From Type II to Z-Scheme: A DFT Study of Enhanced Water Splitting in the SGa2Se/TeMoS Heterojunction
Abstract
1. Introduction
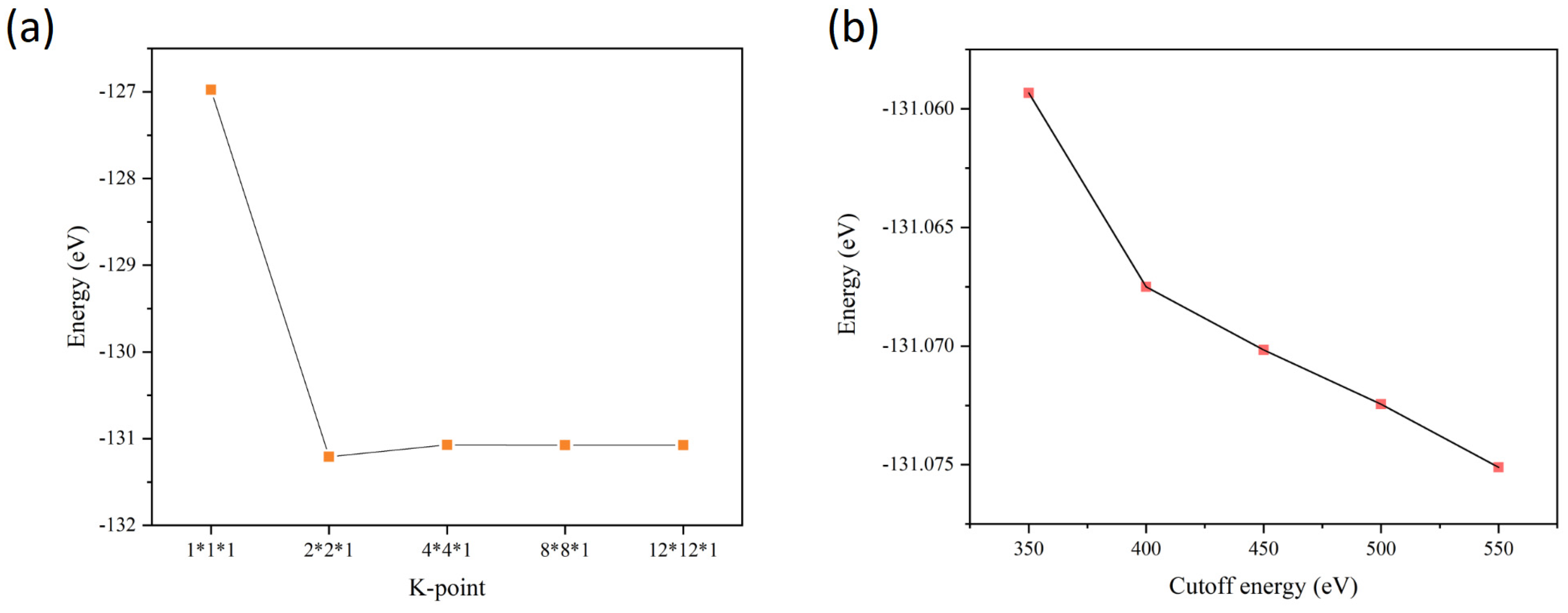
2. Computational Methods
3. Results and Discussion
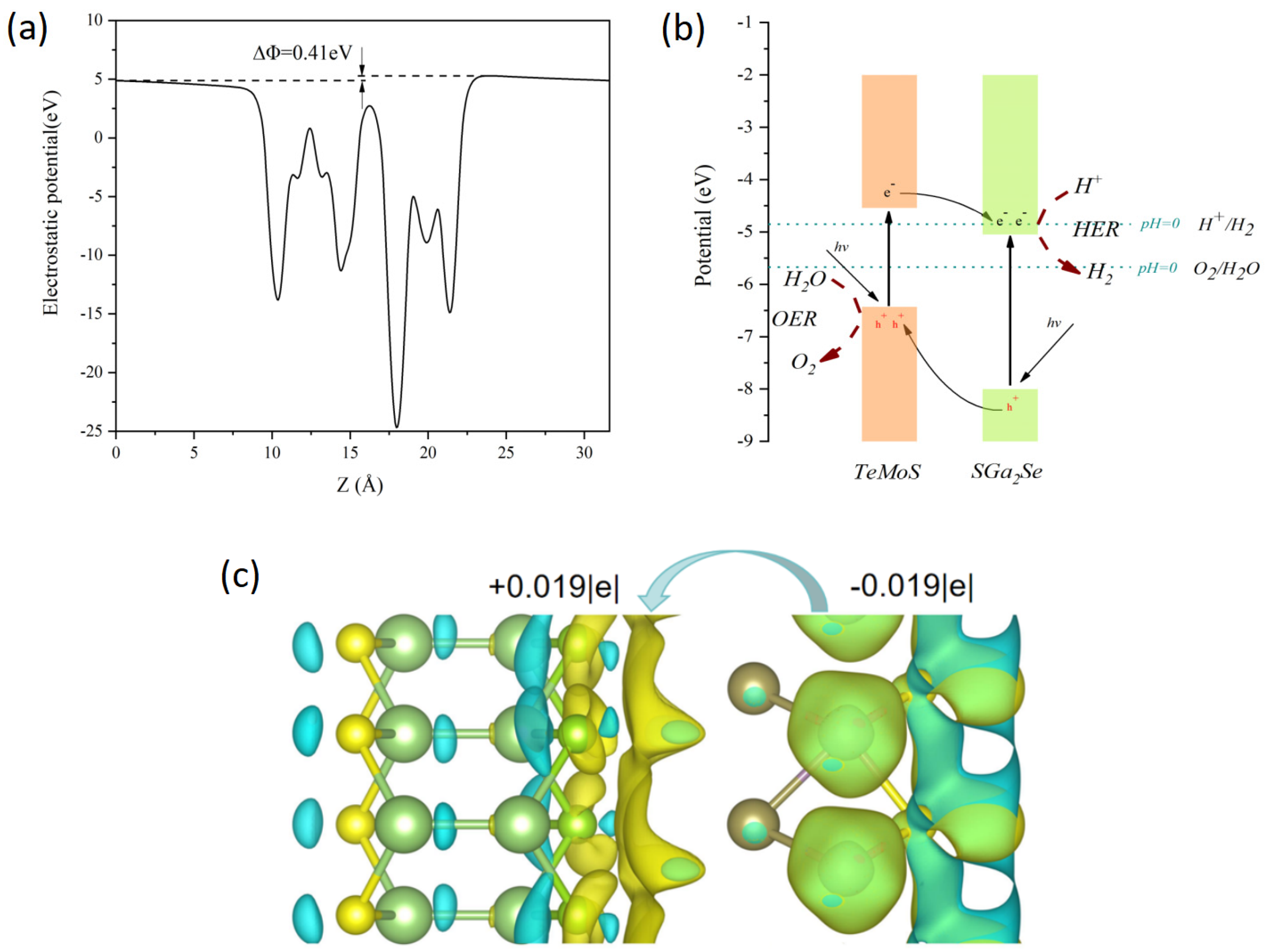
3.1. Geometric and Electronic Structures of the Heterojunction
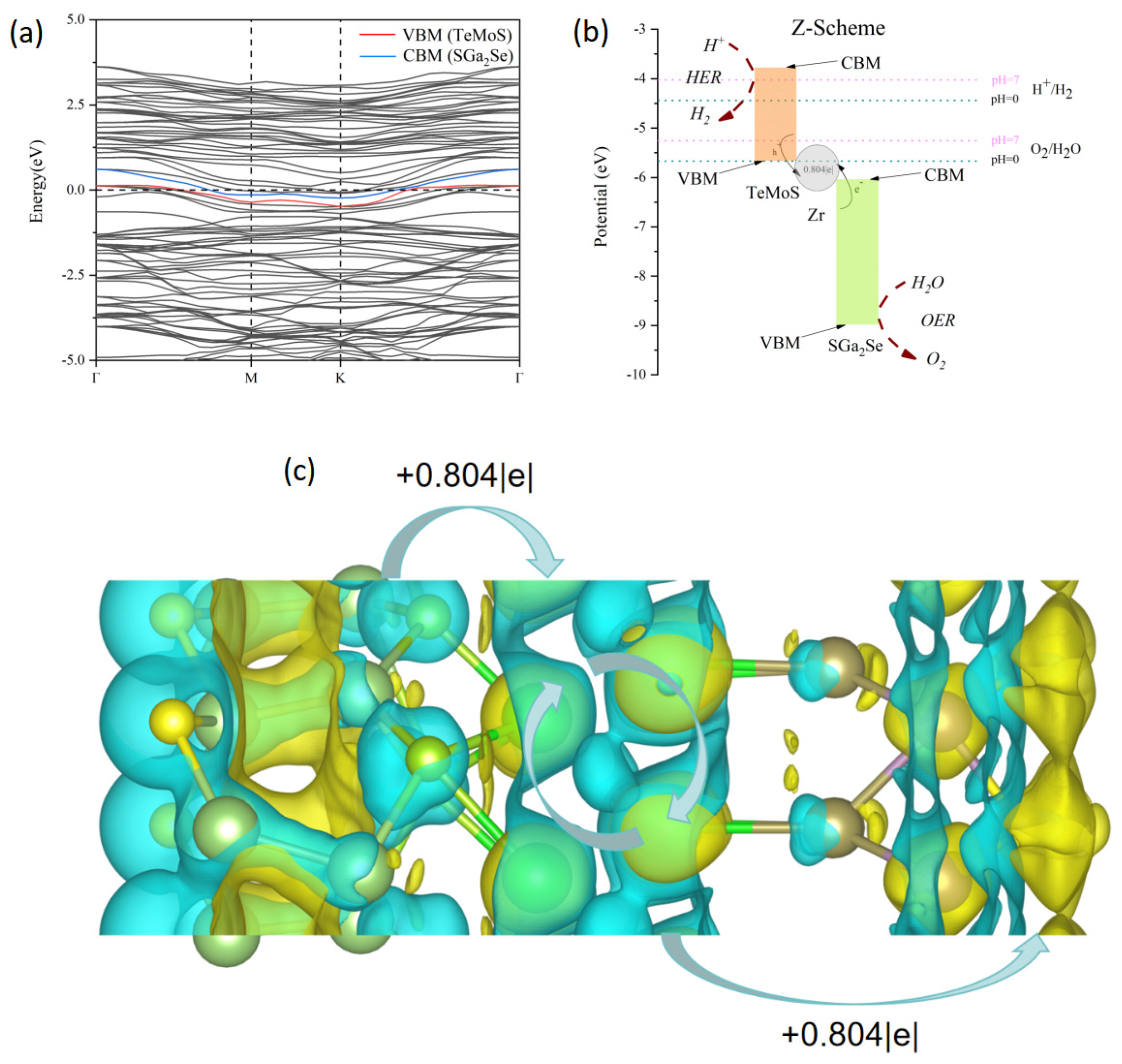
3.2. Mechanism of Transformation from Type-II to Z-Scheme Heterojunction
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A Sustainable Fuel for Future of the Transport Sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.-S.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano-Architecture and Material Designs for Water Splitting Photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Linley, S.; Reisner, E. Solar Reforming as an Emerging Technology for Circular Chemical Industries. Nat. Rev. Chem. 2024, 8, 87–105. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent Advances and Perspectives for Solar-Driven Water Splitting Using Particulate Photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, X. Oxysulfide Semiconductors for Photocatalytic Overall Water Splitting with Visible Light. Angew. Chem. Int. Ed. 2019, 58, 15580–15582. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Slassi, A.; Han, X.; Cornil, D.; Ha-Thi, M.-H.; Pino, T.; Debecker, D.P.; Colbeau-Justin, C.; Arbiol, J.; Cornil, J.; et al. Tuning the Electronic Bandgap of Graphdiyne by H-Substitution to Promote Interfacial Charge Carrier Separation for Enhanced Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2021, 31, 2100994. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Sun, Q.; Li, F.; Huang, B.; Dai, Y. Two-Dimensional Germanium Monochalcogenides for Photocatalytic Water Splitting with High Carrier Mobility. Appl. Catal. B Environ. 2017, 217, 275–284. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.