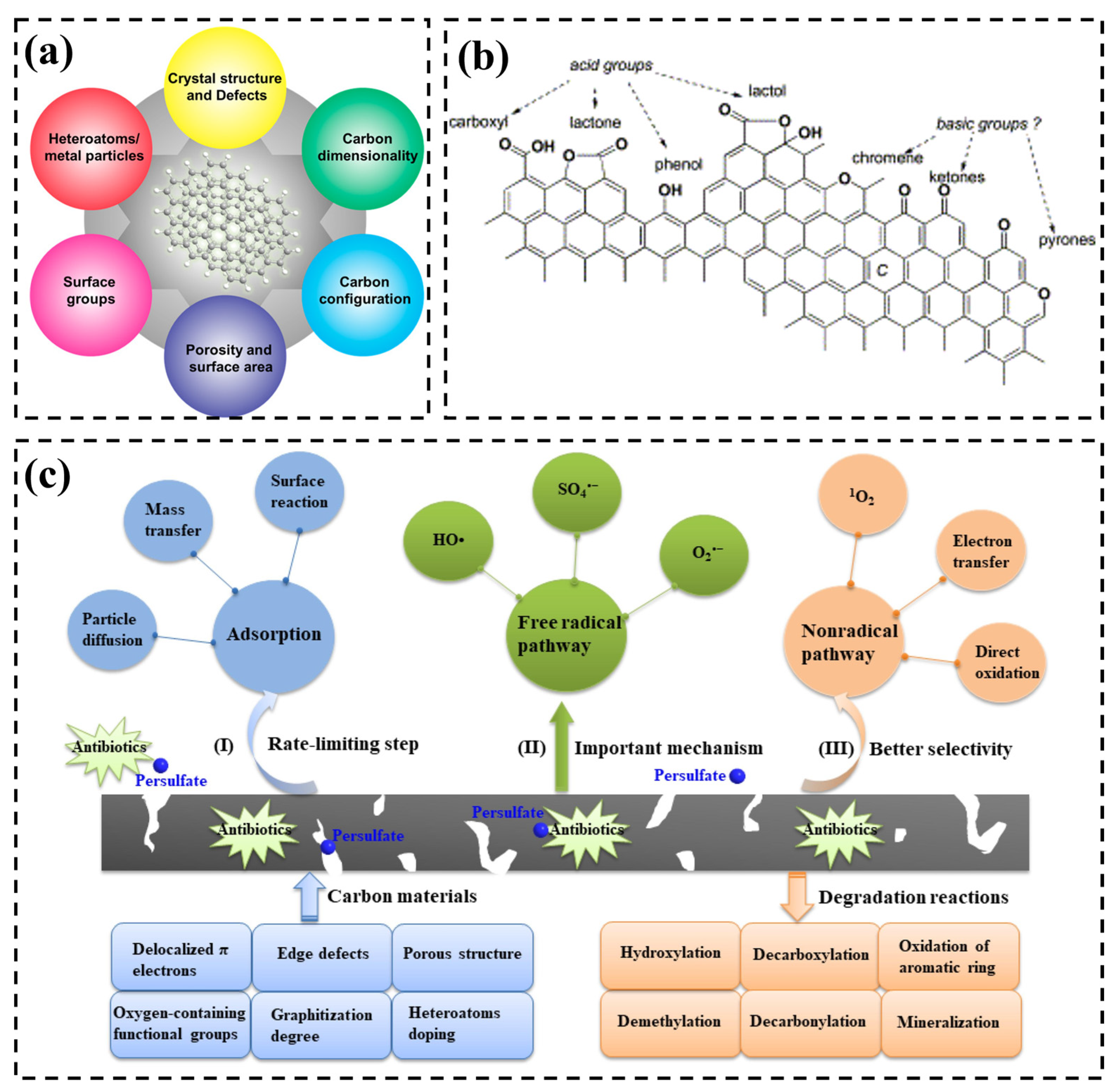
Advances in Activation of Persulfate by Novel Carbon-Based Materials: Degradation of Emerging Contaminants, Mechanisms, and Perspectives
Abstract
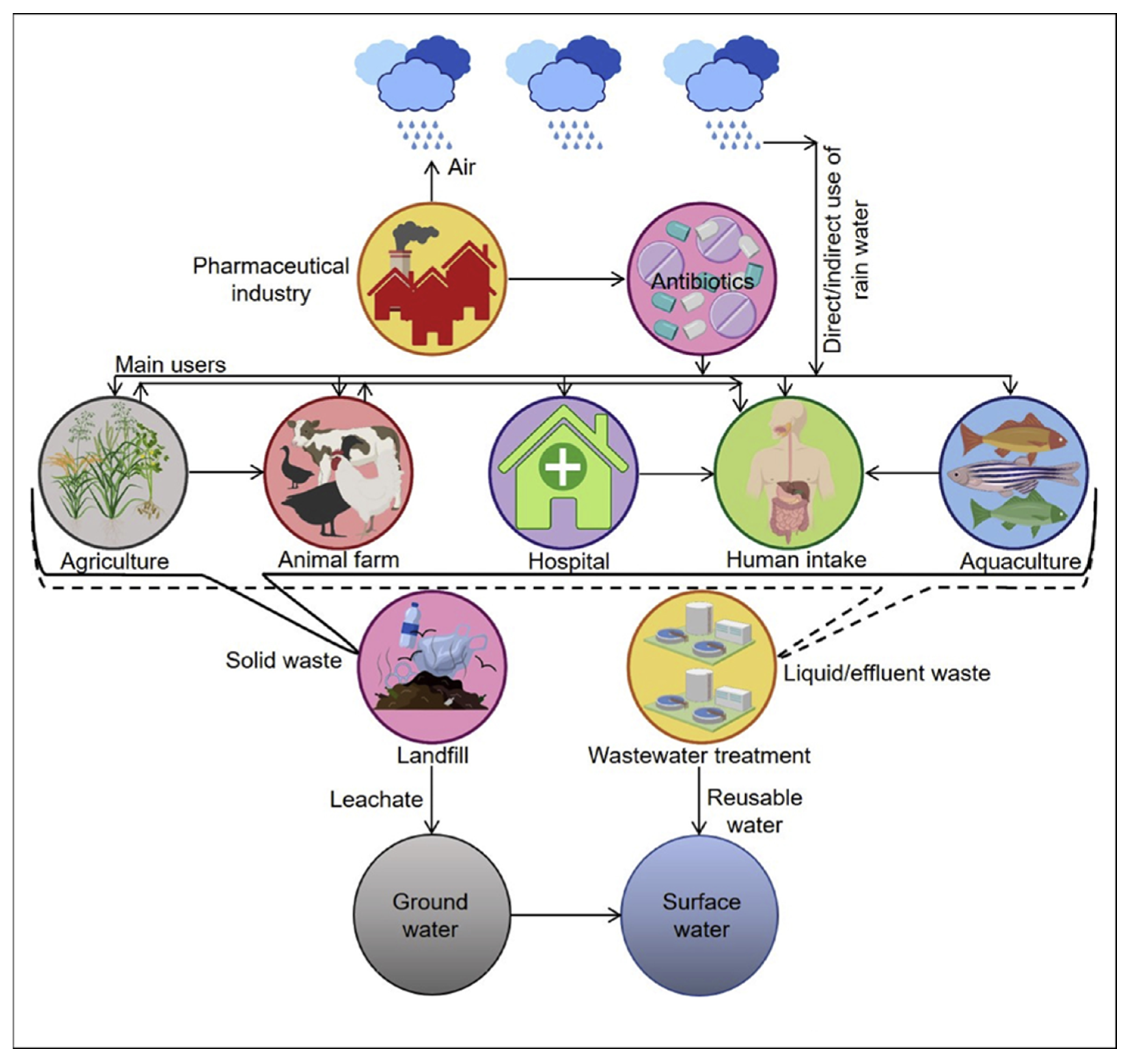
1. Introduction

2. Carbon-Based Material Catalysts
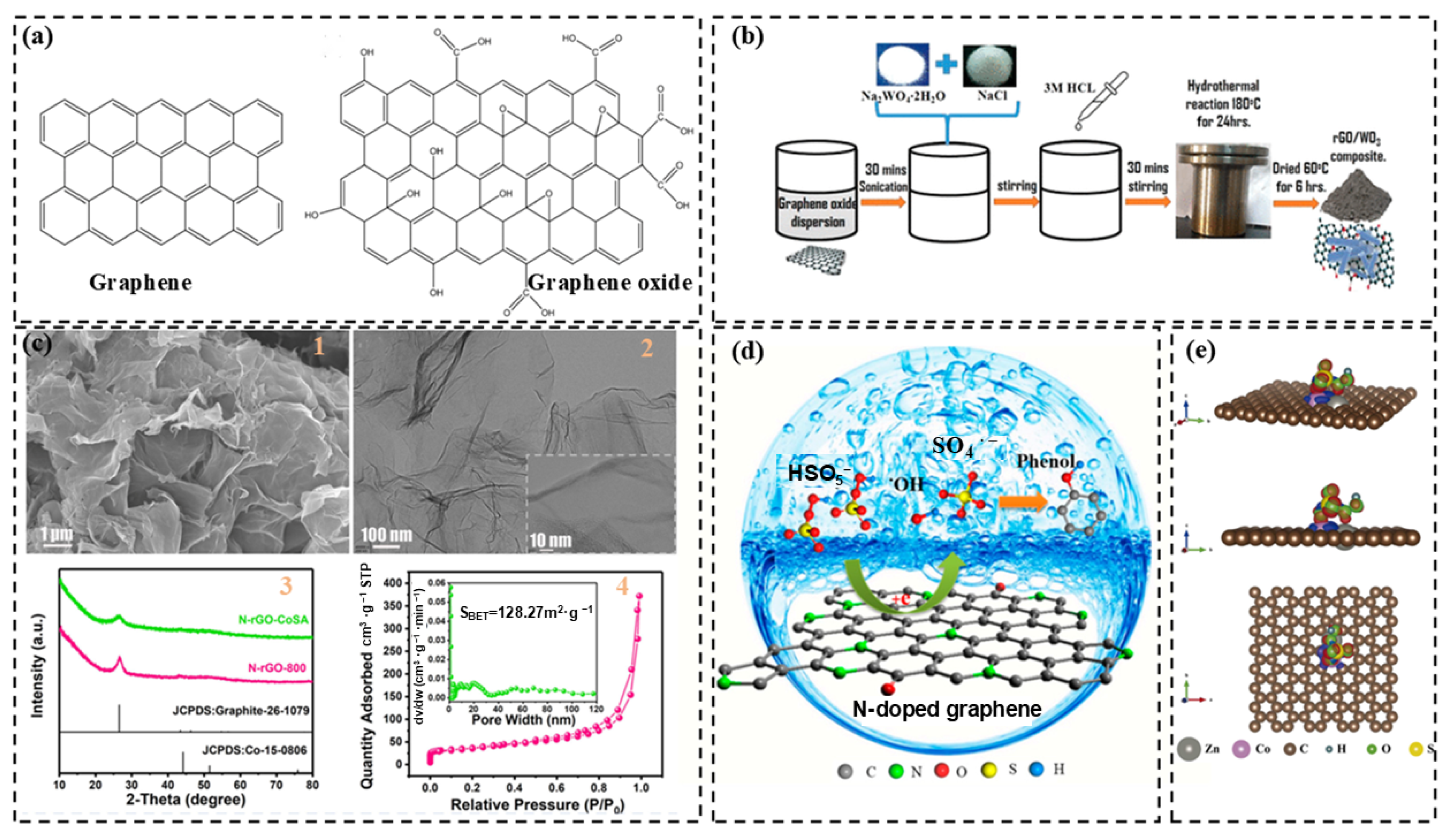
2.1. Graphene and Graphene Oxide
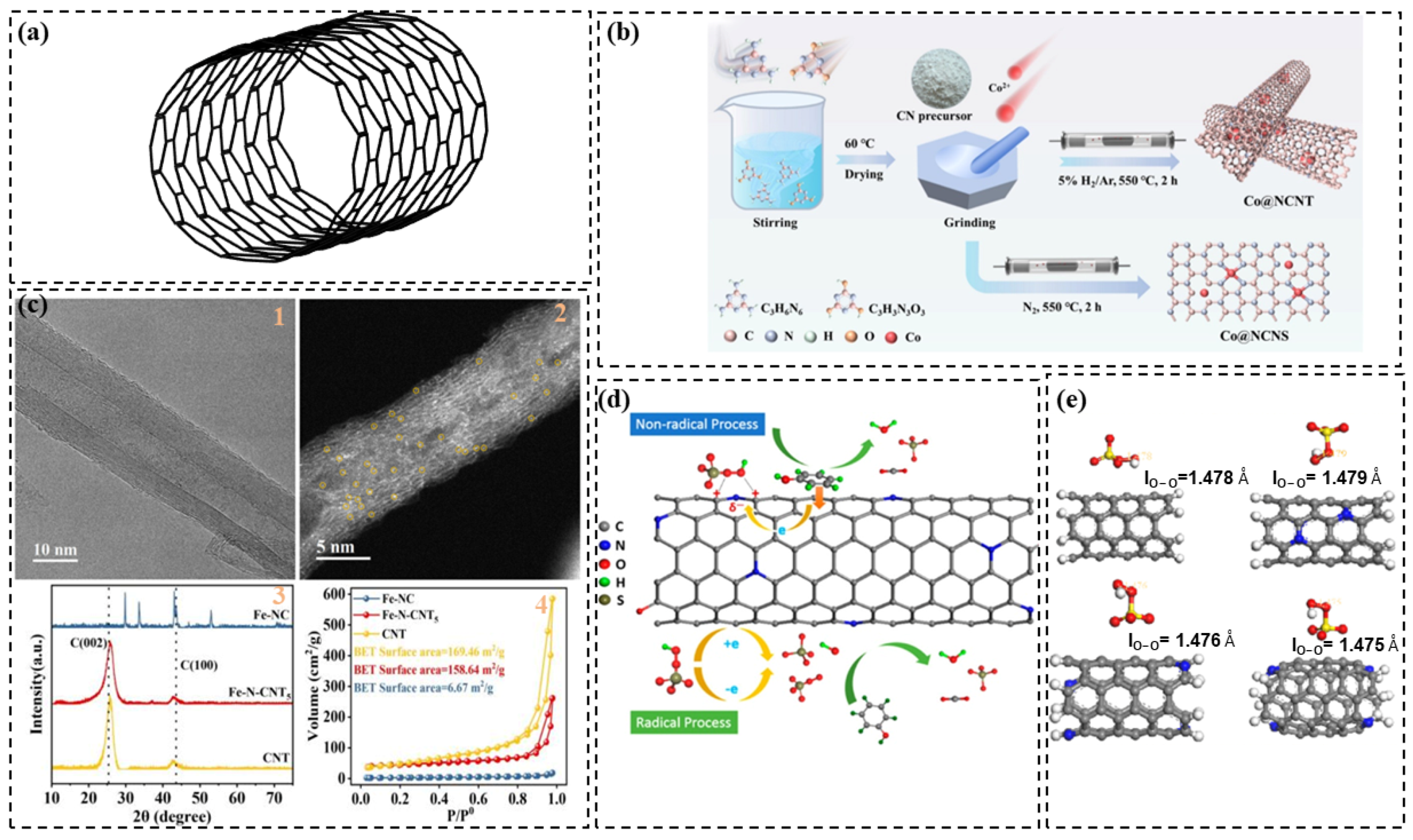
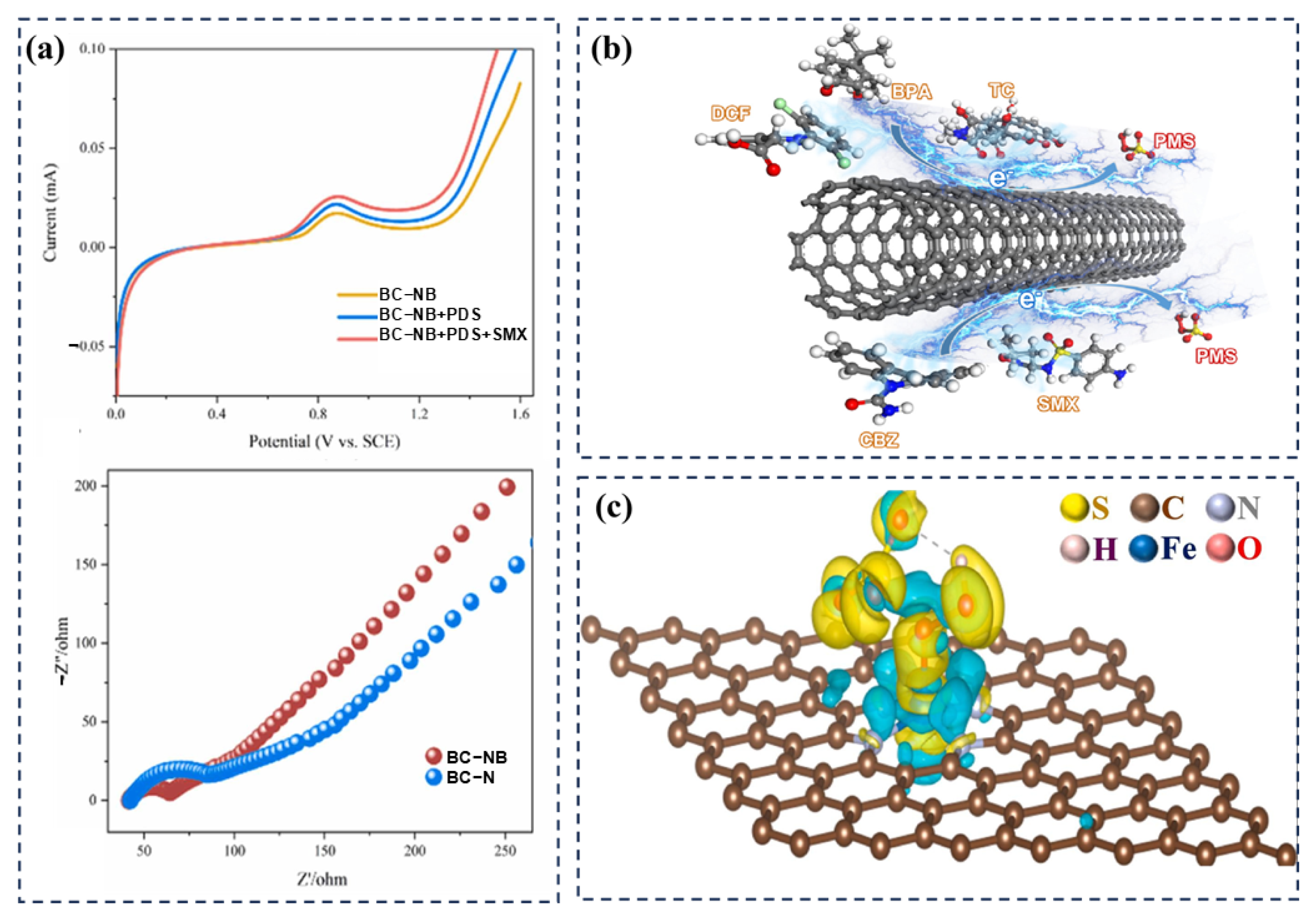
2.2. Carbon Nanotubes
2.3. Biochar
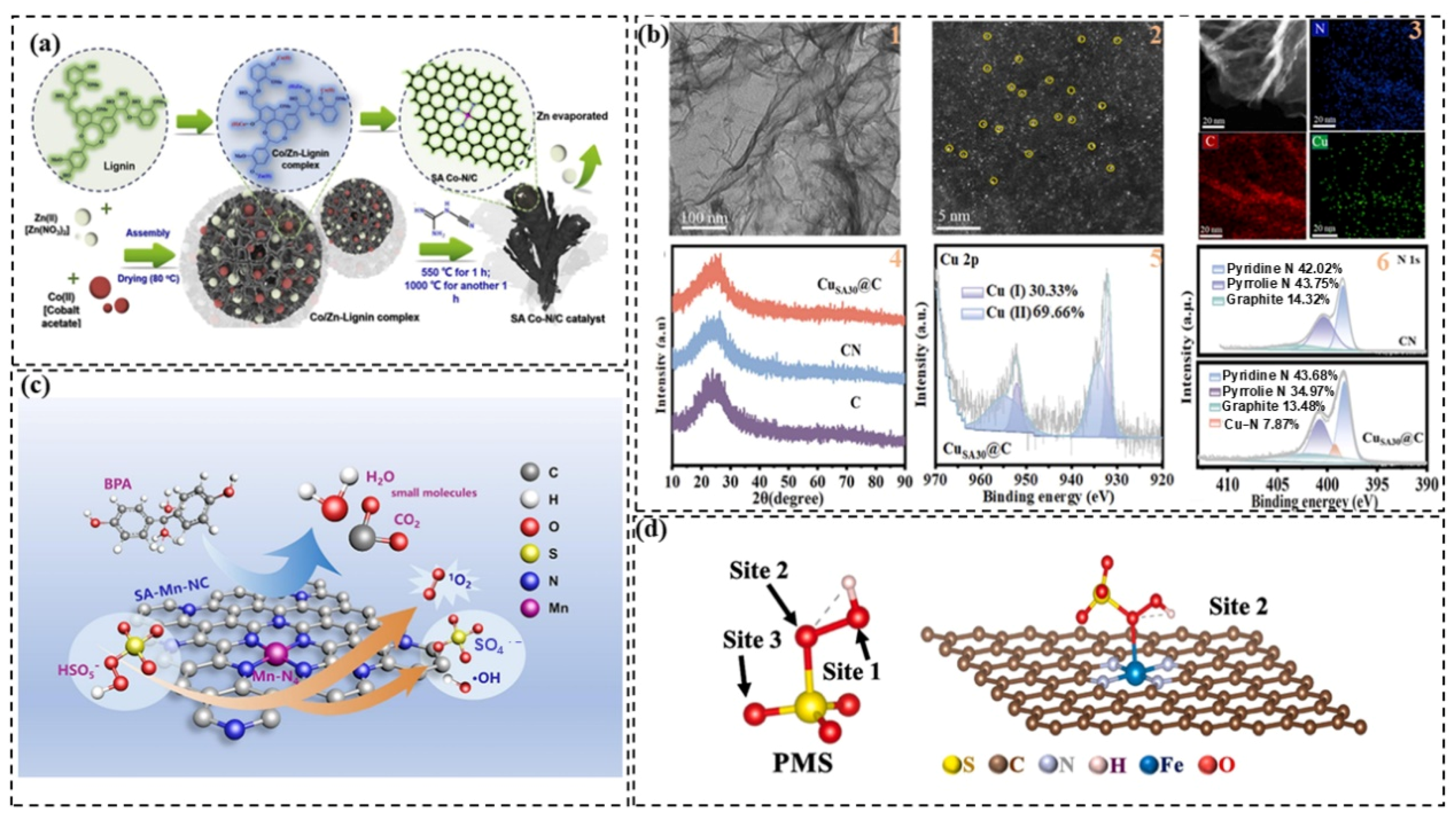
2.4. Other Carbon Materials
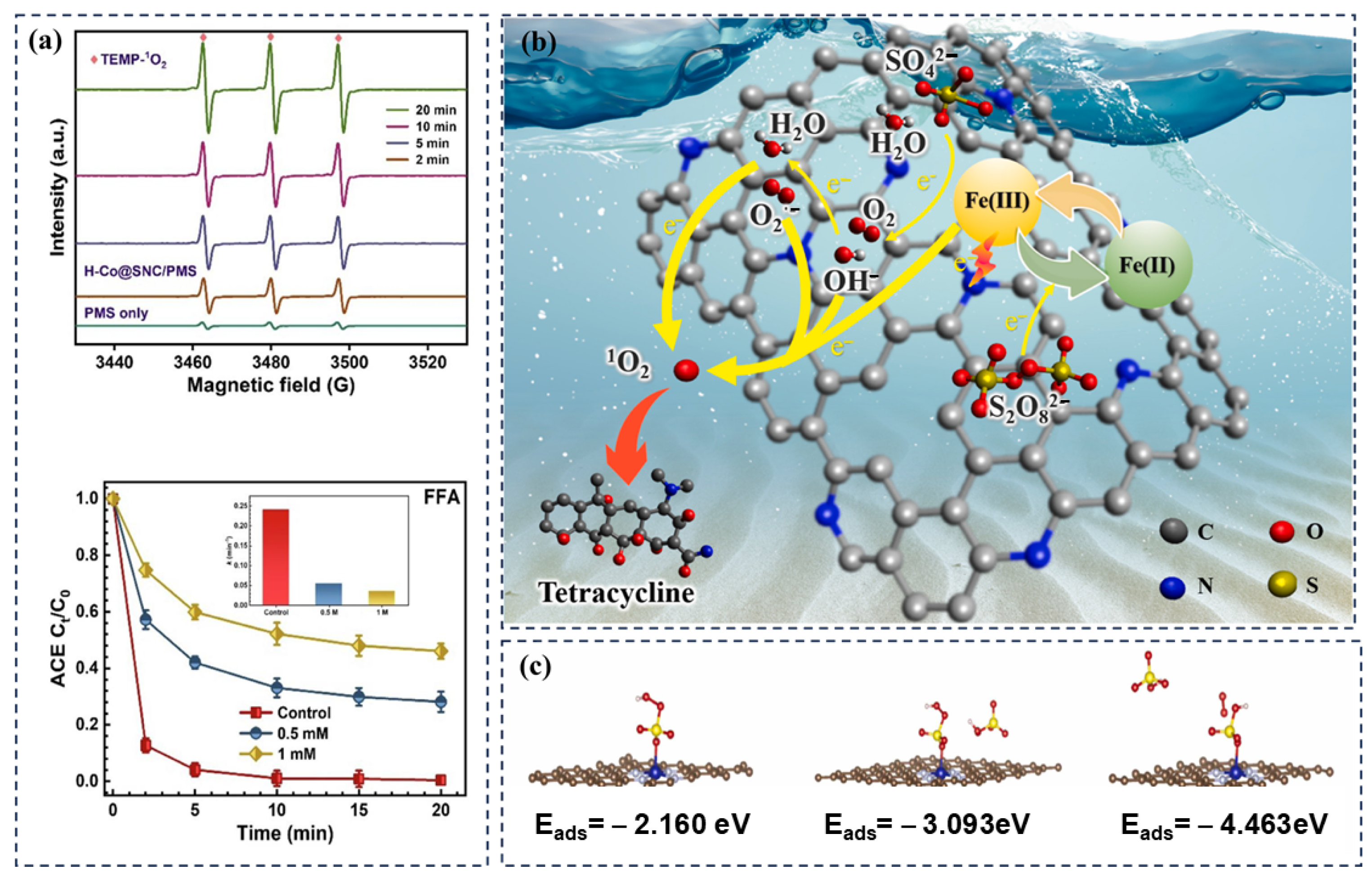
2.5. Single-Atom Catalysts
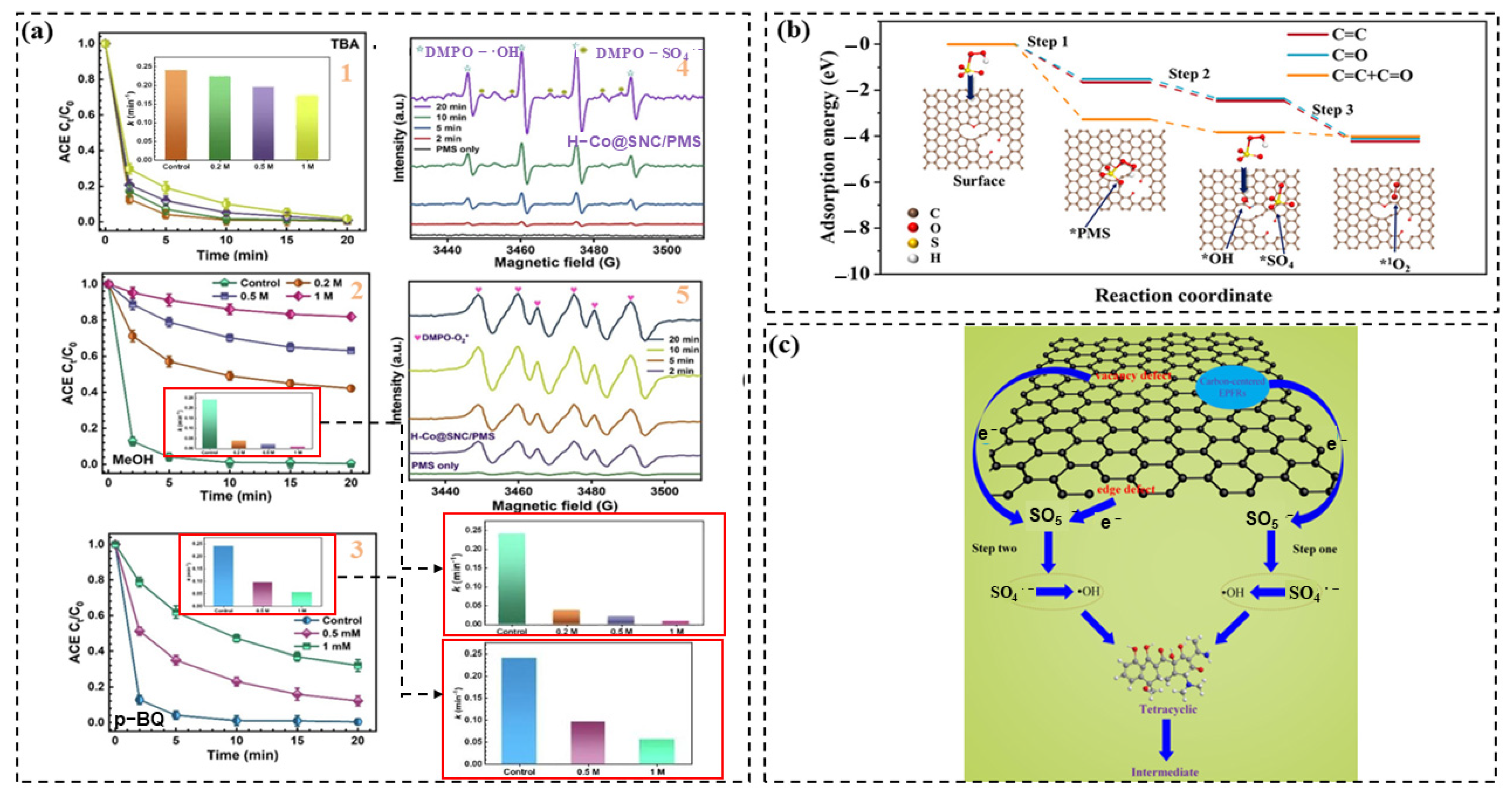
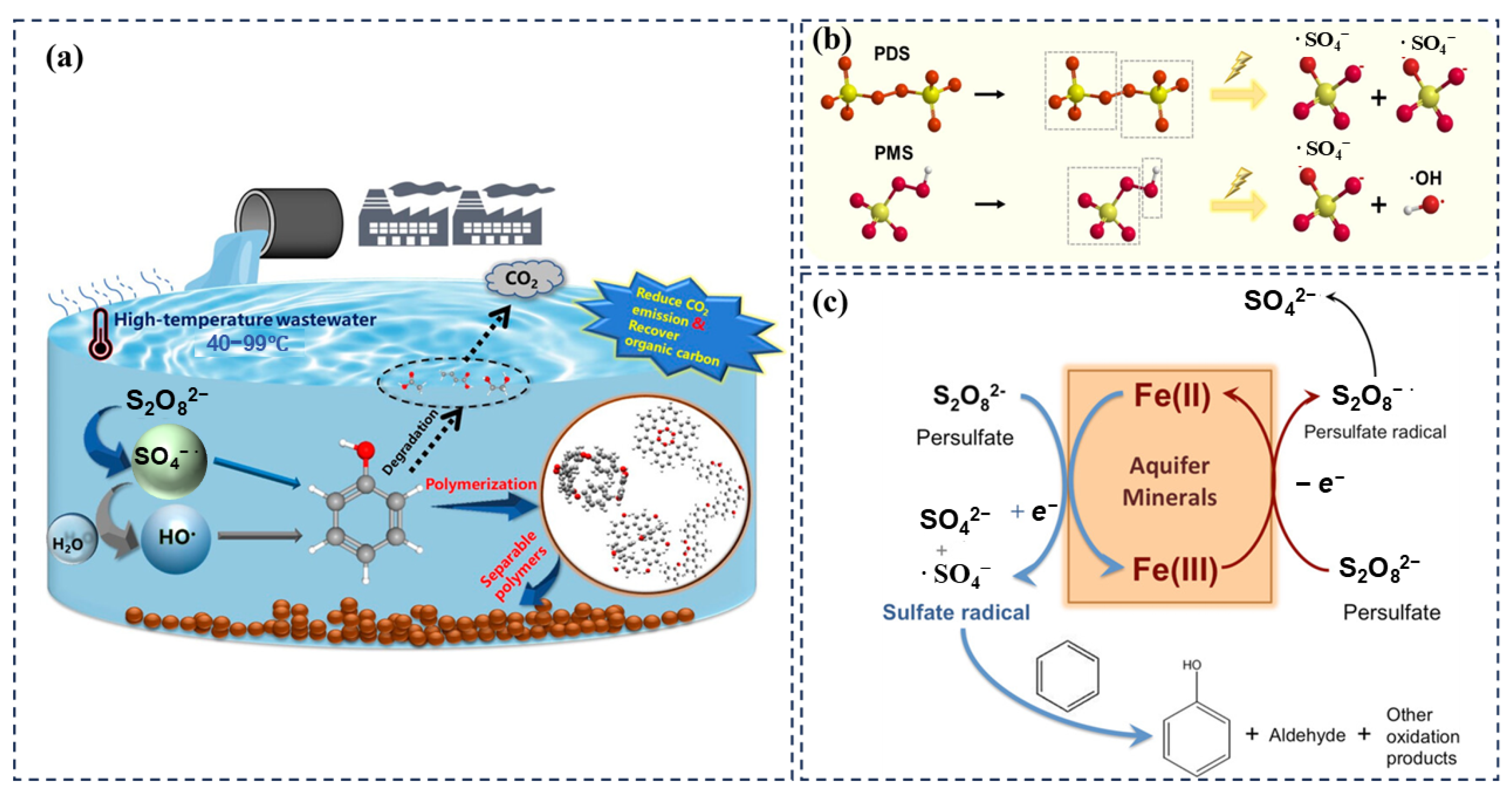
3. Degradation Mechanism
3.1. Radical Pathway
3.2. Non-Radical Pathways
3.2.1. Singlet Oxygen
3.2.2. Direct Oxidation
3.2.3. Electronic Transfer
4. Carbon-Based Catalyst Activation of PS for Degradation of ECs
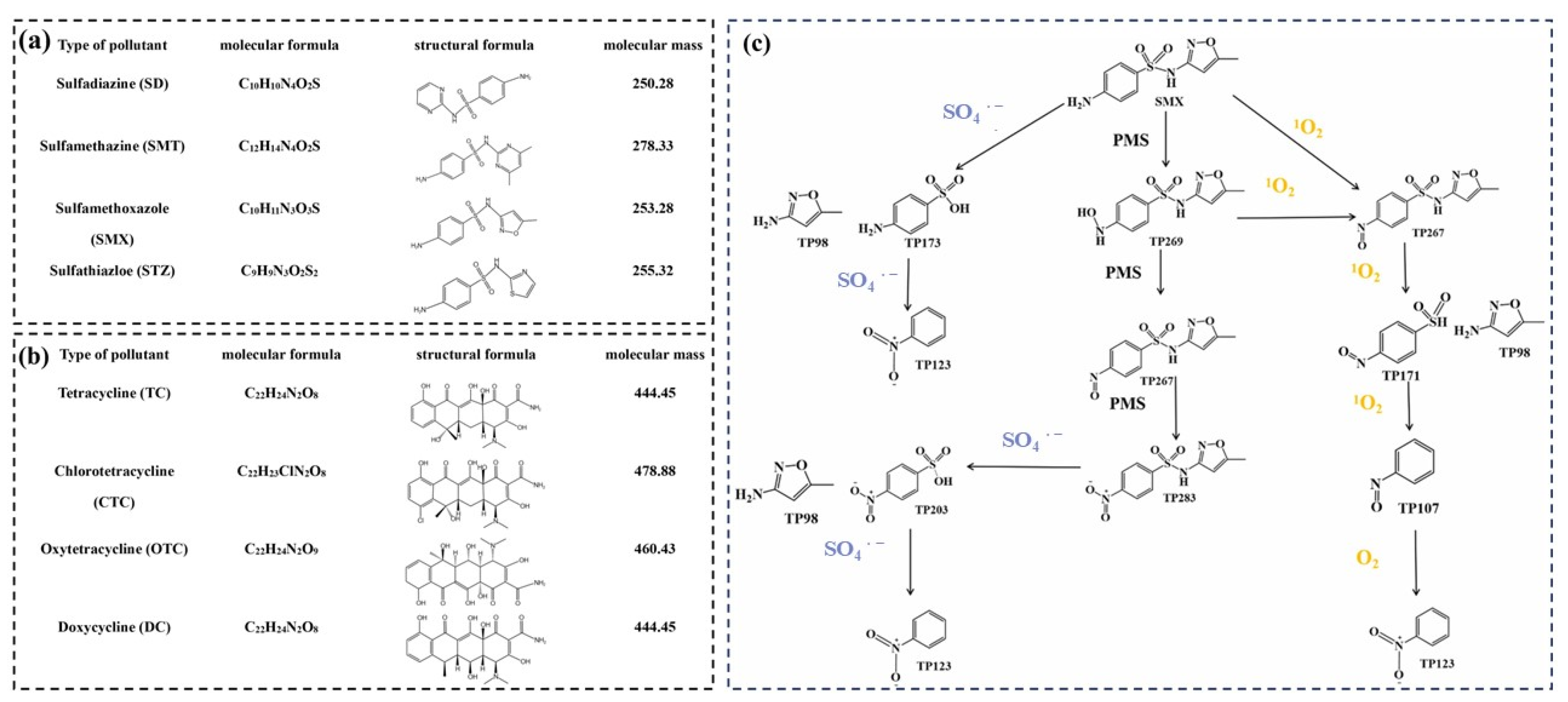
4.1. Antibiotics
4.2. Phenolic Compounds
4.3. Dyes
5. Auxiliary Activation Methods
6. Prospects and Outlook
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pankow, C.A.; Oh, M.; Heath, L.S.; Zhang, L.; Du, P.; Xia, K.; Pruden, A. Effect of antibiotic use and composting on antibiotic resistance gene abundance and resistome risks of soils receiving manure-derived amendments. Environ. Int. 2019, 128, 233–243. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic degradation of sulfamethoxazole using TiO2-based materials—Perspectives for the development of a sustainable water treatment technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Yee Leung, K.M.; Snyder, S.A.; Alvarez, P.J.J. Which Micropollutants in Water Environments Deserve More Attention Globally? Environ. Sci. Technol. 2022, 56, 13–29. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Xu, N.; Pan, B.; Ni, J. Pharmaceuticals and personal care products (PPCPs) in water, sediment and freshwater mollusks of the Dongting Lake downstream the Three Gorges Dam. Chemosphere 2022, 301, 134721. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Östman, M.; Olofsson, U.; Grabic, R.; Fick, J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014, 58, 221–229. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C. Occurrence, fate and health risk assessment of 10 common antibiotics in two drinking water plants with different treatment processes. Sci. Total Environ. 2019, 674, 316–326. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Change 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Achermann, S.; Bianco, V.; Mansfeldt, C.B.; Vogler, B.; Kolvenbach, B.A.; Corvini, P.F.X.; Fenner, K. Biotransformation of Sulfonamide Antibiotics in Activated Sludge: The Formation of Pterin-Conjugates Leads to Sustained Risk. Environ. Sci. Technol. 2018, 52, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, R.; Li, S.; Chen, X.; Ren, N.; Ho, S.-H. Biodegradation of Sulfonamide Antibiotics by Microalgae: Mechanistic Insights into Substituent-Induced Effects. ACS EST Water 2024, 4, 2422–2432. [Google Scholar] [CrossRef]
- Birkigt, J.; Gilevska, T.; Ricken, B.; Richnow, H.-H.; Vione, D.; Corvini, P.F.X.; Nijenhuis, I.; Cichocka, D. Carbon Stable Isotope Fractionation of Sulfamethoxazole during Biodegradation by Microbacterium sp. Strain BR1 and upon Direct Photolysis. Environ. Sci. Technol. 2015, 49, 6029–6036. [Google Scholar] [CrossRef]
- Vega, M.A.P.; Scholes, R.C.; Brady, A.R.; Daly, R.A.; Narrowe, A.B.; Vanzin, G.F.; Wrighton, K.C.; Sedlak, D.L.; Sharp, J.O. Methane-Oxidizing Activity Enhances Sulfamethoxazole Biotransformation in a Benthic Constructed Wetland Biomat. Environ. Sci. Technol. 2023, 57, 7240–7253. [Google Scholar] [CrossRef]
- Jia, X.; Li, S.; Wang, Y.; Wang, T.; Hou, X. Adsorption Behavior and Mechanism of Sulfonamide Antibiotics in Aqueous Solution on a Novel MIL-101(Cr)@GO Composite. J. Chem. Eng. Data 2019, 64, 1265–1274. [Google Scholar] [CrossRef]
- Dai, H.; Li, N.; Cui, Y.; Gao, W.; Peng, X.; Peng, W.; Si, H.; Mu, L.; Shi, Y.; Cheng, Z.; et al. Indispensable Synergy between C=C and C=O Sites in Biochar for Peroxomonosulfate Activation and Sulfamethoxazole Degradation. ACS EST Eng. 2024, 4, 2888–2897. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xiong, B.; Bai, F.; Wang, S.; Wan, Y.; Zhang, L.; Xie, P.; Wiesner, M.R. Application of Cobalt/Peracetic Acid to Degrade Sulfamethoxazole at Neutral Condition: Efficiency and Mechanisms. Environ. Sci. Technol. 2020, 54, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yang, Z.; Yu, X.; Li, M.; Cao, H. The reactivity of organic radicals in the performic, peracetic, perpropionic acids-based advanced oxidation process: A case study of sulfamethoxazole. J. Hazard. Mater. 2024, 476, 135033. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Liu, L.; Wang, Y.; Zhou, S.; Long, X.; Yu, J.; Jiao, F. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Chem. Eng. J. 2021, 421, 127845. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Li, X.; Zeng, L.; Zhu, M. Heterogeneous Photocatalytic Activation of Persulfate for the Removal of Organic Contaminants in Water: A Critical Review. ACS EST Eng. 2022, 2, 527–546. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, J.; Xing, M.; Zhang, Y. Current Mechanism of Peroxymonosulfate Activation by Cobalt-Based Heterogeneous Catalysts in Degrading Organic Compounds. ACS EST Eng. 2024, 4, 19–46. [Google Scholar] [CrossRef]
