Microstructural Investigation and High-Temperature Oxidation Performance of K417G Alloy Prepared by Wide-Gap Brazing
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Sample Preparation
2.2. Oxidation Tests
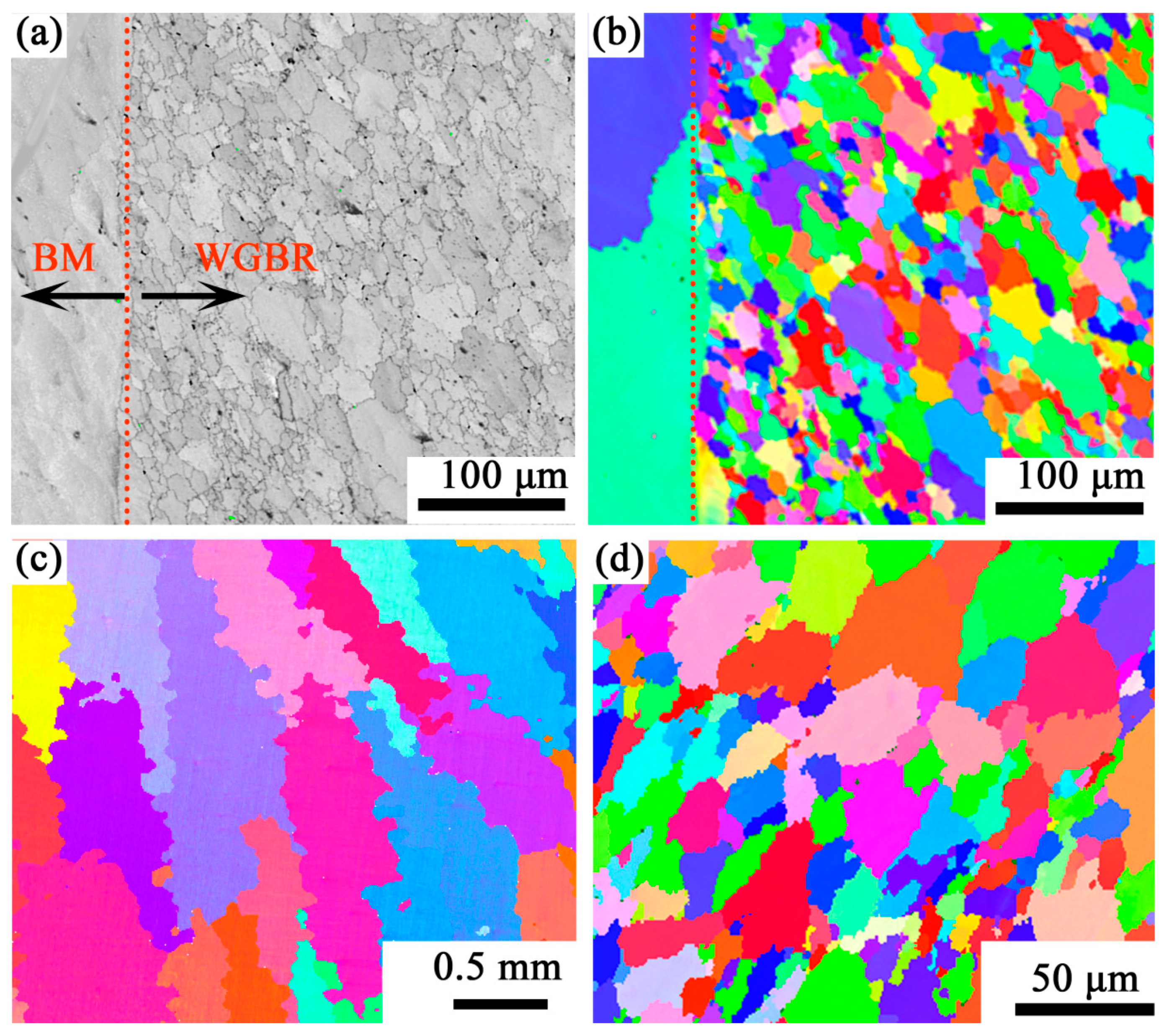
2.3. Microstructural Characterization
3. Results
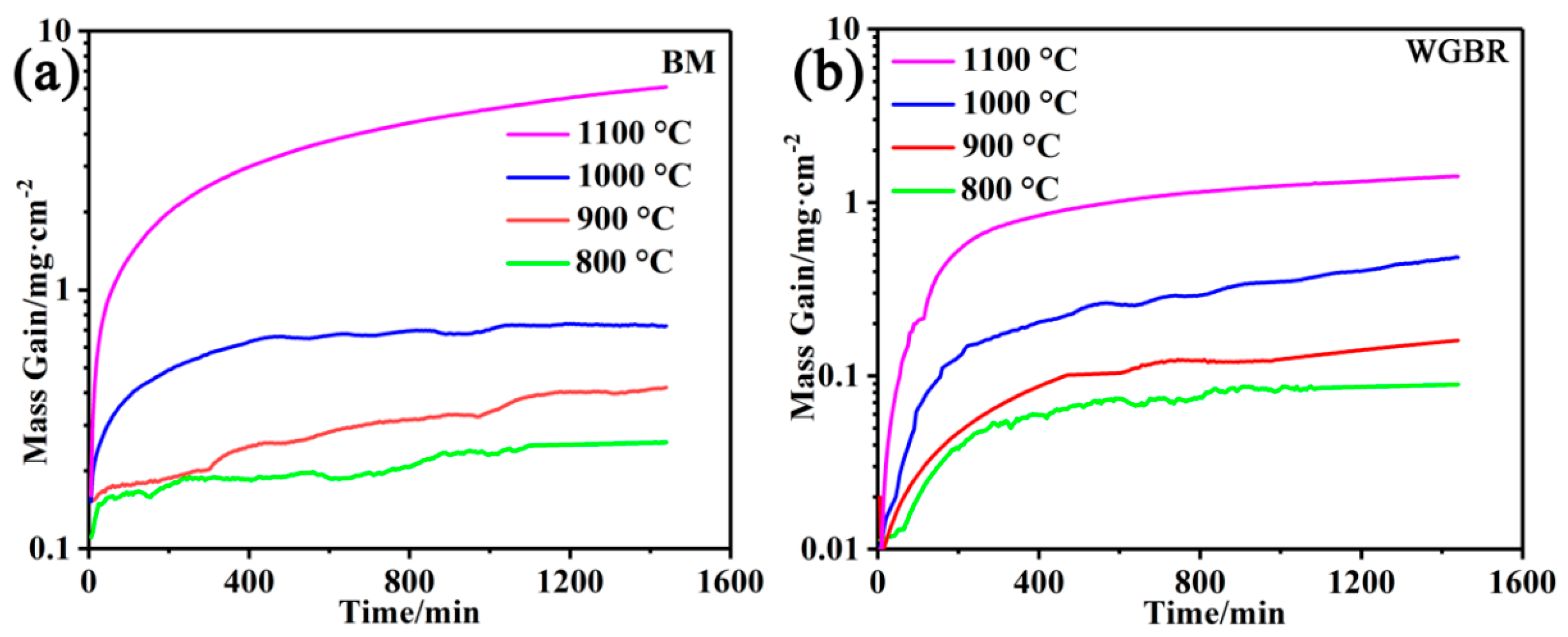
3.1. Oxidation Behaviors at High Temperature
- K0 = Pre-exponential constant;
- Q = Activation energy (430 kJ/mol for the alloy);
- R = Universal gas constant;
- T = Absolute temperature.