-J.; Zhu, H. Engineering Graphene and TMDs Based van Der Waals Heterostructures for Photovoltaic and Photoelectrochemical Solar Energy Conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition Metal Dichalcogenides and Beyond: Synthesis, Properties, and Applications of Single- and Few-Layer Nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.; Sae Hong, S.; Li, Y.; Cui, Y. Physical and Chemical Tuning of Two-Dimensional Transition Metal Dichalcogenides. Chem. Soc. Rev. 2015, 44, 2664–2680. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, J.-H.; Lee, G.-H.; Lee, C.-H. Two-Dimensional Semiconductor Optoelectronics Based on van Der Waals Heterostructures. Nanomaterials 2016, 6, 193. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Huang, B.; Wang, X.; Niu, H.; Guo, Y.; Li, B.; Zheng, R.; Wu, H. Theoretical Study on the Photocatalytic Properties of 2D InX(X = S, Se)/Transition Metal Disulfide (MoS2 and WS2) van Der Waals Heterostructures. Nanoscale 2020, 12, 20025–20032. [Google Scholar] [CrossRef]
- Lin, M.; Chen, H.; Zhang, Z.; Wang, X. Engineering Interface Structures for Heterojunction Photocatalysts. Phys. Chem. Chem. Phys. 2023, 25, 4388–4407. [Google Scholar] [CrossRef]
- Chen, D.; Lei, X.; Wang, Y.; Zhong, S.; Liu, G.; Xu, B.; Ouyang, C. Tunable Electronic Structures in BP/MoSSe van Der Waals Heterostructures by External Electric Field and Strain. Appl. Surf. Sci. 2019, 497, 143809. [Google Scholar] [CrossRef]
- Yang, F.; Record, M.-C.; Boulet, P. Self-Defined Monolayers, Bilayers and Trilayers of Two-Dimensional Janus MoSSe and Ga2SSe van Der Waals Homojunctions as Potential Photocatalysts for Overall Water Splitting. Renew. Energy 2025, 245, 122829. [Google Scholar] [CrossRef]
- Zu, D.; Wei, H.; Lin, Z.; Bai, X.; Ivan, M.N.A.S.; Tsang, Y.H.; Huang, H. The Role of Point Defects in Heterojunction Photocatalysts: Perspectives and Outlooks. Adv. Funct. Mater. 2024, 34, 2408213. [Google Scholar] [CrossRef]
