Determination of the 207Pb Chemical Shift Tensor in Crocoite, PbCrO4, Using Single-Crystal NMR Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
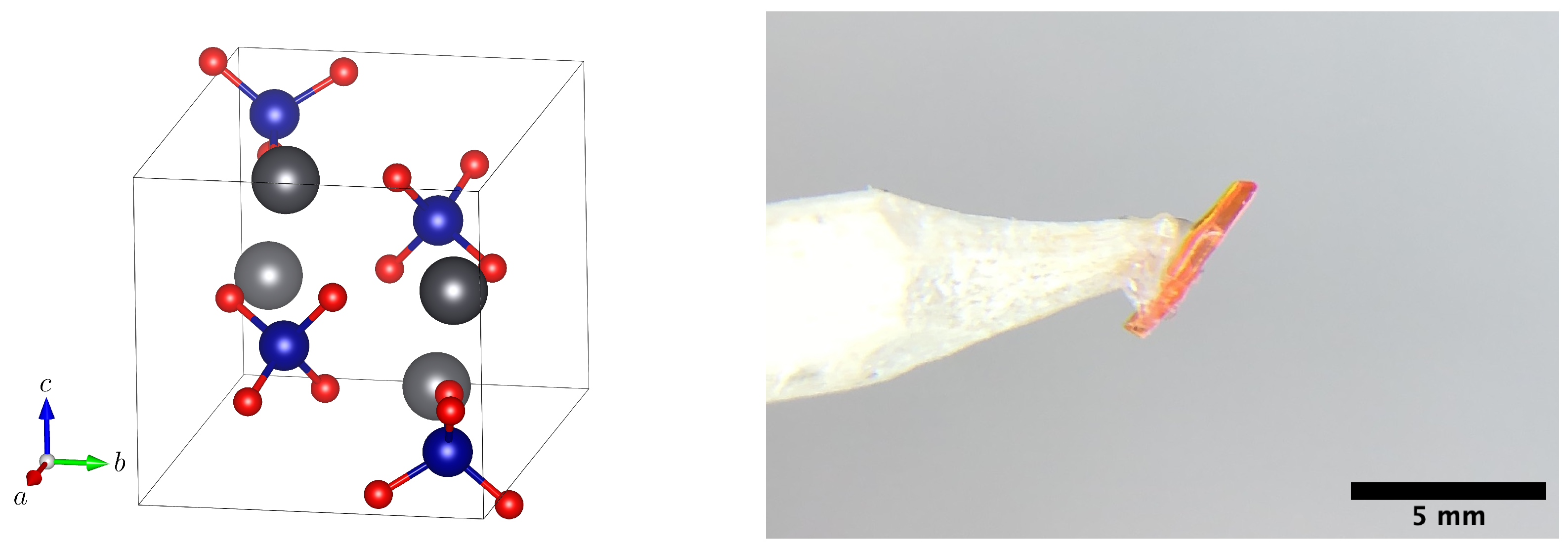
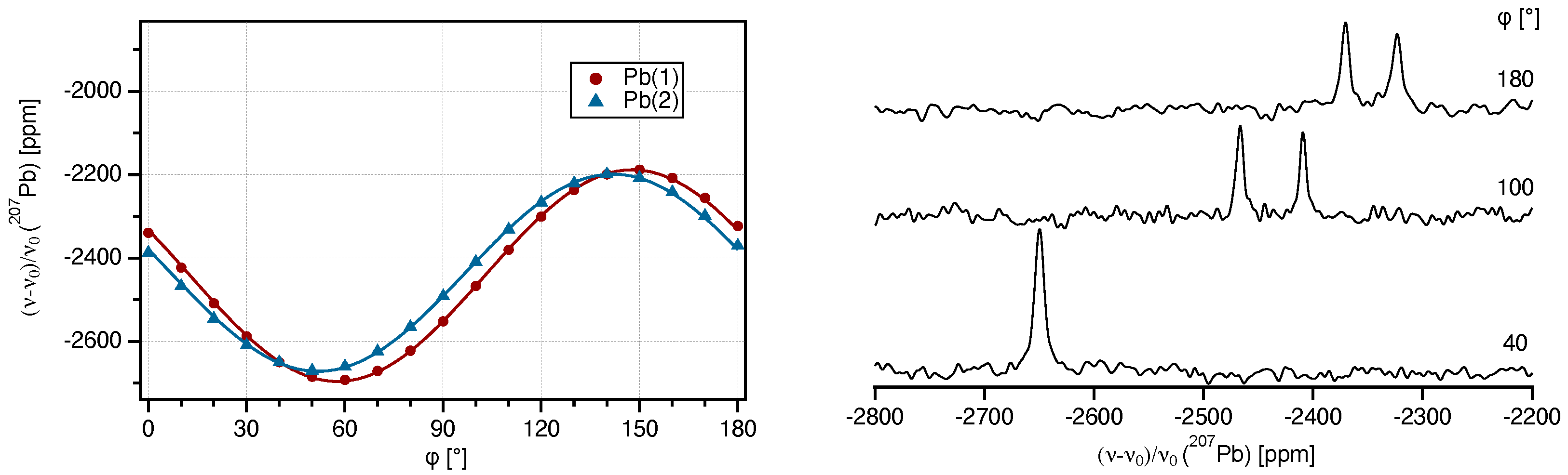
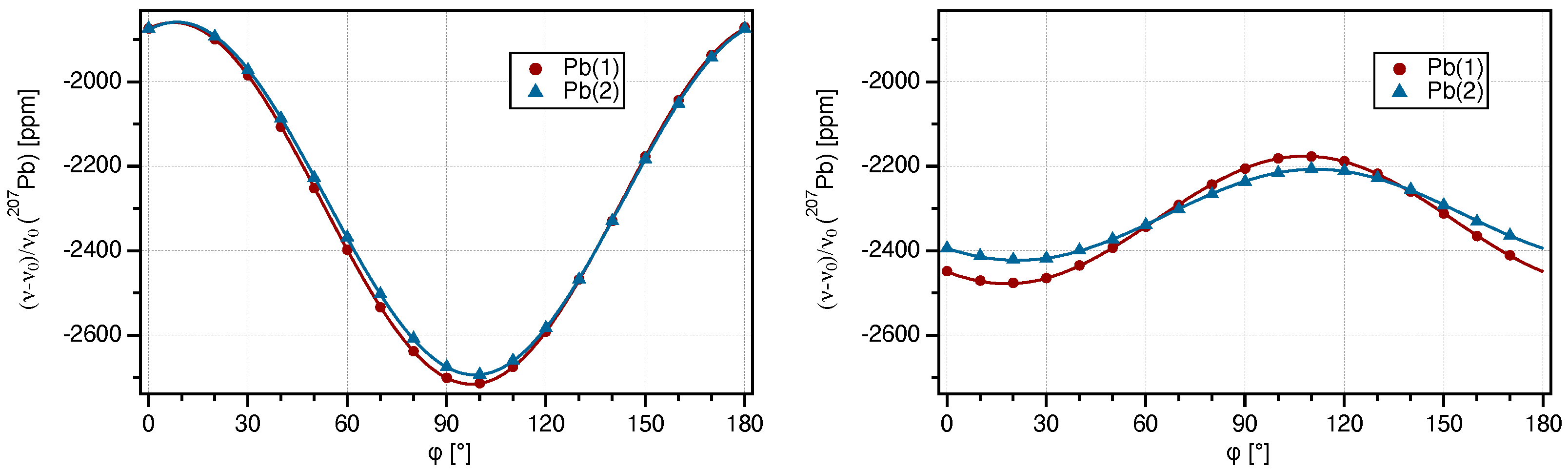
2.1. Single-Crystal 207Pb-NMR of Crocoite
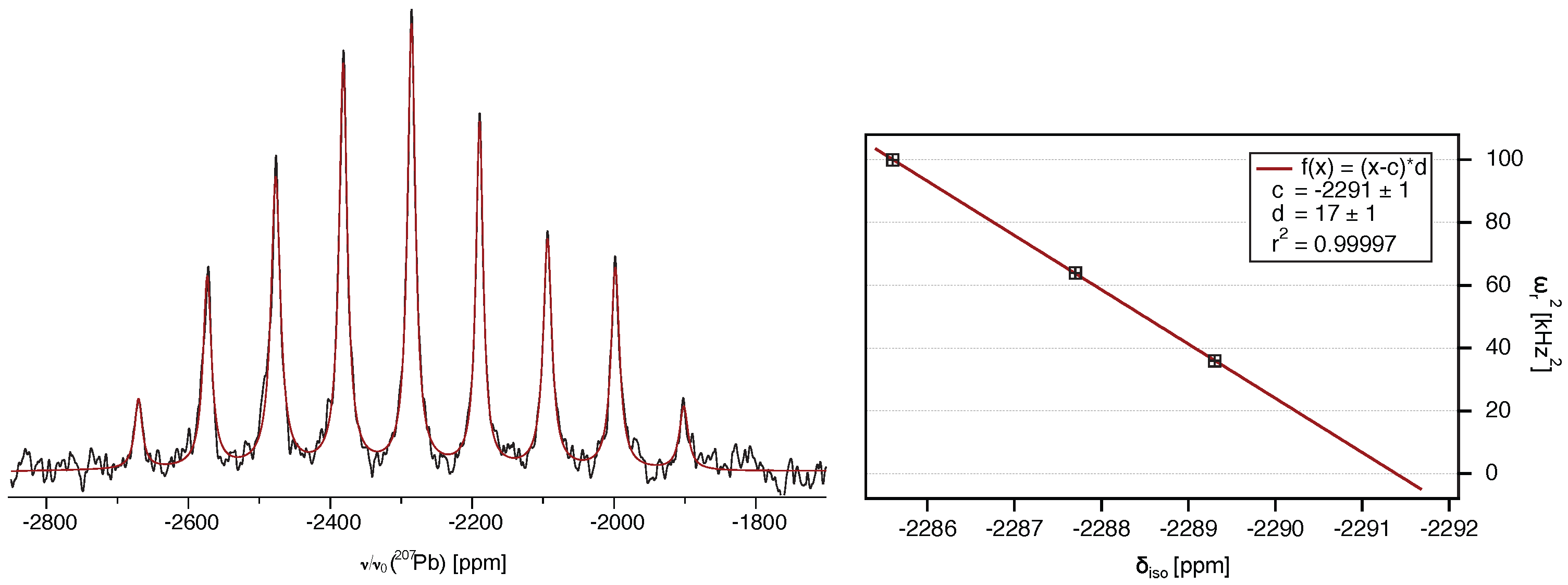
2.2. 207Pb-NMR of Crocoite Under Magic-Angle Spinning (MAS)
2.3. Anisotropy of the 207Pb Chemical Shift Tensor in Pb(II) Compounds
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State NMR of Inorganic Materials; Pergamon Press: Oxford, UK, 2002. [Google Scholar]
- Harris, R.K. NMR crystallography: The use of chemical shifts. Solid State Sci. 2004, 6, 1025–1037. [Google Scholar] [CrossRef]
- Engelhardt, G.; Radeglia, R. A semi-empirical quantum-chemical rationalization of the correlation between SiOSi angles and 29Si NMR chemical shifts of silica polymorphs and framework aluminosilicates (zeolites). Chem. Phys. Lett. 1984, 108, 271–274. [Google Scholar] [CrossRef]
- Dann, S.E.; Weller, M.T. Correlations between 9Be magic-angle spinning nuclear magnetic resonance spectra and the geometry of beryllium containing framework structures. Solid State Nucl. Magn. Reson. 1997, 10, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.V.; Blackwell, C.S. Nuclear magnetic resonance of silica polymorphs. Nature 1983, 303, 223–225. [Google Scholar] [CrossRef]
- Grimmer, A.-R.; Radeglia, R. Correlation between the isotropic 29Si chemical shifts and the mean silicon-oxygen bond lengths in silicates. Chem. Phys. Lett. 1984, 106, 262–265. [Google Scholar] [CrossRef]
- Stebbins, J.F. Cation sites in mixed-alkali oxide glasses: Correlations of NMR chemical shift data with site size and bond distance. Solid State Ionics 1998, 112, 137–141. [Google Scholar] [CrossRef]
