Effect of TiO2 Content on the Corrosion and Thermal Resistance of Plasma-Sprayed Al2O3-TiO2 Coatings
Abstract
1. Introduction
2. Experimental Setup, Materials and Methods
3. Results and Discussion
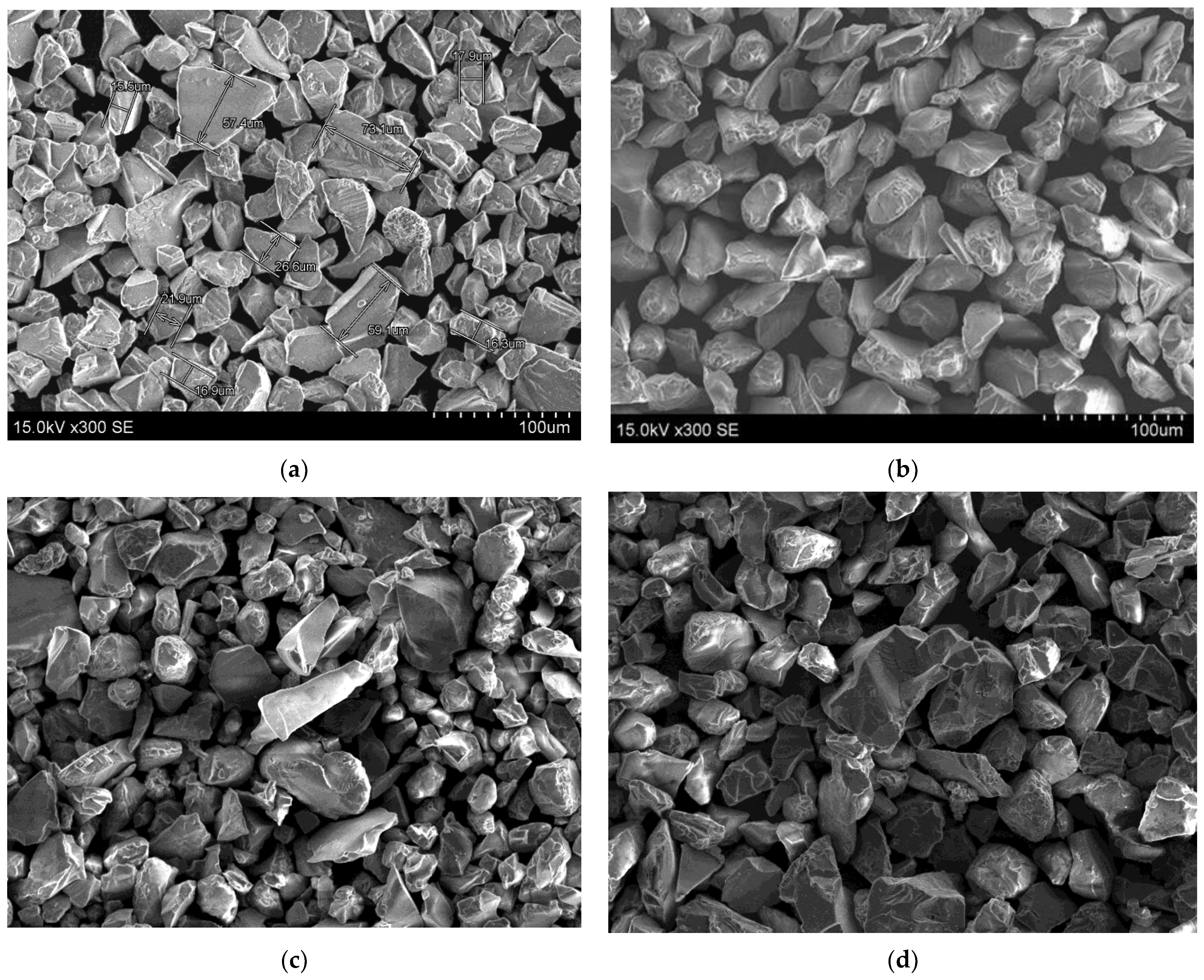
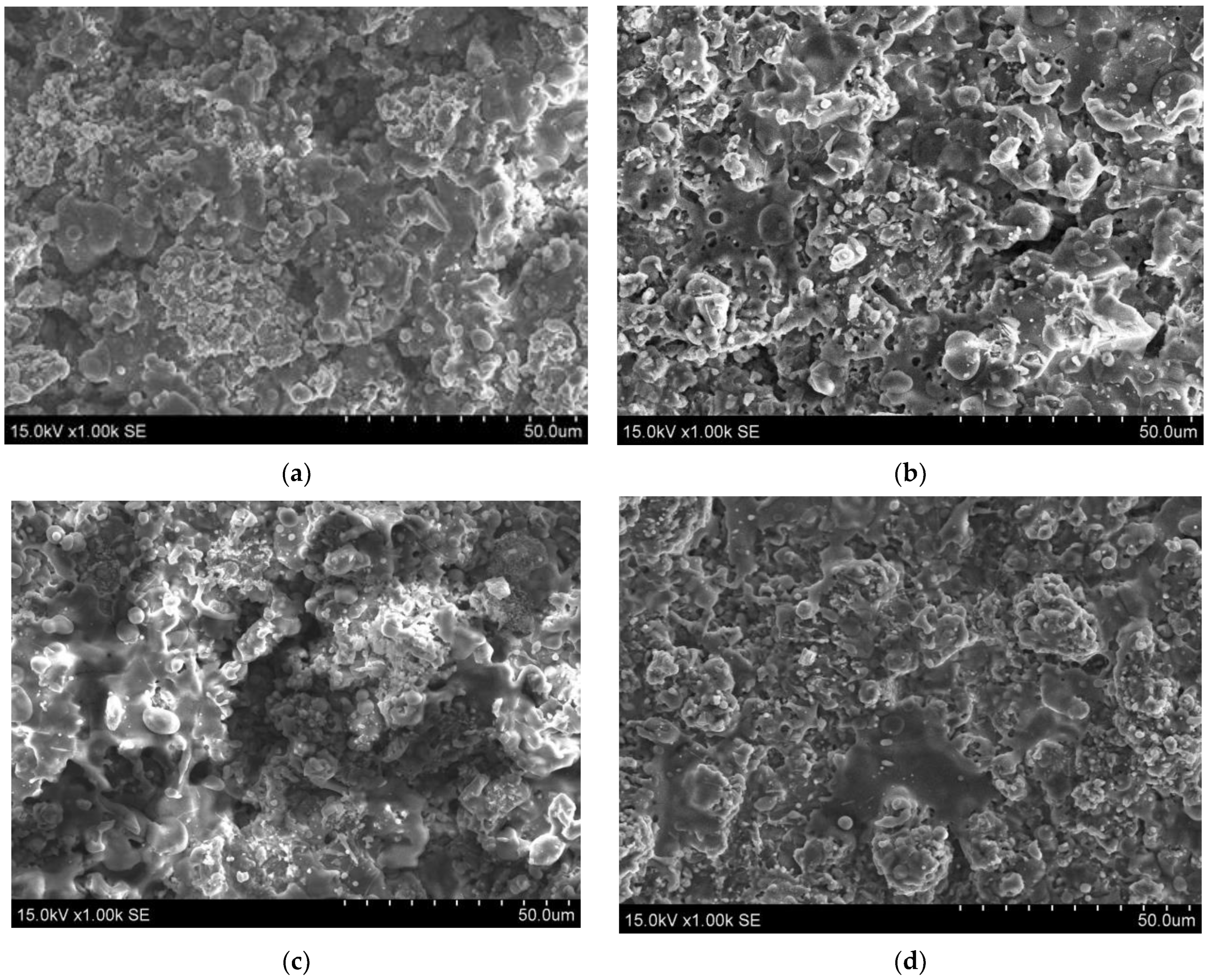
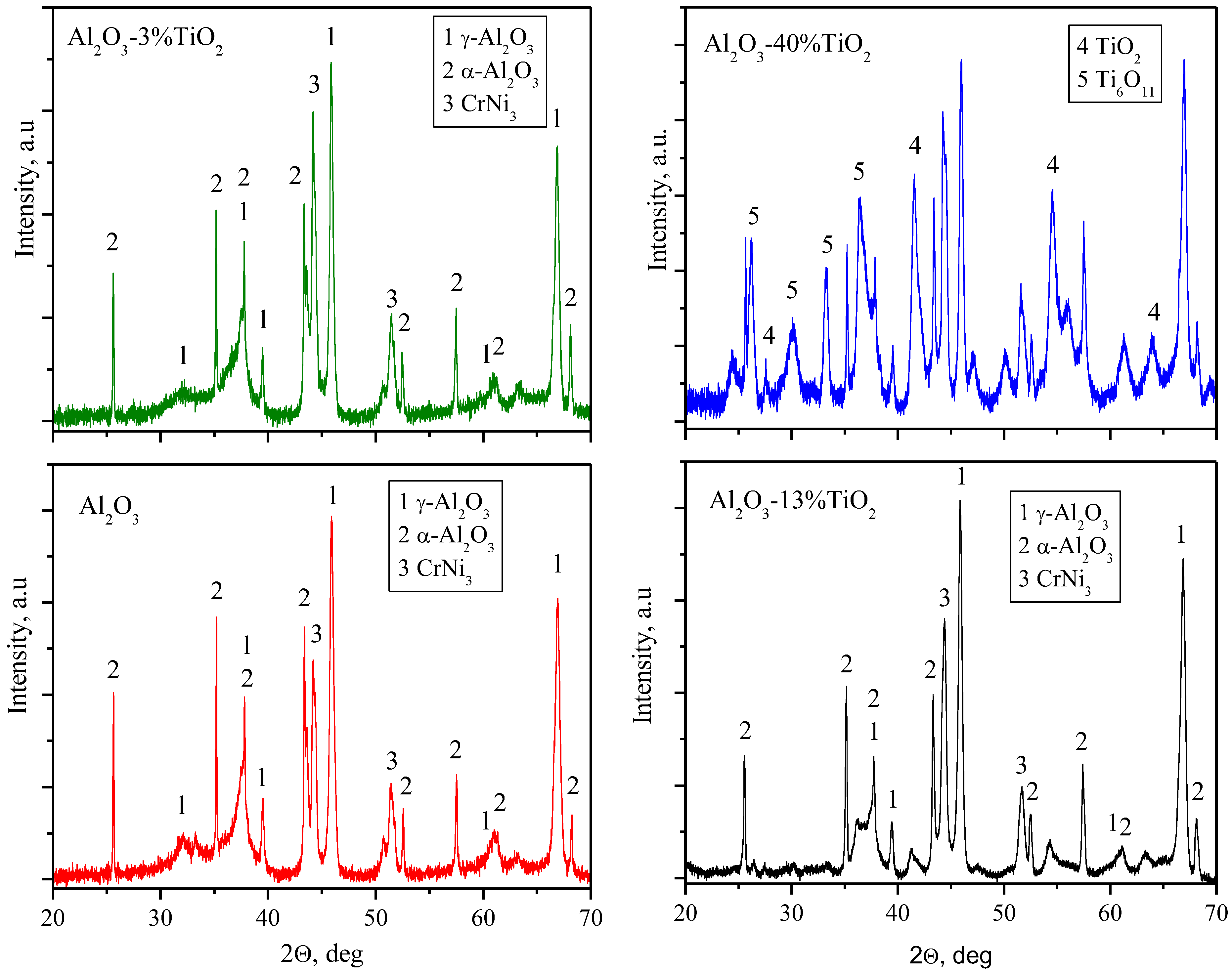
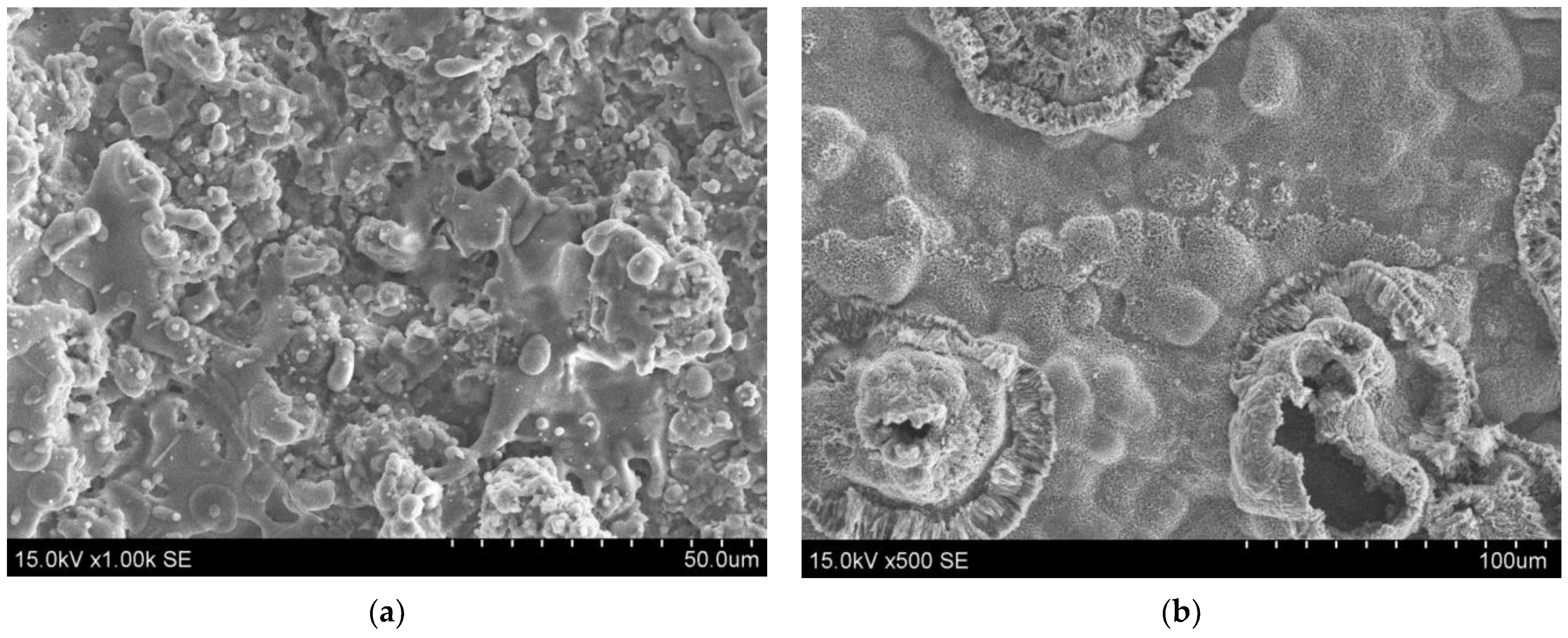
3.1. The Morphology of Deposited Coatings
3.2. The Corrosive–Thermal Resistance of Deposited Coatings
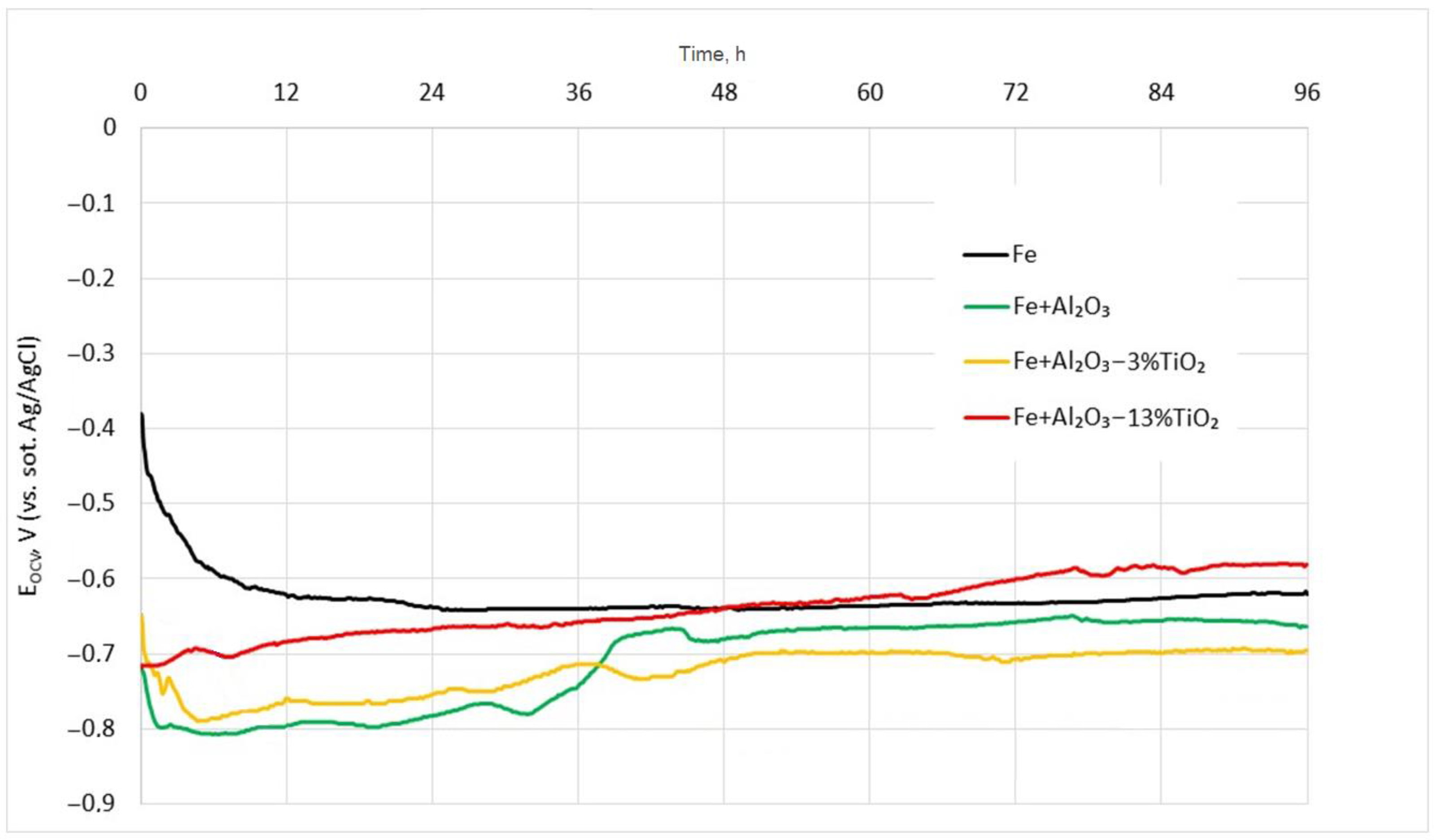
3.3. The Corrosion Behavior of Deposited Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Jiang, S.; Wang, M.; Wang, S.; Xiao, T.D.; Strutt, P.R. Abrasive wear characteristics of plasma sprayed nanostructured alumina/titania coatings. Wear 2000, 237, 176–185. [Google Scholar] [CrossRef]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131–3150. [Google Scholar] [CrossRef]
- Tankal, K.; Güney, B.; Erden, M.A. A comparative study of thermal sprayed Al2O3-TiO2 coatings on PM AISI 316L. Eng. Sci. Technol. Int. J. 2024, 60, 101895. [Google Scholar] [CrossRef]
- Valincius, V.; Krusinskaite, V.; Valatkevicius, P.; Valinciute, V.; Marcinauskas, L. Electric and thermal characteristics of the linear, sectional dc plasma generator. Plasma Sources Sci. Technol. 2004, 13, 199–206. [Google Scholar] [CrossRef]
- Brinkiene, K.; Kezelis, R. Effect of alumina addition on the microstructure of plasma sprayed YSZ. J. Eur. Ceram. Soc. 2005, 25, 2181–2184. [Google Scholar] [CrossRef]
