Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease which has a rather complex pathophysiology. During its course, several neurotransmitter neuronal systems get affected such as acetylcholinergic, glutamatergic, gamma-aminobutyric acid (GABA)ergic systems, etc. Such complex physiology requires a sophisticated approach to pharmaceutical management. Therefore, multi-target drugs seem to be an appealing solution. In the present study, we designed and synthesized a hybrid molecule—N-sinapoylamide of memantine, whose parent molecules memantine (MEM) and sinapic acid have been shown in vivo to impact glutamatergic, acetylcholinergic, and GABA-ergic systems, respectively. In silico comparative testing of these molecules was performed, their patterns of interaction with the target enzymes or molecular complexes were analyzed, and some of the mechanisms of action were proposed. Consequently, in vivo testing was performed on a scopolamine mice model of AD and the results overly confirm part of the in silico findings. Therefore, the hybrid molecule (N-Sinapoyl-memantine) seems to be a potent candidate for further evaluation in the management of AD.
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease worldwide [1]. Among its key disturbances are neuronal loss in the acetylcholinergic system; reduced availability of its neurotransmitter—acetylcholine; and dysregulation of its nicotinic and muscarinic receptors [2]. As a result, many of the researchers have focused on the development of acetylcholinesterase (AChE) inhibitors as a means to combat some of these disturbances. However, the officially approved AChE inhibitors as galantamine, etc., are still scarce and do not stop the disease progression in the long run [2,3,4,5]. Hereto AChE inhibitors are wanted and developed, hopefully with better clinical and pharmaceutical parameters and performance. Another major neurotransmitter system significantly affected by AD is the glutamatergic one. The glutamatergic N-methyl-D-aspartate receptor (NMDAR) ion channels conduct monovalent ions and Ca2+ which elicit not only electric signals but also can trigger Ca2+-dependent intracellular signaling processes; therefore, their dysfunction in AD leads to impairments of memory and learning, increased oxidative stress and impaired Ca2+ homeostasis, etc., ultimately causing neuronal death [6,7]. Therefore, glutamatergic NMDA receptors are another target for the drug designers involved in the research on the management of AD. Currently, the number of approved drugs for these receptors as memantine is rather limited [6,8,9]. The difficulty in good design of such drugs arises from the fact that the NMDA receptors are of pivotal importance and their full inhibition is similarly undesired as their over-excitation [8].
Additionally, growing evidence suggests that the main inhibitory neurotransmitter system in the mammalian CNS is also disturbed in the course of AD. In addition, some studies showed that GABA-ergic system dysfunctions are related to the Aβ depositions and pathology in AD patients—one of the hallmarks of AD [10]. So, the GABA receptors, the GABA-ergic system, and the related symptoms in AD also need to be addressed by the therapeutic interventions and management of AD. One of the ways probably can be by using modulators of the GABAA receptors as the results from a recent study may suggest [11].
Sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid; SINA) belongs to one of the fundamental classes of phenolic acids, namely hydroxycinnamic acids (ferulic-, coumaric-, caffeic acids), which are widely distributed in the plant kingdom [12,13]. Besides its free form of existence, this secondary plant metabolite may also be found as diverse natural or synthetic conjugates (esters, amides, and others).
It has been reported that SINA is active against diverse pathological conditions such as infections [14], oxidative stress [15,16], inflammation [17,18], cancer [19], diabetes [20], neurodegeneration [21,22], and so on. Generally, the pharmaceutical potential of sinapic acid (or other hydroxycinnamic acids) can be attributed to its potent antioxidant ability against various radicals [23,24,25,26,27]. However, when the imbalance between antioxidants and free radicals occurs, the physiological state has been known as “oxidative stress”. The latter is suspected to be associated with the development of various diseases, including neurodegenerative disease, cancer, cardiovascular disorders, and so on. In particular, NO and superoxide being considerable cytotoxic molecules exist at high concentrations during inflammation. Moreover, they are also implicated in the pathology of various brain neurodegenerative disorders, ischemic stroke, aging of the brain, etc. [28]. Thus, peroxynitrite can be easily formed by NO and superoxide which are rather reactive and can provoke protein modifications, leading to the progress of various diseases such as AD and Parkinson’s disease [29,30].
Recent studies found that SINA is able to ameliorate, to some extent, some of the AD symptoms in animal models of the disease [31,32].
Several previous in vitro studies suggest that SINA has a neuroprotective effect through GABA receptor agonistic property [21,33] and peroxynitrite scavenging activity in neurodegenerative diseases. Moreover, the neuroprotective effect of sinapic acid has also been found in a mouse model of amyloid β1–42 protein-induced Alzheimer’s disease (AD) [34].
Considering the involvement of the oxidative stress hypothesis in the complex multifactorial nature of AD, the antioxidant molecules could play a special role in the prevention and therapy of AD. Some recent studies showed that SINA has the potential to mitigate, to some extent, the effects of oxidative stress in experimental animal models and in vitro [32,35].
Currently, memantine (MEM) is well known as the only N-methyl-D-aspartate (NMDA) receptor channel blocker, used for the treatment of moderate-to-severe forms of AD [36,37,38,39,40]. However, besides the other available anti-AD drugs, MEM offers only symptomatic relief to patients and is not able to stop AD progression.
In addition, some recent studies showed that SINA may also have the potential to impact the NMDA receptors [41].
Multi-target-based therapeutic approach has increasingly gained popularity in recent years in pathological conditions with complex symptomatics and physiology as in AD [5,42,43,44]. The combination of cholinesterase inhibitors and NMDA receptor modulators is especially appealing [45,46].
Stepping on this approach, medicinal chemistry goes even further by attempting to design drugs, or more broadly, pharmaceutical agents, with multi-target properties by using parental molecules with proven desired effects upon one or more symptoms which get conjugated to each other or to a common substrate [42]. Based on this approach, in recent years, various hybrid molecules using MEM or adamantine scaffold and AChE inhibitors have been designed and tested with different success [47,48,49]. Many of them are based on the tacrine AChE inhibitor, which, although initially approved for clinical usage, is, nowadays, withdrawn due to hepatotoxicity being found [49]. Alternatively, recent studies showed the positive potential of this approach in AD by using and combining MEM and a close “relative” of SINA, namely the cinnamic acid which has relatively weak to moderate cholinesterase inhibiting effects [50,51].
In our earlier study by Chochkova et al., (2021), we developed a series of antioxidant hydroxycinnamic hybrids based on MEM scaffold. Among all the memantine hybrids, N-sinapoylamide of memantine (N-Sinapoyl-memantine; SINA-MEM) was found to be a highly effective antioxidant in all the tested in vitro radical scavenging tests. Moreover, the single crystal X-ray analysis of SINA-MEM defines that amide crystallizes in a non-centrosymmetric manner in a trigonal crystal system, space group R3. Furthermore, it was found that the aromatic ring system present in the sinapoyl moiety is substantially planar. The neighbor intermolecular groups and bonds (NH; ·CO; ·CH=CH) also seem to stabilize the 3D structure of the new hybrid molecule according to our data and structure analysis [52]. In the testing of anti-Alzheimer effects in vitro (against Aβ toxicity, excitotoxicity, oxidative stress damage, hypoxia injury, and neuroinflammation), the memantine hybrids displayed neuroprotection at moderate levels. Despite the tested memantine hybrids being slightly weaker than the positive control MEM, they may be able to enhance the efficacy of AD therapy in vivo [52,53].
Following our line of research based on a particularly attractive approach for multi-target ligands for AD treatment, herein, we further explore in silico the interaction with a couple of host target molecules of interest and consequently, we check in a lab and in vivo some of the possible resulting neuroprotective activities of a sinapic acid hybrid with memantine from such modeled interactions.
2. Results and Discussion
2.1. Compound Synthesis
In our previous papers, we reported the synthesis of SINA-MEM and other hydroxycinnamoyl hybrids by EDC/HOBt method following the synthetic route (see Figure 1) [52,54]. The crude product was purified by column chromatography on silica gel using hexane/ethylacetate = 1:1 to obtain SINA-MEM as white crystals.
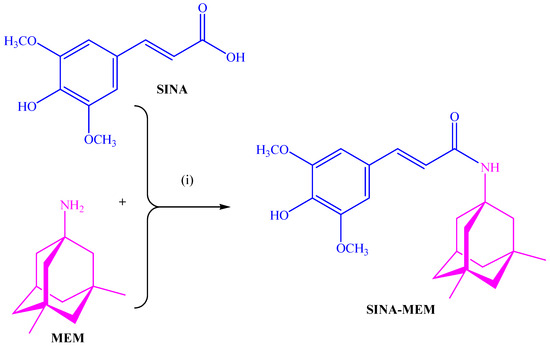
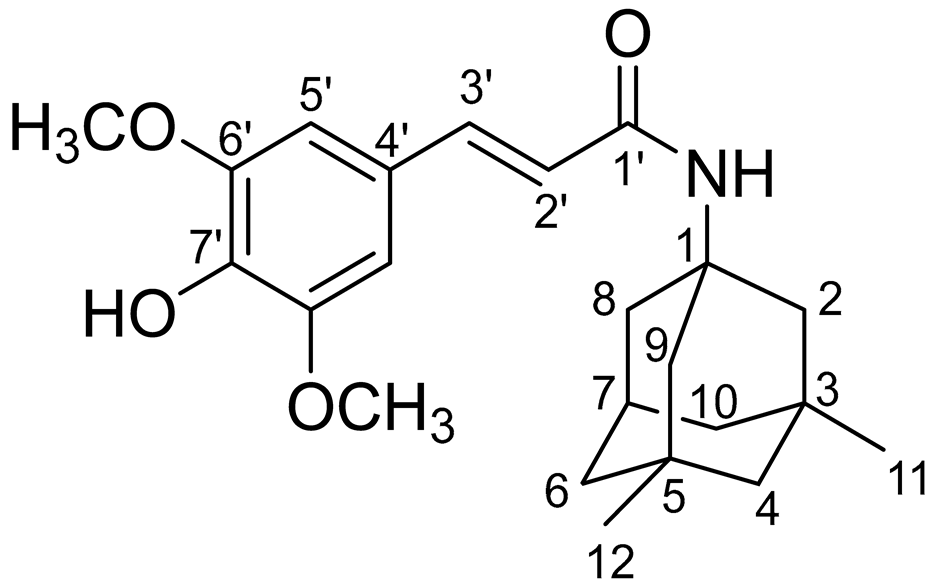
Figure 1.
Synthesis of memantine-based multi-target-directed ligand: (i) EDC/HOBt, NMM, CH2Cl2/DMF, 1 h at 0 °C, and then 23 h at rt.
Based on the manifested in vitro antioxidant and neuroprotection investigations, SINA-MEM was selected in order to study its ability to stop the disease progression in mice.
