, S.; Zhang, B.; Qiao, G.; Liu, J.; Xiao, F. Effects of Niobium on Microstructure, Hardness and Wear Behavior for High-Speed Steel Rolls. Metall. Mater. Trans. A 2023, 54, 3271–3285. [Google Scholar] [CrossRef]
- Ke, C.; Chen, C.; Zhang, M. Effect of Vanadium on Microstructure and Mechanical Properties of M2 High-Speed Steel Prepared by Laser Metal Direct Deposition Forming. Steel Res. Int. 2022, 93, 2100389. [Google Scholar] [CrossRef]
- Zheng, D.; Ma, G.; Li, J.; Cho, J.; Xiang, Z.; Liu, M.; Zhu, J. Effect of cerium on the primary carbides and inclusions in electroslag remelted M35 high speed steel. J. Mater. Res. Technol. 2023, 24, 8252–8266. [Google Scholar] [CrossRef]
- Jiao, W.; Li, H.; Feng, H.; Jiang, Z.; Wang, H.; Zhu, H.; Han, P.; Wu, W. Significant Improvement of Cleanliness and Macro/Microstructure of As-Cast AISI M42 High-Speed Steel by Mg Treatment. Metall. Mater. Trans. B 2022, 53, 1196–1211. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, H.; Sun, X.; Guo, J. Influence of the Nitrogen Content on the Carbide Transformation of AISI M42 High-Speed Steels during Annealing. Sci. Rep. 2018, 8, 4328. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, M.; Xu, B.; Li, D. A method based on semi-solid forming for eliminating Laves eutectic phase of INCONEL 718 alloy. J. Mater. Process Technol. 2017, 249, 202–211. [Google Scholar] [CrossRef]
- Liu, W.; Cao, Y.; Guo, Y.; Sun, M.; Xu, B.; Li, D. Solidification microstructure of Cr4Mo4V steel forged in the semi-solid state. J. Mater. Sci. Technol. 2020, 38, 170–182. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Y.; Sun, M.; Xu, B.; Li, D. Effects of liquid fraction on the microstructure and mechanical properties in forge solidifying 12Cr1MoV steel. J. Mater. Process Technol. 2018, 256, 25–35. [Google Scholar] [CrossRef]
- Zheng, T.; Li, W.; Qi, W.; Xia, Z.; Li, Q.; Shen, Z.; Guo, Y.; Zhong, Y.; Wang, H.; Wang, Q. Morphology transition of eutectic carbide assisted by thermoelectric magnetic force during the directional solidification of M2 high-speed steel. Ironmak. Steelmak. 2021, 48, 885–892. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, W.; Li, Q.L.; Godfrey, A.; Liu, Q. Effect of magnetic field on solidification structure of a centrifugal cast high speed steel roll. Mater. Sci. Technol. 2010, 26, 1177–1183. [Google Scholar] [CrossRef]
- Li, W.; Jiao, S.; Tong, W.; Zang, X.; Wang, G.; Jiang, Z.; Li, H. Effect of Secondary Aerosol Cooling on the Characteristics of Carbides in M42 High-Speed Steel Produced by the Electroslag Remelting Withdrawal Process. Steel Res. Int. 2020, 91, 2000139. [Google Scholar] [CrossRef]
- Luan, Y.; Song, N.; Bai, Y.; Kang, X.; Li, D. Effect of solidification rate on the morphology and distribution of eutectic carbides in centrifugal casting high-speed steel rolls. J. Mater. Process Technol. 2010, 210, 536–541. [Google Scholar] [CrossRef]
- Li, W.; Jiao, S.; Tong, W.; Zang, X.; Li, D.; Jiang, Z.; Li, H.; Jing, Y. An innovative method for calibrating local cooling rate in electroslag remelting of M42 high-speed steel. J. Iron Steel Res. Int. 2021, 28, 990–996. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, Y.; Wan, X.; Li, J.; Li, G. Effect of Cooling Rate on Carbide Characteristics of the High Vanadium High-speed Steel. ISIJ Int. 2022, 62, 524–531. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, W.; Chen, X.; Li, J. Increasing Solidification Rate of M2 High-Speed Steel Ingot by Fusible Metal Mold. Acta Metall. Sin.-Engl. Lett. 2016, 29, 382–387. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.F.; Fang, F.; Li, F.; Jiang, J.Q. Morphology and microstructure of M2C carbide formed at different cooling rates in AISI M2 high speed steel. J. Mater. Sci. 2011, 46, 1196–1202. [Google Scholar] [CrossRef]
- Mao, M.; Guo, H.; Wang, F.; Sun, X. Effect of Cooling Rate on the Solidification Microstructure and Characteristics of Primary Carbides in H13 Steel. ISIJ Int. 2019, 59, 848–857. [Google Scholar] [CrossRef]
- Zhao, G.; Zang, X.; Li, W.; Zhao, Z.; Li, D. Study on primary carbides precipitation in H13 tool steel regarding cooling rate during solidification. China Foundry 2020, 17, 235–244. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Lu, D.; Lu, L.; Jia, J.; Li, H.; Zou, J.; Hu, Q. Microstructure and Mechanical Properties of Modified M2 High-Speed Steel by Adding La2O3 in the Electroslag Casting Process. Steel Res. Int. 2024, 95, 2300035. [Google Scholar] [CrossRef]
- Jiao, W.; Li, H.; Feng, H.; Jiang, Z.; Xia, L.; Zhang, S.; Zhu, H.; Wu, W. Evolutions of Micro- and Macrostructure by Cerium Treatment in As-Cast AISI M42 High-Speed Steel. Metall. Mater. Trans. B 2020, 51, 2240–2251. [Google Scholar] [CrossRef]
- GB/T 9943-2008; High-Speed Tool Steels. China Iron and Steel Industry Association: Beijing, China, 2008.