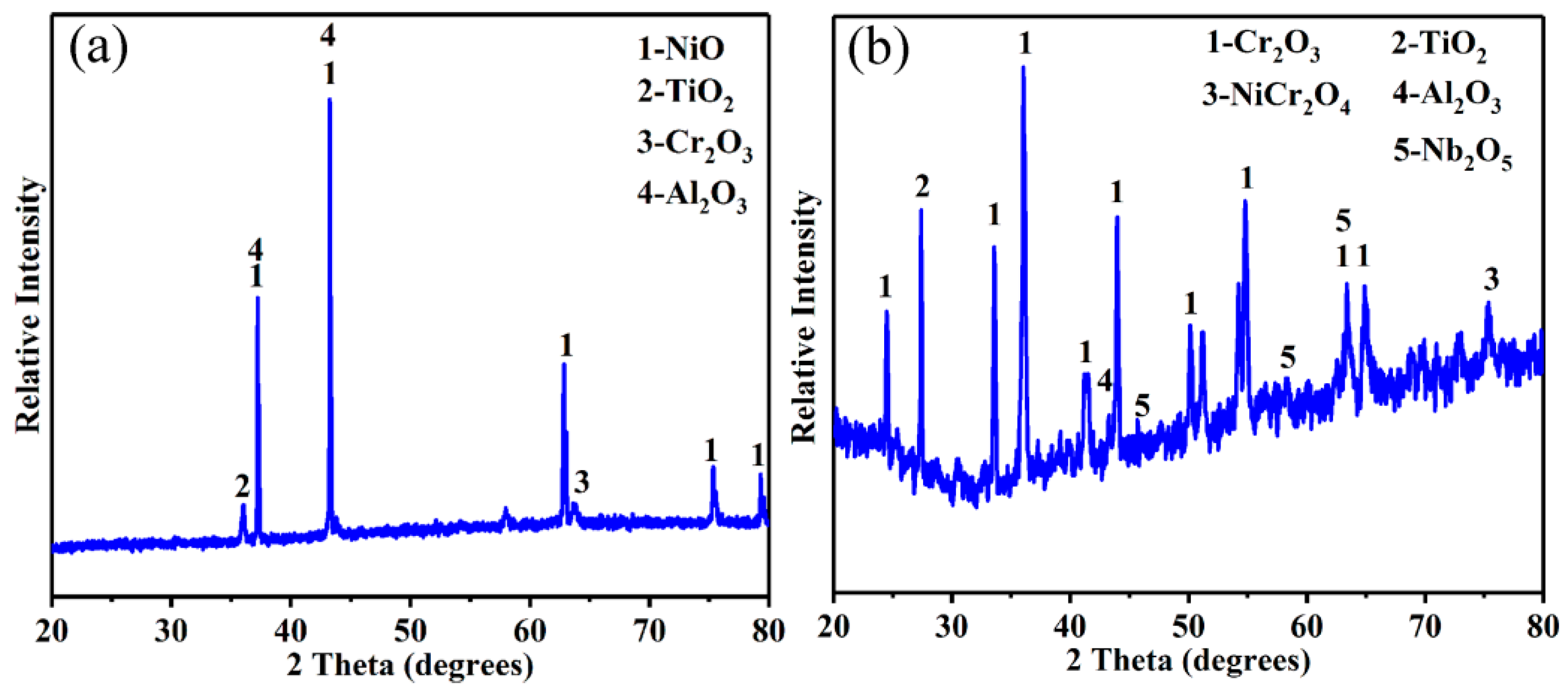
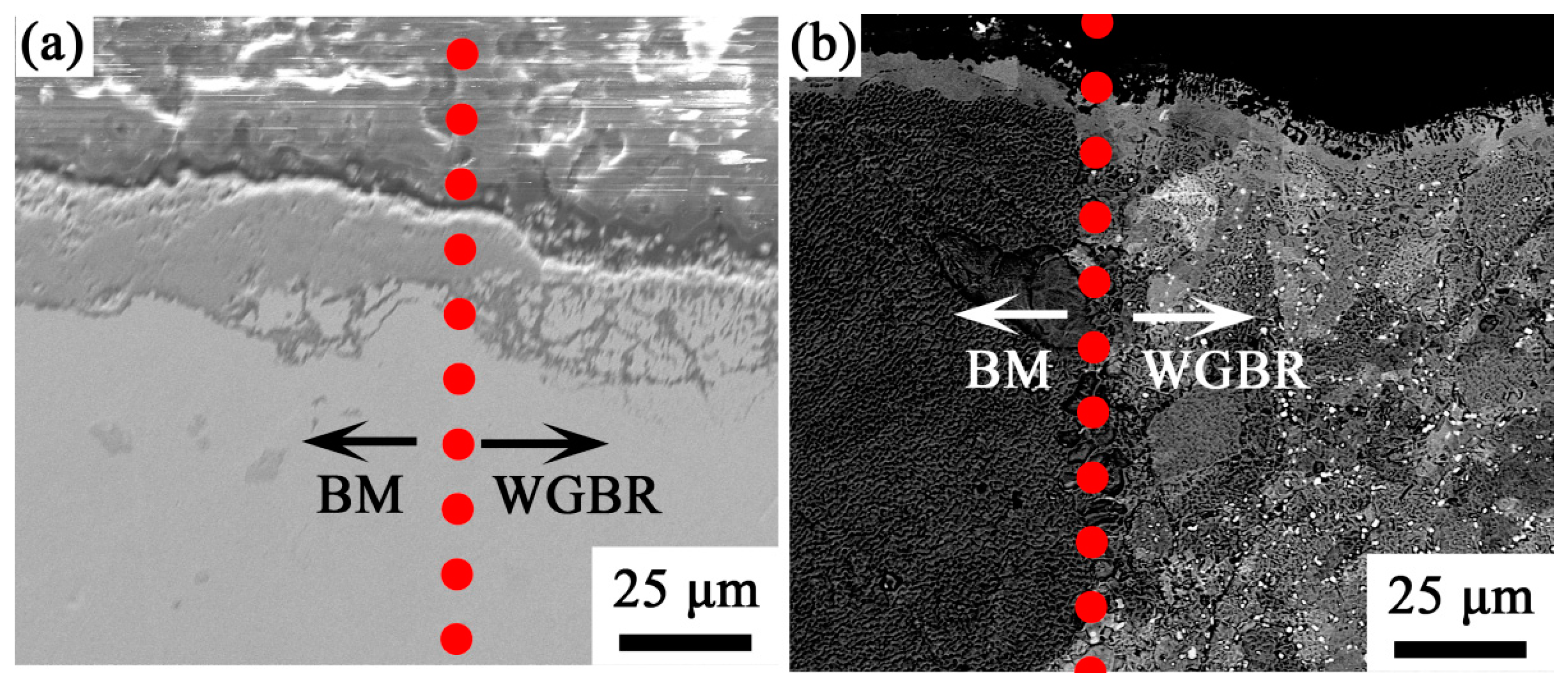
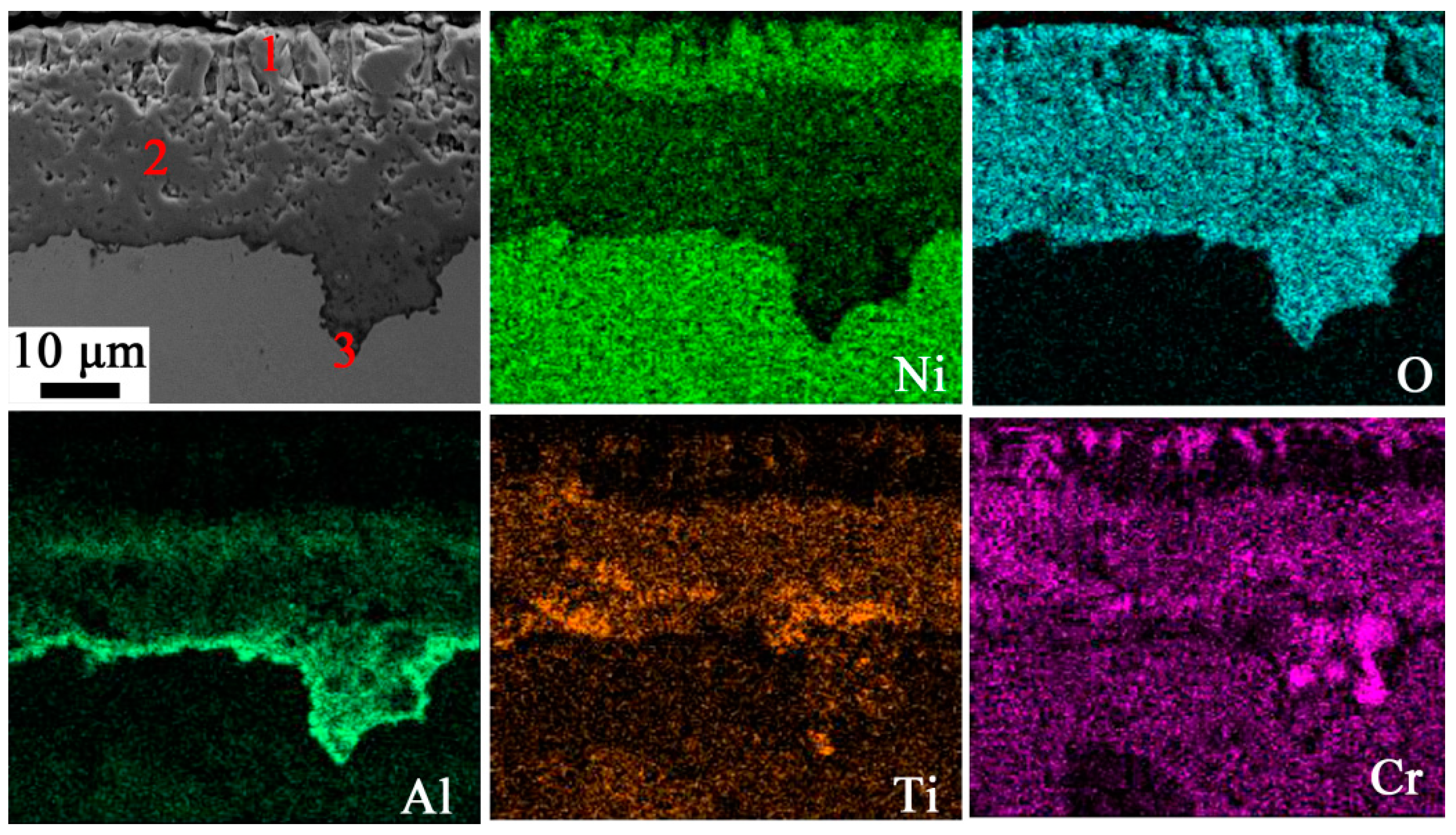
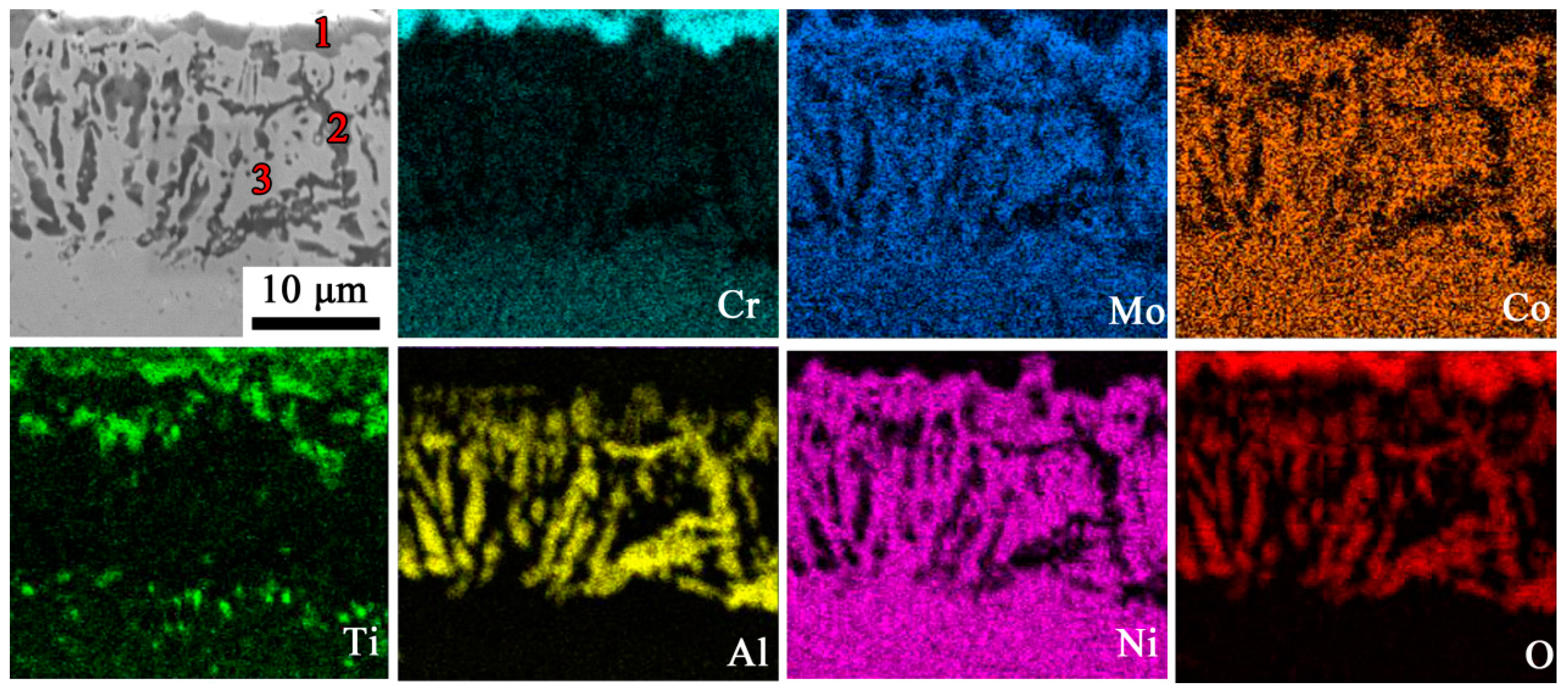
3.2. Oxide Composition
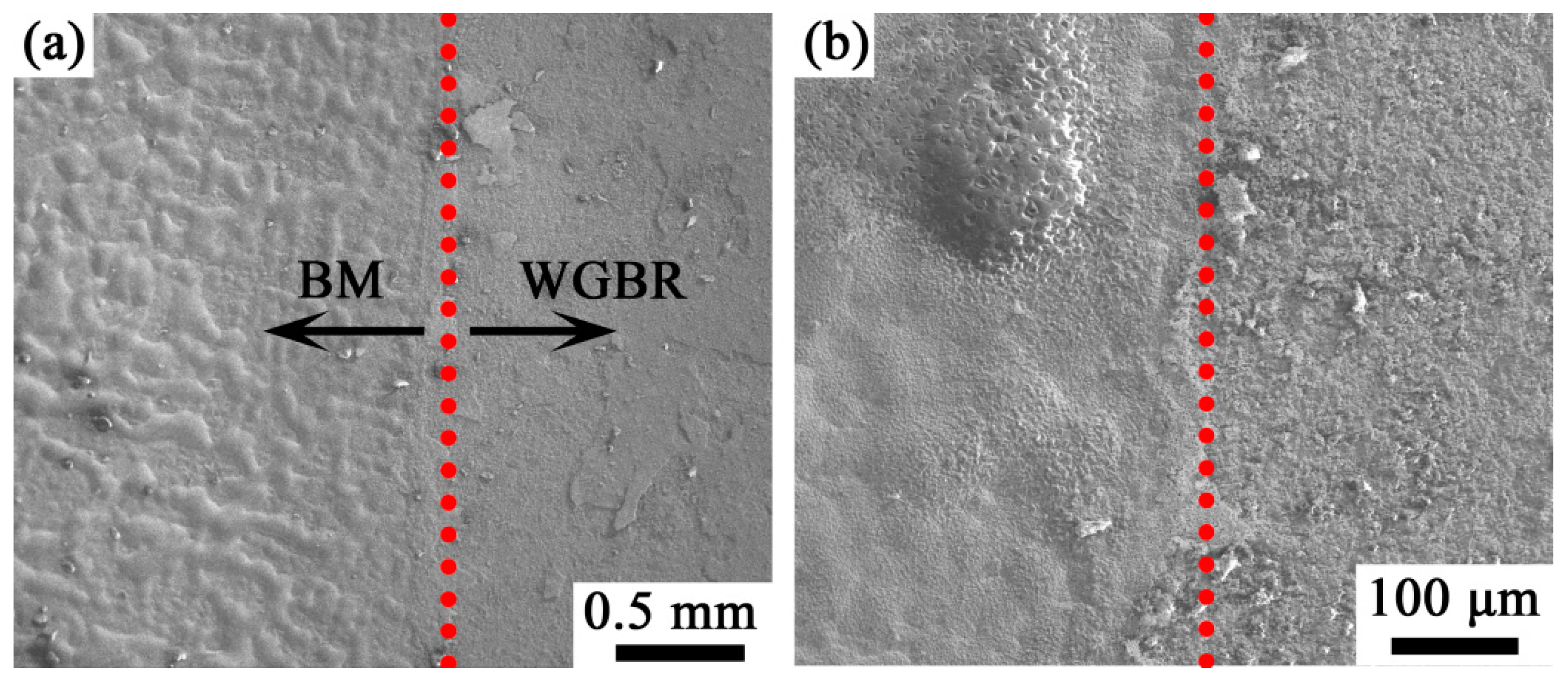
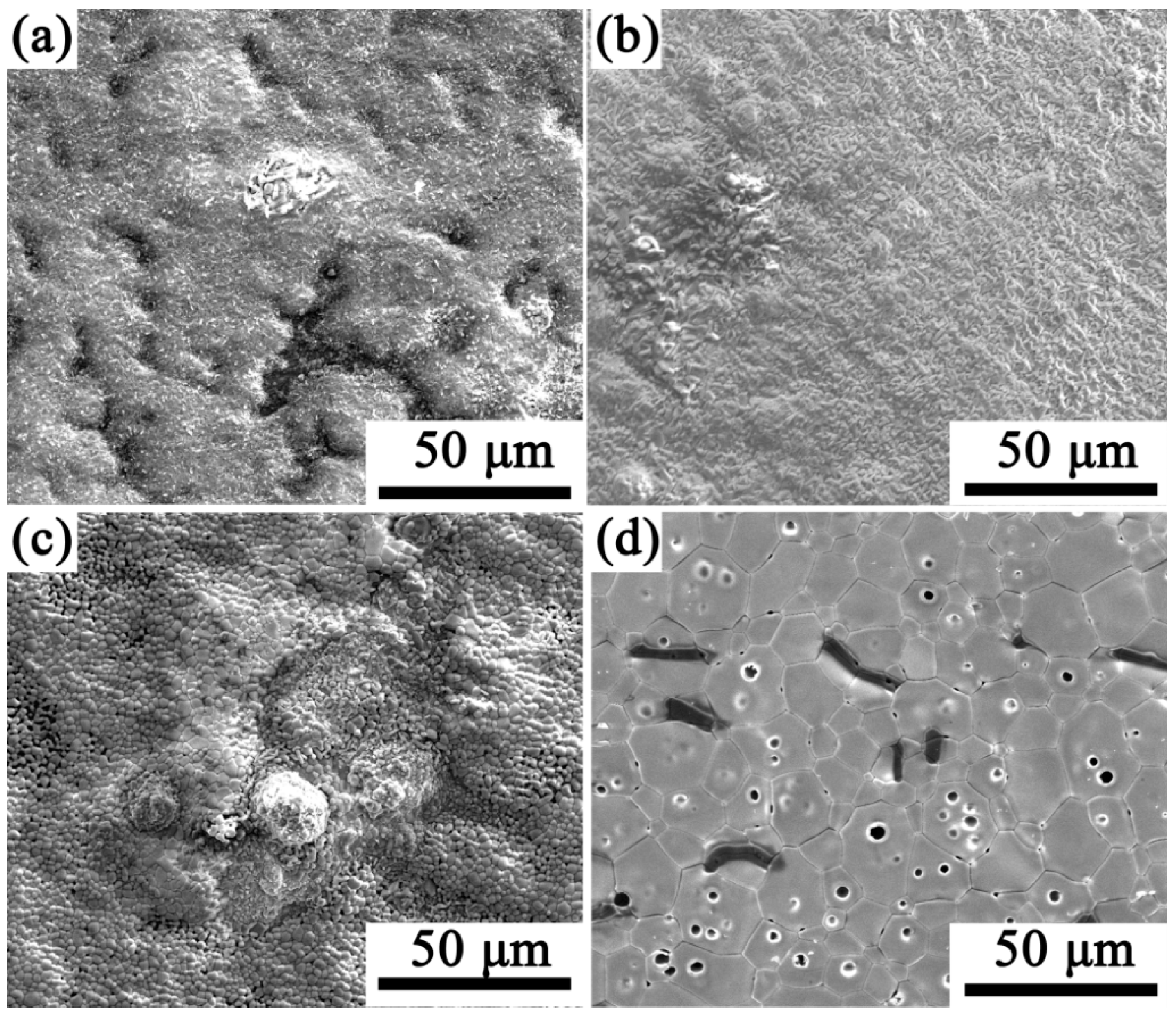
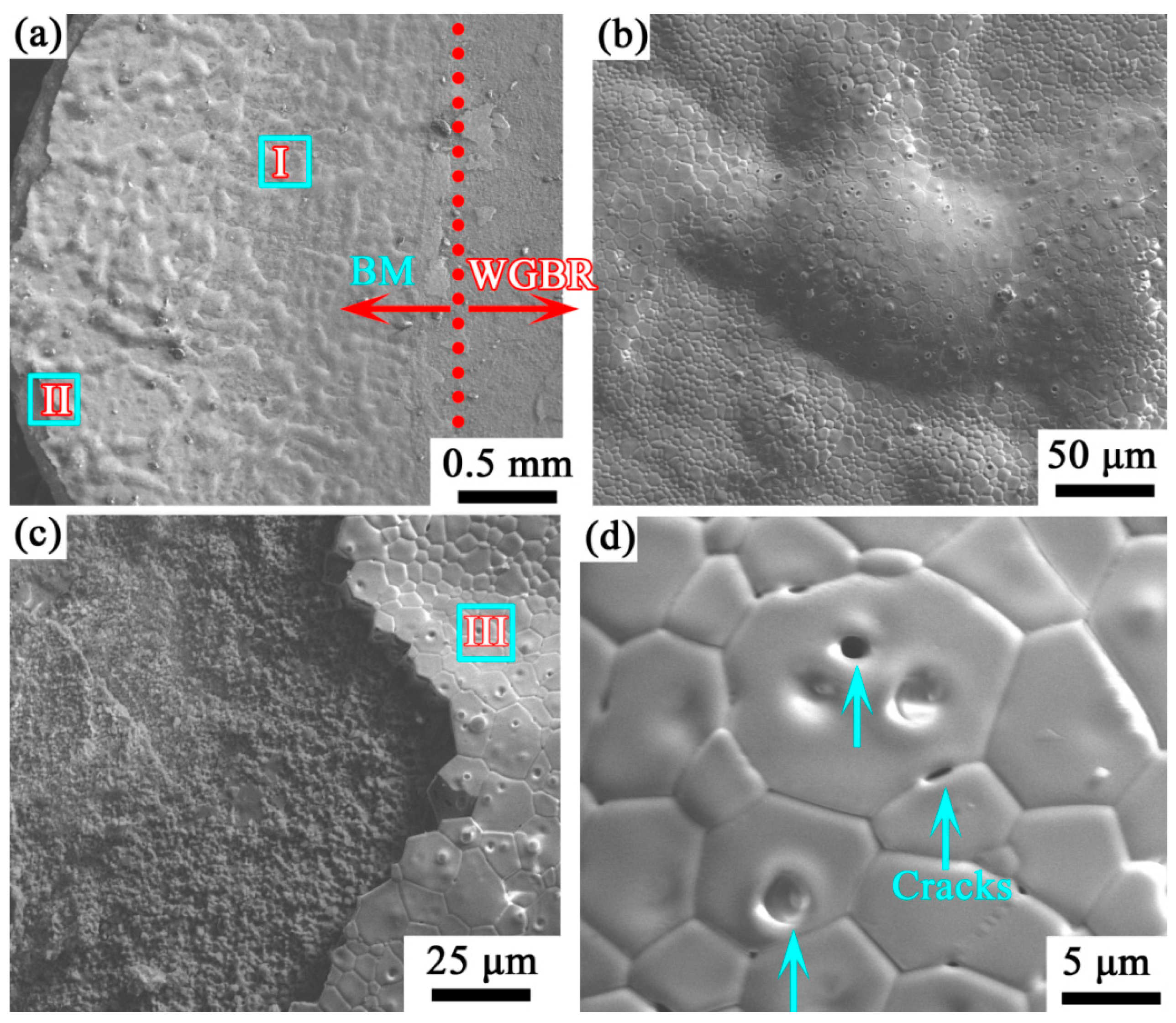
3.3. Surface Morphology of Oxidized Material
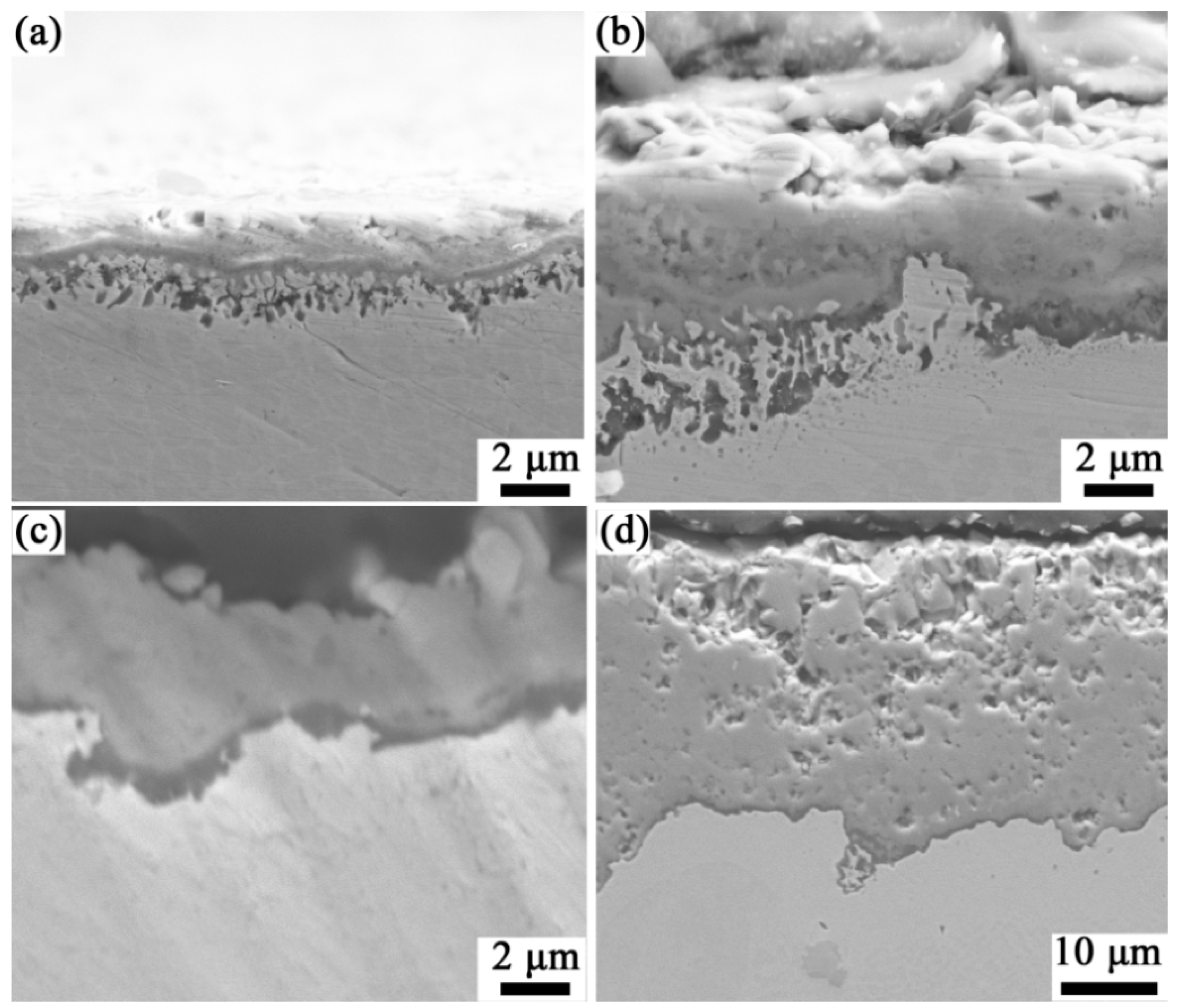
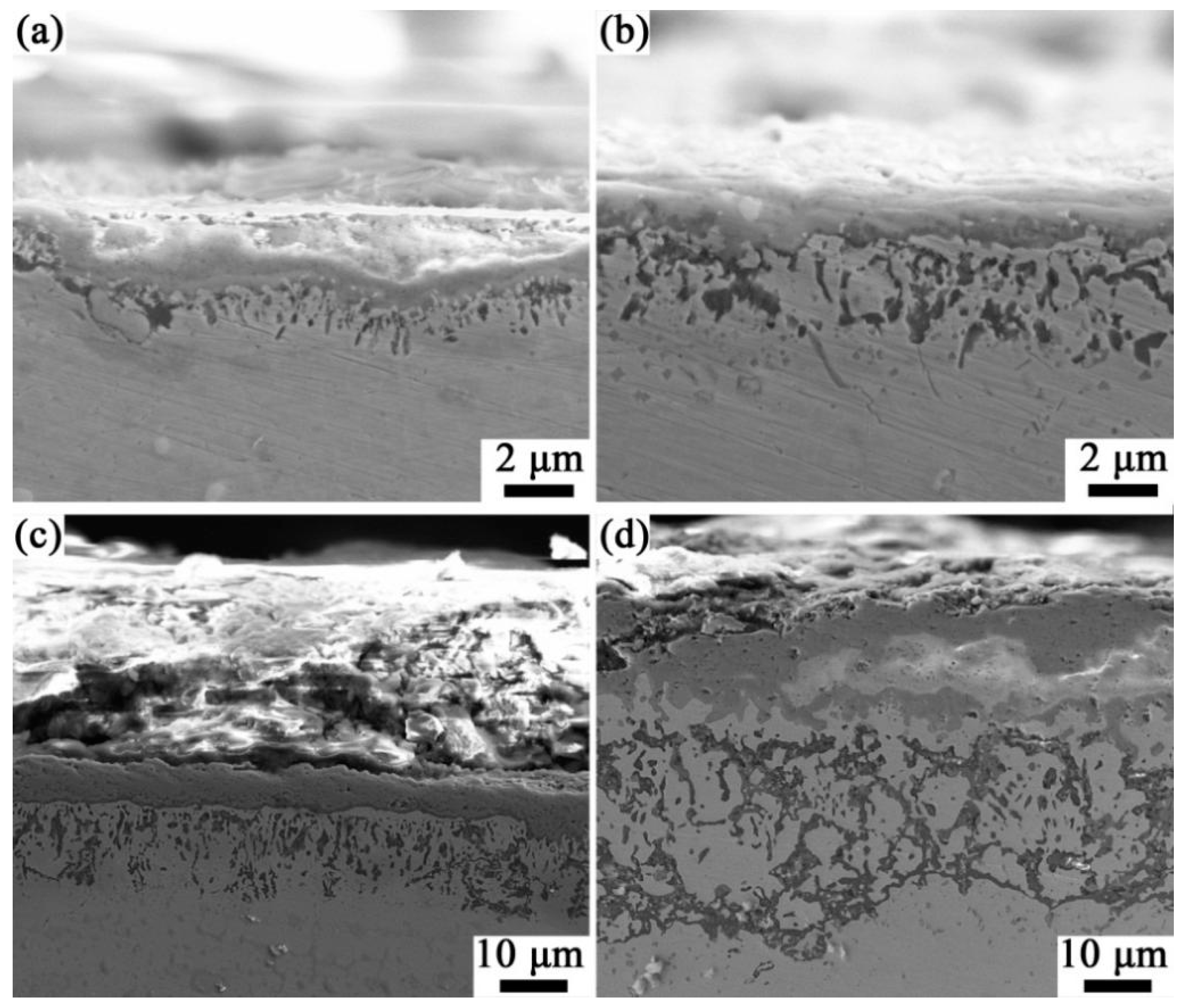
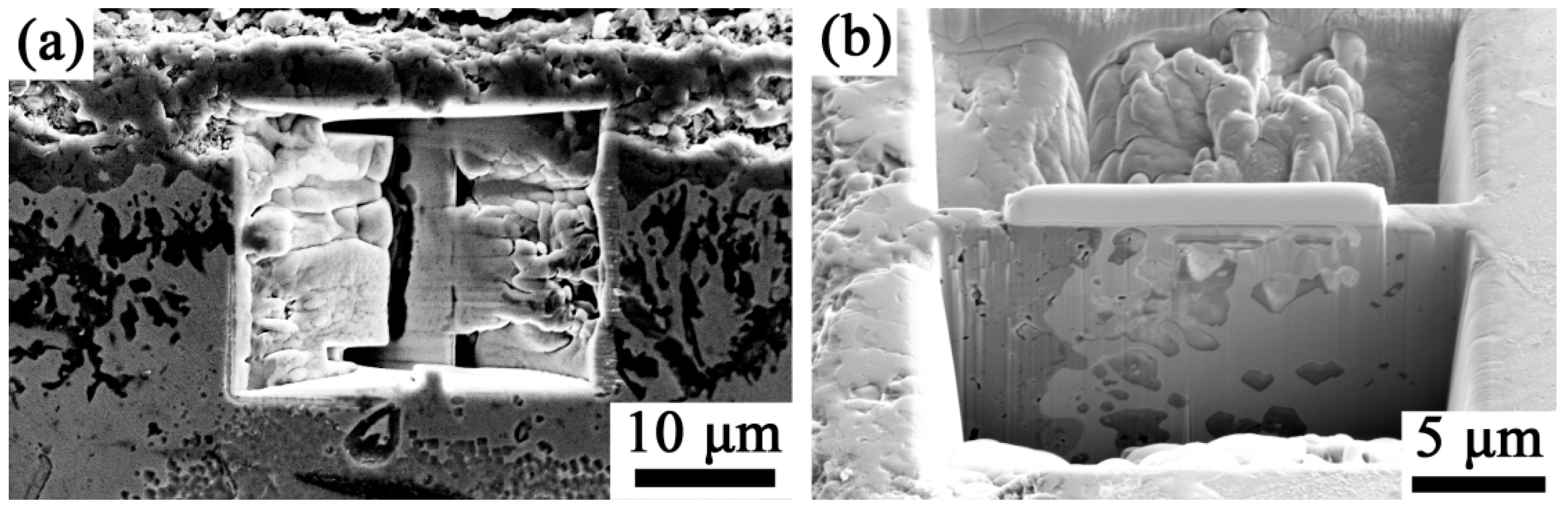
3.4. Cross-Sectional Morphology After High-Temperature Oxidation
4. Discussion
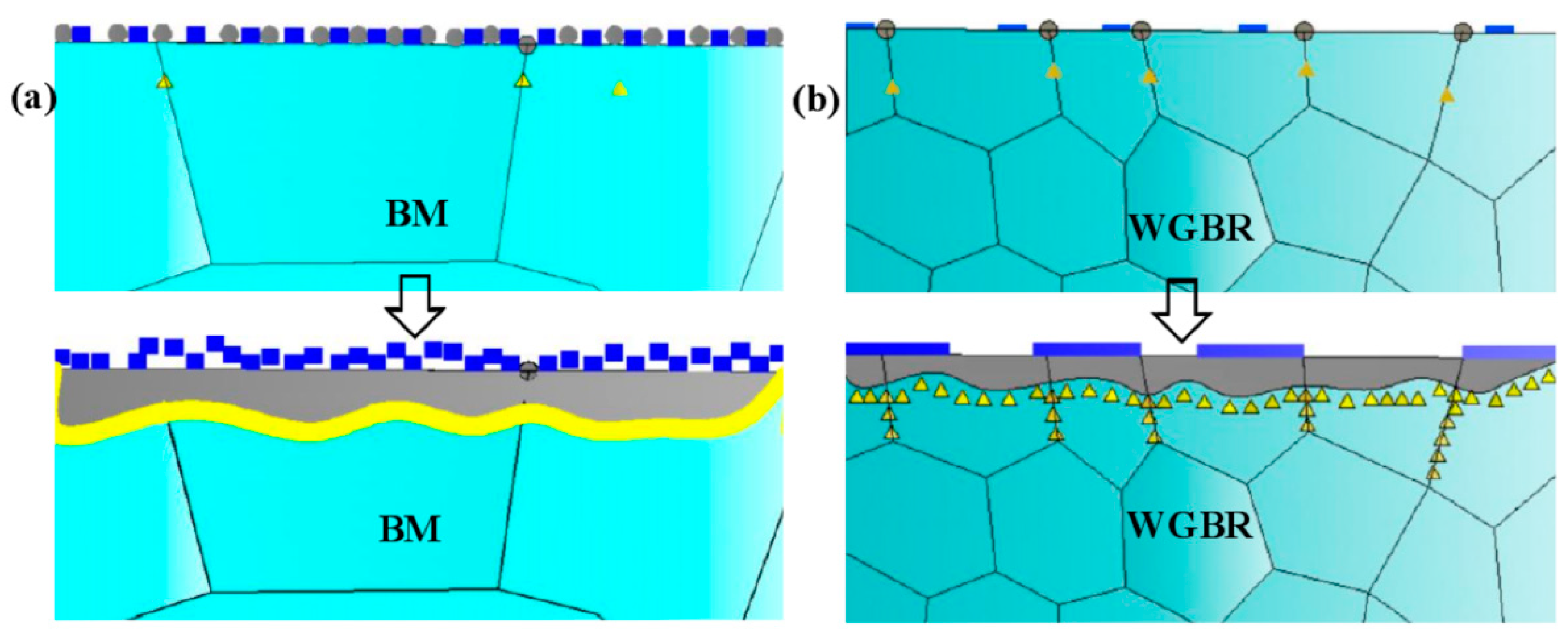
4.1. High-Temperature Oxidation Mechanism
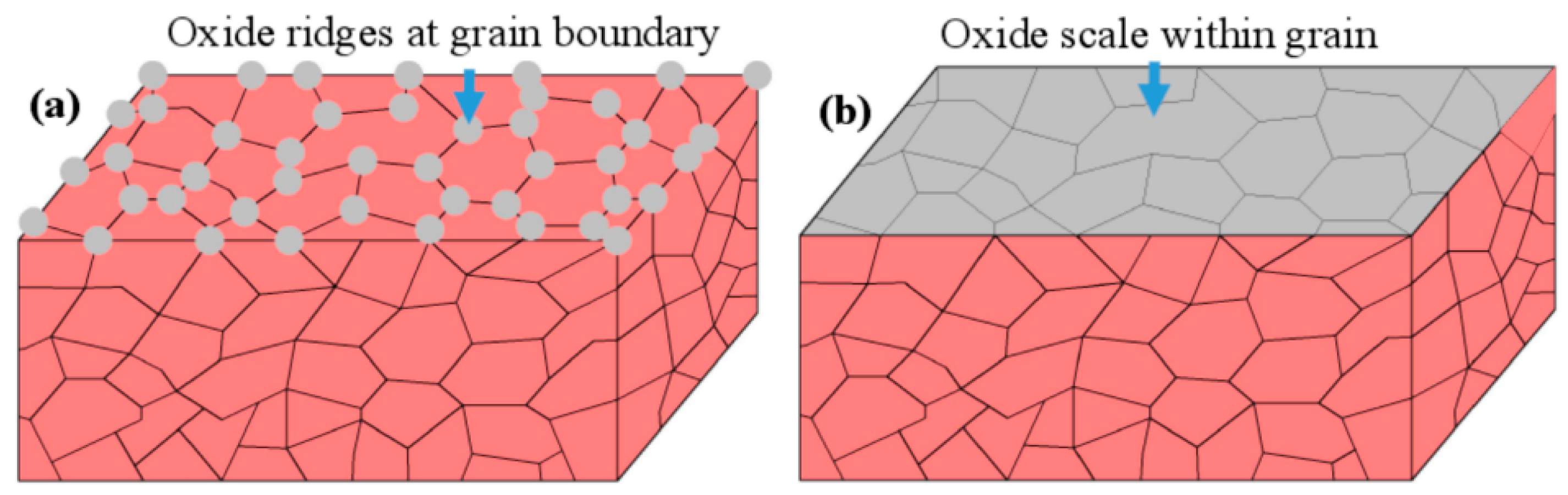
4.2. Oxide Spallation Mechanism Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Z.; Li, X.Q.; Wang, B.; Qu, S.G.; Li, H.Y. M3B2-type borides effect on the wide gap brazing of K417G alloy with mixed powder. J. Alloys Compd. 2020, 821, 153431. [Google Scholar] [CrossRef]
- Yu, X.F.; Wang, S.J.; Zheng, D.Y.; Shen, X.; Li, Y.; Liu, W.; Su, Y.; Liu, F. Effect of heat treatment on rotating bending fatigue properties of K417G nickel-base superalloy. J. Alloys Compd. 2022, 905, 164209. [Google Scholar] [CrossRef]