- Brar, S.S.; Brar, G.S.; Chawla, V. Characterization of D-gun sprayed Al2O3-13%TiO2 and Al2O3-40%TiO2 coatings on ASTM 316 boiler steel and their microstructure. Mater. Today Proc. 2021, 50, 2299–2306. [Google Scholar] [CrossRef]
- Eraslan, F.S.; Gecu, R. Chemical composition optimization of Al2O3-TiO2 composite coatings for enhanced wear and corrosion resistance. Surf. Coat. Technol. 2023, 474, 130053. [Google Scholar] [CrossRef]
- Ghazali, M.J.; Forghani, S.M.; Hassanuddin, N.; Muchtar, A.; Daud, A.R. Comparative wear study of plasma sprayed TiO2 and Al2O3-TiO2 on mild steels. Tribol. Int. 2016, 93, 681–686. [Google Scholar] [CrossRef]
- Wei, Z.; Hong, S.; Wei, Z.; Hu, N.; Ying, G.; Wu, Y. Comparison on long-term corrosion performance of WC-CoCr and Al2O3-TiO2 ceramic coatings in sulphide-containing 3.5 wt% NaCl solution. Int. J. Refract. Met. Hard Mater. 2022, 107, 105906. [Google Scholar] [CrossRef]
- Lan, W.; Chen, Z.; Zhang, X.; Bi, W.; Liu, W. Corrosion resistance analysis of Al2O3-TiO2 composite ceramic coatings on carbon steel pipe surfaces. Alex. Eng. J. 2025, 110, 377–385. [Google Scholar] [CrossRef]
- Eraslan, F.S.; Turu, I.C.; Ozcan, M.; Birol, B.; Gecu, R. Influence of layer number and sintering temperature on microstructural, tribological, and corrosion behavior of Al2O3-TiO2 multilayer coatings. Ceram. Int. 2023, 49, 33226–33235. [Google Scholar] [CrossRef]
- Sreekumar Rajesh, T.; Venkata Rao, R. Experimental Investigation and Parameter Optimization of Al2O3-40% TiO2 Atmospheric Plasma Spray Coating on SS316 Steel Substrate. Mater. Today Proc. 2018, 5, 5012–5020. [Google Scholar] [CrossRef]