2.2. Physico-Chemical Analysis of the Compounds
The comparison of the data from the X-ray diffraction between the parental and the new hybrid compounds (see Table 1 and Figure 2) shows that the lengths of all the bonds are changed. The least pronounced are the changes in the C-H bonds, which change in the range of −0.5–+0.5%, with the exception of the ones of the -OCH3 group where the change can go up to 0.15%. The next least affected type of bonds is the C-C ones from the aromatic rings, which change in the range −1.2–+1.2%. More affected are the C-C bonds from the aliphatic chain which change by 1.9% and −2.48%, respectively. From the C-O bonds, the least affected are the ones in the carbonyl group with a 0.95% reduction. The C-O bonds with carbon atoms from the aromatic ring are increased by slightly more than 1%. More pronounced are the changes in the C-O bonds in the -OCH3 groups whose length can change by up to 2.8%. The most pronounced change is found in the OH bond of the hydroxyl group at the para position of the aromatic ring where a length change of over 7% is observed.

Table 1.
Comparison of the bond lengths as the percentage difference between SINA and SINA-MEM as determined by the X-ray diffraction.
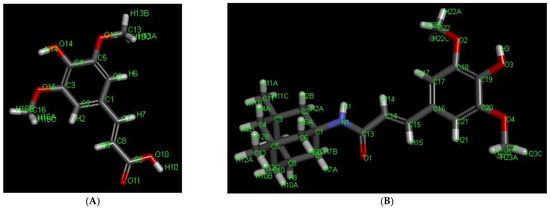
Figure 2.
Three-dimensional structures of the compounds with labeled atoms: (A)—SINA; (B)—SINA-MEM.
The comparison of the data from the X-ray diffraction between the parental MEM and the MEM moiety of the new hybrid compound shows that the lengths of the C-C bonds are changed within the range −0.3% to + 0.5%, greater changes are observed in the bonds with the methyl groups—approximately 0.8%. The change in the bond length with the nitrogen atom is reduced by 0.6%. And, the maximal change in the whole MEM moiety is observed at the N-H bond with a length reduction of over 5.5%.
Comparing the spatial arrangement of the atoms in the new hybrid molecule with the parental SINA, it is found that the planarity is preserved in the respective part of the molecule.
According to the attenuated total reflectance infrared spectra (ATR-IR) spectrum of SINA, the broad peak in the 2400–3500 cm−1 range indicates O-H stretching bands, originating mainly from hydrogen-bonded dimers and also the intramolecular hydrogen bonds between the phenolic OH and OCH3 (as recorded in a solid phase) [55]. However, the smaller “shoulder” related to the dimeric carboxylic species and protruding from 2400 to 2700 cm−1 are absent in the spectrum of the hybrid molecule, which suggests the obtaining of the desired amide (SINA-MEM).
From the literature, it has been known that the salt formation in amines may give rise to a modification of NH stretching absorption [56]. So, the spectrum of MEM hydrochloride clearly shows the occurrence of complex “ammonium” bands between 3200 and 3080 cm−1, 2700 and 2500 cm−1, and near 2055 cm−1, which is associated with NH3+ stretching absorption. Additionally, in the spectral region 1614–1510 cm−1, the bands are assigned to the -NH3+ bending modes.
Contrary to the infrared spectrum of MEM, the vibrational overtone and composite frequencies of the NH3+ band at 2055 cm−1 and about 2700–2500 cm−1 vanish in the spectrum of SINA-MEM. Additionally, the appeared C=O stretching peak (amide I) at 1662 cm−1 and also the stretching vibration of N–H at 3200–3080 cm–1 confirm the amide bond formation in the obtained SINA-MEM. However, the position of NH deformation bands of SINA-MEM (amide II) is strongly influenced by the overlap of the aromatic bands occurring in the same region about 1600–1513 cm−1, and thus it is so complicated to identify [57].
Furthermore, the C-H bending vibrations of SINA and SINA-MEM at 973 cm−1 and 974 cm−1, respectively, define the trans-configurations of the olefinic side chain in these molecules.
Further analyzing comparatively the acquired ATR-IR shows that many of the more pronounced peaks are shifted in the new hybrid molecule in comparison to the parents (see Figure 3 and Table 2). Comparing the parent compound SINA with the corresponding moiety of the new hybrid, it was determined that most of the peaks of the bonds in the aromatic ring decreased with 1–2 cm−1 in the hybrid molecule. On the other hand, the wavenumber of the bonds at the aliphatic chain connecting with MEM moiety increased their absolute value by 2–5 cm−1. However, the biggest observed change was at the most remote group from the MEM moiety, namely the hydroxyl one at the para position of the aromatic ring which shifted by almost 9 cm−1.
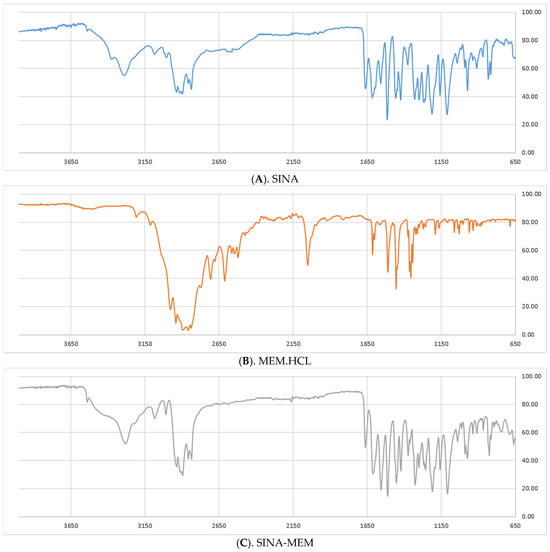
Figure 3.
Comparison of the ATR-IR spectra of the parental and the new hybrid compounds. X axis—wavenumbers; Y axis—transmittance intensity. Legend: (A). SINA (sinapic acid); (B). MEM.HCl (memantine hydrochloride); (C). SINA-MEM (N-Sinapoyl-memantine).

Table 2.
Comparison of the bond wavenumbers of some of the characteristic peaks between SINA and the corresponding moiety of SINA-MEM as determined by the ATR-IR.
The comparison of the data from the ATR-IR between the parental MEM and the MEM moiety of the new hybrid compound was hindered mostly due to the low intensity, overlapping, and masking of some of the characteristic peaks, thus turning it less informative.
One- and two-dimensional nuclear magnetic resonance (1D- and 2D- NMR) techniques were applied to determine the structure of SINA-MEM. The NMR spectra and correlation analysis of the new hybrid compound (see Table 3, Figure 4) further confirm its structure. The more precise identification of the geometry of the double bond in SINA-MEM can be provided by the analysis of the 1D (1H) and 2D (COSY (Correlation Spectroscopy (COSY)), HMBC (heteronuclear multiple bond correlation), and 1H/13C HSQC-heteronuclear single quantum coherence) NMR spectra. As depicted in Table 3, as well as in 1D- and 2D NMR spectra of SINA-MEM, the resonance signals of the vinyl protons appearing as two doublets at 7.20 (H3′) and 6.51 (H2′) ppm with a large vicinal spin–spin coupling constant (3J) around 15.6 Hz, confirming the E-pi diastereoisomeric form of the synthesized hybrid.

Table 3.
Assignment of 1H and 13C NMR spectra for SINA-MEM (DMSO-d6).
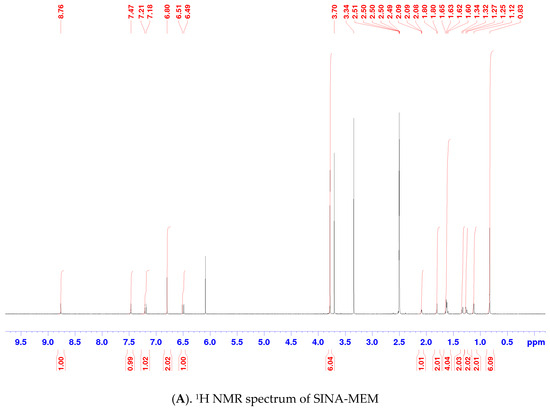
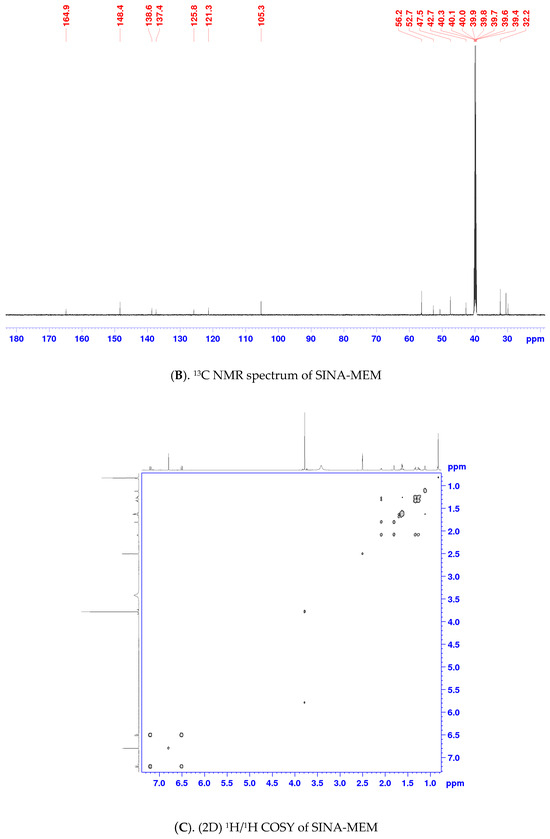
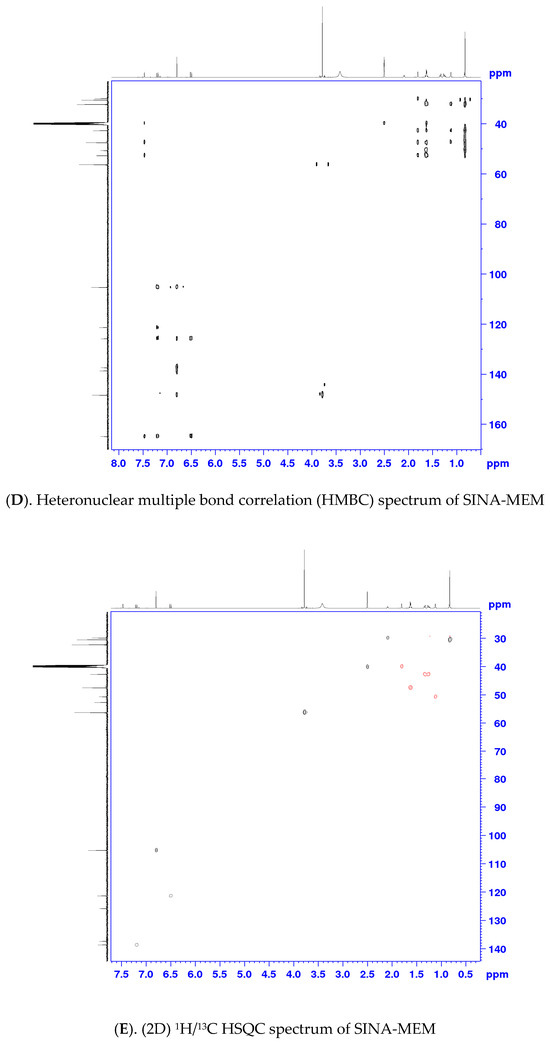
Figure 4.
NMR spectra and correlation analysis of the hybrid compound. Legend: (A). 1H NMR—proton nuclear magnetic resonance spectrum; (B). 13C NMR—carbon nuclear magnetic resonance spectrum; (C). 1H/1H COSY—Correlation Spectroscopy; (D). HMBC—heteronuclear multiple bond correlation; (E). 1H/13C HSQC—heteronuclear single quantum coherence.