- Ren, K.; Wang, K.; Cheng, Y.; Tang, W.; Zhang, G. Two-Dimensional Heterostructures for Photocatalytic Water Splitting: A Review of Recent Progress. Nano Futures 2020, 4, 032006. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-Light Driven Heterojunction Photocatalysts for Water Splitting—A Critical Review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Wu, J. Construction of a Z-Scheme Heterojunction for High-Efficiency Visible-Light-Driven Photocatalytic CO2 Reduction. Nanoscale 2021, 13, 4359–4389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, X.; Shen, J.; Zhang, Z.; Wang, D.; Lin, J.; Wu, J.C.S.; Fu, X.; Wang, X.; Li, C. Direct and Indirect Z-Scheme Heterostructure-Coupled Photosystem Enabling Cooperation of CO2 Reduction and H2O Oxidation. Nat. Commun. 2020, 11, 3043. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.; Hong, L.; Ji, X.; Lin, Z.; Li, Z.; Pan, W. A Review of Metal Oxide-Based Z-Scheme Heterojunction Photocatalysts: Actualities and Developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Abdul Nasir, J.; Munir, A.; Ahmad, N.; Haq, T.U.; Khan, Z.; Rehman, Z. Photocatalytic Z-Scheme Overall Water Splitting: Recent Advances in Theory and Experiments. Adv. Mater. 2021, 33, 2105195. [Google Scholar] [CrossRef]
- Ng, B.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient Photocatalytic Overall Water Splitting Induced by the Giant Internal Electric Field of a g-C3 N4/rGO/PDIP Z-Scheme Heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, J.; Zhang, B.; Cao, S.; Wu, Y.; Gao, Z.; Nie, X.; Sun, L. Two-Dimensional Janus Heterostructures for Superior Z-Scheme Photocatalytic Water Splitting. Nano Energy 2019, 59, 537–544. [Google Scholar] [CrossRef]
- Stojilovic, N.; Bender, E.T.; Ramsier, R.D. Surface Chemistry of Zirconium. Prog. Surf. Sci. 2005, 78, 101–184. [Google Scholar] [CrossRef]