- Yugeswaran, S.; Selvarajan, V.; Vijay, M.; Ananthapadmanabhan, P.V.; Sreekumar, K.P. Influence of critical plasma spraying parameter (CPSP) on plasma sprayed Alumina-Titania composite coatings. Ceram. Int. 2010, 36, 141–149. [Google Scholar] [CrossRef]
- Lu, Y.; Peng, Y.; Shi, Z. Plasma sprayed Al2O3–40%TiO2 coating by laser remelting: Structural evolution, tribological properties and DFT calculation. Tribol. Int. 2023, 189, 109009. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, M.; Fan, Z.; Yin, Y.; Lin, W.; Huang, H. Laser surface engineering of Ti–6Al–4V with TiO2/Al2O3 composite powder for improved wear resistance. Smart Mater. Manuf. 2023, 1, 100015. [Google Scholar] [CrossRef]
- Hanifi, D.; Hakimi, N.; Ali, R.; Huang, T.; Huma, T.; Bakhshyar, D.; Afzali, N.; Shafi, M.; Babeker, H.; Lu, J.; et al. The fracture toughness and tribological performance of NiAl/Al2O3-40 Wt.% TiO2 coating generated by air plasma spraying. Ceram. Int. 2024, 50, 1533–1546. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, H.; Jin, G.; Ma, G.; Zhang, J.; Chen, S. Tribological properties and solid particle erosion wear behavior of Al2O3-13 wt%TiO2/Al2O3-PF composite coatings prepared on resin matrix by supersonic plasma spraying. Ceram. Int. 2024, 50, 29987–29996. [Google Scholar] [CrossRef]
- Chang, C.S.T.; Wyss, M.; Andrzejewski, M.; Darut, G.; Graf, L.; Novak, V.; Olbinado, M.; Erpel, S.; Vogel, A.; Bode, S.; et al. Microstructures, phase and mechanical characterisation of Al2O3-ZrO2-TiO2 coating produced by atmospheric plasma spraying. Open Ceram. 