- Zheng, Z.; Min, J.; Wang, X.; Lung, C.W.; Shih, K.; Lo, I.M.C. Directional Separation of Highly Reductive Electrons to the Reactive Center in a Magnetic S-Scheme ZnFe2O4/A-MoS2 Heterojunction for Enhanced Peroxymonosulfate Activation toward Pharmaceuticals and Personal Care Product Removal. Environ. Sci. Technol. 2023, 57, 8414–8425. [Google Scholar] [CrossRef]
- Fang, G.; Wu, W.; Liu, C.; Dionysiou, D.D.; Deng, Y.; Zhou, D. Activation of persulfate with vanadium species for PCBs degradation: A mechanistic study. Appl. Catal. B Environ. 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Ait El Fakir, A.; Anfar, Z.; Enneiymy, M.; Jada, A.; El Alem, N. Conjugated polymers templated carbonization to design N, S co-doped finely tunable carbon for enhanced synergistic catalysis. Appl. Catal. B Environ. 2022, 300, 120732. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, L.; Song, C.; Chen, Z.; Meng, F.; Song, M. Selective Degradation of Electron-Rich Organic Pollutants Induced by CuO@Biochar: The Key Role of Outer-Sphere Interaction and Singlet Oxygen. Environ. Sci. Technol. 2022, 56, 10710–10720. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Xie, Y.; Yu, G.; Wang, Y. Clarification of the role of singlet oxygen for pollutant abatement during persulfate-based advanced oxidation processes: Co3O4@CNTs activated peroxymonosulfate as an example. Water Res. 2023, 244, 120480. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Liu, Z.; Tang, W.; Xiao, S.; Shao, B.; Liang, Q.; He, Q.; Pan, Y.; Zhao, C.; et al. Carbon nanotube-based materials for persulfate activation to degrade organic contaminants: Properties, mechanisms and modification insights. J. Hazard. Mater. 2022, 431, 128536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Leong, S.Y.; Li, Q. Modified biochar for removal of antibiotics and antibiotic resistance genes in the aqueous environment: A review. J. Water Process Eng. 2023, 55, 104222. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zhou, G.; Ang, H.M.; Tadé, M.O.; Wang, S. Reduced Graphene Oxide for Catalytic Oxidation of Aqueous Organic Pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Liu, S.; Wang, D.; Zhou, X.; Zhang, M.; Li, L.; Li, X.; Xu, F.; Nie, J. Metal-carbon hybrid materials induced persulfate activation: Application, mechanism, and tunable reaction pathways. Water Res. 2023, 234, 119808. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Li, J.; Wu, J.; Yao, X.; Zhang, T. Graphitic carbon nitride-based materials in activating persulfate for aqueous organic pollutants degradation: A review on materials design and mechanisms. Chemosphere 2021, 262, 127675. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-H.; Pei, D.-S. A review on efficient removal of phthalic acid esters via biochars and transition metals-activated persulfate systems. Chemosphere 2021, 277, 130256. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, L.; Zhang, S.; Tang, J. Nitrogen-doped biochar (N-doped BC) and iron/nitrogen co-doped biochar (Fe/N co-doped BC) for removal of refractory organic pollutants. J. Hazard. Mater. 2023, 446, 130727. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Li, J.-Y.; Liu, Z.-Q.; Cui, Y.-H.; Yang, S.-Q.; Gu, J.; Ma, J. Abatement of Aromatic Contaminants from Wastewater by a Heat/Persulfate Process Based on a Polymerization Mechanism. Environ. Sci. Technol. 2023, 57, 18575–18585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, Z.; Huang, D.; Zhao, D.; Wang, M. Photoactivated persulfate for water and wastewater remediations: Mechanisms, applications, and catalysts. Sep. Purif. Technol. 2025, 356, 129829. [Google Scholar]
- Liu, H.; Bruton, T.A.; Li, W.; Buren, J.V.; Prasse, C.; Doyle, F.M.; Sedlak, D.L. Oxidation of Benzene by Persulfate in the Presence of Fe(III)- and Mn(IV)-Containing Oxides: Stoichiometric Efficiency and Transformation Products. Environ. Sci. Technol. 2016, 50, 890–898. [Google Scholar] [CrossRef]
- Xue, X.; Xue, N.; Ouyang, D.; Yang, L.; Wang, Y.; Zhu, H.; Aihemaiti, A.; Yin, J. Biochar-Based Single-Atom Catalyst with Fe-N3O-C Configuration for Efficient Degradation of Organic Dyes by Peroxymonosulfate Activation. ACS Appl. Mater. Interfaces 2023, 15, 57003–57014. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pulimi, M. Sulfate Radical-Based Degradation of Organic Pollutants: A Review on Application of Metal-Organic Frameworks as Catalysts. ACS Omega 2023, 8, 34262–34280. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H. Structure–Function Correlations of Carbonaceous Materials for Persulfate-Based Advanced Oxidation. Langmuir 2021, 37, 13969–13975. [Google Scholar] [CrossRef] [PubMed]