- Padro, D.; Howes, A.P.; Smith, M.E.; Dupree, R. Determination of titanium NMR parameters of ATiO3 compounds: Correlations with structural distortion. Solid State Nucl. Magn. Reson. 2000, 15, 231–236. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Steinadler, J.; Hochleitner, R.; Bräuniger, T. Determination of the full 207Pb chemical shift tensor of anglesite, PbSO4, and correlation of the isotropic shift to lead-oxygen distance in natural minerals. Crystals 2019, 9, 43. [Google Scholar] [CrossRef]
- Steinadler, J.; Zeman, O.E.O.; Bräuniger, T. Correlation of the isotropic NMR chemical shift with oxygen coordination distances in periodic solids. Oxygen 2022, 2, 327–336. [Google Scholar] [CrossRef]
- Steinadler, J.; Krach, G.; Schnick, W.; Bräuniger, T. Investigation of the binary nitrides YN, LaN and LuN by solid-state NMR spectroscopy. Molecules 2024, 29, 5572. [Google Scholar] [CrossRef]
- Andrew, E.R. Magic angle spinning in solid state n.m.r. spectroscopy. Phil. Trans. R. Soc. A 1981, 299, 505–520. [Google Scholar]
- Van Bramer, S.E.; Glatfelter, A.; Bai, S.; Dybowski, C.; Neue, G. Data acquisition and analysis of broad chemical-shift powder patterns from solids with spin-echo techniques. Concepts Magn. Reson. 2002, 14, 365–387. [Google Scholar] [CrossRef]
- Herzfeld, J.; Berger, A.E. Sideband intensities in NMR spectra of samples spinning at the magic angle. J. Chem. Phys. 1980, 73, 6021–6030. [Google Scholar] [CrossRef]
- Hodgkinson, P.; Emsley, L. The reliability of the determination of tensor parameters by solid-state nuclear magnetic resonance. J. Chem. Phys. 1997, 107, 4808–4816. [Google Scholar] [CrossRef]
- Purcell, E.M.; Bloembergen, N.; Pound, R.V. Resonance absorption by nuclear magnetic moments in a single crystal of CaF2. Phys. Rev. 1946, 70, 988. [Google Scholar] [CrossRef]
- Volkoff, G.M.; Petch, H.E.; Smellie, D.W.L. Nuclear electric quadrupole interactions in single crystals. Can. J. Phys. 1952, 30, 270–289. [Google Scholar] [CrossRef]
- Vosegaard, T. Single-crystal NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 123, 51–72. [Google Scholar] [CrossRef]
- Bräuniger, T. High-Precision Determination of NMR Interaction Parameters by Measurement of Single Crystals: A Review of Classical and Advanced Methods. Molecules 2024, 29, 4148. [Google Scholar] [CrossRef]