Meanwhile, in the 1H-NMR spectrum of SINA-MEM, the amide proton appears as a singlet signal at 7.47 ppm (Figure 4A), whereas the amide carbonyl group is evident in 13C-NMR at 164.9 ppm (Figure 4B). As shown in Figure 4D, the HMBC experiments displayed correlations between the NH proton of SINA-MEM and the neighboring carbonyl carbon (NH/C-1′) and also with C-1 (NH/C-1) from the memantine nucleus.
2.3. QSAR and Docking Results
The results of the ADMET/QSAR analysis and evaluation are presented in Table 4 for each compound, where the first column presents in binary form the relevance of this parameter/property, and in the right adjacent column is a quantitate evaluation of the probability of this parameter/property. The evaluation of the SINA-MEM compound compared to its parent molecules shows that it has no expected mutagenicity and carcinogenicity. All of the compounds are predicted to have hepatotoxicity with moderate to high probability—the lowest being for the SINA-MEM compound (0.567) and the highest for the parent SINA (0.702). All of the compounds are predicted to have class III acute oral toxicity (LD50 in the range of 500–5000 mg/kg) with similar probability for SINA (0.45) and SINA-MEM (0.4847), while MEM has higher (0.7138). It has a high probability of intestinal absorption (0.9434) as its parents but a lower probability for the oral bioavailability parameter (0.5571). The compound has a high probability of crossing the blood–brain barrier (0.9426) as its parents. Its subcellular localization is expected to be the mitochondria.

Table 4.
QSAR predicted the pharmacological values of the tested compounds. Legend: Some of the ADMET results/evaluations are presented as binary output; for example, the presence/relevance of some parameter/property is marked as “+” in the columns labeled “PROBABILITY”, and the respective absence/irrelevance is marked with “-”. (a): Acute Oral Toxicity is classified into four categories based on their LD50 values (Category I contains compounds with LD50 ≤ 50 mg/kg. Category II with LD50 50–500 mg/kg. Category III with LD50 500–5000 mg/kg. Category IV with LD50 > 5000 mg/kg).
The in silico experiments with molecular docking revealed that all of the tested substances can interact with the active site of one of the key target enzymes in the management of AD—acetylcholinesterase (AChE).
The active site of AChE is an unusual one and is deeply recessed at the bottom of a relatively long gorge (approximately 20A) [58]. It can be divided into several subsites each with different amino acid residues, namely the following: (a). catalytic triad, also called esteratic subsite, encompassing the residues Ser203, His447, and Glu334; (b). acyl binding pocket (ABP)—Trp236, Phe295, Phe297, and Phe338; (c). peripheral anionic subsite (PAS)—Asp74, Tyr124, Ser125, Trp286, Tyr337, and Tyr341; (d). anionic subsite—Trp86, Tyr133, Glu202, Gly448, and Ile451; (e). oxyanion hole—Gly121, Gly122, Ala204, and other residues of the omega loop (Thr83, Asn87, and Pro88) (see Figure 5). The omega loop is a disulfide-linked loop (Cys69–Cys96) that covers the active site of AChE, which is buried at the bottom of the gorge.
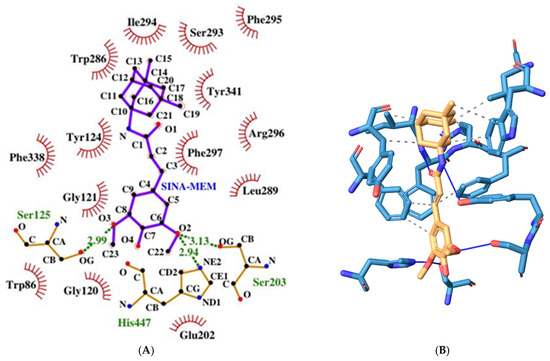
Figure 5.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of SINA-MEM with the AChE active site.
In order to compare the docking result parameters and how the studied substances interact and probably can inhibit the AChE enzyme, we used one of the established and clinically approved AChE inhibitors—galantamine—as a referent molecule [59].
The hybrid molecule has a relatively low binding energy of −8.61 kcal/mol and forms several hydrogen bonds with few amino acid residues which are part of the active sites of the enzyme. All of these hydrogen bonds are formed with the participation of the oxygen atoms from the sinapoyl moiety. Two of these bonds are with amino acid residues His447 and Ser203 and are part of the catalytic triad, and the other hydrogen bond is with Ser125 (PAS).
The molecule also engages in several hydrophobic interactions with the following AChE amino acid residues (some of them are part of its active sites): Trp286 (PAS), Leu289, Ile294, Arg296, Phe297 (ABP), Phe338 (ABP), and Tyr341 (PAS).
The SINA has a binding energy of −5.36 kcal/mol which is higher than the one of the hybrid molecule. However, it forms more hydrogen bonds with the surrounding amino acid residues at the AChE catalytic center (see Figure 6). These amino acids are Tyr124 (PAS), Ser293, Phe295 (ABP), Arg296, Tyr337 (PAS). It engages in hydrophobic interactions with the following amino acid residues: Phe297 (ABP), Phe338 (ABP), and Tyr341 (PAS).
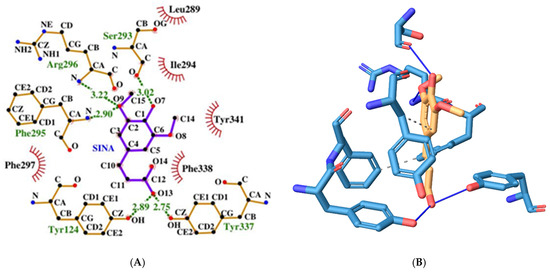
Figure 6.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of SINA with AChE active site.
MEM has a relatively low binding energy of −8.02 kcal/mol. It forms two hydrogen bonds with Ser293 and Tyr341 (PAS) through its only nitrogen atom (see Figure 7). It also engages in hydrophobic interactions with Trp286 (PAS), Ile294, Phe297 (ABP), Phe338 (ABP), and Tyr341 (PAS).
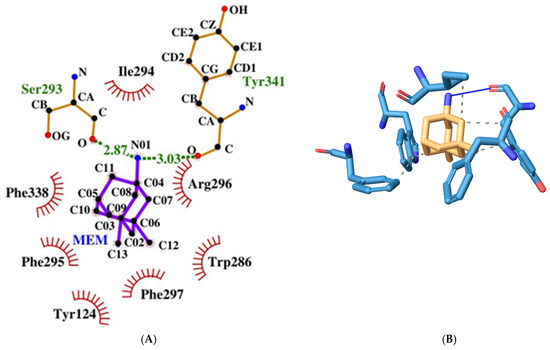
Figure 7.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of MEM with AChE active site.
Galantamine has a binding energy of −8.79 kcal/mol which is the lowest among the tested molecules. It forms three hydrogen bonds (but longer than 3.19A, and therefore, probably not counted by the 2D plotting software). These bonds are with the amino acid moieties Tyr72, Tyr124 (PAS), and Tyr337 (PAS) (see Figure 8). It engages in hydrophobic interactions with Phe338 (ABP) and Tyr341 (PAS). However, unlike the other molecules, it also interacts through pi-stacking.
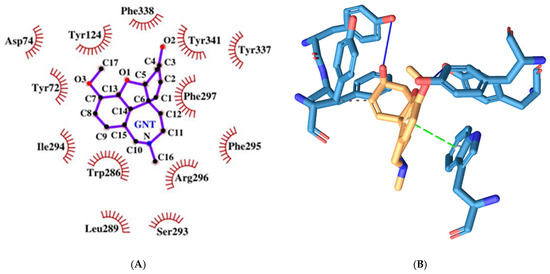
Figure 8.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of GNT with AChE active site.
As we can see, all of the molecules, regardless of their similarities, interact differently with the active site of AChE. SINA-MEM forms hydrogen bonds with the residues from CT and PAS, while SINA forms such bonds with the residues from ABP and PAS, and MEM only with the residues from PAS. The pattern of hydrophobic interactions also differs. The common residues for SINA-MEM, MEM, and SINA are Phe297 (ABP), Phe338 (ABP), and Tyr341 (PAS). SINA-MEM interacts with four more residues than SINA and with two more than Mem.
As for the galantamine, additionally, it differs from the rest by the pi-stacking interaction. All of the tested molecules have hydrophobic interactions with Phe338 (ABP) and Tyr341 (PAS). And none of them interacts with the oxyanion hole, anionic subsite, and omega loop. Only SINA-MEM interacts with the catalytic triad.
The detailed results of the re-docking experiments generated a similar profile of the interaction of the amino acid residues (see Figure 9).
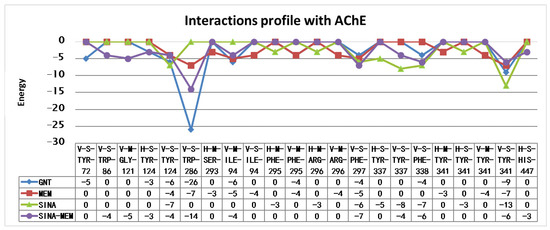
Figure 9.
Amino acid interaction profiles of the compounds with AChE active site derived from the re-docking experiments (V—Van der Waals interaction; H—hydrogen bond; S—strong; M—medium strength). Only interactions with a value below −2.5 are shown.
Most of the tested compounds have relatively low binding energy below −8.0 kcal/mol. Only SINA has a higher value of −5.36 kcal/mol. In comparison, the acquired conformations of ACH have binding energies in the range above −4.80 kcal/mol (see Table 5).

Table 5.
Calculated energies and inhibitory constants from the molecular docking of the tested compounds.
According to Silman and Sussman [60], there is a narrowing in the middle of the active site gorge produced by the juxtaposition of Phe330 and Gly121, whose cross-section they estimate to be ~5 Å. So, molecules bigger than acetylcholine molecules can probably pass through such obstacles, but only if their 3D structure is planar and/or sufficiently flexible. MEM and its synthesized analog SINA-MEM are the bulkiest molecules among the tested batch. The approximate distance between the carbon atoms of its methyl moieties is 5 A, similar to the one between the nitrogen in the amino group and the carbons from the methyl groups.
So, regardless that the MEM has a rather low value of the binding energy of the interaction with the active site of AChE, in reality, we cannot benefit from such affinity simply because the molecule is spatially obstructed from access to this site.
However, the situation with the other bulky molecule—the MEM hybrid SINA-MEM—is different. This molecule comprises two bulky moieties connected by a long carbon chain. Howbeit, one of these bulky moieties—the aromatic ring from the sinapic acid—has mostly planar structure and is less likely to be obscured by the gorge narrowing. In fact, in all of the found docking conformations for this molecule that bind to the amino acid residues in the AChE active site, this moiety is the one inserted in it, while the MEM one remains outside or at its entrance. As it can be seen from the 2D plotting diagram, the sinapic acid moiety is behind the Gly121 amino acid residue, while the MEM one remains outside the active center.