2024, 20, 100698. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, Y.; Deng, C.; Fan, X.; Li, S.; Wang, C.; Yang, Y.; Zhang, Y.; He, C. Preparation, corrosion and tribological properties evolution of self-lubricating Al2O3-TiO2 coatings with superior anti-corrosion. Surf. Coat. Technol. 2025, 498, 131849. [Google Scholar] [CrossRef]
- Girisha, K.; Sreenivas Rao, K.V.; Durga Prasad, C. Slurry Erosion Resistance of Martenistic Stainless Steel with Plasma Sprayed Al2O3-40%TiO2 Coatings. Mater. Today Proc. 2018, 5, 7388–7393. [Google Scholar] [CrossRef]
- Sharma, V.; Kazi, S. Oxidation behaviour of D-gun sprayed Al2O3-3 wt% SiC coating. Surf. Coat. Technol. 2020, 383, 125238. [Google Scholar] [CrossRef]
- Łatka, L.; Michalak, M.; Jonda, E. Atmospheric Plasma Spraying of Al2O3 + 13% TiO2 Coatings Using External and Internal Injection System. Adv. Mater. Sci. 2019, 19, 5–17. [Google Scholar] [CrossRef]
- Lu, X.; Yan, D.; Yang, Y.; Dong, Y.; He, J.; Zhang, J. Phase evolution of plasma sprayed Al2O3−13%TiO2 coatings derived from nanocrystalline powders. Trans. Nonferrous Met. Soc. China 2013, 23, 2951–2956. [Google Scholar] [CrossRef]
- Wang, D.S.; Qu, G.; Su, J.L. Thermal Barrier Effects Comparison of Plasma-Sprayed and Laser-Remelted Al2O3-13% TiO2 Ceramic Coatings. Adv. Mater. Res. 2014, 978, 40–43. [Google Scholar] [CrossRef]
