A First-Principles Study of Sn Dimer Adsorbed on MgO Surface
Abstract
1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, C.R. Surface Studies of Supported Model Catalysts. Surf. Sci. Rep. 1998, 31, 235–325. [Google Scholar] [CrossRef]
- Corain, B.; Schmid, G.; Toshima, N. Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Anderson, J.A.; García, M.F. Supported Metals in Catalysis, 2nd ed.; Catalytic Science Series; Imperial College Press: London, UK, 2012; Volume 11. [Google Scholar]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical Synthesis of Single Atomic Site Catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ye, C.; Yan, H.; Li, L.; He, H.; Wang, D.; Li, Y. Single-Atom Site Catalysts for Environmental Catalysis. Nano Res. 2020, 13, 3165–3182. [Google Scholar] [CrossRef]
- Fuente, S.A.; Ferullo, R.M.; Domancich, N.F.; Castellani, N.J. Interaction of NO with Au Nanoparticles Supported on (100) Terraces and Topological Defects of MgO. Surf. Sci. 2011, 605, 81–88. [Google Scholar] [CrossRef]
- Pacchioni, G.; Freund, H. Electron Transfer at Oxide Surfaces. The MgO Paradigm: From Defects to Ultrathin Films. Chem. Rev. 2013, 113, 4035–4072. [Google Scholar] [CrossRef]
- Hemmingson, S.L.; Campbell, C.T. Trends in Adhesion Energies of Metal Nanoparticles on Oxide Surfaces: Understanding Support Effects in Catalysis and Nanotechnology. ACS Nano 2017, 11, 1196–1203. [Google Scholar] [CrossRef]
- Groß, A. Theoretical Surface Science. A Microscopic Perspective; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gong, N.; He, H.; Wan, H.; Hou, H.; Zhou, Z.; Yang, Y.; Qin, G.; Yin, A.; Cai, Y.; Sun, X.; et al. Insights into the Electronic Modulation of Bimetallic Pt–Sn Cluster for the Selective Hydrogenation of 1,3-Butadiene. Catal. Sci. Technol. 2023, 13, 3313–3320. [Google Scholar] [CrossRef]
- Recchia, S.; Dossi, C.; Poli, N.; Fusi, A.; Sordelli, L.; Psaro, R. Outstanding Performances of Magnesia-Supported Platinum–Tin Catalysts for Citral Selective Hydrogenation. J. Catal. 1999, 184, 1–4. [Google Scholar] [CrossRef]
- Virnovskaia, A.; