The Electrochemical Characteristics and Corrosion Resistance of a Low-Melting-Point Al49Sn21Zn16Pb14 Alloy in NaCl Solution
Abstract
1. Introduction
2. Experiments
2.1. Experimental Materials
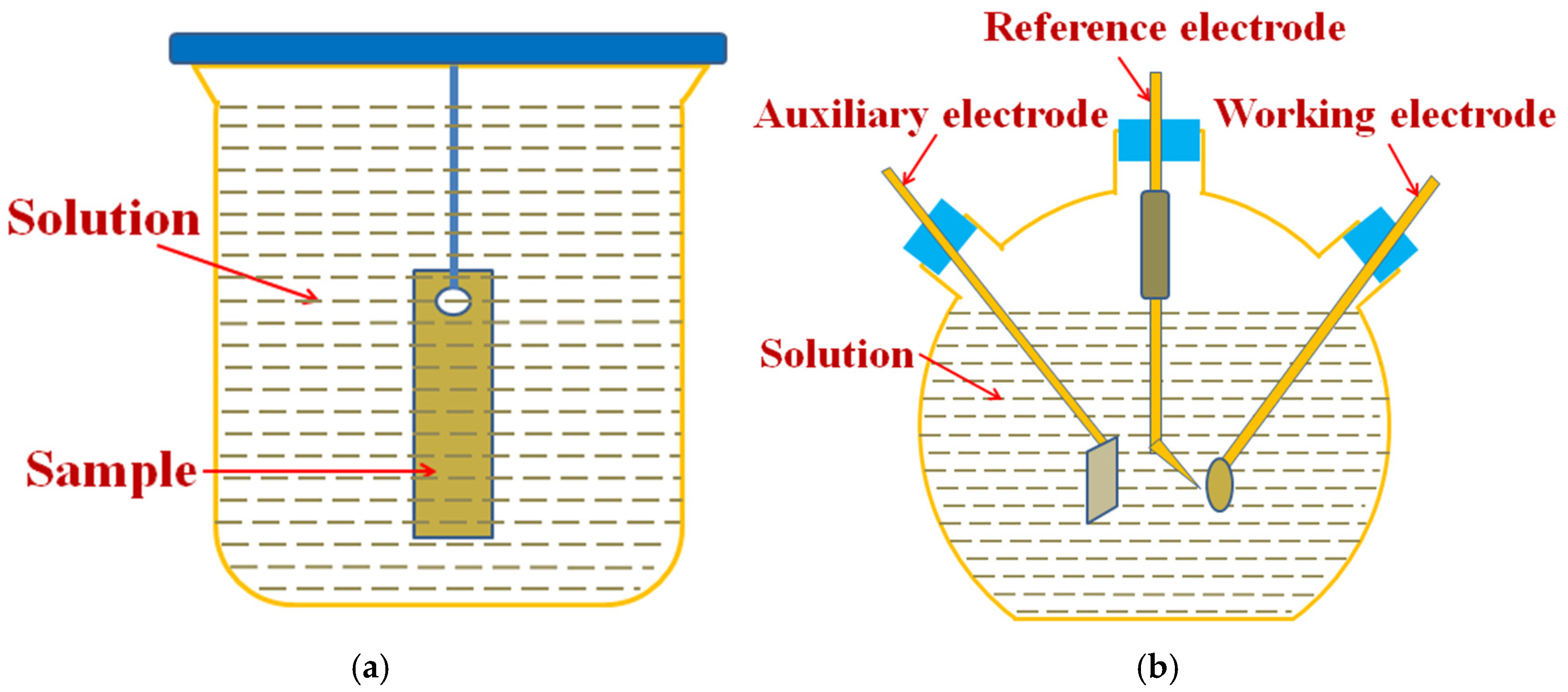
2.2. Corrosion Test
2.3. Material Characterization and Property Testing
3. Results and Discussion
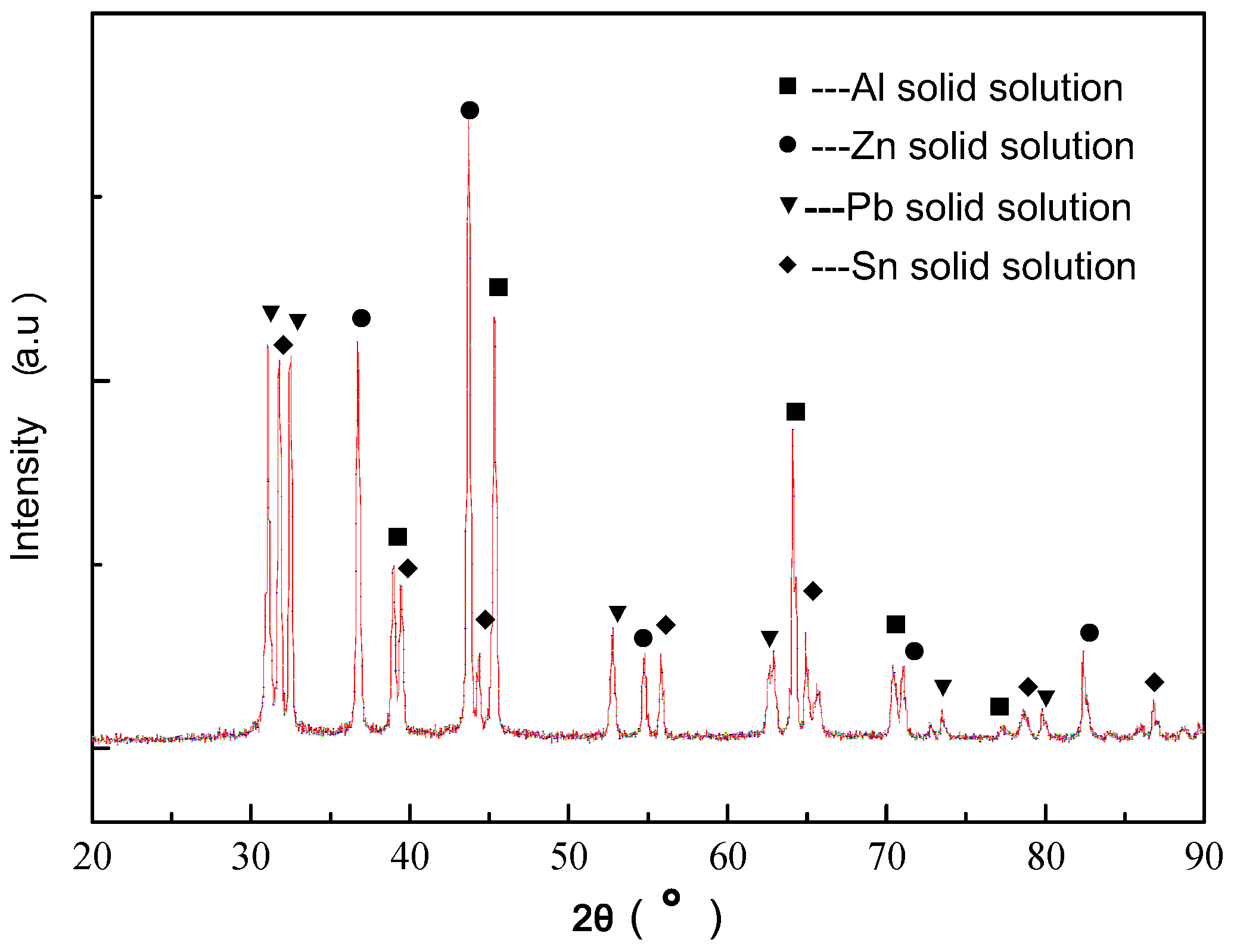
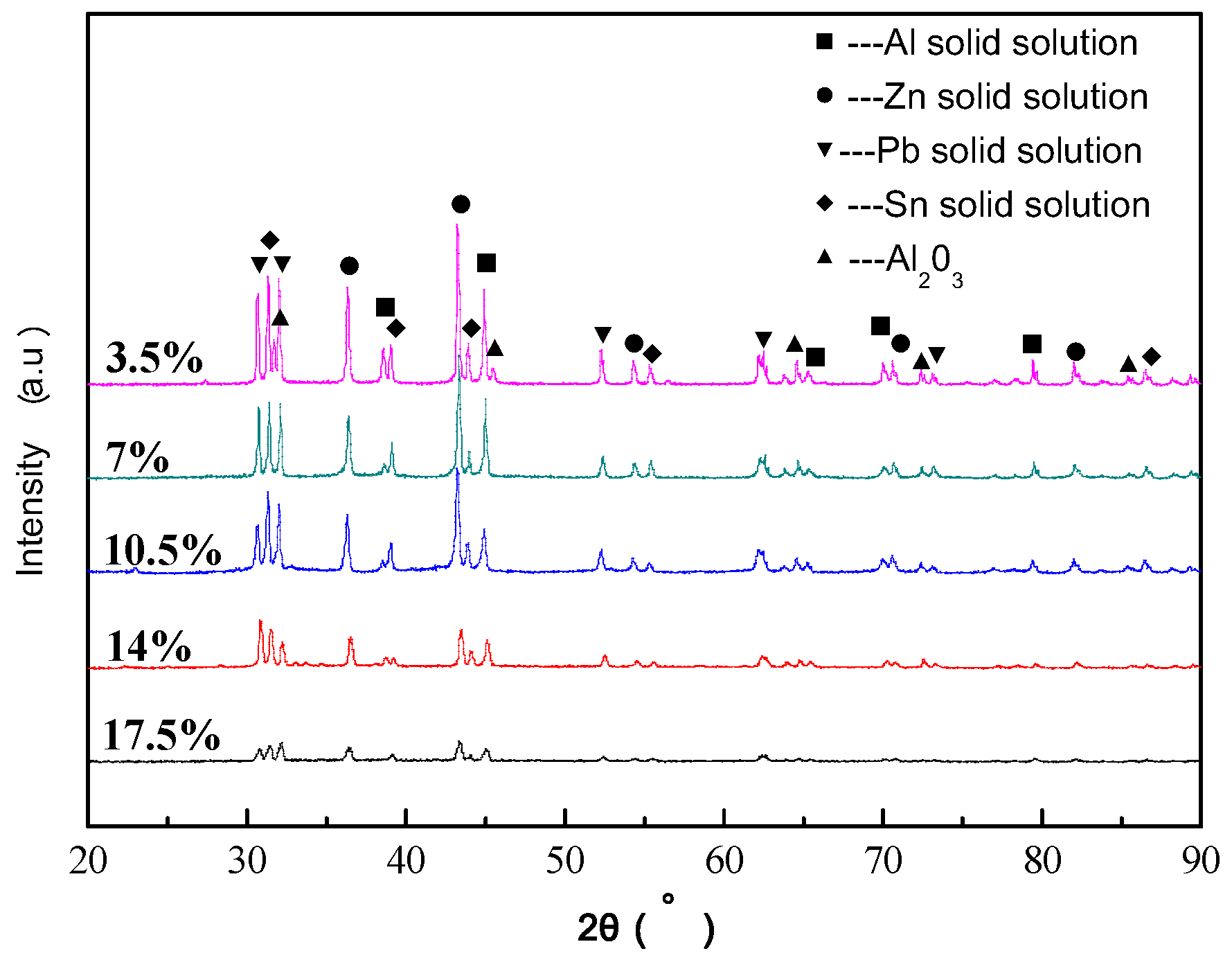
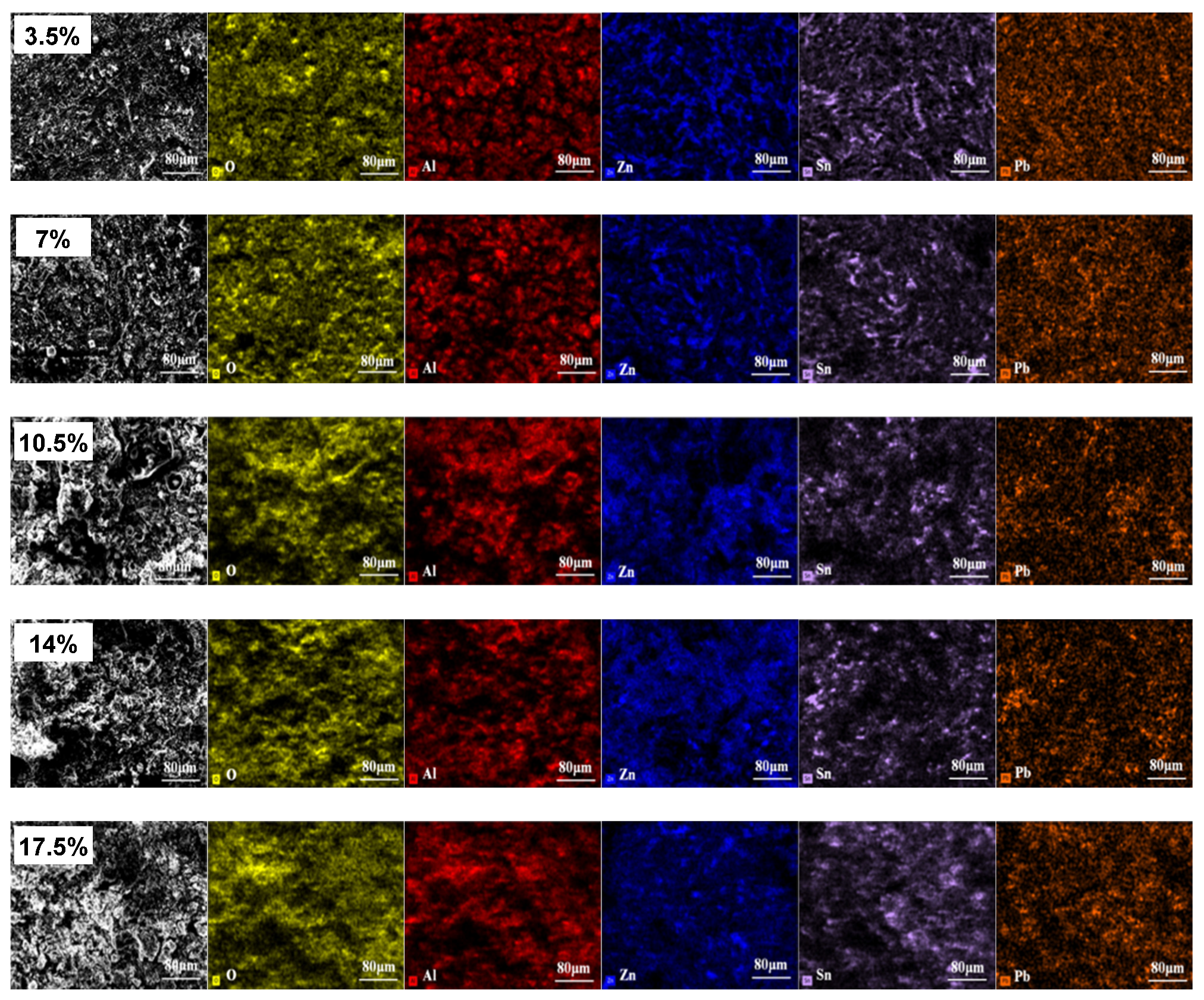
3.1. Microstructure and Phase
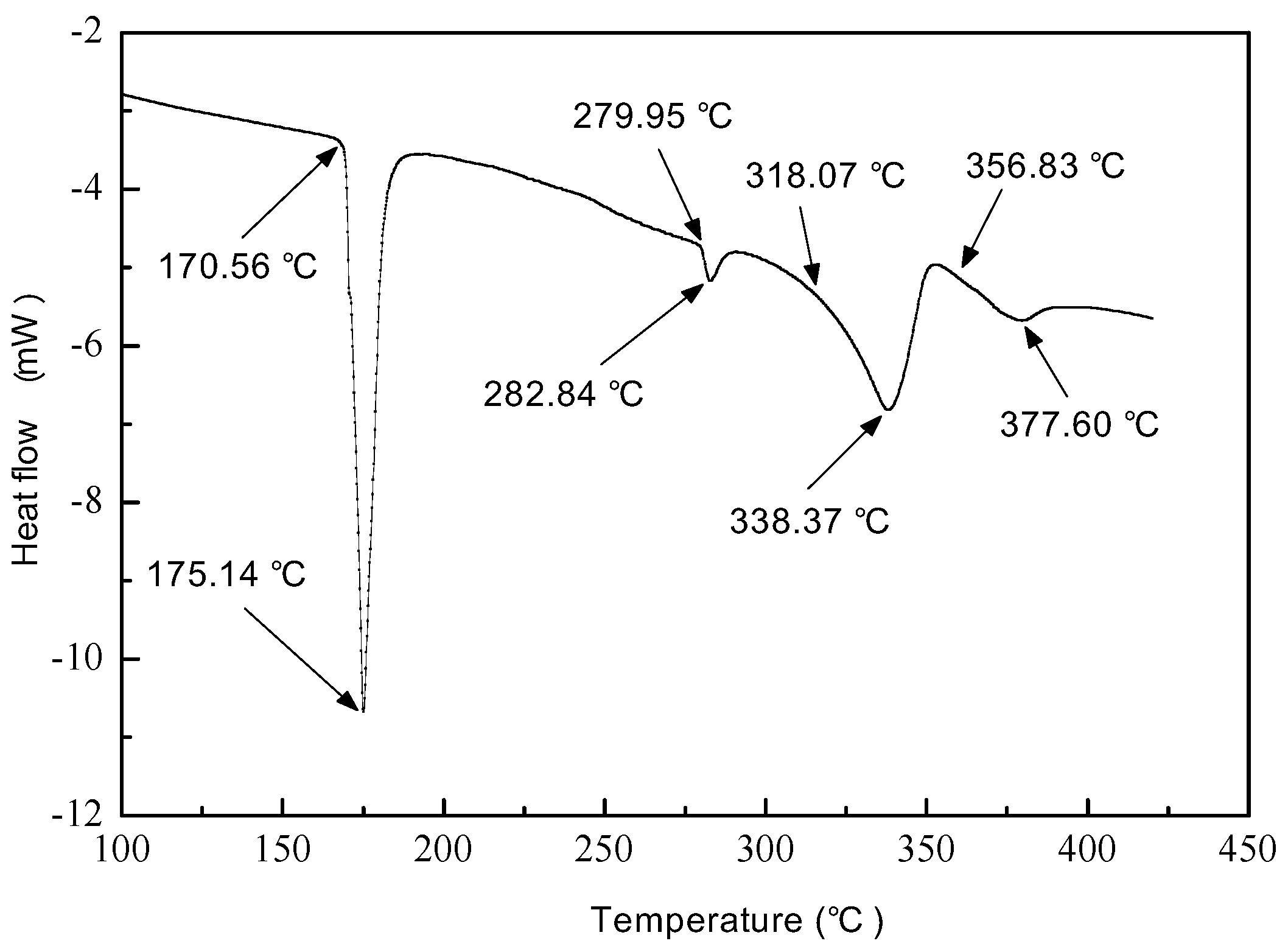
3.2. Melting Point Analysis
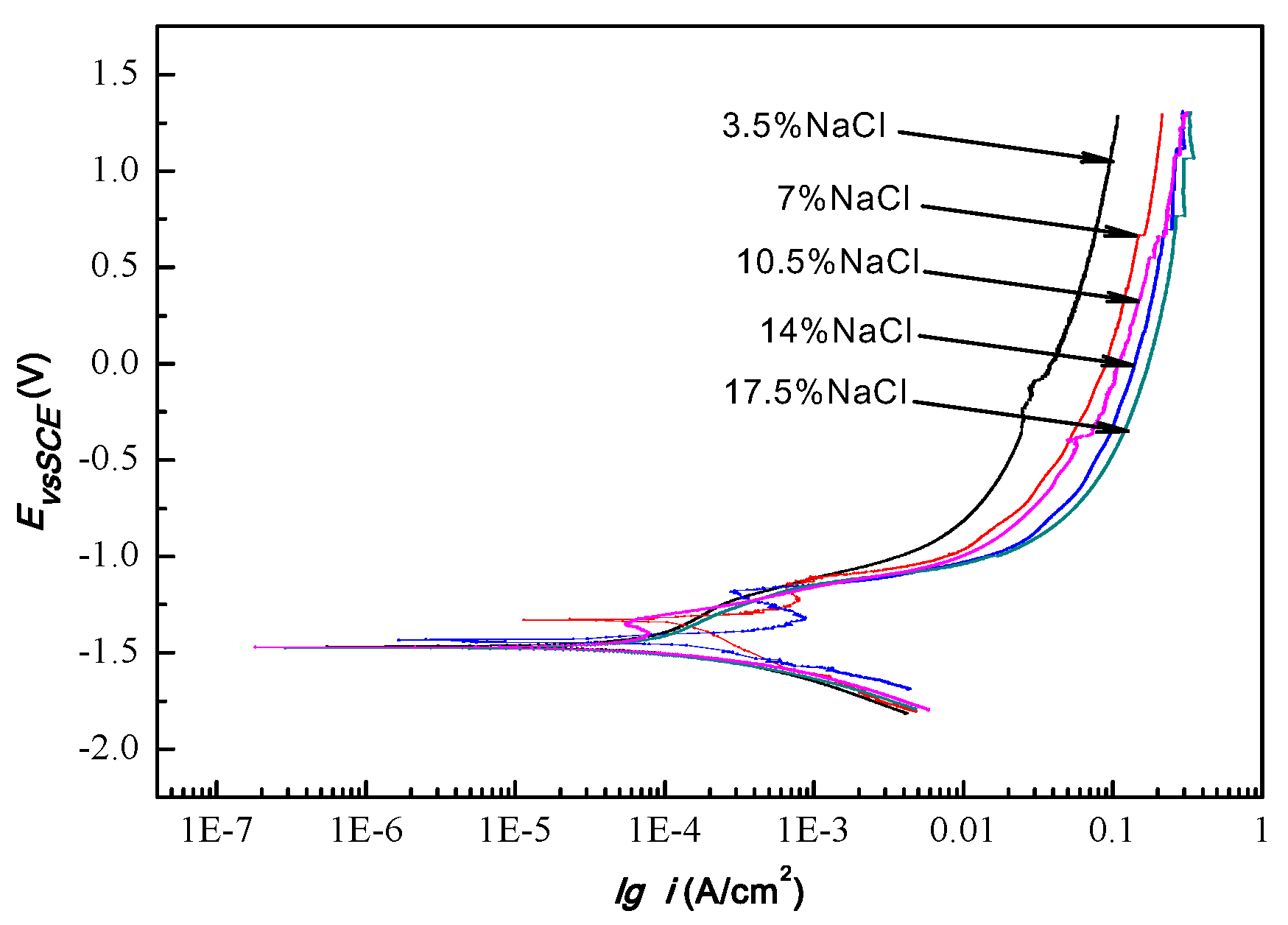
3.3. Potentiodynamic Polarization
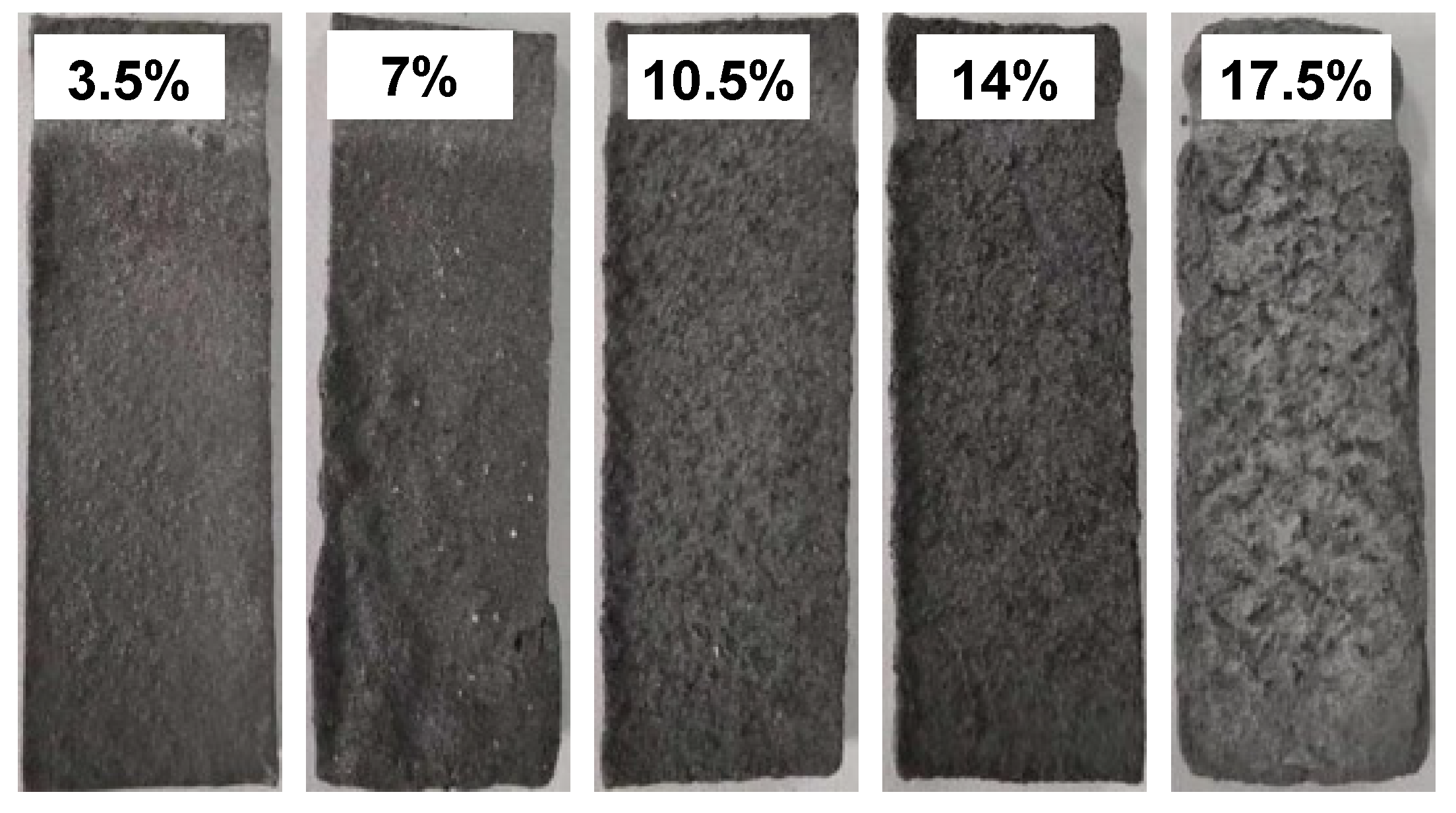
3.4. Immersion Corrosion Morphologies
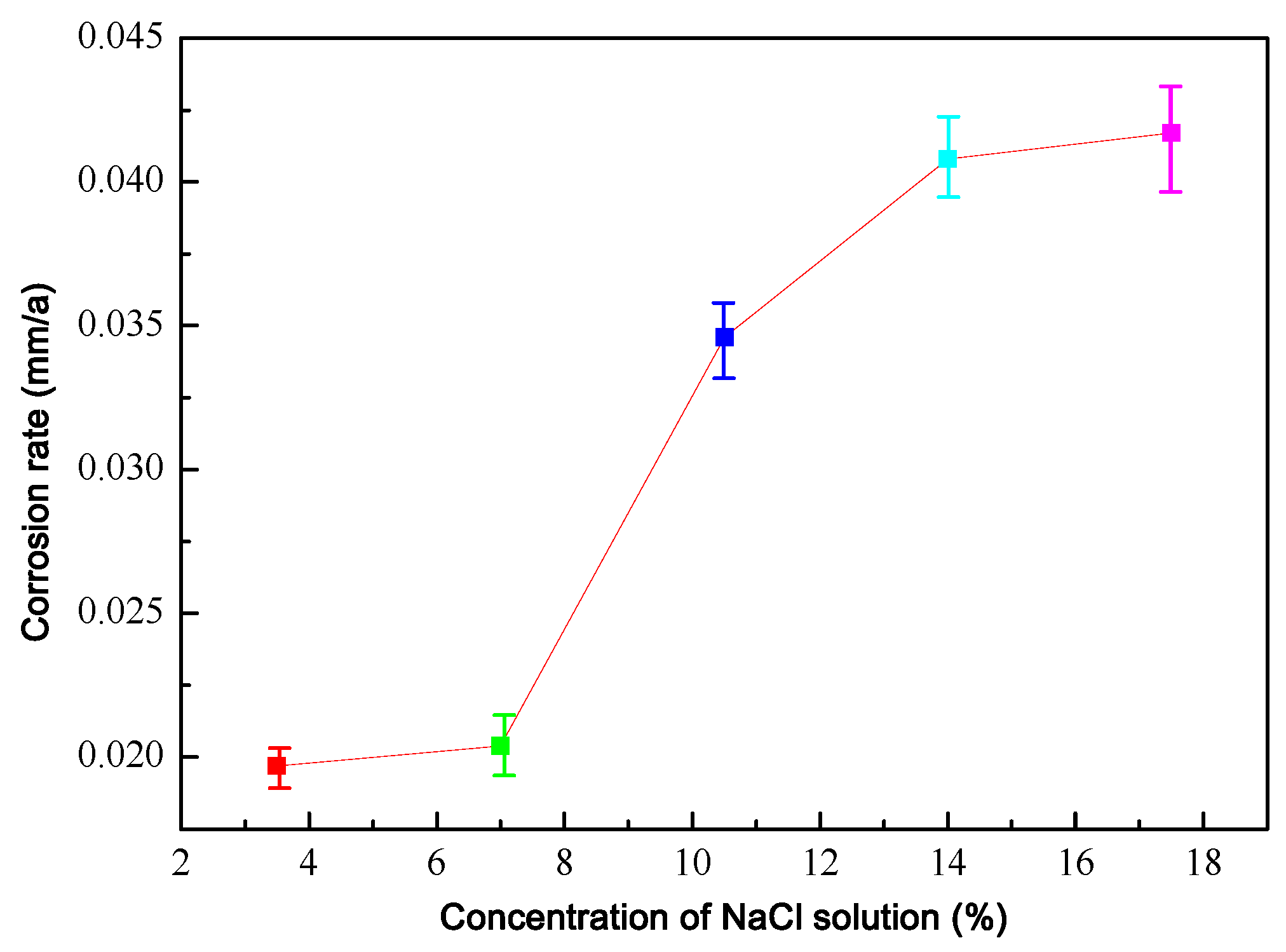
3.5. Corrosion Rate
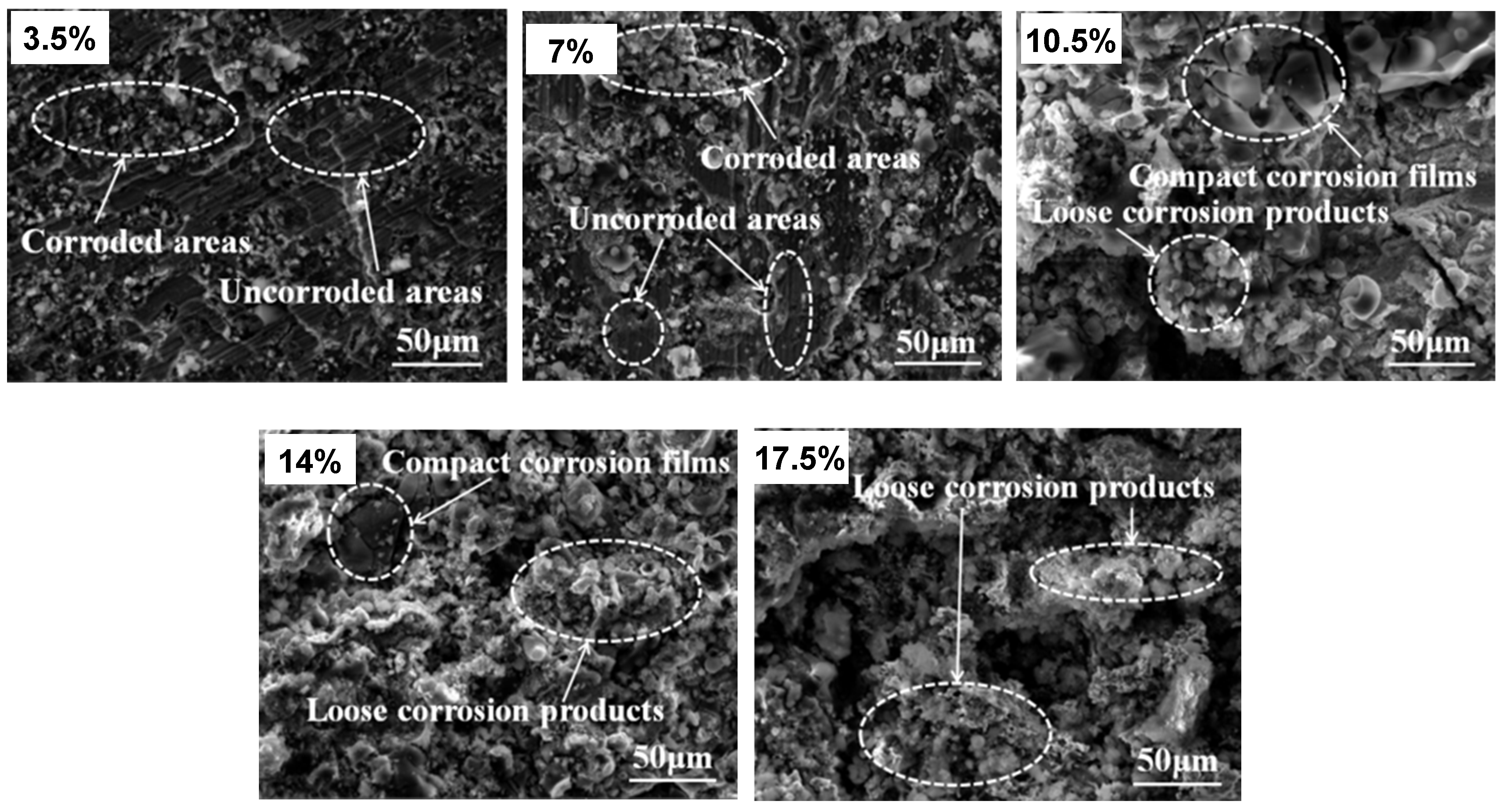
3.6. Corrosion Products
3.7. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Li, H.; Hu, X.; Shi, C. Evaluation on steel corrosion risk of seawater sea sand concrete with low water-to-binder ratio by electrochemical measurement. Constr. Build. Mater. 2024, 417, 135326. [Google Scholar] [CrossRef]
- Gadioli, A.O.; de Souza, L.M.; Pereira, E.C.; Monteiro, S.N.; Azevedo, A.R. Imidazolium-based ionic liquids as corrosion inhibitors for stainless steel in different corrosive media: An overview. J. Mater. Res. Technol. 2024, 29, 803–823. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Wang, C. Corrosion behavior of steel exposed to “zero” pollution atmosphere environment for 20 years. J. Mater. Res. Technol. 2024, 30, 2388–2396. [Google Scholar] [CrossRef]
- Ni, Q.; Zhu, M.; Yuan, Y.; Guo, S. Corrosion behavior and mechanism of FeCoNi high-entropy alloy compared with X100 pipeline steel in a simulated alkaline soil environment. Mater. Today Commun. 2024, 39, 108846. [Google Scholar] [CrossRef]