- Nguyen Van, T.; Nguyen, T.A.; Pham Thi, H.; Pham Thi, L.; Nguyen Thi, P.; Le Thu, Q. Effect of heat treatment on corrosion resistance of Al2O3-TiO2 ceramic coating impregnated with aluminum phosphate. Vietnam J. Sci. Technol. 2024, 62, 463–474. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, P.; Fang, H.; Wang, Y.; Pu, J. Effects of the TiO2 content on the mechanical properties and galvanic corrosion resistance of Al2O3 coatings. Ceram. Int. 2023, 49, 38593–38601. [Google Scholar] [CrossRef]
- Kawase, M.; Ido, A.; Morinaga, M. Development of SiO2/TiO2/Al2O3-based/TiO2 coating for preventing sulfide corrosion in thermal power plant boilers. Appl. Therm. Eng. 2019, 153, 242–249. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Zhang, T.; Yang, Y. Microstructure, spallation and corrosion of plasma sprayed Al2O3–13%TiO2 coatings. Corros. Sci. 2009, 51, 2924–2931. [Google Scholar] [CrossRef]
- Celik, E.; Ozdemir, I.; Avci, E.; Tsunekawa, Y. Corrosion behaviour of plasma sprayed coatings. Surf. Coat. Technol. 2005, 193, 297–302. [Google Scholar] [CrossRef]
- Šuopys, A.; Marcinauskas, L.; Grigaitienė, V.; Kėželis, R.; Aikas, M.; Uscila, R.; Tučkutė, S.; Lelis, M. The Effect of Heat Treatment on the Microstructure and Phase Composition of Plasma Sprayed Al2O3 and Al2O3-TiO2 Coatings for Applications in Biomass Firing Plants. Coatings 2021, 11, 1289. [Google Scholar] [CrossRef]
- Šuopys, A.; Grigaitienė, V.; Marcinauskas, L.; Kėželis, R.; Uscila, R.; Aikas, M. Influence of Plasma Torch Power on the Plasma Jet Properties and Microstructure of Alumina Coatings. Coatings 2022, 12, 934. [Google Scholar] [CrossRef]