- Orio, M.; Pantazis, D.A.; Neese, F. Density Functional Theory. Photosynth. Res. 2009, 102, 443–453. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio Simulations of Materials Using VASP: Density-functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Cooper, V.R. Van Der Waals Density Functional: An Appropriate Exchange Functional. Phys. Rev. B 2010, 81, 161104. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Moellmann, J.; Grimme, S. DFT-D3 Study of Some Molecular Crystals. J. Phys. Chem. C 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Bučko, T.; Hafner, J.; Lebègue, S.; Ángyán, J.G. Improved Description of the Structure of Molecular and Layered Crystals: Ab Initio DFT Calculations with van Der Waals Corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef]
- Heyd, J.; Peralta, J.E.; Scuseria, G.E.; Martin, R.L. Energy Band Gaps and Lattice Parameters Evaluated with the Heyd-Scuseria-Ernzerhof Screened Hybrid Functional. J. Chem. Phys. 2005, 123, 174101. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-Based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.; Barbosa, R.; Mançano, R.R.; Durães, N.; Pontes, R.B.; Miwa, R.H.; Fazzio, A.; Padilha, J.E. Metal Chalcogenides Janus Monolayers for Efficient Hydrogen Generation by Photocatalytic Water Splitting. ACS Appl. Nano Mater. 2019, 2, 890–897. [Google Scholar] [CrossRef]
- Liu, L.-L.; Shen, L.-L.; Yan, X.-J.; Li, W.-Y.; Nan, G.-T.; Wang, S.-F.; Wei, Y.; Yang, C.; Hu, L. Single-Layer and Bilayer MoSTe for Photocatalytic Water Splitting: Role of Optical Absorption Correction and Band Edge Distribution. Results Phys. 2022, 42, 106033. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, J. Proposed Photosynthesis Method for Producing Hydrogen from Dissociated Water Molecules Using Incident Near-Infrared Light. Phys. Rev. Lett. 2014, 112, 018301. [Google Scholar] [CrossRef]
- Zhang, W.X.; Hou, J.T.; Bai, M.; He, C.; Wen, J.R. Construction of Novel ZnO/Ga2SSe (GaSe) vdW Heterostructures as Efficient Catalysts for Water Splitting. Appl. Surf. Sci. 2023, 634, 157648. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Shi, J.; Cen, Y.; Wu, M. Improvement of Visible-Light Photocatalytic Efficiency in a Novel InSe/Zr2 CO2 Heterostructure for Overall Water Splitting. J. Phys. Chem. C 2019, 123, 12781–12790. [Google Scholar] [CrossRef]