- Bielecki, A.; Burum, D.P. Temperature dependence of 207Pb MAS spectra of solid lead nitrate. An accurate, sensitive thermometer for variable-temperature MAS. J. Magn. Reson. A 1995, 116, 215–220. [Google Scholar] [CrossRef]
- van Gorkom, L.C.M.; Hook, J.M.; Logan, M.B.; Hanna, J.V.; Wasylishen, R.E. Solid-state lead-207 NMR of lead(II) nitrate: Localized heating effects at high magic angle spinning speeds. Magn. Reson. Chem. 1995, 33, 791–795. [Google Scholar] [CrossRef]
- Milder, T.; Ernst, H.; Freude, D. 207Pb NMR detection of spinning-induced temperature gradients in MAS rotors. Solid State Nucl. Magn. Reson. 1995, 5, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, O.; Bai, S.; Beckmann, P.A.; van Bramer, S.; Vega, A.J.; Dybowski, C. The relationship between 207Pb NMR chemical shift and solid-state structure in Pb(II) compounds. J. Phys. Chem. A 2008, 112, 3046–3052. [Google Scholar] [CrossRef]
- Alkan, F.; Dybowski, C. Chemical-shift tensors of heavy nuclei in network solids: A DFT/ZORA investigation of 207Pb chemical shift tensors using the bond-valence method. Phys. Chem. Chem. Phys. 2015, 17, 25014–25026. [Google Scholar] [CrossRef]
- Alkan, F.; Dybowski, C. Effect of co-ordination chemistry and oxidation state on the 207Pb magnetic shielding tensor: A DFT/ZORA investigation. J. Phys. Chem. A 2016, 120, 161–168. [Google Scholar] [CrossRef]
- Krivdin, L.B. Computational NMR of heavy nuclei involving 109Ag, 113Cd, 119Sn, 125Te, 195Pt, 199Hg, 205Tl, and 207Pb. Russ. Chem. Rev. 2021, 90, 1166–1212. [Google Scholar] [CrossRef]
- Nolle, A. 207Pb magnetic shielding anisotropy in Pb(NO3)2, PbCO3, PbCrO4, PbMoO4 and PbWO4 by Fourier transform NMR. Z. Naturforsch. 1977, 32, 964–967. [Google Scholar] [CrossRef]
- Neue, G.; Dybowski, C.; Smith, M.L.; Hepp, M.A.; Perry, D.L. Determination of 207Pb2+ chemical shift tensors from precise powder lineshape analysis. Solid State Nucl. Magn. Reson. 1996, 6, 241–250. [Google Scholar] [CrossRef]
- Siegel, R.; Nakashima, T.T.; Wasylishen, R.E. Application of multiple-pulse experiments to characterize broad NMR chemical-shift powder patterns from spin-1/2 nuclei in the solid state. J. Chem. Phys. B 2004, 108, 2218–2226. [Google Scholar] [CrossRef]
- Van Bramer, S.E.; Glatfelter, A.; Bai, S.; Dybowski, C.; Neue, G.; Perry, D.L. Solid-state 207Pb NMR studies of lead-group 16 and mixed transition-metal/lead-group 16 element-containing materials. Magn. Reson. Chem. 2006, 44, 357–365. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Hoch, C.; Hochleitner, R.; Bräuniger, T. NMR interaction tensors of 51V and 207Pb in vanadinite, Pb5(VO4)3Cl, determined from DFT calculations and single-crystal NMR measurements, using only one general rotation axis. Solid State Nucl. Magn. Reson. 2018, 89, 11–20. [Google Scholar] [CrossRef]
- Zeman, O.E.O. Single-Cystal NMR Spectroscopy—Method Development and Application to Inorganic Solids. Ph.D. Thesis, LMU Munich, Munich, Germany, 2020. Available online: https://edoc.ub.uni-muenchen.de/26813/ (accessed on 13 May 2025).
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Effenberger, H.; Pertlik, F. Four monazite type structures: Comparison of SrCrO4, SrSeO4, PbCrO4 (crocoite), and PbSeO4. Z. Kristallogr. Cryst. Mater. 1986, 176, 75–83. [Google Scholar] [CrossRef]
- Buckingham, A.D.; Malm, S.M. Asymmetry in the nuclear magnetic shielding tensor. Mol. Phys. 1971, 22, 1127–1130. [Google Scholar] [CrossRef]
- Anet, F.A.L.; O’Leary, D.J. The shielding tensor. Part I: Understanding its symmetry properties. Concepts Magn. Reson. 1991, 3, 193–214. [Google Scholar] [CrossRef]
- Eichele, K.; Wasylishen, R.E. 31P NMR study of powder and single-crystal samples of ammonium dihydrogen phosphate: Effect of homonuclear dipolar coupling. J. Phys. Chem. 1994, 98, 3108–3113. [Google Scholar] [CrossRef]
- Weil, J.A.; Anderson, J.H. Determination of the g Tensor in Paramagnetic Resonance. J. Chem. Phys. 1958, 28, 864–866. [Google Scholar] [CrossRef]
- Weil, J.A. Use of symmetry-related crystal sites for measuring tensor properties in magnetic resonance. J. Magn. Reson. 1973, 10, 391–393. [Google Scholar] [CrossRef]
- Eichele, K. HBA 1.7.5; University of Tübingen: Tübingen, Germany, 2015. [Google Scholar]
- Zhao, P.; Prasad, S.; Huang, J.; Fitzgerald, J.J.; Shore, J.S. Lead-207 NMR spectroscopic study of lead-based electronic materials and related lead oxides. J. Chem. Phys. B 1999, 103, 10617–10626. [Google Scholar] [CrossRef]
- Greer, G.J.; Michaelis, V.K.; Katz, M.J.; Leznoff, D.B.; Schreckenbach, G.; Kroeker, S. Characterising lone-pair activity of lead(II) by 207Pb solid-state NMR spectroscopy: Coordination polymers of [N(CN)2]− and [Au(CN)2]− with terpyridine ancillary ligands. Chem. Eur. J. 2011, 17, 3609–3618. [Google Scholar] [CrossRef]
- Lutz, O.; Nolle, A. Nuclear magnetic shielding tensors of 207Pb2+ in Pb(NO3)2. Z. Phys. B 1980, 36, 323–328. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Moudrakovski, I.L.; Hoch, C.; Hochleitner, R.; Schmahl, W.W.; Karaghiosoff, K.; Bräuniger, T. Determination of the 31P and 207Pb Chemical Shift Tensors in Pyromorphite, Pb5(PO4)3Cl, by Single-Crystal NMR Measurements and DFT Calculations. Z. Anorg. Allg. Chem. 2017, 643, 1635–1641. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Steinadler, J.; Hochleitner, R.; Bräuniger, T. Characterisation of contact twinning for cerussite, PbCO3, by single-crystal NMR spectroscopy. Phys. Chem. Mineral. 2021, 48, 40. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Steinadler, J.; Hochleitner, R.; Bräuniger, T. Single-crystal 207Pb-NMR of wulfenite, PbMoO4, aided by simultaneous measurement of phosgenite, Pb2Cl2CO3. Solid State Nucl. Magn. Reson. 2019, 103, 17–24. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Hochleitner, R.; Schmahl, W.W.; Karaghiosoff, K.; Bräuniger, T. Relationship between 207Pb NMR chemical shift and the morphology and crystal structure for the apatites Pb5(AO4)3Cl, vanadinite (A=V), pyromorphite (A=P) and mimetite (A = As). Am. Mineral. 2021, 106, 541–548. [Google Scholar] [CrossRef]
- Kunwar, A.C.; Turner, G.L.; Oldfield, E. Solid-state spin-echo Fourier transform NMR of 39K and 67Zn salts at high field. J. Magn. Reson. 1986, 69, 124–127. [Google Scholar] [CrossRef]
| Sample and Method | Ref. | ||||
|---|---|---|---|---|---|
| (in ppm) | |||||
| powder, static | [27] | ||||
| powder, static | [28] | ||||
| powder, static | [29] | ||||
| powder, static | [30] | ||||
| powder, MAS | this work | ||||
| single crystal, static | this work | ||||
| Compound (Mineral) | Wyckoff Position | Ref. | ||
|---|---|---|---|---|
| (in ppm) | ||||
| PbSO4 | 577 | [9] | ||
| (anglesite) | ||||
| Pb(NO3)2 | 53 | a | [43] | |
| b | [28] | |||
| Pb5(PO4)3Cl | 180 | [44] | ||
| (pyromorphite) | 1149 | |||
| PbCO3 | 756 | [45] | ||
| (cerussite) | ||||
| PbCrO4 | 890 | this work | ||
| (crocoite) | ||||
| PbMoO4 | 176 | [46] | ||
| (wulfenite) | ||||
| Pb2Cl2CO3 | 1252 | [46] | ||
| (phosgenite) | ||||
| Pb5(VO4)3Cl | 1200 | [31] | ||
| (vanadinite) | 1800 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kläger, S.; Zeman, O.E.O.; Bräuniger, T. Determination of the 207Pb Chemical Shift Tensor in Crocoite, PbCrO4, Using Single-Crystal NMR Spectroscopy. Crystals 2025, 15, 480. https://doi.org/10.3390/cryst15050480
Kläger S, Zeman OEO, Bräuniger T. Determination of the 207Pb Chemical Shift Tensor in Crocoite, PbCrO4, Using Single-Crystal NMR Spectroscopy. Crystals. 2025; 15(5):480. https://doi.org/10.3390/cryst15050480
Chicago/Turabian StyleKläger, Sebastian, Otto E. O. Zeman, and Thomas Bräuniger. 2025. "Determination of the 207Pb Chemical Shift Tensor in Crocoite, PbCrO4, Using Single-Crystal NMR Spectroscopy" Crystals 15, no. 5: 480. https://doi.org/10.3390/cryst15050480
APA StyleKläger, S., Zeman, O. E. O., & Bräuniger, T. (2025). Determination of the 207Pb Chemical Shift Tensor in Crocoite, PbCrO4, Using Single-Crystal NMR Spectroscopy. Crystals, 15(5), 480. https://doi.org/10.3390/cryst15050480