- Zhang, G.T.; Liu, W.J.; Bian, H.Y.; Wang, C.; Li, Q.; Wang, H. Evolutionary mechanism analysis of Laves phase precipitation behavior and wear resistance of laser-directed energy deposited T-800 alloy coatings on K417G alloy at various aging temperatures. Mater. Lett. 2025, 382, 137938. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.S.; Gao, X.Y.; Wen, X.; Qiao, S.; Yuan, C. Insights into process optimization and induction preheating for crack-free laser metal deposition of nickel-based superalloy K417G. J. Mater. Res. Technol. 2024, 29, 2035–2050. [Google Scholar] [CrossRef]
- Zhang, L.H.; Liu, Y.H.; Guo, H.M.; Xing, W. Effects of pouring temperature on microstructure and properties of K417G superalloy in thermally-controlled solidification process. Rare Met. Mater. Eng. 2024, 53, 2546–2554. [Google Scholar]
- Kalfhaus, T.; Schneider, M.; Ruttert, B.; Sebold, D.; Hammerschmidt, T.; Frenzel, J.; Drautz, R.; Theisen, W.; Eggeler, G.; Guillon, O.; et al. Repair of Ni-based single-crystal superalloys using vacuum plasma spray. Mater. Des. 2019, 168, 107656. [Google Scholar] [CrossRef]
- Way, M.; Willingham, J.; Goodall, R. Brazing filler metals. Int. Mater. Rev. 2019, 65, 1–29. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, X.Q.; Qu, S.G.; Zhu, D.; Li, H. Microstructural evolution and mechanical properties of wide gap brazing of K417G superalloy. Rare Met. Mater. Eng. 2021, 50, 3262–3269. [Google Scholar]
- Ren, X.Y.; Wang, H.; Li, W.W.; Dong, Q.; Chen, B.; Mao, W. Efect of holding time on interfacial evolution and mechanical strength of wide-gap brazed K417G superalloy joints. Weld. World 2024, 68, 3079–3084. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Y.; You, X.Q.; Wang, D.; Hu, Y.; Song, S.; Qiang, J. Effect of trace impurity elements on the high-temperature oxidation resistance of a high-purity nickel-based superalloy. Corros. Sci. 2023, 211, 110904. [Google Scholar] [CrossRef]
- Miller, C.F.; Simmons, G.W.; Wei, R.P. High temperature oxidation of Nb, NbC and Ni3Nb and oxygen enhanced crack growth. Scr. Mater. 2000, 42, 227–232. [Google Scholar] [CrossRef]
- Zhao, D.; Xuan, W.D.; Shao, G.L.; Che, X.; Wang, S.; Qi, J.; Ren, Z. Unveiling the short-term oxidation behavior of nickel-base superalloy CMSX-4 in high-temperature air. Corros. Sci. 2025, 251, 112915. [Google Scholar] [CrossRef]
- Khedr, M.; Li, W.; Min, N. Strengthening contributions of mechanical twinning and dislocations to the flow stress of hadfield high-manganese steel: Quantitative analysis. J. Mater. Eng. Perform. 2022, 32, 501–511. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, X.Q.; Zhang, M.A.; Qu, S.; Li, H. Wide-gap brazing of K417G alloy assisted by in situ precipitation of M3B2 boride particle. Materials 2020, 13, 3140. [Google Scholar] [CrossRef]