- Montes-Morán, M.A.; Suárez, D.; Menéndez, J.A.; Fuente, E. On the nature of basic sites on carbon surfaces: An overview. Carbon 2004, 42, 1219–1225. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lim, T.-T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, J.; Guo, X.; Cui, Y.; Zhang, Z.; Zhang, X.; Hu, S.; Yang, C.; Chi, B.; Noborio, K.; et al. Activation of peroxymonosulfate by a CuCo-NC diatomic catalyst on N-doped graphene for selective generation of 1O2 towards efficient organic pollutant treatment. J. Environ. Chem. Eng. 2024, 12, 112151. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Pan, B. Development of Fe-doped g-C3N4/graphite mediated peroxymonosulfate activation for degradation of aromatic pollutants via nonradical pathway. Sci. Total Environ. 2019, 675, 62–72. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Ao, Z.; Zhou, L.; Wang, G.; Wang, S. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Kuang, C.; Wu, Y.; Zeng, G.; Zhou, Y.; Hu, Z.; Li, D.; Zhong, J.; Wang, R.; Yang, Y.; Li, C. Edge state engineering of nitrogen/phosphorus co-doped graphene nanoribbons towards electron-transfer-based peroxydisulfate activation. Sep. Purif. Technol. 2024, 350, 127842. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and Graphene Oxide-Based Composites for Removal of Organic Pollutants: A Review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Ramar, V.; Balasubramanian, K. Reduced Graphene Oxide/WO3 Nanorod Composites for Photocatalytic Degradation of Methylene Blue under Sunlight Irradiation. ACS Appl. Nano Mater. 2021, 4, 5512–5521. [Google Scholar] [CrossRef]
- Long, Y.; Dai, J.; Zhao, S.; Su, Y.; Wang, Z.; Zhang, Z. Atomically Dispersed Cobalt Sites on Graphene as Efficient Periodate Activators for Selective Organic Pollutant Degradation. Environ. Sci. Technol. 2021, 55, 5357–5370. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-Doped Graphene for Generation and Evolution of Reactive Radicals by Metal-Free Catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Lin, P.; Shi, Q.; Bai, H.; Zhang, H.; Zhu, W. A metal-organic framework (MOF) and graphene oxide (GO) based peroxymonosulfate (PMS) activator applied in pollutant removal. Process Saf. Environ. Prot. 2023, 171, 847–858. [Google Scholar] [CrossRef]
- Cui, J.; Shao, S.; Li, L.; Zhang, P.; Cui, J.; Hu, C.; Zhao, Y. Nanoconfinement-Regulated Peroxymonosulfate Activation via an Anomalously Efficient Mediated Electron-Transfer Pathway on Cobalt. ACS EST Eng. 2022, 2, 2014–2022. [Google Scholar] [CrossRef]
- Xie, X.; Xiao, F.; Zhan, S.; Zhu, M.; Xiang, Y.; Zhong, H.; Huang, H. Deep Oxidation of Chlorinated VOCs by Efficient Catalytic Peroxide Activation over Nanoconfined Co@NCNT Catalysts. Environ. Sci. Technol. 2024, 58, 1625–1635. [Google Scholar] [CrossRef]
- Fu, S.; Xu, Z.; Yang, H.; Pang, Y.; Wang, Y.; Zou, Y.; Li, S.; Xiao, Y.; Lu, Y.; Zhang, T. Iron Single-Atom Catalyst Actuates PMS/O3 Activation Process: Nonradical Generation Path for Synergistic Multiperoxides. ACS Appl. Eng. Mater. 2025, 3, 202–213. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Kang, J.; Wang, S. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Liu, S.; Pan, Q.; Li, J.; Wang, M.; Zhang, J.; Song, Y.; Zhao, C.; Shi, J.; Deng, H. Enhanced Mediated Electron Transfer Pathway of Peroxymonosulfate Activation Dominated with Graphitic-N for the Efficient Degradation of Various Organic Contaminants in Multiple Solutions. ACS EST Water 2022, 2, 817–829. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Liu, L.; Ye, Y.; Xie, X. Electron transfer mechanism of chitosan-modified natural manganese ore-cornstalk biochar composites with activated peroxymonosulfate: The role of functional groups on the surface of biochar-based composites. Sep. Purif. Technol. 2022, 302, 122107. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Jia, P.; Wang, X.; Liu, S.; Hua, Y.; Zhou, S.; Jiang, Z. Combined use of biochar and microbial agent can promote lignocellulose degradation and humic acid formation during sewage sludge-reed straw composting. Bioresour. Technol. 2023, 370, 128525. [Google Scholar] [CrossRef]
- Tan, J.; Chen, X.; Shang, M.; Cui, J.; Li, D.; Yang, F.; Zhang, Z.; Zhang, H.; Wu, Q.; Li, Y.; et al. N-doped biochar mediated peroxydisulfate activation for selective degradation of bisphenol A: The key role of potential difference-driven electron transfer mechanism. Chem. Eng. J. 2023, 468, 143476. [Google Scholar] [CrossRef]
- Qi, Y.; Ge, B.; Zhang, Y.; Jiang, B.; Wang, C.; Akram, M.; Xu, X. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: Role of graphitic N and radicals transformation. J. Hazard. Mater. 2020, 399, 123039. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Li, S.; Liu, J.; Deng, H.; Xia, D. Novel sludge-sugarcane bagasse mixed biochar as an efficient activator for peroxymonosulfate to degrade bisphenol AF. Chem. Eng. J. 2023, 462, 142114. [Google Scholar] [CrossRef]
- Ta, M.; Bai, H.; Wang, T.; Guo, J.; Zhen, Y.; Zhao, C.; Jing, Y.; Liu, Z.; Liu, G.; Zhang, F. Asymmetrically coordinated Co-N2S1 sites anchored on biochar regulating the free radical activation of peroxymonosulfate for efficient ofloxacin degradation. Chem. Eng. J. 2025, 505, 159899. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Jin, L.; Lu, Z.; Xiang, M.; Wang, C.; Wang, W.; Zhang, J.; Li, C.; Xie, H. Activation of peroxymonosulfate by magnetic Fe3S4/biochar composites for the efficient degradation of 2,4,6-trichlorophenol: Synergistic effect and mechanism. J. Environ. Chem. Eng. 2022, 10, 107085. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process. J. Hazard. Mater. 2021, 409, 124893. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, J.; Huang, L.; Liu, L.; Qiu, S.; Ding, J.; Liu, X.; Zhang, J. Activation of PMS by MIL-53(Fe)@AC composites contributes to tetracycline degradation: Properties and mechanisms. Surf. Interfaces 2024, 51, 104521. [Google Scholar] [CrossRef]