- Mahade, S.; Mulone, A.; Björklund, S.; Klement, U.; Joshi, S. Incorporation of graphene nano platelets in suspension plasma sprayed alumina coatings for improved tribological properties. Appl. Surf. Sci. 2021, 570, 151227. [Google Scholar] [CrossRef]
- Marcinauskas, L.; Mathew, J.S.; Milieška, M.; Aikas, M.; Kalin, M. Influence of graphite content on the tribological properties of plasma sprayed alumina-graphite coatings. Surf. Interfaces 2023, 38, 102763. [Google Scholar] [CrossRef]
- Song, E.P.; Ahn, J.; Lee, S.; Kim, N.J. Effects of critical plasma spray parameter and spray distance on wear resistance of Al2O3–8 wt.%TiO2 coatings plasma-sprayed with nanopowders. Surf. Coat. Technol. 2008, 202, 3625–3632. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Havaldar, S.S.; Hiriyannaiah, A.; Keshavamurthy, R. Investigation of Corrosion Characteristics of Plasma-Sprayed Composite Coating on Bearing Steel Through Electrochemical and Salt Spray Test. J. Bio-Tribo-Corros. 2022, 8, 106. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhang, H.; Li, M.; Li, T.; Wang, Z. Fabrication and Corrosion Resistance of Plasma-Sprayed Glass-Powder-Doped Al2O3-13 wt.%TiO2 Coatings. J. Therm. Spray Technol. 2020, 29, 500–509. [Google Scholar] [CrossRef]
- Bayram, T.; Karabas, M.; Kayali, Y. Deposition and study of plasma sprayed Al2O3-TiO2 coatings on AZ31 magnesium alloy. Eur. Mech. Sci. 2023, 7, 35–40. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A.; Lu, X.; Blawert, C.; Zheludkevich, M.L. The effect of pulse waveforms on surface morphology, composition and corrosion behavior of Al2O3 and Al2O3 /TiO2 nano-composite PEO coatings on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 208–221. [Google Scholar] [CrossRef]