- He, W.B.; Xue, Z.L.; Guo, R.; Chen, N. On machining K417 superalloy with sliding self-propelled rotary tools: Simulation and experimental investigation. Int. J. Adv. Manuf. Technol. 2023, 126, 1669–1680. [Google Scholar] [CrossRef]
- Li, X.Q.; Cheng, Z.; Qu, S.G.; Zhu, D.; Li, H. Effect of filler metal on the microstructural evolution and mechanical properties of wide gap brazed K417G superalloy joints. Vacuum 2021, 184, 109967. [Google Scholar] [CrossRef]
- Jang, C.; Kim, D.; Kim, D.; Sah, I.; Ryu, W.S.; Yoo, Y.S. Oxidation behaviors of wrought nickel-based superalloys in various high temperature environments. Trans. Nonferr. Met. Soc. China 2011, 21, 1524–1531. [Google Scholar] [CrossRef]
- Liu, B.C.; Li, H.; Lou, L.H. Isothermal Oxidation Behavior of Single-Crystal Nickel-Base Superalloy DD10. Rare Met. Mater. Eng. 2010, 39, 1407–1410. [Google Scholar]
- Xu, C.; Nai, Q.L.; Yao, Z.H.; Dong, J. Grain Boundary Oxidation Effect of GH4738 Superalloy on Fatigue Crack Growth. Acta Metall. Sin. 2017, 53, 44–51. [Google Scholar]
- Wang, Y.L.; Tan, Y.; Liu, L.B.; Li, P.; Li, X. Oxidation behavior and mechanism of GH4975 superalloy prepared electron beam smelting between 900 °C and 1100 °C in air. Vacuum 2024, 219, 112752. [Google Scholar] [CrossRef]
- Wang, Q.; Shuai, S.X.; Lu, G.X.; Attard, B.; Ma, R.; Gao, S.; Rochman, A.; Cassar, G. Residual stress release and corresponding microstructural changes in high-energy impact-modified GH4169 after aging at 425 °C and 650 °C. J. Mater. Res. Technol. 2024, 33, 6461–6466. [Google Scholar] [CrossRef]
- Shi, Z.X.; Yan, X.F.; Duan, C.H. Isothermal oxidation behavior of newly developed superalloy GH4282. Trans. Nonferr. Met. Soc. China. 2019, 44, 45–53. [Google Scholar]
- Gao, S.; Hou, J.S.; Yang, F.; Wang, C.S.; Zhou, L.Z. High Temperature Oxidation Behaviors of Two Cast Ni-based Superalloys. Rare Met. Mater. Eng. 2019, 48, 960–966. [Google Scholar]
- Li, Y.; Liu, X.G.; Guo, J.T.; Yuan, C.; Yang, H.C. Isothermal oxidation behavior of cast Ni-base superalloy K44. Trans. Nonferr. Met. Soc. China. 2006, 16, 1967–1972. [Google Scholar]
- Zheng, L.; Zhang, M.C.; Dong, J. Oxidation behavior and mechanism of powder metallurgy Rene95 nickel based superalloy between 800 and 1000 C. Appl. Surf. Sci. 2010, 256, 7510–7515. [Google Scholar] [CrossRef]
- Kumar, D.; Adharapurapu, R.R.; Pollock, T.M.; Was, G.S. High-Temperature Oxidation of Alloy 617 in Helium Containing Part-Per-Million Levels of CO and CO2 as Impurities. Met. Mater. Trans. A 2011, 42, 1245–1265. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.; Chellali, R.; Dong, J. Investigations on the growing, cracking and spalling of oxides scales of powder metallurgy Rene95 nickel-based superalloy. Appl. Surf. Sci. 2011, 257, 9762–9767. [Google Scholar] [CrossRef]