Docking simulations were also performed with another possible molecular target in the brain—the GABAA receptors. These receptors are part of the GLIC family and form ion channels in the neuronal membranes. Since this type of protein complexes was notoriously hard to study until recently, there was no 3D protein structure of them available for the scientific community. Recently, the 3D structure of human GABAA receptors was published [61,62], followed by chimera 3D assembly for mice [63].
The docking with the classic benzodiazepine flumazenil revealed that the ligand binds to the benzodiazepine binding site of GABAA receptors with a binding energy of −7.55 kcal/mol.
Flumazenil forms with three hydrogen bonds with both protein chains (α and γ) with the following amino acid residues: Thr142γ, Ser205α, and Ser206α. It also has 7 hydrophobic interactions with Tyr58γ, Phe77γ, Tyr160α, and Tyr210α (see Figure 10).
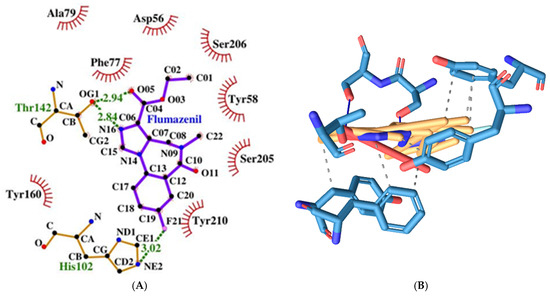
Figure 10.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of Flumazenil with GABA A receptor’s benzodiazepine active site.
MEM has a slightly higher binding energy of −7.37 kcal/mol than flumazenil. It forms fewer hydrogen bonds amino acids from both protein chains, namely the following: Asp56γ and Ser206α (see Figure 11). It has four hydrophobic interactions with amino acids from the γ protein chain: Tyr58, Phe77, and Ala79.
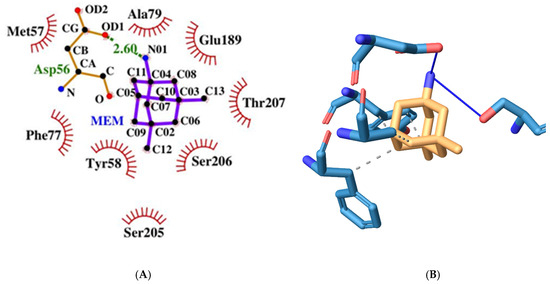
Figure 11.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of MEM with GABA A receptor’s benzodiazepine active site.
SINA has the highest binding energy among the tested substances at −5.76 kcal/mol. It forms more hydrogen bonds with amino acids but only from the α-protein chain, namely the following: His102, Ser159, Ala161, and Ser205 (see Figure 12). It has the fewest hydrophobic interactions with amino acids only from the γ protein chain: Phe77. And unlike the previous compounds, it interacts through π-stacking with Tyr210α.
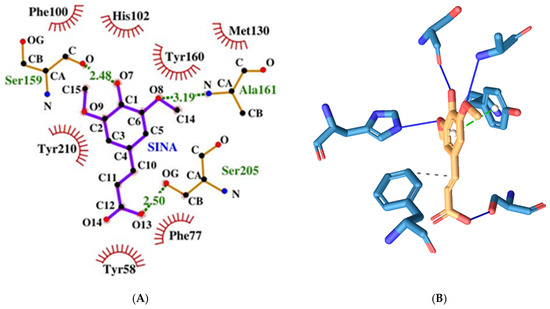
Figure 12.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of SINA with GABA A receptor’s benzodiazepine active site.
The derivative SINA-MEM scores second in binding energy after flumazenil with a value of −7.5 kcal/mol. It forms three hydrogen bonds with amino acids only from the α-protein chain, namely the following: Lys156 and Ser205 (see Figure 13). It has four hydrophobic interactions with amino acids from both protein chains: Phe77γ, Tyr160α, and Tyr210α.
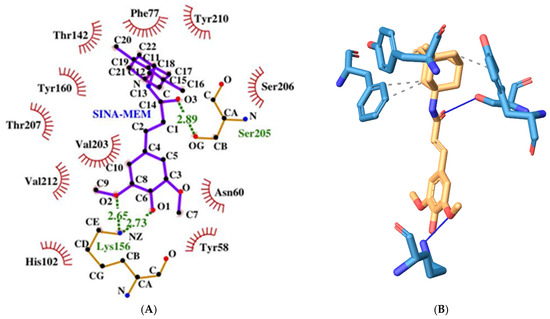
Figure 13.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of SINA-MEM with GABA A receptor benzodiazepine active site.
All of the tested compounds form hydrogen bonds with one of the polar serine residues at α-chain (Ser205 or Ser206). And, all of them have hydrophobic interaction with Phe77γ, which is an important amino acid residue in the benzodiazepine binding pocket [62]. Among them, SINA is the compound with the weakest inhibitory potential, while the rest have estimated inhibitory constants Ki in the range 2.9–4.0 (see Table 6).

Table 6.
Summary of the parameters of the compound interactions with GABA A receptor’s benzodiazepine active site.
The detailed results of the re-docking experiments generated a similar profile of the interaction of the amino acid residues (see Figure 14).
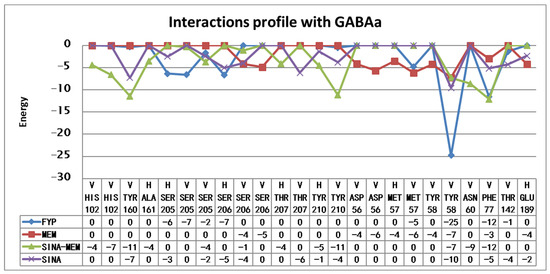
Figure 14.
Amino acid interaction profiles of the compounds with GABA A receptor’s benzodiazepine active site derived from the re-docking experiments (V—Van der Waals interaction; H—hydrogen bond). Only interactions with a value below −1 are shown.
Since MEM is among the very few approved drugs for the management of AD as an NMDA receptor antagonist and is part of the hybrid molecule, it was decided to perform a docking simulation with the active site of the NMDA receptor.
MEM has a relatively weak to moderate binding energy of −6.13 kcal/mol. It forms two hydrogen bonds amino acids from both protein chains, namely the following: Asn599d and Asn606a (see Figure 15). It has six hydrophobic interactions with amino acids from two protein chains: Asn598d, Asn599d, Ala622d, Val623d, Leu626d, and Asn606a.
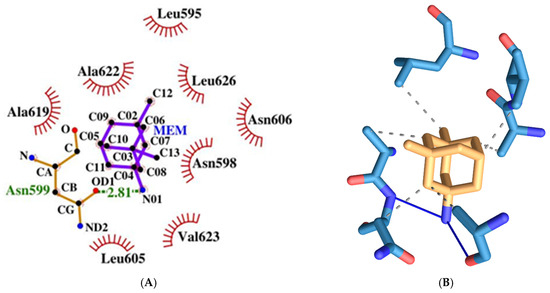
Figure 15.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of MEM with NMDA receptor ion channel gate.
SINA has a relatively weak binding energy of −4.59 kcal/mol. It forms four hydrogen bonds amino acids from both protein, chains namely the following: Leu595d, Asn598d, Asn599d, and Leu604a (see Figure 16). It has only one hydrophobic interaction with amino acids from protein chain d: Val623d.
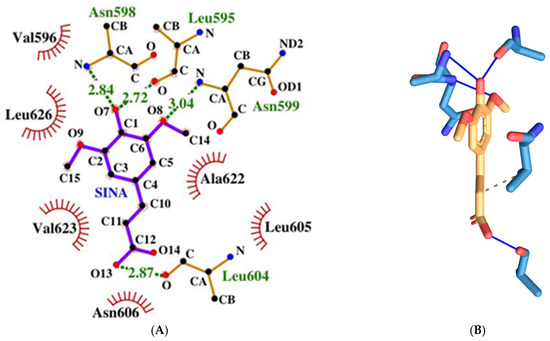
Figure 16.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of SINA with NMDA receptor ion channel gate.
The memantine-based hybrid molecule has the lowest binding energy among the tested compounds with a value of −8.91 kcal/mol, which corresponds to the strongest binding affinity to the respective receptor. It forms two hydrogen bonds amino acids from both protein chains, namely the following: Asn599d and Leu605a (see Figure 17). It has six hydrophobic interactions with amino acids from one protein chain: Leu595d, Asn598d, Asn599d, Ala622d, Val623d, and Leu626d.
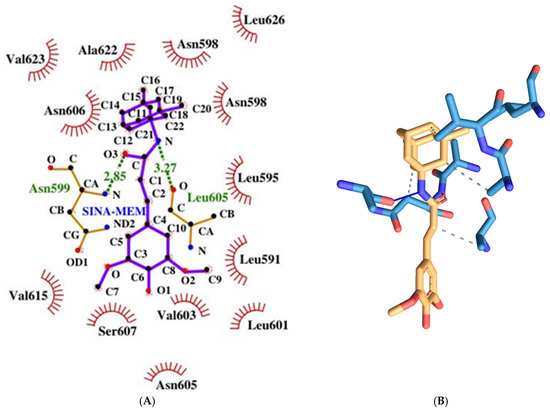
Figure 17.
Two-dimensional (A) and three-dimensional (B) diagrams of the interactions of SINA-MEM with NMDA receptor ion channel gate.
It seems that MEM and SINA-MEM rely more on the hydrophobic interactions for binding to the ion channel protein chains and they share most of the amino acid residues they interact with, while the SINA relies more on forming hydrogen bonds with the surrounding amino acid residues. All of the compounds form hydrogen bonds with the important-for-the-ion-channel-blockage Asn residues or/and they have hydrophobic interactions with them (Asn598d, Asn599d, and Asn606a).
The detailed results of the re-docking experiments generated a similar profile of the interaction of the amino acid residues (see Figure 18).
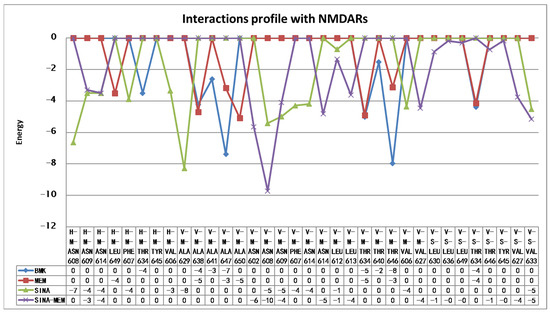
Figure 18.
Amino acid interaction profiles of the compounds with NMDA receptor ion channel gate derived from the re-docking experiments (V—Van der Waals interaction; H—hydrogen bond; S—strong; M—medium strength). Only interactions with a value below −1 are shown.
Sinapic acid is the weakest NMDAR ion channel inhibitor with the highest binding energy (−4.59 kcal/mol) and estimated inhibitory constant (428.54 µM), while the hybrid molecule SINA-MEM is the most potent inhibitor with the lowest binding energy (−8.91 kcal/mol) and estimated inhibitory constant (294.18 nM) (see Table 7).

Table 7.
Summary of the parameters of the compound interactions with the NMDAR ion channel.
It is noteworthy to mention that both MEM and SINA-MEM show a preference for binding at the ion channel opening vicinity, while the SINA shows no such trend.