- Li, S.; Chen, Y.; Gong, T.; Chen, X.; Fu, P.; Li, D. Effect of Cooling Rate on the Precipitation Mechanism of Primary Carbide During Solidification in High Carbon-Chromium Bearing Steel. Acta Metall. Sin. 2022, 58, 1024–1034. [Google Scholar]
- Yin, F.; Su, M.; Ji, F.; Tian, Q.; Bai, Y.; Feng, J.; Xiao, Z. Effect of melting rate on microsegregation and primary MC carbides in M2 high-speed steel during electroslag remelting. China Foundry 2021, 18, 163–169. [Google Scholar] [CrossRef]
- Boccalini, M.; Goldenstein, H. Solidification of high speed steels. Int. Mater. Rev. 2001, 46, 92–115. [Google Scholar] [CrossRef]
- Zhou, X.F.; Fang, F.; Jiang, J.Q.; Zhu, W.L.; Xu, H.X. Refining carbide dimensions in AISI M2 high speed steel by increasing solidification rates and spheroidising heat treatment. Mater. Sci. Technol. 2014, 30, 116–122. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Z.; Ma, C.; Li, G. Precipitation Behavior and Elemental Distribution of MC Carbides in High Carbon and Vanadium High-Speed Steel. J. Mater. Eng. Perform. 2022, 31, 4444–4458. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Zhu, W.; Fang, F.; Tu, Y.; Jiang, J. Morphology, microstructure and decomposition behavior of M2C carbides in high speed steel. J. Iron Steel Res. Int. 2017, 24, 43–49. [Google Scholar] [CrossRef]
- Liu, W.; Guo, Y.; Cao, Y.; Wang, J.; Hou, Z.; Sun, M.; Xu, B.; Li, D. Transformation behavior of primary MC and M2C carbides in Cr4Mo4V steel. J. Alloys Compd. 2021, 889, 161755. [Google Scholar] [CrossRef]
- Mao, M.; Guo, H.; Wang, F.; Sun, X. Chemical composition and structural identification of primary carbides in as-cast H13 steel. Int. J. Min. Met. Mater. 2019, 26, 839–848. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Shi, C.; Huang, J. Growth and agglomeration behaviors of eutectic M7C3 carbide in electroslag remelted martensitic stainless steel. J. Mater. Res. Technol. 2021, 11, 1490–1505. [Google Scholar] [CrossRef]
- Schaffnit, P.; Stallybrass, C.; Konrad, J.; Stein, F.; Weinberg, M. A Scheil-Gulliver model dedicated to the solidification of steel. Calphad 2015, 48, 184–188. [Google Scholar] [CrossRef]
- Ren, J.; Teng, Y.; Liu, X.; Xu, X.; Li, H.; Han, K.; Zhai, Q. In-situ observation and analysis of high temperature behavior of carbides in GCr15 bearing steel by confocal laser scanning microscopy. J. Iron Steel Res. Int. 2025, 32, 409–417. [Google Scholar] [CrossRef]
- Attallah, M.M.; Terasaki, H.; Moat, R.J.; Bray, S.E.; Komizo, Y.; Preuss, M. In-Situ observation of primary γ′ melting in Ni-base superalloy using confocal laser scanning microscopy. Mater. Charact. 2011, 62, 760–767. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Deng, Z.; Qiu, S.; Zhang, P.; Zheng, G. Peritectic Solidification Characteristics and Mechanism of 15CrMoG Steel. Acta Metall. Sin. 2020, 56, 1335–1342. [Google Scholar]
- Wang, W.; An, Z.; Luo, S.; Zhu, M. In-situ observation of peritectic solidification of Fe-Mn-Al-C steel with medium manganese. J. Alloys Compd. 2022, 909, 164750. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Galenko, P.K. Model of Eutectic Transformation Involving Intermetallic Compound. Acta Metall. Sin. 2021, 57, 1320–1332. [Google Scholar]
- Cui, C.; Ren, C.; Liu, Y.; Wang, S.; Su, H. Directional solidification of Fe-Al-Ta eutectic by electron beam floating zone melting. J. Alloys Compd. 2019, 785, 62–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).