- Zeng, Q.; Zheng, C.; Wei, X. Study of the corrosion behaviors of M50 steel in brine-contaminated lubricating oil. Int. J. Electrochem. Sci. 2024, 19, 100572. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, L.; Li, W.; Cui, S.; Shang, L.; Wang, C.; Song, Q.; Zhang, C. Comparative study of anti-corrosion properties in different CrN/WC-Co duplex coatings processed by PVD/HVOF. Surf. Coat. Technol. 2024, 483, 130799. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, W.; Zhou, M.; Qiu, X.; Zhou, Y.; Zhong, Y.; Liu, N.; Yang, J. Effect of Al content on the microstructure, mechanical property and lead-bismuth eutectic corrosion resistance of refractory AlxTiNbZrMoV coatings produced by magnetron sputtering. Mater. Today Commun. 2024, 38, 108569. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Z.; Lu, Z.; Ma, L. Carbon dot/nickel nanocomposite coating for wear and corrosion control of Mg alloy: Experimental and theoretical studies. Appl. Surf. Sci. 2024, 659, 159845. [Google Scholar] [CrossRef]
- Ding, K.; Wu, Y.; Wei, X.; Zhu, X. Preparation and corrosion resistance mechanism of chromium-free ZnAl+CeO2 composite coatings. Mater. Chem. Phys. 2024, 312, 128704. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J. One-step preparation of color-tuned surface coatings on magnesium alloy with corrosion resistance. J. Alloys Compd. 2024, 976, 173300. [Google Scholar] [CrossRef]
- Xue, F.; Wang, J.; Hou, Y.; Hu, W. Preparation of ZnO resistive switching film and its corrosion behavior in 3.5 wt.% NaCl solution. Appl. Surf. Sci. 2024, 649, 159105. [Google Scholar] [CrossRef]