2.4. Biochemical Testing Results
The results of the AChE activity assay show that the treatment with SINA has the strongest impact among the tested compounds in the scopolamine (SCO) model of AD, followed by GNT, and then SINA-MEM in reducing the AChE activity (see Figure 19), which is in concordance with the results from a study of AD-related pathological conditions as the induced brain hypoxia [64]. Another in vitro study shows that sinapic acid is a potent AChE inhibitor [65], which may explain our results; however, our study further shows that the N-Sinapoyl-memantine has a weaker impact on the AChE activity which probably can be accounted to the additional memantine group. Since memantine is an NMDA receptor antagonist, it has no inhibitory effects upon acetylcholinesterase activity as our study confirms, and in the new hybrid molecule memantine moiety is rather bulky, which may hinder or obstruct the inhibitory action of the sinapic acid moiety in the synthesized molecule.
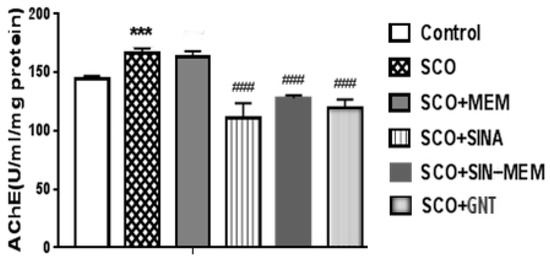
Figure 19.
Impact upon the acetylcholine esterase (AChE) activity of the tested compounds. Treatment of all the groups continued for 11 consecutive days. Control (saline 0.1 mL/10 mg i.p.); SCO (scopolamine 1 mg/kg i.p.); SCO+MEM (scopolamine 1 mg/kg i.p. plus memantine 20 mg/kg i.p.); SCO+SINA (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg i.p.); SCO+SINA-MEM (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg, i.p. plus memantine 20 mg/kg); SCO+GNT (scopolamine 1 mg/kg i.p. plus new hybrid molecule 20 mg/kg i.p). AChE activity was measured in brain homogenates. The results are expressed as a mean value ± SEM (n = 8 animals per group). Statistical analysis: one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. Significance vs. saline-treated group: *** p < 0.001; significance vs. Sco-treated group ### p < 0.01.
Part of these results may superficially seem to contradict some of the findings from the molecular docking. For example, SINA showed the most pronounced effect upon AChE activity in our in vitro testing, but has the weakest score in the docking results among the tested compounds, which is comparable with ACH itself. However, when ACH enters the AChE active site compartment, it is catalyzed rather quickly to smaller products, some of which are removed either through the backdoor in the active site bottom as Silman and Sussman [60] suggest, or they diffuse through the AChE gorge entrance. The situation with the SINA is different. When it enters the AChE active site compartment through the narrow opening it actually gets trapped there. Additionally, it has three oxygen atoms that can induce strong polarization, which is a predisposition for various relatively strong non-covalent interactions with the amino acid residues. We presented here in detail only the best conformation of the interaction of the ligand with the protein. However, our molecular docking results show that SINA is capable of interacting with many different residues in the AChE active site vicinity. So, once it gets detached from one conformation, it is very possible to bind to another part of the active site instead of leaving the active site completely.
The hybrid compound SINA-MEM, on the other hand, has rather low binding energy but does not perform so well in the in vitro assay (see Figure 19). This, to some extent, can be attributed to the fact that less than half of the molecule enters the AChE active site compartment while the bigger part of it remains outside the narrowing of the gorge. Here, we should take into account that in reality the enzyme actually “breathes” as Silman and Sussman [60] suggest. With such a long bulky tail, it is easier for the enzyme to exhale the inhibiting molecule at some point regardless of its good binding capacities. And also, it is probably more difficult for such a long and bulky molecule to enter the gorge and pass through the mentioned narrowing.
2.5. Behavioral Testing Results
The animals with the AD model show higher anxiety than the control group, while the tested compounds reduce significantly this parameter at p < 0.001, with the best results with sinapic acid, which nearly restores it to the level of the control group (see Figure 20). This corresponds to the results from previous studies with oral treatment with sinapic acid in mice [33]. This effect probably is mediated by action upon the GABA receptors, which are known to be involved in anxiety-related states [33]. The SINA-MEM hybrid molecule shows less pronounced anxiolytic effects than the parent SINA molecule, but still higher than the other parent molecule—MEM which is also known to have some anxiolytic effects in rodents and to be an NMDA receptor antagonist [66]. Therefore, the anxiolytic action of the hybrid molecule probably can be attributed to its impact on GABA.
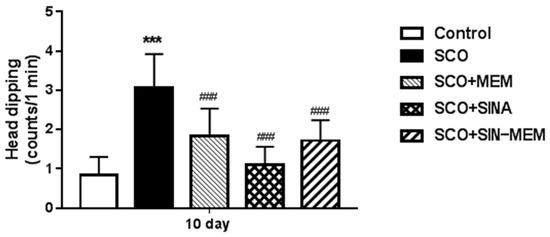
Figure 20.
Effects of the tested compounds on the performance of the animals in the hole-board test. Treatment of all the groups continued for 11 consecutive days. Control (saline 0.1 mL/10 mg i.p.); SCO (scopolamine 1 mg/kg i.p.); SCO+MEM (scopolamine 1 mg/kg i.p. plus memantine 20 mg/kg i.p.); SCO+SINA (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg i.p.); SCO+SINA-MEM (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg, i.p. plus memantine 20 mg/kg). The results are expressed as mean values ± SEM (n = 8 animals per group). Statistical analysis: one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. Significance vs. saline-treated group: *** p < 0.001; significance vs. Sco-treated group ### p < 0.001.
Glutamatergic neurotransmission in regard to the NMDA receptors is classically accounted to be involved in some cognitive processes such as memory and learning, development, etc.; however, these receptors are found to modify also some affective states such as depression. So the antagonists of the NMDA ion channels can have anti-depressant-like effects or potentiate the effects of classic anti-depressants such as lithium [67,68,69]. Some studies showed that MEM can significantly alleviate some depressive symptoms in dose dose-dependent way in rodent animal models [68]. Our results confirm such action of MEM in the SCO model in the forced swim testing (see Figure 21). Close but slightly better is the performance of the hybrid molecule SINA-MEM and worst is the performance of the parent SINA, which somewhat resembles our findings in the in silico experiments.
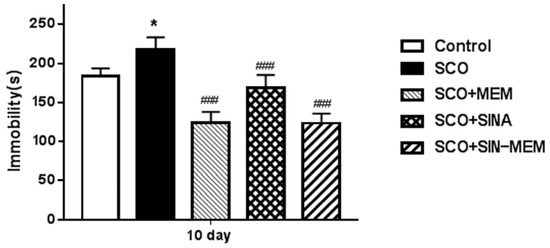
Figure 21.
Effects of the tested compounds on the performance of the animals in the forced swim test. Treatment of all the groups continued for 11 consecutive days. Control (saline 0.1 mL/10 mg i.p.); SCO (scopolamine 1 mg/kg i.p.); SCO+MEM (scopolamine 1 mg/kg i.p. plus memantine 20 mg/kg i.p.); SCO+SINA (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg i.p.); SCO+SINA-MEM (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg, i.p. plus memantine 20 mg/kg). The results are expressed as mean values ± SEM (n = 8 animals per group). Statistical analysis: one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. Significance vs. saline-treated group: * p < 0.05; significance vs. Sco-treated group ### p < 0.001.
Further in the previous century, systematic studies on the glutamatergic neurotransmission by the NMDA receptors found that it is involved in the pain perception and management during the late tonic phase of locally induced pain in many mammal species such as cats, rats, mice, primates, etc., [70]. As a result, several NMDA antagonists were shown to have analgesic effects through “pre-conditioning” the nervous system towards injuring pain-inducing stimuli [71]. Here, we used the pain-inducing formalin test to evaluate the antagonistic effects of the studied compounds on the NMDA receptors. The results of the treatment with the compounds before the pain induction in the animals show that the best-performing NMDA channel inhibitor is the SINA-MEM, followed by the parent MEM (see Figure 22). The other parent SINA has the least effect, which is in concordance with the in silico results. The treatment with the compounds after the pain induction did not produce significant changes in the tonic pain mitigation and differences between the groups, which is in agreement with previous studies with such mode of action of the NMDA antagonists [71].
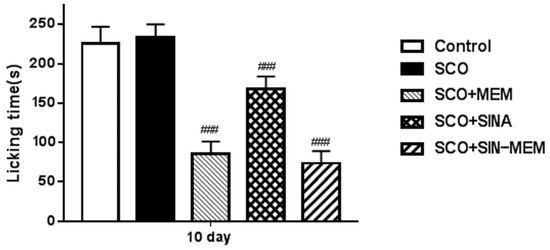
Figure 22.
Effects of the tested compounds on the licking behavior of the animals in the formalin pain test during the late tonic phase. Treatment of all the groups was performed on the 10th day 20 min before the formalin injection. Control (saline 0.1 mL/10 mg i.p.); SCO (scopolamine 1 mg/kg i.p.); SCO+MEM (scopolamine 1 mg/kg i.p. plus memantine 20 mg/kg i.p.); SCO+SINA (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg i.p.); SCO+SINA-MEM (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg, i.p. plus memantine 20 mg/kg). The results are expressed as mean values ± SEM (n = 8 animals per group). Statistical analysis: one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. Significance vs. Sco-treated group ### p < 0.001.
The performance on the passive avoidance test has some mixed effects (see Figure 23). There was an improvement in all of the tested compounds which is in concordance with previous studies with the parental molecules in AD animal models [72,73]; however, only the synthesized molecule had statistically significant (at p < 0.05) improvement in the learning and memory performance in the Sco AD model with levels comparable to the control group and the already approved AChE inhibitor GNT. Such less pronounced effects in the case with the parental molecules may be attributed to the relatively low doses in this particular model of AD since another study showed a dose dependence in the observed cognitive ameliorative effects [73].
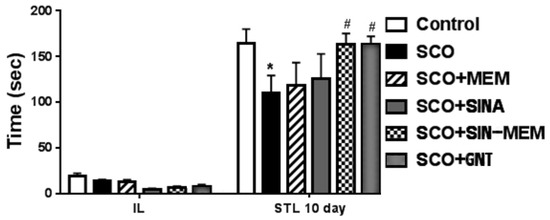
Figure 23.
Effects of the tested compounds on the performance of the animals in the step-through test. Treatment of all the groups continued for 11 consecutive days. Control (saline 0.1 mL/10 mg i.p.); SCO (scopolamine 1 mg/kg i.p.); SCO+MEM (scopolamine 1 mg/kg i.p. plus memantine 20 mg/kg i.p.); SCO+SINA (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg i.p.); SCO+SINA-MEM (scopolamine 1 mg/kg i.p. plus sinapic acid 10 mg/kg, i.p. plus memantine 20 mg/kg); SCO+GNT (scopolamine 1 mg/kg i.p. plus new hybrid molecule 20 mg/kg i.p). Initial latency (IL) was measured during the training session. Step-through latency (STL) was measured 10 days after training. The results are expressed as mean values ± SEM (n = 8 animals per group). Statistical analysis: one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. Significance vs. saline-treated group: * p < 0.05; significance vs. Sco-treated group # p < 0.05.