- Tian, M.; Ren, X.; Ding, S.; Fu, N.; Wei, Y.; Yang, Z.; Yao, X. Effective degradation of phenol by activating PMS with bimetallic Mo and Ni Co-doped g C3N4 composite catalyst: A Fenton-like degradation process promoted by non-free radical 1O2. Environ. Res. 2024, 243, 117848. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Huang, H.; Li, J.; Zhang, H.; Li, J. Pore structure-domoinated nonradical oxidation activity of self-dispersed Fe doped mesoporous-carbon for boosting antibiotics degradation efficiency. Sep. Purif. Technol. 2025, 362, 131742. [Google Scholar] [CrossRef]
- Chen, M.; Yang, C.; Yu, M.; Han, M.; Meng, Z.; Zhao, T.; Niu, J.; Mu, S.; Zhang, J.; Ma, J.; et al. N-doped carbon achieved by pyrolyzing urea and active carbon for the capacitive deionization coupled PMS oxidation system. Desalination 2024, 587, 117918. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, H.; Yao, Y.; Xiao, C.; Qi, J.; Zhou, Y.; Yang, Y.; Zhu, Z.; Li, J. Stabilizing atomic Co on 2D ordered mesoporous carbon sandwiched MXene for peroxymonosulfate activation: Enhanced performance and electron-transfer mechanism. Appl. Catal. B Environ. Energy 2024, 358, 124432. [Google Scholar] [CrossRef]
- Peng, X.; Luo, W.; Wu, J.; Hu, F.; Hu, Y.; Xu, L.; Xu, G.; Jian, Y.; Dai, H. Carbon quantum dots decorated heteroatom co-doped core-shell Fe0@POCN for degradation of tetracycline via multiply synergistic mechanisms. Chemosphere 2021, 268, 128806. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Bai, X.; Du, X.; Wang, Y.; Jin, P. Persulfate activation towards organic decomposition and Cr(VI) reduction achieved by a novel CQDs-TiO2−x/rGO nanocomposite. Chem. Eng. J. 2019, 373, 238–250. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Cheng, Z.; Xu, Q.; Zhang, X.; Liu, Y.; Qiu, F. Peroxymonosulfate (PMS) activated by magnetic Fe3O4 doped carbon quantum dots (CQDs) for degradation of Rhodamine B (RhB) under visible light: DFT calculations and mechanism analysis. J. Clean. Prod. 2023, 426, 139202. [Google Scholar] [CrossRef]
- He, D.; Zhu, K.; Huang, J.; Shen, Y.; Lei, L.; He, H.; Chen, W. N, S co-doped magnetic mesoporous carbon nanosheets for activating peroxymonosulfate to rapidly degrade tetracycline: Synergistic effect and mechanism. J. Hazard. Mater. 2022, 424, 127569. [Google Scholar] [CrossRef]
- Burbano, E.A.; Vallejo, C.A.; Ramirez, J.D.; Hidalgo-Troya, A.; Galeano, L.A. Heterogeneous Fenton oxidation of phenol photo-assisted with visible radiation in the presence of g-C3N4 catalysts modified with different iron phases. Chem. Eng. J. 2024, 485, 149766. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Lu, H.; Liu, l.; Tang, J. Silver and g-C3N4 co-modified biochar (Ag-CN@BC) for enhancing photocatalytic/PDS degradation of BPA: Role of carrier and photoelectric mechanism. Environ. Res. 2024, 262, 119972. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, H.; Zhang, Q.; Wang, D. Efficient activation of peroxomonosulfate (PMS) by co-doped g-C3N4/β-Bi2O3 heterojunction for the degradation of 2,4-dichlorophenol under visible light. J. Water Process Eng. 2025, 70, 107034. [Google Scholar] [CrossRef]
- Li, X.; Bu, W.; Zhu, K.; Chen, Y.; Liang, X.; Wang, B.; Wang, Y.; Yan, K. Fe-nanocluster embedded biomass-derived carbon for efficient photo-Fenton-like activity in water purification. Sep. Purif. Technol. 2024, 337, 126382. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, W.; Wu, D.; Wu, Q.; Hu, H. Highly selective production of singlet oxygen by manipulating the spin state of single-atom Co–N moieties and electron localization. Appl. Catal. B Environ. 2023, 324, 122248. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Su, J.; Chen, J.; Yu, Z.; Liu, L.; Yu, D.; Dong, S. A urea-based approach to synthesize single atom iron catalysts for catalyzing peroxymonosulfate to degrade antibiotics. Sep. Purif. Technol. 2024, 347, 127681. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, H.; Dong, W.; Du, T.; Li, X.; Wang, F.; Zhao, Z. Electron transfer-mediated peroxymonosulfate activation through monoatomic Fe–pyridinic N4 moiety in biochar-based catalyst for electron-rich pollutants degradation in groundwater. Chem. Eng. J. 2024, 492, 152158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Su, Y.; Wan, J. Asymmetric coordination structure constructed by Co doping enhances Fe–N–C catalyst to activate peroxymonosulfate for Bisphenol AF degradation. Chem. Eng. J. 2025, 504, 158891. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Yang, X.; Dai, Z.; Sun, Y.; Liu, H.; Hu, Z.; Zheng, X. Ru single atom catalyst with dual reaction sites for efficient fenton-like degradation of organic contaminants. Appl. Catal. B Environ. 2023, 320, 121952. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, S.; Yuan, Y.; Yu, W.; Fang, S.; Kong, F.; Qiu, J.; Wang, S.; Zhang, J. Coordination dependent photocatalytic peroxymonosulfate activation on biomass derived Fe single atom catalysts for atrazine degradation. Chem. Eng. J. 2024, 499, 156625. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Zhang, Y.; Cao, Q.; Si, Y.; Wu, Z.; Akram, M.; Xu, X. Novel lignin-based single atom catalysts as peroxymonosulfate activator for pollutants degradation: Role of single cobalt and electron transfer pathway. Appl. Catal. B Environ. 2021, 286, 119910. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Fan, Z.; Duan, P.; Wang, L.; Pan, J.; Gao, B. Redox potentials of sulfonamide antibiotics mediating the electron transfer process in single-atom Cu catalyst/peroxymonosulfate system: Selective removal mechanisms for sulfonamides. J. Hazard. Mater. 2025, 485, 136880. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Xue, Y.; Fan, J. Efficient activation of peroxymonosulfate by Mn single-atom: Critical role of Mn-N4 coordination for generating singlet oxygen. Sep. Purif. Technol. 2024, 335, 126129. [Google Scholar] [CrossRef]
- An, L.; Wang, J.; Wang, J.; Liu, H.; Wu, F.; Hu, T.; Qian, X.; Zhang, L.; Sun, Y.; Wang, X.; et al. Efficient peroxymonosulfate activation by iron-cobalt bimetallic biochar for rapid removal of antibiotic resistant bacteria, antibiotic resistance genes, and ampicillin: The coexisting of free-radical and non-radical pathways. Sep. Purif. Technol. 2024, 342, 127025. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, H.; Zhao, Z.; Xu, L.; Li, M.; Liu, W.; Lai, B.; Vithanage, M.; Pu, S. Electron transfer tuning for persulfate activation via the radical and non-radical pathways with biochar mediator. J. Hazard. Mater. 2025, 486, 136825. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xiao, H.; Liu, C.; Li, Y.; Li, D.; Li, M.; Liu, Y. Efficient degradation of tetracycline with N-rGO/CuO catalysts under high salinity condition via persulfate activation dominated by non-radical pathways. Sep. Purif. Technol. 2023, 327, 124936. [Google Scholar] [CrossRef]
- Wang, J.; Lv, H.; Tong, X.; Ren, W.; Shen, Y.; Lu, L.; Zhang, Y. Modulation of radical and nonradical pathways via modified carbon nanotubes toward efficient oxidation of binary pollutants in water. J. Hazard. Mater. 2023, 459, 132334. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
- He, Q.; Zhao, C.; Tang, L.; Liu, Z.; Shao, B.; Liang, Q.; Wu, T.; Pan, Y.; Wang, J.; Liu, Y.; et al. Peroxymonosulfate and peroxydisulfate activation by fish scales biochar for antibiotics removal: Synergism of N, P-codoped biochar. Chemosphere 2023, 326, 138326. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Xu, J.; Qi, W.; Hao, R.; Lin, D.; Li, D.; Zhang, J. Magnetic biochar based on platanus leaves and iron sludge for persulfate activation and catalytic degradation of tetracycline. J. Clean. Prod. 2022, 370, 133336. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Choi, J.; Kim, M.; Kim, M.S.; Lee, D.; Bang, W.H.; Yun, E.-T.; Lee, H.; Lee, J.-H.; Lee, C.; et al. Chloride-Mediated Enhancement in Heat-Induced Activation of Peroxymonosulfate: New Reaction Pathways for Oxidizing Radical Production. Environ. Sci. Technol. 2021, 55, 5382–5392. [Google Scholar] [CrossRef]
- Tuan, D.D.; Tsai, Y.-c.; Linh, H.X.; Thanh, D.V.; Khiem, T.C.; Lin, K.-Y.A. MOF-derived hollow Co@N-doped carbon as an efficient peroxymonosulfate activator for acetaminophen degradation: Dominant sulfate radical-induced degradation pathway. Sep. Purif. Technol. 2025, 354, 129381. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Liang, S.; Feng, Z.; Zhao, J. Mechanism of persulfate activation by biochar for the catalytic degradation of antibiotics: Synergistic effects of environmentally persistent free radicals and the defective structure of biochar. Sci. Total Environ. 2021, 794, 148707. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Hu, Y.; Lv, Y.; Liu, Y.; Ye, X.; Lin, C.; Song, L.; Tian, C.; Yang, G.; Liu, M. Non-free radical regulation mechanism based on pH in the peroxymonosulfate activation process mediated by single-atom Co catalyst for the specific rapid degradation of emerging pollutants. J. Colloid Interface Sci. 2025, 687, 617–629. [Google Scholar] [CrossRef]
- Liang, J.; Chen, K.; Duan, X.; Zhao, L.; Qiu, H.; Xu, X.; Cao, X. pH-dependent generation of radical and nonradical species for sulfamethoxazole degradation in different carbon/persulfate systems. Water Res. 2022, 224, 119113. [Google Scholar] [CrossRef]
- Ma, D.; Mei, J.; Liang, Q.; Jiao, Y.; Hu, T.; Chen, J.; Wang, J.; Zhou, H.; Lian, Q.; Sun, M.; et al. Recent advances in nanomaterial-enhanced persulfate activation for organic pollutants removal: Electron transfer, surface reactions, and radical generation. J. Environ. Chem. Eng. 2023, 11, 111511. [Google Scholar] [CrossRef]
- Qi, Z.; Wu, X.; Li, Q.; Lu, C.; Carabineiro, S.A.C.; Zhao, Z.; Liu, Y.; Lv, K. Singlet oxygen in environmental catalysis: Mechanisms, applications and future directions. Coord. Chem. Rev. 2025, 529, 216439. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Yang, Q.; Guo, W.; Ren, X. Surface-regulated FexOy/NG catalysts enhancing persulfate activation to remove tetracycline via a singlet oxygen-dominated pathway. Sep. Purif. Technol. 2025, 361, 131570. [Google Scholar] [CrossRef]
- Zheng, Q.; Guo, J.; Ren, X.; Zhang, W.; Qin, H.; Zhang, P.; Zhang, S.; Xu, X. Singlet oxygen triggered by carbon doped boron nitride nanosheets for enhanced removal of tetracycline by peroxymonosulfate activation. J. Environ. Chem. Eng. 2023, 11, 110863. [Google Scholar] [CrossRef]