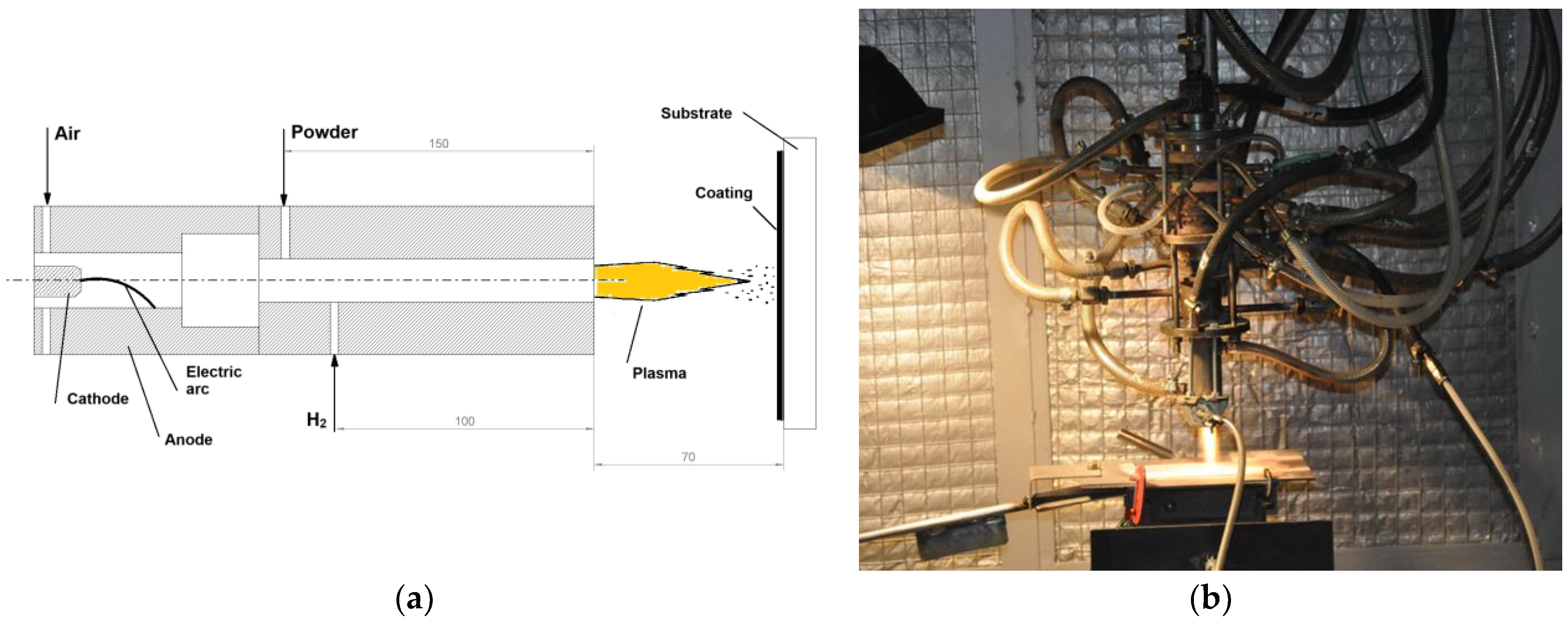




| Arc Current | Voltage | Mean Plasma Jet Temperature | Mean Plasma Jet Speed | Spray Distance | Primary Gas Flow Rate | Powder Carrier Gas Flow Rate | Secondary Gas Flow Rate | Spray Duration |
|---|---|---|---|---|---|---|---|---|
| 200 A | 200–203 V | 3200 K | 1060 m/s | 70 mm | 4.2 g/s | 0.75 g/s | 0.06 g/s | 60 s |
| Samples | ||||
|---|---|---|---|---|
| Composition | Al2O3 | Al2O3 + 3%TiO2 | Al2O3 + 13%TiO2 | Al2O3 + 40%TiO2 |
| Oxygen, at.% | 60 | 58 | 60 | 50 |
| Carbon, at.% | 1 | 1 | 1.2 | 0.7 |
| Nickel, at.% | 1.4 | 4 | 1.8 | 1.2 |
| Chromium, at.% | 0.4 | 1.5 | 0.4 | 0.6 |
| Aluminum, at.% | 36 | 32 | 30 | 23 |
| Titanium, at.% | - | 1.7 | 6 | 24 |
| Copper, at.% | 1 | |||
| Sample | Time, h | Ecorr., mV | icorr., μA/cm2 | βa, mV/Decade | βk, mV/Decade |
|---|---|---|---|---|---|
| Fe (substrate) | 1 | −474.9 | 23.4 | 111.5 | 869.8 ** |
| 12 | −643.9 | 21.6 | 95.5 | 454.7 ** | |
| 24 | −661.4 | 20.8 | 97.1 | 370.9 ** | |
| 48 | −680.0 | 15.5 | 100.6 | 169.2 ** | |
| 72 | −678.1 | 18.2 | 103.7 | 140.1 ** | |
| 96 | −655.3 | 29.4 | 104.7 | 155.5 ** | |
| Fe + Al2O3 | 1 | −756.9 | 0.6 | 99.6 | 121.1 ** |
| 12 | −834.3 | 4.7 | 222.6 * | 133.9 | |
| 24 | −810.8 | 5.2 | 248.9 * | 156.5 | |
| 48 | −687.3 | 7.8 | 119.2 | 184.2 ** | |
| 72 | −653.3 | 11.4 | 130.0 | 188.9 ** | |
| 96 | −662.6 | 8.4 | 140.7 | 184.4 ** | |
| Fe + Al2O3—3%TiO2 | 1 | −789.1 | 18.08 | 429.8 * | 158.9 |