Additionally, these in vivo results only partly confirm the in silico findings, showing the SINA-MEM compound as the most potent NMDA ion channel inhibitor among the tested ones. Such subpar results can be attributed to the sophisticated involvement of the NMDAR in the pathophysiology of AD and its complex molecular mechanisms of regulation as shown in recent molecular studies of the inhibition and control of these channels with MEM and other agents [37,74].
3. Discussion
The results from the NMR and ATR-IR spectroscopy and X-ray diffraction analysis revealed that the new hybrid molecule inherits the planarity of the SINA parent and to a significant extent the 3D structure of the MEM parent. However, the addition of the MEM moiety to SINA has some more pronounced far-reaching impact due to the resonant properties of the formed conjugated quantum electronic system. Thus, the most pronounced changes were observed besides near the -C-N-C- bonds, but also at the most distant ones from them—the hydroxyl group at the aromatic ring, which shortens the overall length of the molecule. This means that the charge distribution, polarization pattern, and dipole moment are tweaked to various extents. And, this is a predisposition for different pharmacological behavior in the delicate fine-tuned microenvironment of the active centers and other functional sites of the target biomolecules as enzymes and receptors. Such modifications of the electronic shield are probably to be blamed for the differences in the interaction profiles since the overall spatial changes in the atoms in the new hybrid molecule are often less than 0.01 A.
Its polarized aromatic ring paired with a specific aliphatic chain that further extends the conjugated quantum system enables the new hybrid molecule to pair with other aromatic amino acid moieties through π-stacking and pairing and other weak interactions as the one with Tyrosine which is significantly present in the vicinity of the active sites of AChE and the GABAA receptor. On the other hand, the presence of strongly electronegative atoms as oxygen facilitates the Van der Waals interactions and H-bond formation with the respective polarized amino acid moieties which are also present within and around the active sites. Thus, the new hybrid compound can interact with most of the important amino acid moieties in the active sites and partly mimics the interaction of the established pharmacological agents. These observations are in concordance with the in vivo and biochemical results where it scored admirably, being on par with GNT on the in vivo testing.
As for the effects on the important NMDARs, the results are less straightforward. Although the new hybrid molecule has the best binding energy according to the docking results, it performed only slightly differently than the established pharmacological agent in the management of these receptors—MEM. Among the possible reasons for these discrepancies in the results is that the NMDARs have relatively complex and dynamic mechanisms of their regulation as recent studies with molecular dynamics and other biophysical methods revealed [37,74]. And especially for their usage in Alzheimer’s disease management, the “good” agent for NMDAR is the one that only partly inhibits their function, and not in a static fashion but in a dynamic one. And that is among the reasons why MEM performs so well there—because it has low polarizability but due to its specific cyclic geometric shape, it has a specific polarized charge distribution pattern that facilitates weak interactions in a dynamic way which is necessary to control the micro-currents in the vicinity of the NMDAR ion channel. Therefore, although the new hybrid molecule has the MEM moiety with relatively preserved geometry and charge distribution, it can have not so pronounced in vivo effect as the in silico results would suggest. Because for this particular receptor, the ideal pharmacological agent must be able to interact dynamically in the channel core—not too strongly, but not too weekly either. MEM performs relatively well in this regard, while its child SINA-MEM performs slightly subpar probably due to its slightly stronger interaction than the optimal level for this pathological physiological condition.
Overall, the QSAR analysis and the in vivo studies show that the new hybrid molecule with its low toxicity and low to medium penetration and bioavailability has significant pharmacological potential in the management of Alzheimer’s disease.
4. Conclusions
The synthesized N-Sinapoyl-memantine molecule showed better ameliorative effects in the key AD symptom as the cognitive decline than the parental molecules. Additionally, it has good anxiolytic properties and excellent antioxidant potency, which is also important since it, is known that AD is an oxidative stress-related disease. Therefore, the synthesized compound deserves further exploration and testing as a prospective AD drug.
5. Materials and Methods
5.1. Reagents
(E)-3,5-dimethoxy-4-hydroxycinnamic (sinapic acid, SINA), 1-[3-(dimethylamino)propyl]-3-ethyl carbodiimide hydrochloride (EDC), HOBt (N-hydroxybenzotriazole), NMM (N-methylmorpholine),hexane, ethylacetate and dichloromethane were purchased from Sigma-Aldrich (now part of Merck KGaA, Darmstadt, Germany). All the solvents were reagent grade and used without further purification.
5.2. Chemical and Structural Analysis
5.2.1. X-Ray Diffraction Analysis
To obtain single crystals of sufficient grade, the compound dry powder was dissolved in acetone at 290 K. Slow evaporation produced large crystals (0.3 × 0.2 × 0.15 mm3) appropriate for single crystal X-ray experiments in 2–3 days.
From each compound a single crystal was put on a glass capillary, and all the data were acquired at 290 K using an Oxford diffraction Supernova diffractometer (Yarnton, UK) utilizing Mo-K α radiation (λ = 0.71013 Å) from a micro-focus source. CrysalisPro 1.171 [75] was used to determine unit cell characteristics, integrate data, scale, and make absorption adjustments. The structures were solved using direct techniques (ShelxS-2018 [76]). The structures were refined over numerous cycles using full-matrix least-squares on F 2 with the ShelxL-2018 package [76]. The N hydrogen atoms were positioned using the difference Fourier map, whereas all the other hydrogen atoms were placed in idealized places. The non-hydrogen atoms were refined anisotropically, whereas hydrogen atoms were refined using the riding model. ORTEP [77] was used to create visual representations of the molecules in the asymmetric unit, and Mercury [78] was used to depict 3D packing and hydrogen bonding interactions. The Crystallographic Information File’s data (coordinates, structure factors, etc.) were checked by IUCr checkCIF/PLATON [79]. Crystallographic data (excluding structure factors) for the structural investigation of the novel hybrid compound were deposited with the Cambridge Crystallographic Data Centre, CCDC No. Deposition Number 1,957,027.
Further analysis of the data was performed with software packages such as Vesta and Biovia Studio [80,81].
5.2.2. ATR-IR Spectroscopy
Attenuated total reflectance infrared spectroscopy (ATR-IR) measurements were provided at 293 K using the Thermo Scientific Nicolet iS10 FT-IR spectrometer (Madison, WI, USA) equipped with ID5 ATR accessory (diamond crystal). The FT-IR spectra of the tested compounds were recorded at 4 cm−1 resolution and 32 scans over the range 4000–650 cm−1. All the probes were examined in a solid state without any FTIR sample preparation or alteration.
5.2.3. NMR Spectroscopy
The sample was prepared as follows: a stock solution of trimethoxybenzene (TMB) in DMSO-d6 at a concentration of 8.44 mM (7.1 mg per 5 mL) was produced. Weighted quantities of the compounds were dissolved in 0.6 mL stock solution, and 1H spectra were obtained.
The Bruker Avance II + spectrometer (14.09 T magnet) with a 5 mm BBO probe and z-gradient coil was used to record all the NMR spectra. It operated at 600.01 MHz 1H frequencies. A Bruker B-VT 3000 temperature unit with an airflow of 535 L/h is used to keep the temperature at 293 K. Tetramethylsilane (TMS, 0.00 ppm) or the residual solvent signal of 2.5 ppm for DMSO are used as references for all the chemical shifts, which are expressed in parts per million (ppm).
The Bruker pulse sequences cosygpmfqf and hsqcedetgpsp.3 were used to capture two-dimensional (2D) 1H/1H COSY and (2D) 1H/13C HSQC spectra.
5.2.4. Animals
The experimental male IRC mice (18–22 g) were maintained in a controlled environment (20 ± 2 °C, 50 ± 10% relative humidity, 12 h light/dark cycle) housed in plastic cages where free access to food and water was provided. Each of the experimental groups consisted of 8–10 animals. Compliance of the experiments with the guidelines and practice established by the Ethical Committees of the Institute of Neurobiology, Sofia, Bulgaria, and in conformance with the European Convention on Animal Protection and Guidelines on Research Animal Use was strictly followed.
5.2.5. Experimental Protocol of the AD Model
The model of AD was induced in the experimental animals through daily treatment with a single i.p. injection of 1 mg/kg scopolamine for a duration of 11 days [82]. The model was verified by using cognitive and biochemical assays and markers, such as learning and memory behavioral tasks and acetylcholine esterase (AChE) activity in the brain.
5.2.6. Drug Treatment and Experimental Design
The mice were separated into the following groups: control group; SCO group; MEM group; SINA group; and SINA-MEM group. The control group was treated daily with single saline injections (saline 0.1 mL/10 g, i.p). The other groups received daily injections of scopolamine (scopolamine solution 1 mg/kg, i.p) plus the specific substance for each group with doses as follows: SCO group (scopolamine solution 1 mg/kg, i.p); MEM group (memantine 20 mg/kg, i.p) + (scopolamine 1 mg/kg, i.p); SINA group (sinapic acid 10 mg/kg, i.p) + (scopolamine 1 mg/kg, i.p); and SINA-MEM group (SINA-MEM 20 mg/kg, i.p) + (scopolamine 1 mg/kg, i.p). The daily treatment with the respective substances for all the groups continued for 11 days.
The selected doses of the used compounds were chosen on the basis of our previous preliminary studies (data not published) and data from the literature [83,84].
5.3. Behavioral Tests
5.3.1. Step-Through Passive Avoidance Learning Test
The passive avoidance learning test (step-through test) was utilized to evaluate the learning and memory performance in the mice [85]. The utilized apparatus in this test consisted of two compartments: the first compartment was illuminated and the other one was kept dark. The floor of the later compartment was built of stainless steel rods through which it delivered an electrical shock to the animals. These compartments were kept separated by a wall with a guillotine door. During the acquisition phase, every animal was placed in the lightened compartment. Shortly after the animal entered the dark compartment, they received an electrical foot shock (0.5 mA, 1 s). Therefore, during this phase, the rodent learned that the entrance into the dark compartment is related to negative consequences. In this phase, it was recorded the initial latency (IL, acquisition latency time) of the entrance into the dark chamber, and mice with ILs > 60 s were excluded from the study. The test phase was performed 1 h after the acquisition phase and also on the 6th and 10th day, during which each animal was placed in the lightened chamber for evaluation of passive avoidance. The measured parameter (named step-through latency (STL)) was the duration between the placement in the illuminated compartment and the entry into the dark compartment. The behavioral tests were carried out between 9 a.m. to 12 a.m.
After the completion of the planned behavioral tests, the animals were euthanized with CO2 followed by quick removal of their brains and further biochemical analyses.
5.3.2. Hole-Board Test
For the test, we used a special apparatus comprising perforated floorboard which was made of plastic (45 cm × 45 cm × 25 cm), and it was drilled with 16 evenly spaced holes (3 cm in diameter); the board was delimited by a square box having 50 cm in height made of plexiglass [86]. The registered parameter for each animal was the number of head-dips during a 3 min trial.
The testing was performed 1 h and also on the 5th and 10th day after the treatment with scopolamine/saline of the animals.
5.3.3. Forced Swim Test
The test was performed according to the guidance in previous studies with mice [67]. In brief, each mouse was put in an open cylindrical container with a diameter of 10 cm and a height of 25 cm. The container held a 19 cm level (measured from the bottom) of water at 25 ± 1 °C. The mice were given 6 min to swim. When a mouse stopped struggling and stayed still in the water, it was considered immobile; just the motions required to maintain its head above water were considered immobile. During the final 4 min of the test, the length of immobility was noted.