- Xue, F.; He, W.; Wang, J.; Hu, W. Preparation of AlxZn1−xO resistive switching film by sol–gel method and its corrosion behavior in 3.5 wt% NaCl solution. Appl. Surf. Sci. 2023, 615, 156279. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, F.; Wang, J.; Hu, W. Preparation of Zn1−xCrxO resistive switching film and its corrosion behavior in 3.5 wt.% NaCl solution. Appl. Surf. Sci. 2023, 609, 155359. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Q.; Wang, J.; Hu, W. Preparation and corrosion resistance of ZnO films under different annealing temperatures and their resistive switching behaviours. Int. J. Electrochem. Sci. 2021, 16, 210654. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Luo, C.; Yu, C.; Li, M.; Han, W. Effect of pre-oxidation and sea salt on the hot corrosion behavior of MCrAlY coatings and Alsingle bondSi coatings. Surf. Coat. Technol. 2024, 477, 130354. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Huang, R.; Sun, P.; Zheng, S.; Li, M.; Wang, X.; Du, Q. Investigation of microstructure and corrosion resistance of an Al-Zn-Mg-Cu alloy under various ageing conditions. Corros. Sci. 2024, 227, 111719. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; Bai, J.; Niu, J.; Ma, R.; Du, A.; Zhao, X.; Fan, Y.; Li, G. Study on the structure and corrosion behavior of hot-dipped Zn–6Al–3Mg alloy coating in chlorine-containing environment. Corros. Sci. 2024, 231, 112001. [Google Scholar] [CrossRef]
- Chen, T.-C.; Yung, T.-Y.; Chou, C.-C.; Wang, Y.-M. Investigating the corrosion resistance of Zn and Al coating deposited by arc thermal spraying process. Surf. Coat. Technol. 2024, 483, 130684. [Google Scholar] [CrossRef]
- Yang, K.; Yan, J.; Wang, Q.; Ding, J.; Xu, Q.; Wen, Z.; Zeng, R.; Lu, C.; Fan, T.; Gong, M.; et al. Revealing microstructure and the associated corrosion mechanism of Al/amorphous Al2O3/Al tri-layer coating deposited on depleted uranium by magnetron sputtering. Appl. Surf. Sci. 2024, 659, 159911. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhou, T.; Yin, B.; Yin, S.; Zhang, Y. Effect of tin on the corrosion behavior of sea-water corrosion-resisting steel. Mater. Des. 2015, 84, 1–9. [Google Scholar] [CrossRef]