- Xiong, S.; Deng, Y.; Gong, D.; Tang, R.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Liao, C.; Yang, L. Magnetically modified in-situ N-doped Enteromorpha prolifera derived biochar for peroxydisulfate activation: Electron transfer induced singlet oxygen non-radical pathway. Chemosphere 2021, 284, 131404. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; He, H.; Cai, A.; Xi, S.; Du, J.; Zhang, F.; Fan, X.; Peng, W.; Li, Y. Wastewater flocculation substrate derived three-dimensional ordered macroporous Co single-atom catalyst for singlet oxygen-dominated peroxymonosulfate activation. Appl. Catal. B Environ. 2023, 335, 122886. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Li, Y.-Q.; Hayat, F.; Liu, C.; Li, J.; Chen, M. Multi-targeted removal of coexisted antibiotics in water by the synergies of radical and non-radical pathways in PMS activation. Sep. Purif. Technol. 2023, 305, 122475. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Xu, S.; Huang, J.; Li, F.; Wang, C. Selective oxidation of organic pollutants by unactivated and carbonate-activated peroxymonosulfate (PMS): Mechanism, kinetics, and transformation pathway. Sep. Purif. Technol. 2025, 356, 129472. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Selective degradation of sulfonamide antibiotics by peroxymonosulfate alone: Direct oxidation and nonradical mechanisms. Chem. Eng. J. 2018, 334, 2539–2546. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Zhang, W.; Shao, W.; Wang, L.; Jia, Q.; Zhou, L.; Xiu, G. Biochar-supported MnS nanocrystals as peroxymonosulfate activator to remove levofloxacin in water: Dominated by electron-transfer regime. J. Environ. Chem. Eng. 2025, 13, 116075. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Li, Y.; Zhao, M.; Kang, H.; Liu, Y.; Jiang, H.; Chen, L.; Wang, J.; Zhou, W.; et al. Nitrogen-doped carbon modified with boron atoms activating peroxydisulfate for sulfamethoxazole degradation: The electron transfer-dominated pathway. J. Environ. Chem. Eng. 2025, 13, 116080. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Y.; Li, Z.; Zhang, S.; Du, C. Nitrogen-Doped Biochar for Enhanced Peroxymonosulfate Activation to Degrade Phenol through Both Free Radical and Direct Oxidation Based on Electron Transfer Pathways. Langmuir 2024, 40, 8520–8532. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Wang, L.; Chen, Z.; Cheng, D.; Liu, H.; Ngo, H.H.; Li, J.; Cao, X.; Wang, Y.; Zhang, J. A novel layered porous Fe, Cu dual-loading biochar heterogeneous catalyst to guided non-free radical pathway for peroxymonosulfate activation. J. Colloid Interface Sci. 2025, 689, 137262. [Google Scholar] [CrossRef]
- Chen, F.; Wu, X.-L.; Yang, L.; Chen, C.; Lin, H.; Chen, J. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst. Chem. Eng. J. 2020, 394, 124904. [Google Scholar] [CrossRef]
- Kayani, K.F. Tetracycline in the environment: Toxicity, uses, occurrence, detection, and removal by covalent organic frameworks—Recent advances and challenges. Sep. Purif. Technol. 2025, 364, 132418. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, G.; Shao, P.; Ren, W.; Li, M.; Hu, Y.; Yang, L.; Shi, H.; Luo, X. A comparison of SMX degradation by persulfate activated with different nanocarbons: Kinetics, transformation pathways, and toxicity. Appl. Catal. B Environ. 2022, 310, 121345. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ji, Y.; Shen, T. Reduced graphene oxide-supported hollow Co3O4@N-doped porous carbon as peroxymonosulfate activator for sulfamethoxazole degradation. Chem. Eng. J. 2022, 430, 132951. [Google Scholar] [CrossRef]
- Hou, S.; Hu, H.; Fu, Q.; Xiao, T.; Xie, J.-Q.; Chan, S.-H.; He, M.; Miao, B.; Zhang, L. Undaria pinnatifida (wakame)-derived Fe, N co-doped graphene-like hierarchical porous carbon as highly efficient catalyst for activation of peroxymonosulfate (PMS) toward degradation of tetracycline (TC). Sep. Purif. Technol. 2024, 333, 125980. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Chen, J.; Wu, T.; Wang, J.; Zhao, X.; Sun, W.; Lin, H.; Lv, S. Graphene/Fe@N-doped carbon hybrid derived from spent MOF adsorbent as efficient persulfate activator for degradation of tetracycline hydrochloride. Chem. Eng. J. 2022, 431, 133443. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yan, Y.; Wang, F.; Feng, L.; Chen, Y. Nitrogen-doped graphene for tetracycline removal via enhancing adsorption and non-radical persulfate activation. Environ. Res. 2023, 235, 116642. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of sulfamethoxazole from water via activation of persulfate by Fe3C@NCNTs including mechanism of radical and nonradical process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, C.; Li, X.; Qi, K.; Gao, L. Degradation of tetracycline by peroxymonosulfate activated with Mn0.85Fe2.15O4-CNTs: Key role of singlet oxygen. Environ. Res. 2023, 227, 115750. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Zhao, J.; Zhang, B.-T. Peroxymonocarbonate activation by Co in N-doped carbon for efficient quinolone degradation. Process Saf. Environ. Prot. 2025, 195, 106790. [Google Scholar] [CrossRef]
- Pang, S.; Zhou, C.; Sun, Y.; Zhang, K.; Ye, W.; Zhao, X.; Cai, L.; Hui, B. Natural wood-derived charcoal embedded with bimetallic iron/cobalt sites to promote ciprofloxacin degradation. J. Clean. Prod. 2023, 414, 137569. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhong, Y.; Lim, T.-T. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity. Chem. Eng. J. 2019, 372, 420–428. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Li, J.; Zhang, M.; Zhang, Y.; Zhan, R.; Li, J.; Wang, Z. Insights into the practicability of electrochemical enhanced heterogeneous activation of peroxymonosulfate for the treatment of liquid waste during penicillin g production. Chem. Eng. J. 2023, 455, 140590. [Google Scholar] [CrossRef]