- Jiang, P.; Record, M.-C.; Boulet, P. Electron Density and Its Relation with Electronic and Optical Properties in 2D Mo/W Dichalcogenides. Nanomaterials 2020, 10, 2221. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Wang, K.; Cao, A.; Wei, J.; Li, C.; Jia, Y.; Li, Z.; Li, X.; Wu, D. Graphene-On-Silicon Schottky Junction Solar Cells. Adv. Mater. 2010, 22, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.-E.; Chung, H.-J.; Lee, J.; Yang, H.; Song, H.J.; Heo, J.; Seo, D.H.; Park, S.; Hwang, S.W.; Yoo, I.; et al. Graphene for True Ohmic Contact at Metal–Semiconductor Junctions. Nano Lett. 2013, 13, 4001–4005. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Wang, H.; Wang, Y. A Janus MoSSe Monolayer: A Potential Wide Solar-Spectrum Water-Splitting Photocatalyst with a Low Carrier Recombination Rate. J. Mater. Chem. A 2018, 6, 2295–2301. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Record, M.-C.; Boulet, P. From Type II to Z-Scheme: A DFT Study of Enhanced Water Splitting in the SGa2Se/TeMoS Heterojunction. Crystals 2025, 15, 442. https://doi.org/10.3390/cryst15050442
Yang F, Record M-C, Boulet P. From Type II to Z-Scheme: A DFT Study of Enhanced Water Splitting in the SGa2Se/TeMoS Heterojunction. Crystals. 2025; 15(5):442. https://doi.org/10.3390/cryst15050442
Chicago/Turabian StyleYang, Fan, Marie-Christine Record, and Pascal Boulet. 2025. "From Type II to Z-Scheme: A DFT Study of Enhanced Water Splitting in the SGa2Se/TeMoS Heterojunction" Crystals 15, no. 5: 442. https://doi.org/10.3390/cryst15050442
APA StyleYang, F., Record, M.-C., & Boulet, P. (2025). From Type II to Z-Scheme: A DFT Study of Enhanced Water Splitting in the SGa2Se/TeMoS Heterojunction. Crystals, 15(5), 442. https://doi.org/10.3390/cryst15050442