- Athreya, C.N.; Deepak, K.; Kim, D.I.; de Boer, B.; Mandal, S.; Sarma, V.S. Role of grain boundary engineered microstructure on high temperature steam oxidation behaviour of Ni based superalloy alloy 617. J. Alloys Compd. 2019, 778, 224–233. [Google Scholar] [CrossRef]
- Ma, J.Y.; Jiang, W.X.; Wang, J. Initial oxidation behavior of a single crystal superalloy during stress at 1150 °C. Sci. Rep. 2020, 10, 3089. [Google Scholar] [CrossRef]
- Bitara, T.E.; Meligya, M.E.; Khedrb, M. Investigation of exhaust valve failure in a marine diesel engine. Eng. Fail. Anal. 2020, 114, 104574. [Google Scholar] [CrossRef]




| Sample | Oxidation Rate (mg·cm−2·h−1) | |||
| 800 °C | 900 °C | 1000 °C | 1100 °C | |
| BM | 0.18 × 10−3 | 0.29 × 10−3 | 0.50 × 10−3 | 42.24 × 10−3 |
| WGBR | 0.06 × 10−3 | 0.16 × 10−3 | 0.35 × 10−3 | 1.00 × 10−3 |
| Sample | Kp (mg2·cm−4·min−1) | |||
| 800 °C | 900 °C | 1000 °C | 1100 °C | |
| BM | 1.01 × 10−5 | 1.85 × 10−5 | 1.31 × 10−4 | 2.78 × 10−2 |
| WGBR | 2.07 × 10−6 | 2.25 × 10−5 | 2.73 × 10−4 | 1.38 × 10−3 |
| Spots | Element (at.%) | ||||||
| O | Al | Ti | Cr | Co | Ni | Mo | |
| 1 | 21.17 | 1.03 | 3.37 | 7.16 | 7.47 | 37.07 | 1.31 |
| 2 | 47.72 | 11.72 | 5.83 | 8.32 | 5.86 | 19.58 | 0.98 |
| 3 | 50.30 | 38.38 | 0.61 | 1.77 | 0.98 | 7.24 | 2.87 |