- Dou, M.; Wang, J.; Gao, B.; Ma, Z.; Huang, X. A novel in-situ chemical oxidation channel—Selective pH-dependence of refractory β-lactam antibiotics in the synergistic mechanism of persulfate and g-C3N4 under visible light. Chem. Eng. J. 2020, 394, 124899. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, S.; Guo, C.; Yang, W.; Ma, J. Amorphous carbon from coal slime for efficient degradation of phenol by non-free radical activation of peroxymonosulfate. Fuel 2024, 373, 132285. [Google Scholar] [CrossRef]
- Cao, B.; Qu, J.; Bian, W.; Hu, Q.; Fu, X.; Zhang, G.; Zhang, Y.; Tao, Y.; Jiang, Z.; Zhang, Y. Porous hydrochar loaded nZVI as an efficient catalyst to activate persulfate for phenol degradation: Performance and mechanism. J. Clean. Prod. 2024, 444, 141221. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, N.; Wang, D.; Wang, L.; Song, Y.; Chen, Z.; Ma, J.; Zhang, T. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways. Appl. Catal. B Environ. 2020, 263, 118350. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.-J.; Jeong, J.; Lee, J.; Park, N.-B.; Lee, C. Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 2015, 266, 28–33. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Fang, G.; Zhang, M.; Kong, L.; Zhu, F.; Ma, L.; Zhou, D.; Zhan, J. Biomass Schiff base polymer-derived N-doped porous carbon embedded with CoO nanodots for adsorption and catalytic degradation of chlorophenol by peroxymonosulfate. J. Hazard. Mater. 2020, 384, 121345. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Dong, H.; Li, Y.; Xiao, J.; Dong, Q.; Xiang, S.; Chu, D. Activation of persulfate by graphene/biochar composites for phenol degradation: Performance and nonradical dominated reaction mechanism. J. Environ. Chem. Eng. 2023, 11, 109348. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.G.; Ko, S.O. Catalytic degradation of acetaminophen by Fe and N Co-doped multi-walled carbon nanotubes. Environ. Res. 2021, 201, 111535. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.-G.; Ko, S.-O. Advanced oxidative degradation of acetaminophen by carbon catalysts: Radical vs non-radical pathways. Environ. Res. 2020, 188, 109767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Y.; Duan, X.; Zhou, S.; Niu, Y.; Sun, H.; Zhi, L.; Wang, S. Unzipping carbon nanotubes to nanoribbons for revealing the mechanism of nonradical oxidation by carbocatalysis. Appl. Catal. B Environ. 2020, 276, 119146. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.; Chen, Z.; Chen, Z. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Liang, C.; Sun, H.; Ling, C.; Liu, X.; Li, M.; Zhang, X.; Guo, F.; Zhang, X.; Shi, Y.; Cao, S.; et al. Pyrolysis temperature-switchable Fe-N sites in pharmaceutical sludge biochar toward peroxymonosulfate activation for efficient pollutants degradation. Water Res. 2023, 228, 119328. [Google Scholar] [CrossRef]
- Abu-Nada, A.; Abdala, A.; McKay, G. Removal of phenols and dyes from aqueous solutions using graphene and graphene composite adsorption: A review. J. Environ. Chem. Eng. 2021, 9, 105858. [Google Scholar] [CrossRef]
- Dhila, H.; Bhapkar, A.; Bhame, S. Metal oxide/biochar hybrid nanocomposites for adsorption and photocatalytic degradation of textile dye effluents: A review. Desalination Water Treat. 2025, 321, 101004. [Google Scholar] [CrossRef]
- Xu, H.; Xiong, J.; Zhang, Y.; Cheng, J.; Yang, B.; He, S. Floating La modified g-C3N4 photocatalytic sponge mesh for sustainable dye degradation under sunlight irradiation. Inorg. Chem. Commun. 2024, 163, 112327. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Du, Y.; Zhan, W.; Zhang, T.C.; Du, D. Highly efficient degradation of rhodamine B by carbon nanotubes-activated persulfate. Sep. Purif. Technol. 2021, 256, 117788. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R.-a.; Huang, C.P.; Chen, C.-W.; Dong, C.-D. Activation of persulfate by CoO nanoparticles loaded on 3D mesoporous carbon nitride (CoO@meso-CN) for the degradation of methylene blue (MB). Sci. Total Environ. 2019, 675, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, X.; Li, J.; Yan, Y.; Li, J.; Chen, K.; Guo, A.; Liu, H.; Liu, D. Rapid preparation of N,P co-doped carbon for advanced oxidative degradation of wastewater. Surf. Interfaces 2024, 55, 105347. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Huang, T.; Li, W.; Wang, Y.; Wang, Z. Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism. J. Hazard. Mater. 2016, 320, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Zhang, H.; Tong, X.; Feng, J. Fabrication of sewage sludge-derived magnetic nanocomposites as heterogeneous catalyst for persulfate activation of Orange G degradation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 856–863. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Duan, X.; Sarmah, A.K.; Zhao, X. Remediation of environmentally persistent organic pollutants (POPs) by persulfates oxidation system (PS): A review. Sci. Total Environ. 2023, 863, 160818. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, P.; Huo, X.; Liu, Y.; Liu, Y.; Zhang, Y. Enhancement of bisphenol A degradation by accelerating the Fe(III)/Fe(II) cycle in graphene oxide modified Fe(III)/peroxymonosulfate system under visible light irradiation. J. Colloid Interface Sci. 2020, 580, 540–549. [Google Scholar] [CrossRef]