5.3.4. Formalin Pain Test
The test was carried out in accordance with the guidelines from earlier mammal and mouse research [87,88]. A transparent cylinder of 25 cm in diameter and 30 cm in height was used to monitor the mice. One hind paw (at the plantar area) received a subcutaneous injection of 20 microliters of 5% formalin. During the early phase (0–10 min after formalin injection) and late phase (30–40 min after formalin injection), the amount of time the animal spent licking the formalin-injected paw was measured in order to assess the level of discomfort. The animals were treated with the studied compounds either 20 min (on the 10th day) before the formalin injection or 10 min afterward (on the 11th day). The used doses were as follows: SCO group (scopolamine solution 1 mg/kg, i.p); MEM group (memantine 20 mg/kg, i.p) + (scopolamine 1 mg/kg, i.p); SINA group (sinapic acid 10 mg/kg, i.p) + (scopolamine 1 mg/kg, i.p); and SINA-MEM group (SINA-MEM 20 mg/kg, i.p) + (scopolamine 1 mg/kg, i.p).
5.3.5. Determination of Acetylcholinesterase (AChE) Activity
AChE activity was measured in the whole brain as in our previous studies [82]. The enzyme hydrolyses the substrate acetylthiocholine which results in thiocholine formation which product reacts with Ellman’s reagent 5,5-Dithiobis 2-nitrobenzoic acid (DTNB). A total of 10% brain homogenates, prepared in 0.1 M phosphate buffer (pH 8; 1000 g), was centrifuged at 4500 g for 10 min. In a test tube, 2900 µL of 0.1 M phosphate buffer, 100 µL of 0.1 M DTNB, 20 µL of 0.075 M freshly prepared acetylthiocholine iodide, and 100 µL of supernatant were added. A total of 500 µL of the reaction mixture was injected into the Semiauto Chemistry Analyzer and the absorbance was measured at 412 nm every 10 s for 3 min.
5.3.6. Molecular Docking and Screening
For the in silico molecular docking experiments, two software packages AutoDock Tools 4.2.6 and iGemdock 2.1 were used, which use different scoring algorithms for cross-validation purposes according to the protocols by Shlyahtun et al. and Azad et al. [89,90,91,92]. Briefly, with custom scripts, screening of the active sites of the target molecular complexes was performed with a box of 20A. The most prospective conformations were selected and further evaluated at ligand–amino acid residue level by using software tools such as Ligplot 2.2 and protein–ligand interaction profiler [93,94]. The target molecular complexes were acquired from the publicly available protein data banks. Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA) and RStudio 2019 packages were used for the data processing [95]. The ADMET evaluation and analysis of some of the basic molecular, biological activity, and pharmacokinetic properties of the ligands as absorption, distribution, metabolism, excretion, and general and organ-specific toxicity were evaluated and predicted through the available ADMET webtools and services such as admetSAR 2.0 and Admetlab 2018 [96,97].
Author Contributions
Investigation, L.T.; molecular docking, data processing and analysis, and writing—original draft, A.P.; writing—review and editing, R.K.; synthesis and writing, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Bulgarian National Science Fund (BNSF), grant number KP-06-Russia/7-2019.
Institutional Review Board Statement
The animal study protocol was approved by the Commission of Bioethics (CBE) at the Institute of Neurobiology, Bulgarian Academy of Sciences—BAS/CBE/018/2020.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors express their deepest gratitude to Yavor Mitrev (Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev Str. Bl. 9, Sofia 1113, Bulgaria) for his help and assistance with the acquisition and processing of the NMR spectra and data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, J.; Gao, Y.; Gao, Y.; Munsell, B.C.; Shen, D. Detecting anatomical landmarks for fast Alzheimer’s disease diagnosis. IEEE Trans. Med. Imaging 2016, 35, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Jann, M.W.; Shirley, K.L.; Small, G.W. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin. Pharmacokinet. 2002, 41, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Koola, M.M. Galantamine-Memantine combination in the treatment of Alzheimer’s disease and beyond. Psychiatry Res. 2020, 293, 113409. [Google Scholar] [CrossRef]
- Vishwas, S.; Awasthi, A.; Corrie, L.; Singh, S.K.; Gulati, M. Multiple target-based combination therapy of galantamine, me-mantine and lycopene for the possible treatment of Alzheimer’s disease. Med. Hypotheses 2020, 143, 109879. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Feng, J.; Wu, M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 2016, 37, 1039–1047. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Limapichat, W.; Yu, W.Y.; Branigan, E.; Lester, H.A.; Dougherty, D.A. Key binding interactions for memantine in the NMDA receptor. ACS Chem. Neurosci. 2013, 4, 255–260. [Google Scholar] [CrossRef]
- Al_hussaniy, H.A.; Alkhafaje, Z.; Altamimi, Z.S.; Oraibi, A.I.; Abdalhassan, A.H.; Abdulhamza, H.M.; AL-Zobaidy, M.J. Me-mantine and its role in parkinsonism, seizure, depression, migraine headache, and Alzheimer’s disease. Pharmacia 2023, 70, 291–297. [Google Scholar] [CrossRef]
- Garcia-Marin, V.; Blazquez-Llorca, L.; Rodriguez, J.R.; Boluda, S.; Muntane, G.; Ferrer, I.; DeFelipe, J. Diminished perisomat-ic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front. Neuroanat. 2009, 3, 1102. [Google Scholar] [CrossRef]
- Petrache, A.L.; Rajulawalla, A.; Shi, A.; Wetzel, A.; Saito, T.; Saido, T.C.; Harvey, K.; Ali, A.B. Aberrant excitatory–inhibitory synaptic mechanisms in entorhinal cortex microcircuits during the pathogenesis of Alzheimer’s disease. Cereb. Cortex 2019, 29, 1834–1850. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Hameed, H.; Aydin, S.; Başaran, N. Sinapic acid: Is it safe for humans. FABAD J. Pharm. Sci. 2016, 41, 39. [Google Scholar]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidi-osa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Shahid, M.; Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Ansari, M.A.; Ahad, A.; Alkharfy, K.M.; Alaofi, A.L.; Al-Jenoobi, F.I. Sin-apic acid ameliorates acetic acid-induced ulcerative colitis in rats by suppressing inflammation, oxidative stress, and apop-tosis. Molecules 2022, 27, 4139. [Google Scholar] [CrossRef]
- Zou, Y.; Kim, A.R.; Kim, J.E.; Choi, J.S.; Chung, H.Y. Peroxynitrite scavenging activity of sinapic acid (3, 5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea. J. Agric. Food Chem. 2002, 50, 5884–5890. [Google Scholar] [CrossRef]
- Yun, K.J.; Koh, D.J.; Kim, S.H.; Park, S.J.; Ryu, J.H.; Kim, D.G.; Lee, J.-Y.; Lee, K.T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. J. Agric. Food Chem. 2008, 56, 10265–10272. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phe-nols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Kanchana, G.; Shyni, W.J.; Rajadurai, M.; Periasamy, R. Evaluation of antihyperglycemic effect of sinapic acid in normal and streptozotocin-induced diabetes in albino rats. Glob. J. Pharmacol. 2011, 5, 33–39. [Google Scholar]
- Kim, D.H.; Yoon, B.H.; Jung, W.Y.; Kim, J.M.; Park, S.J.; Park, D.H.; Huh, Y.; Park, C.; Cheong, J.H.; Lee, K.-T.; et al. Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology 2010, 59, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxidative Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, L.; Chochkova, M.; Totseva, I.; Seizova, K.; Marinova, E.; Ivanova, G.; Ninova, M.; Najdenski, H.; Milkova, T. Anti-tyrosinase, antioxidant and antimicrobial activities of hydroxycinnamoylamides. Med. Chem. Res. 2013, 22, 4173–4182. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1, 1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Hotta, H.; Nagano, S.; Ueda, M.; Tsujino, Y.; Koyama, J.; Osakai, T. Higher radical scavenging activities of polyphenolic antioxidants can be ascribed to chemical reactions following their oxidation. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2002, 1572, 123–132. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Interactions between nitric oxide, oxygen, reactive oxygen species and reactive nitrogen species. Biochem. Soc. Trans. 2006, 34 Pt 5, 953–956. [Google Scholar] [CrossRef]
- Torreilles, F.; Salman-Tabcheh, S.; Guérin, M.C.; Torreilles, J. Neurodegenerative disorders: The role of peroxynitrite. Brain Res. Rev. 1999, 30, 153–163. [Google Scholar] [CrossRef]
- Klotz, L.O.; Schroeder, P.; Sies, H. Peroxynitrite signaling: Receptor tyrosine kinases and activation of stress-responsive pathways. Free Radic. Biol. Med. 2002, 33, 737–743. [Google Scholar] [CrossRef]
- Lee, I.S.; Choi, G.Y.; Sreelatha, I.; Yoon, J.W.; Youn, S.H.; Maeng, S.; Park, J.H. Effect of sinapic acid on scopolamine-induced learning and memory impairment in SD rats. Brain Sci. 2023, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Singh, D.; Kh, R. Sinapic acid alleviates oxidative stress and neuro-inflammatory changes in sporadic model of Alzheimer’s disease in rats. Brain Sci. 2020, 10, 923. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jung, J.W.; Lee, J.-J.; Cho, Y.-W.; Jang, C.-G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef]
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1–42 protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266. [Google Scholar] [CrossRef]
- Poyraz, F.S.; Akbaş, G.; Duranoğlu, D.; Acar, S.; Mansuroğlu, B.; Ersöz, M. Sinapic-Acid-Loaded Nanoparticles Optimized via Experimental Design Methods: Cytotoxic, Antiapoptotoic, Antiproliferative, and Antioxidant Activity. ACS Omega 2024, 9, 40329–40345. [Google Scholar] [CrossRef]
- Xia, P.; Chen, H.S.V.; Zhang, D.; Lipton, S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef]
- Song, X.; Jensen, M.Ø.; Jogini, V.; Stein, R.A.; Lee, C.-H.; Mchaourab, H.S.; Shaw, D.E.; Gouaux, E. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature 2018, 556, 515–519. [Google Scholar] [CrossRef]
- Tang, B.C.; Wang, Y.T.; Ren, J. Basic information about memantine and its treatment of Alzheimer’s disease and other clini-cal applications. Ibrain 2023, 9, 340–348. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Krasny, M. Virtual therapy with the NMDA antagonist memantine in hippocampal models of moderate to severe Alzheimer’s disease, in silico trials. Pharmaceuticals 2022, 15, 546. [Google Scholar] [CrossRef]