| 12 | −809.5 | 18.32 | 381.7 * | 179.4 | |
| 24 | −790.7 | 17.80 | 281.8 * | 166.7 | |
| 48 | −750.0 | 14.18 | 346.6 * | 163.8 | |
| 72 | −748.5 | 16.05 | 347.6 * | 168.4 | |
| 96 | −725.9 | 11.67 | 203.5 * | 154.2 | |
| Fe + Al2O3—13%TiO2 | 1 | −754.0 | 12.4 | 151.9 | 147.0 * |
| 12 | −697.4 | 12.8 | 82.2 | 149.8 * | |
| 24 | −662.1 | 13.0 | 68.9 | 210.8 * | |
| 48 | −640.1 | 13.5 | 138.3 | 191.4 * | |
| 72 | −608.6 | 17.3 | 196.4 | 231.7 * | |
| 96 | −589.1 | 17.4 | 118.9 | 215.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigaitienė, V.; Marcinauskas, L.; Šuopys, A.; Kėželis, R.; Griškonis, E. Effect of TiO2 Content on the Corrosion and Thermal Resistance of Plasma-Sprayed Al2O3-TiO2 Coatings. Crystals 2025, 15, 439. https://doi.org/10.3390/cryst15050439
Grigaitienė V, Marcinauskas L, Šuopys A, Kėželis R, Griškonis E. Effect of TiO2 Content on the Corrosion and Thermal Resistance of Plasma-Sprayed Al2O3-TiO2 Coatings. Crystals. 2025; 15(5):439. https://doi.org/10.3390/cryst15050439
Chicago/Turabian StyleGrigaitienė, Viktorija, Liutauras Marcinauskas, Airingas Šuopys, Romualdas Kėželis, and Egidijus Griškonis. 2025. "Effect of TiO2 Content on the Corrosion and Thermal Resistance of Plasma-Sprayed Al2O3-TiO2 Coatings" Crystals 15, no. 5: 439. https://doi.org/10.3390/cryst15050439
APA StyleGrigaitienė, V., Marcinauskas, L., Šuopys, A., Kėželis, R., & Griškonis, E. (2025). Effect of TiO2 Content on the Corrosion and Thermal Resistance of Plasma-Sprayed Al2O3-TiO2 Coatings. Crystals, 15(5), 439. https://doi.org/10.3390/cryst15050439