- Cherifi, Y.; Addad, A.; Vezin, H.; Barras, A.; Ouddane, B.; Chaouchi, A.; Szunerits, S.; Boukherroub, R. PMS activation using reduced graphene oxide under sonication: Efficient metal-free catalytic system for the degradation of rhodamine B, bisphenol A, and tetracycline. Ultrason. Sonochemistry 2019, 52, 164–175. [Google Scholar] [CrossRef]
- Wang, T.; Ta, M.; Guo, J.; Liang, L.-E.; Bai, C.; Zhang, J.; Ding, H. Insight into the synergy between rice shell biochar particle electrodes and peroxymonosulfate in a three-dimensional electrochemical reactor for norfloxacin degradation. Sep. Purif. Technol. 2023, 304, 122354. [Google Scholar] [CrossRef]
- Yao, B.; Yu, Y.; Wang, Z.; Yang, J.; Zhou, Y.; Dionysiou, D.D. Electro-induced activation of persulfate over N-doped porous carbon decorated with Fe/Ni bimetals for organic pollutants enhanced degradation: Synergism of electro-activation and catalytic activation. Chem. Eng. J. 2023, 476, 146769. [Google Scholar] [CrossRef]

| Catalysts | Specific Surface Area | Antibiotics | Removal | Activator | Refer |
|---|---|---|---|---|---|
| CNT = 0.1 g·L−1 | 297 m2·g−1 | SMX = 0.15 mM | 99% in 30 min | PMS = 0.5 mM | [121] |
| Co3O4@NPC/rGO = 15 mg·L−1 | 40.6 m2·g−1 | SMX = 25 mg·L−1 | 100% in 30 min | PMS = 0.2 mM | [122] |
| Fe-N/BC = 0.05 g·L−1 | 292.87 m2·g−1 | TC = 30 mg·L−1 | 89.9% in 60 min | PMS = 0.5 g·L−1 | [123] |
| FS-BC = 0.40 g·L−1 | \ | TC = 5 mg·L−1 | 99.71% in 180 min | PMS = 0.40 g·L−1 | [99] |
| MOF-N/C = 0.3 g·L−1 | 264 m2·g−1 | TCH = 96.2 mg·L−1 | 91% in 10 min | PS = 1 mM | [124] |
| NG = 0.1 g·L−1 | 580.7 m2·g−1 | TC = 35 mg·L−1 | 100% in 120 min | PS = 0.75 mM | [125] |
| CuO/N-rGO = 0.2g·L−1 | \ | TC = 20 mg·L−1 | 97% in 120 min | PS = 1 mM | [96] |
| Fe3C@CNT = 0.1 g·L−1 | 374.03 m2·g−1 | SMX = 5 mg·L−1 | 91% in 90 min | PS = 1 mM | [126] |
| Mn0.85Fe2.15O4-CNTs = 0.4 g·L−1 | 116.9 m2·g−1 | TC = 20 mg·L−1 | 95.8% in 60 min | PMS = 0.8 mM | [127] |
| Sludge-derived BC = 2 g·L−1 | \ | SMX = 0.15 mM | 94.6% in 180 min | PDS = 1.5 mM | [128] |
| Catalysts | Specific Surface Area | Phenolic | Removal | Activator | Refer |
|---|---|---|---|---|---|
| Co-N-C = 0.0005 g·L−1 | \ | 2,4-DCP = 5 μM | 80% in 30 min | PMS = 5 μM | [136] |
| Biochar = 0.2 g·L−1 | 594 m2·g−1 | 2,4-DCP = 0.1 g·L−1 | 98% in 120 min | PDS = 0.5 g·L−1 | [137] |
| CNT = 0.1 g·L−1 | 497.7m2·g−1 | Phenol = 0.1 mM | 100% in 60 min | PDS/PMS = 1 mM | [138] |
| CoO–N-C = 0.3 g·L−1 | 453.75m2·g−1 | 4-CP = 50 mg·L−1 | 100% in 30 min | PMS = 1.5 mM | [139] |
| GBC = 0.15 g·L−1 | 307.73 m2·g−1 | Phenol = 5 mg·L−1 | 100% in 30 min | PS = 2 mM | [140] |
| Fe/N-CNT = 0.05 g·L−1 | 220.68 m2·g−1 | ACT = 10 mg·L−1 | 98% in 30 min | PS = 0.08 mM | [141] |
| CNT = 0.3 g·L−1 | 939.38 m2·g−1 | ACT = 10 mg·L−1 | 100% in 10 min | PS = 0.21 mM | [142] |
| NS-CNT = 0.1 g·L−1 | 228 m2·g−1 | BPA = 20 mg·L−1 | 100%in 30 min | PDS = 1.5 mM | [143] |
| Cu-rGO = 0.25 g·L−1 | 148.69 m2·g−1 | BPA = 0.09 mM | 99% in 40 min | PMS = 3 mM | [144] |
| N-biochar = 0.5 g·L−1 | \ | BPA = 10 mg·L−1 | 100% in 5 min | PMS = 2.0 mM | [145] |
| PSBC = 0.4 g·L−1 | 106.8 m2·g−1 | 4-CP = 0.1 mM | 100% in 10 min | PMS = 1.0 mM | [146] |
| Catalysts | Specific Surface Area | Dyes | Removal | Activator | Refer |
|---|---|---|---|---|---|
| Fe@NCNT-BC-80 = 0.05 g·L−1 | 225.4 m2·g−1 | RhB = 20 mg·L−1 | 100% in 10 min | PS = 5 mM | [151] |
| CNT = 0.2 g·L−1 | 104 m2·g−1 | RhB = 20 mg·L−1 | 100% in 210 min | PMS = 119.0 mg·L−1 | [152] |
| CoO@meso-CN = 0.2 g·L−1 | 569 m2·g−1 | MB = 0.1 mM | 99% in 15 min | PS = 20 mM | [153] |
| EC-PN = 0.2 g·L−1 | 783.4 m2·g−1 | MB = 100 mg·L−1 | 100% in 60 min | PMS = 0.15 mM | [154] |
| CNTs = 0.1 g·L−1 | \ | AO7 = 0.057 mM | 100% in 60 min | PMS = 1.14 mM | [155] |
| MnFe2O4-SAC = 0.2 g·L−1 | \ | OG = 20 m g·L−1 | 100% in 30 min | PS = 0.5 g·L−1 | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Liu, D.; Han, R.; Yin, A.; Gong, G.; Li, S.; Chen, R.; Yang, J.; Liu, Z.; Zhi, K. Advances in Activation of Persulfate by Novel Carbon-Based Materials: Degradation of Emerging Contaminants, Mechanisms, and Perspectives. Crystals 2025, 15, 432. https://doi.org/10.3390/cryst15050432
Guo L, Liu D, Han R, Yin A, Gong G, Li S, Chen R, Yang J, Liu Z, Zhi K. Advances in Activation of Persulfate by Novel Carbon-Based Materials: Degradation of Emerging Contaminants, Mechanisms, and Perspectives. Crystals. 2025; 15(5):432. https://doi.org/10.3390/cryst15050432
Chicago/Turabian StyleGuo, Lianghui, Dong Liu, Runyao Han, Aoxiang Yin, Guifan Gong, Shi Li, Ruixuan Chen, Jianyu Yang, Zimeng Liu, and Keke Zhi. 2025. "Advances in Activation of Persulfate by Novel Carbon-Based Materials: Degradation of Emerging Contaminants, Mechanisms, and Perspectives" Crystals 15, no. 5: 432. https://doi.org/10.3390/cryst15050432
APA StyleGuo, L., Liu, D., Han, R., Yin, A., Gong, G., Li, S., Chen, R., Yang, J., Liu, Z., & Zhi, K. (2025). Advances in Activation of Persulfate by Novel Carbon-Based Materials: Degradation of Emerging Contaminants, Mechanisms, and Perspectives. Crystals, 15(5), 432. https://doi.org/10.3390/cryst15050432