- Świetlik, D.; Kusiak, A.; Ossowska, A. Computational modeling of therapy with the NMDA antagonist in neurodegenera-tive disease: Information theory in the mechanism of action of Memantine. Int. J. Environ. Res. Public Health 2022, 19, 4727. [Google Scholar] [CrossRef]
- Ghasemi, M.; Amini-Khoei, H.; Bijad, E.; Rafieian-Kopaei, M.; Sureda, A.; Lorigooini, Z. Sinapinic acid as a potential thera-peutic agent for epilepsy through targeting NMDA receptors and nitrite level. Sci. Rep. 2024, 14, 24941. [Google Scholar] [CrossRef] [PubMed]
- Marotta, G.; Basagni, F.; Rosini, M.; Minarini, A. Memantine derivatives as multitarget agents in Alzheimer’s disease. Molecules 2020, 25, 4005. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Liu, R.; Huang, Y.; Zhang, N.; Zhang, R. Memantine, donepezil, or combination therapy—What is the best therapy for Alzheimer’s disease? A network meta-analysis. Brain Behav. 2020, 10, e01831. [Google Scholar] [CrossRef] [PubMed]
- Veysanoglu, S.; Ertas, B.; Guler, E.; Topal, F.; Ozcan, G.S.; Duruksu, G.; Ece, B.; Cam, C.S.; Aydemir, O.; Cam, M.E. In vitro and in vivo evaluation of multi-target-directed Rivastigmine/Memantine/Gingko biloba-loaded nanofibers against Alz-heimer’s disease. J. Drug Deliv. Sci. Technol. 2023, 86, 104691. [Google Scholar] [CrossRef]
- Balazs, N.; Bereczki, D.; Kovács, T. Cholinesterase inhibitors and memantine for the treatment of Alzheimer and non-Alzheimer dementias. Clin. Neurosci./Ideggyogy. Szle. 2021, 74, 379–387. [Google Scholar] [CrossRef]
- Yunusa, I.; Alsahali, S.; Rane, A.; Eguale, T. Comparative value of cholinesterase inhibitors and memantine in persons with moderate-to-severe Alzheimer’s disease in the United States: A cost-effectiveness analysis. J. Alzheimer’s Dis. Rep. 2021, 5, 705–713. [Google Scholar] [CrossRef]
- Kaniakova, M.; Nepovimova, E.; Kleteckova, L.; Skrenkova, K.; Holubova, K.; Chrienova, Z.; Hepnarova, V.; Kucera, T.; Kobrlova, T.; Vales, K.; et al. Combination of memantine and 6-chlorotacrine as novel multi-target compound against Alz-heimer’s disease. Curr. Alzheimer Res. 2019, 16, 821–833. [Google Scholar] [CrossRef]
- Spilovska, K.; Korabecny, J.; Horova, A.; Musilek, K.; Nepovimova, E.; Drtinova, L.; Gazova, Z.; Siposova, K.; Dolezal, R.; Jun, D.; et al. Design, synthesis and in vitro testing of 7-methoxytacrine-amantadine analogues: A novel cholinesterase in-hibitors for the treatment of Alzheimer’s disease. Med. Chem. Res. 2015, 24, 2645–2655. [Google Scholar] [CrossRef]
- Jevtić, I.I.; Suručić, R.V.; Tovilović-Kovačević, G.; Zogović, N.; Kostić-Rajačić, S.V.; Andrić, D.B.; Penjišević, J.Z. Multi-target potential of newly designed tacrine-derived cholinesterase inhibitors: Synthesis, computational and pharmacological study. Bioorganic Med. Chem. 2024, 101, 117649. [Google Scholar] [CrossRef]
- Naki, T.; Matshe, W.M.R.; Balogun, M.O.; Sinha Ray, S.; Egieyeh, S.A.; Aderibigbe, B.A. Polymer drug conjugates containing memantine, tacrine and cinnamic acid: Promising nanotherapeutics for the treatment of Alzheimer’s disease. J. Microencap-Sul. 2023, 40, 15–28. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Chochkova, M.; Jiang, H.; Kyoseva, R.; Stoykova, B.; Tsvetanova, E.; Alexandrova, A.; Liu, R.; Li, Z.; Mitrev, Y.; Dimi-trova-Sbirkova, H.; et al. Cinnamoyl-memantine hybrids: Synthesis, X-ray crystallography and biological activities. J. Mol. Struct. 2021, 1234, 130147. [Google Scholar] [CrossRef]
- Chochkova, M.; Georgieva, A.; Ilieva, T.; Andreeva, M.; Pramatarov, G.; Petek, N.; Petrova, P.; Štícha, M.; Mitrev, Y.; Svete, J. Hybridization of Aminoadamantanes with cinnamic acid analogues and elucidation of their antioxidant profile. J. Chem. 2022, 2022, 7582587. [Google Scholar] [CrossRef]
- Spasova, M.; Kortenska-Kancheva, V.; Totseva, I.; Ivanova, G.; Georgiev, L.; Milkova, T. Synthesis of cinnamoyl and hy-droxycinnamoyl amino acid conjugates and evaluation of their antioxidant activity. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2006, 12, 369–375. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: London, UK, 2001. [Google Scholar]
- Heacock, R.A.; Marion, L. The infrared spectra of secondary amines and their salts. Can. J. Chem. 1956, 34, 1782–1795. [Google Scholar] [CrossRef]
- Nakanishi, K. Infrared Absorption Spectroscopy: Practical; Holden-Day: San Francisco, CA, USA, 1962. [Google Scholar]
- Deb, P.K.; Sharma, A.; Piplani, P.; Akkinepally, R.R. Molecular docking and receptor-specific 3D-QSAR studies of acetylcho-linesterase inhibitors. Mol. Divers. 2012, 16, 803–823. [Google Scholar] [CrossRef]
- Atanasova, M.; Yordanov, N.; Dimitrov, I.; Berkov, S.; Doytchinova, I. Molecular docking study on galantamine derivatives as cholinesterase inhibitors. Mol. Inform. 2015, 34, 394–403. [Google Scholar] [CrossRef]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: How is structure related to function? Chem.-Biol. Interact. 2008, 175, 3–10. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Laverty, D.; Thomas, P.; Field, M.; Andersen, O.J.; Gold, M.G.; Biggin, P.C.; Gielen, M.; Smart, T.G. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol. 2017, 24, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Bais, S.; Kumari, R.; Prashar, Y. Therapeutic effect of Sinapic acid in aluminium chloride induced dementia of Alzheimer’s type in rats. J. Acute Dis. 2017, 6, 154–162. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Minkeviciene, R.; Banerjee, P.; Tanila, H. Cognition-enhancing and anxiolytic effects of memantine. Neuropharmacology 2008, 54, 1079–1085. [Google Scholar] [CrossRef]
- Ghasemi, M.; Raza, M.; Dehpour, A.R. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J. Psychopharmacol. 2010, 24, 585–594. [Google Scholar] [CrossRef]
- Réus, G.Z.; Stringari, R.B.; Kirsch, T.R.; Fries, G.R.; Kapczinski, F.; Roesler, R.; Quevedo, J. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: Further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res. Bull. 2010, 81, 585–589. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA Receptors in the Central Nervous System. In NMDA Receptors. Methods in Molecular Biology; Burnashev, N., Szepetowski, P., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1677. [Google Scholar]
- Coderre, T.J.; Melzack, R. The role of NMDA receptor-operated calcium channels in persistent nociception after forma-lin-induced tissue injury. J. Neurosci. 1992, 12, 3671–3675. [Google Scholar] [CrossRef]
- Vaccarino, A.L.; Marek, P.; Kest, B.; Weber, E.; Keana, J.F.; Liebeskind, J.C. NMDA receptor antagonists, MK-801 and ACEA-1011, prevent the development of tonic pain following subcutaneous formalin. Brain Res. 1993, 615, 331–334. [Google Scholar] [CrossRef]
- Karakida, F.; Ikeya, Y.; Tsunakawa, M.; Yamaguchi, T.; Ikarashi, Y.; Takeda, S.; Aburada, M. Cerebral protective and cogni-tion-improving effects of sinapic acid in rodents. Biol. Pharm. Bull. 2007, 30, 514–519. [Google Scholar] [CrossRef]
- Abdel-Aal, R.A.; Assi, A.A.A.; Kostandy, B.B. Memantine prevents aluminum-induced cognitive deficit in rats. Behav. Brain Res. 2011, 225, 31–38. [Google Scholar] [CrossRef]
- Chou, T.H.; Epstein, M.; Michalski, K.; Fine, E.; Biggin, P.C.; Furukawa, H. Structural insights into binding of therapeutic channel blockers in NMDA receptors. Nat. Struct. Mol. Biol. 2022, 29, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. P. CrysAlis; Version 171.36.20; Agilent Technologies UK Ltd.: Yarnton, UK, 2011. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. Appl. Crys-tallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Biovia, D.S. Biovia Studio; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Tancheva, L.P.; Popatanasov, A.B.; Dragomanova, S.T.; Tzvetanova, E.R.; Aleksandrova, S.M.; Alova, L.G.; Stefanova, M.; Kalfin, R.E. New mechanisms in preventive effect of ellagic acid on cognition in mice with Alzheimer’s disease type de-mentia. Bulg. Chem. Commun. 2018, 50, 20–24. [Google Scholar]
- Li, F.; Chen, X.; Wang, F.; Xu, S.; Chang, L.; Anwyl, R.; Wang, Q. Chronic pre-treatment with memantine prevents amy-loid-beta protein-mediated long-term potentiation disruption. Neural Regen. Res. 2013, 8, 49–55. [Google Scholar]
- de Moura, F.C.; Godinho, W.D.; Vieira, L.L.; da Silva, C.; Martins, P.M.S.; de Castro Brito, G.A. Current Research on Therapy in Alzheimer’s Disease Experimental Model: Beta-Amyloid1-42 Induction. J. Adv. Res. Pharm. Biol. Sci. 2015, 2208, 2360. [Google Scholar] [CrossRef]
- Jarvik, M.E.; Kopp, R. An improved one-trial passive avoidance learning situation. Psychol. Rep. 1967, 21, 221–224. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; Martínez, A.L.; González-Trujano, M.E.; Moreno, J.; Vibrans, H.; Soto-Hernández, M. Pharmacolog-ical evaluation of the anxiolytic and sedative effects of Tilia americana L. var. mexicana in mice. J. Ethnopharmacol. 2007, 109, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Hsu, K.C.; Chen, Y.F.; Lin, S.R.; Yang, J.M. iGEMDOCK: A graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinform. 2011, 12, 1–11. [Google Scholar] [CrossRef]
- Shlyahtun, A.G.; Raduta, E.F.; Sutko, I.P.; Kasper, E.V.; Bogdevich, E.V.; Sevko, A.A. Screening of molecular targets that de-termine the hypoglycemic effect of lupane triterpenoids. In Proceedings of the International Scientific Conference “Physi-cochemical Biology as the Basis of Modern Medicine”, Minsk, Belarus, 21 May 2021; Khrustalev, V.V., Taganovich, A.D., Khrustaleva, T.A., Eds.; BGMU Press: Minsk, Belarus, 2021; pp. 362–363. [Google Scholar]
- Azad, I.; Khan, T.; Maurya, A.K.; Irfan Azad, M.; Mishra, N.; Alanazi, A.M. Identification of severe acute respiratory syn-drome coronavirus-2 inhibitors through in silico structure-based virtual screening and molecular interaction studies. J. Mol. Recognit. 2021, 34, e2918. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019. [Google Scholar]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.-N.; Yao, Z.-J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.-P.; Cao, D.-S. ADMETlab: A platform for system-atic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).