| Spots | Element (at.%) | ||||||||
| O | Al | Ti | Cr | Nb | Ni | Zr | Co | Mo | |
| 1 | 54.44 | 0.93 | 5.70 | 33.06 | 12.22 | 2.02 | 1.31 | -- | -- |
| 2 | 59.91 | 21.55 | 11.43 | 3.64 | -- | 2.50 | 0.96 | -- | -- |
| 3 | -- | 5.91 | 3.27 | 11.36 | -- | 66.15 | -- | 9.74 | 3.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Lai, X.; He, J.; Li, X.; Fan, J.; Lai, F. Microstructural Investigation and High-Temperature Oxidation Performance of K417G Alloy Prepared by Wide-Gap Brazing. Crystals 2025, 15, 434. https://doi.org/10.3390/cryst15050434
Cheng Z, Lai X, He J, Li X, Fan J, Lai F. Microstructural Investigation and High-Temperature Oxidation Performance of K417G Alloy Prepared by Wide-Gap Brazing. Crystals. 2025; 15(5):434. https://doi.org/10.3390/cryst15050434
Chicago/Turabian StyleCheng, Zhun, Xin Lai, Jing He, Xiaoqiang Li, Jiafeng Fan, and Fuqiang Lai. 2025. "Microstructural Investigation and High-Temperature Oxidation Performance of K417G Alloy Prepared by Wide-Gap Brazing" Crystals 15, no. 5: 434. https://doi.org/10.3390/cryst15050434
APA StyleCheng, Z., Lai, X., He, J., Li, X., Fan, J., & Lai, F. (2025). Microstructural Investigation and High-Temperature Oxidation Performance of K417G Alloy Prepared by Wide-Gap Brazing. Crystals, 15(5), 434. https://doi.org/10.3390/cryst15050434