- Mitsunobu, T.; Tokuda, K.; Shimoda, N. Peritectic structure evolution in hot dip Zn-Al alloy coatings. Tetus Hagane—J. Iron Steel Inst. Jpn. 2020, 106, 254–261. [Google Scholar] [CrossRef]
- Chu, R.; Wang, Z.; Sun, N.; Deng, X.; Xu, C.; Guo, Z. The fundamental research on suspended dross in hot dip Al-Zn galvanising process. Mater. Sci. Technol. 2024, 40, 552–560. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Ambaryan, G.N.; Suleimanov, M.Z.; Tarasenko, A.B.; Vlaskin, M.S. Enhanced hydrogen generation from magnesium–aluminum scrap ball milled with low melting point solder alloy. Materials 2023, 16, 4450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-X.; Shen, Y.-F.; Li, C.-T.; Dai, X.; Xiao, G.-H. Effect of rare earth content on microstructure and corrosion resistance of hot-dip Zn-5Al alloy coated steel wire for bridge cables. J. Iron Steel Res. Int. 2023, 30, 2101–2110. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Shang, T.; Liu, W.; Jiang, G.; Liu, C.; Cheng, X.; Zhang, X.; Li, X. Influence mechanism of different elements and alloy phases on the corrosion resistance of Zn-Al-Mg coated steel in the atmospheric environment: A review. Corros. Commun. 2024, 13, 49–59. [Google Scholar] [CrossRef]
- Gao, Y.; Su, R.; Liu, Z.; Chen, K.; Cong, S.; Zhang, L.; Guo, X. High-resolution characterization reveals the role of Al content in the evolution of oxide scales formed on alumina-forming alloy exposed to supercritical water. Corros. Sci. 2024, 231, 111968. [Google Scholar] [CrossRef]
- Liu, L.; Shi, C.; Zhang, C.; Su, R.; Zhang, H.; Voyles, P.M.; Perepezko, J.H. The effect of Al on the oxidation behavior of Mo-6Si-12B-(1,2,4,8)Al alloys. Corros. Sci. 2022, 208, 110677. [Google Scholar] [CrossRef]
- Guan, Q.; Hang, C.; Li, S.; Tang, X.; Yao, G.; Yu, D.; Ding, Y.; Wang, X.; Zhang, W. Effect of gamma irradiation on microstructural evolution and mechanical properties of SnPb eutectic solder joints. J. Mater. Res. Technol. 2023, 27, 2929–2938. [Google Scholar] [CrossRef]
- Dong, S.; Na, R.; Qu, Y.; Chen, R.; Li, G.; Zhang, W.; Zhang, S.; Liu, S. Hot processing performance and fracture toughness control of AlxCrFeNiCu1.5 (x = 0, 0.2, 0.4, 0.6, 0.8) high entropy alloys. Mater. Today Commun. 2024, 38, 108566. [Google Scholar] [CrossRef]
- Yao, X.; Wang, W.; Qi, X.; Lv, Y.; Yang, W.; Li, T.; Chen, J. Effects of heat treatment cooling methods on precipitated phase and mechanical properties of CoCrFeMnNi–Mo5C0.5 high entropy alloy. J. Mater. Res. Technol. 2024, 29, 3566–3574. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, Z.; Shi, Y.; Ren, X.; Liang, L.; Shao, Q.; Zhu, K. Enhancement of oxygen evolution reaction performance of FeCoNiCrMn high entropy alloy thin film electrodes through in-situ reconstruction. J. Alloys Compd. 2023, 947, 169699. [Google Scholar] [CrossRef]
- Akinwande, A.A.; Kumar, M.S.; Adesina, O.S.; Adediran, A.A.; Romanovski, V.; Salah, B. Tribological performance of a novel 7068-aluminium/lightweight-high-entropy-alloy fabricated via powder metallurgy. Mater. Chem. Phys. 2023, 308, 128207. [Google Scholar] [CrossRef]
- Zhuo, L.; Sun, J.; Chen, B.; Zhan, M.; Jiang, C. Microstructure and mechanical performances of novel multi-phase refractory high entropy alloys in Ti-Zr-V-Mo fabricated by vacuum arc melting. Vacuum 2023, 217, 112544. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Y.; Liang, S.; Xie, W. The effects of non-isothermal aging on the strength and corrosion behavior of AlZnMgCu alloy. J. Alloys Compd. 2016, 681, 57–65. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Liu, S.-D.; Jiang, J.-Y.; Wei, W.-C. Effect of Cu content on intergranular corrosion and exfoliation corrosion susceptibility of Al−Zn−Mg−(Cu) alloys. Trans. Nonferrous Met. Soc. China 2023, 33, 1963–1976. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, F.; Zhang, C.; Jia, Q.; Sun, K.; Duan, D.; Jiang, H.; Li, G. Effect of carbon addition on the microstructure and corrosion resistance of the CoCrFeNi high-entropy alloy. Corros. Sci. 2024, 231, 111965. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Z.; Ma, L.; Lin, Y.; Li, G.; Hailun, W. Effect of SiC particles on the corrosion behaviour of 6.5% SiCp/Al-Cu-Mg-Zn composites. Int. J. Electrochem. Sci. 2017, 12, 11006–11016. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, B.; Li, Q.; Xie, J. Effect of 0.3 at. % Ce on the corrosion resistance of Fe40Ni20Co20Cr20 high-entropy alloy in 3.5 wt% NaCl solution. Mater. Lett. 2024, 364, 136310. [Google Scholar] [CrossRef]
- Chang, W.; Wang, X.; Qian, H.; Chen, X.; Lou, Y.; Zhou, M.; Guo, D.; Kwok, C.T.; Tam, L.M.; Zhang, D. Effect of Sn addition on microstructure, hardness and corrosion behavior of CoCrFeNiSnx high entropy alloys in chloride environment. Corros. Sci. 2024, 227, 111808. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Sun, R.; Chang, C.; Liu, B.; Ma, X. Effect of Mn addition on microstructure and corrosion behavior of AlCoCrFeNi high-entropy alloy. Intermetallics 2024, 167, 108236. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Gao, X.; Peng, L.; Qiao, Q.; Xiao, J.; Guo, F.; Wang, R.; Yu, J. Electrochemical deposition and corrosion resistance characterization of FeCoNiCr high-entropy alloy coatings. Coatings 2023, 13, 1167. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Jie, J.; Li, T.; Yuan, Z.; Cui, H.; Liu, J. Effects of Ti addition on microstructure, mechanical properties and corrosion resistance of the Cu-Zn-Ni alloy. Metals 2024, 14, 1360. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Q.; Gong, M.; Fu, Q. Corrosion performances of selective laser melting Ti6Al4V alloy in different solutions. Metals 2023, 13, 192. [Google Scholar] [CrossRef]
- Wang, S.; Cao, Y.; Liu, X.; Cai, G. In situ electrochemical monitoring of the crevice corrosion process of the 7075-T651 aluminium alloy in acidic NaCl and NaNO3 solution. Materials 2023, 16, 2812. [Google Scholar] [CrossRef]
- Yao, X.; Xia, S.; Lv, Y.; Yang, W.; Chen, J. Effects of hot dipping temperature on microstructure and mechanical properties of Pb40Sn60 alloy coating on copper wires. Mater. Sci. Eng. A 2021, 810, 140991. [Google Scholar] [CrossRef]
- Lee, C.; Chung, J.; Lee, J.; Yoon, W. Refining behavior of aluminum alloyed metallurgical grade silicon during fractional melting process. J. Nanosci. Nanotechnol. 2013, 13, 7161–7164. [Google Scholar] [CrossRef]

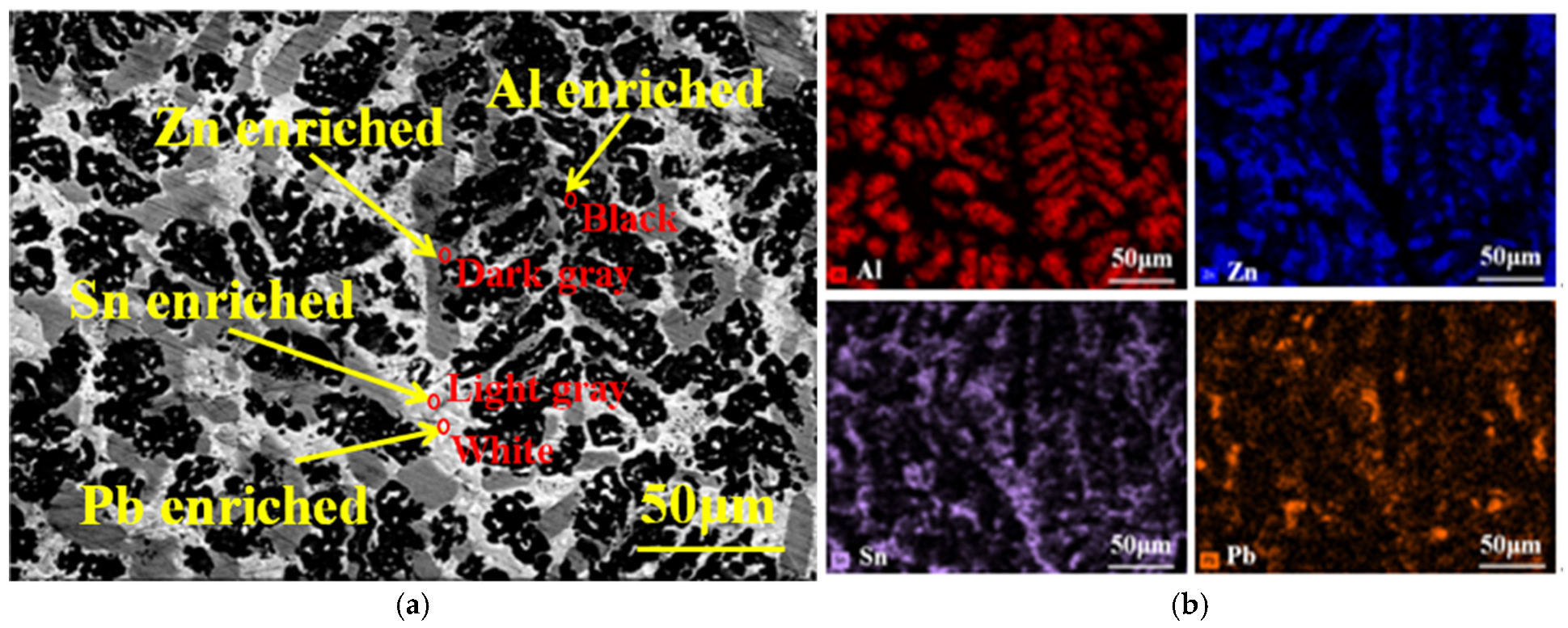

| Position | Element Content (wt.%) | |||
|---|---|---|---|---|
| Al | Zn | Pb | Sn | |
| Black | 61.1 | 26.3 | 8.6 | 4.0 |
| Dark gray | 4.94 | 64.77 | 17.46 | 12.83 |
| Light gray | 2.43 | 4.81 | 13.05 | 79.71 |
| White | 2.07 | 3.61 | 86.96 | 7.36 |
| Area scanned | 50.27 | 16.82 | 12.73 | 20.18 |
| Concentration of NaCl Solution (%) | Self-Corrosion Potential Ecorr (V) | Self-Corrosion Current Density Icorr (A/cm2) | Passivation Potential Interval (V) |
|---|---|---|---|
| 3.5 | −1.33 | 2.3 × 10−5 | / |
| 7 | −1.45 | 2.4 × 10−5 | −1.27–−1.13 |
| 10.5 | −1.45 | 2.6 × 10−5 | −1.33–−1.18 |
| 14 | −1.46 | 3.4 × 10−5 | −1.41–−1.35 |
| 17.5 | −1.46 | 8.4 × 10−5 | / |
| Concentration of NaCl Solution (%) | Position | Element Content (at. %) | ||||
|---|---|---|---|---|---|---|
| O | Al | Zn | Sn | Pb | ||
| 3.5 | Grayness | 42.7 | 7.6 | 48.2 | 1.2 | 0.3 |
| Whiteness | 70.1 | 26.3 | 1.9 | 1.7 | 0.0 | |
| 7 | Grayness | 40.5 | 3.6 | 53.8 | 1.3 | 0.8 |
| Whiteness | 50.1 | 42.2 | 7.2 | 0.4 | 0.1 | |
| 10.5 | Grayness | 58.2 | 6.0 | 30.3 | 4.7 | 0.8 |
| Whiteness | 63.3 | 24.4 | 10.6 | 1.4 | 0.2 | |
| 14 | Grayness | 71.6 | 2.3 | 26.0 | 0.0 | 0.0 |
| Whiteness | 66.9 | 27.3 | 5.4 | 0.4 | 0.0 | |
| 17.5 | Grayness | 68.4 | 7.5 | 22.7 | 3.3 | 0.0 |
| Whiteness | 61.6 | 25.4 | 8.6 | 3.9 | 0.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Wang, W.; Qi, X.; Lv, Y.; Yang, W.; Ma, Y.; Chen, J. The Electrochemical Characteristics and Corrosion Resistance of a Low-Melting-Point Al49Sn21Zn16Pb14 Alloy in NaCl Solution. Crystals 2025, 15, 425. https://doi.org/10.3390/cryst15050425
Yao X, Wang W, Qi X, Lv Y, Yang W, Ma Y, Chen J. The Electrochemical Characteristics and Corrosion Resistance of a Low-Melting-Point Al49Sn21Zn16Pb14 Alloy in NaCl Solution. Crystals. 2025; 15(5):425. https://doi.org/10.3390/cryst15050425
Chicago/Turabian StyleYao, Xiaofei, Weihua Wang, Xiaoling Qi, Yunkun Lv, Wei Yang, Yufei Ma, and Jian Chen. 2025. "The Electrochemical Characteristics and Corrosion Resistance of a Low-Melting-Point Al49Sn21Zn16Pb14 Alloy in NaCl Solution" Crystals 15, no. 5: 425. https://doi.org/10.3390/cryst15050425
APA StyleYao, X., Wang, W., Qi, X., Lv, Y., Yang, W., Ma, Y., & Chen, J. (2025). The Electrochemical Characteristics and Corrosion Resistance of a Low-Melting-Point Al49Sn21Zn16Pb14 Alloy in NaCl Solution. Crystals, 15(5), 425. https://doi.org/10.3390/cryst15050425





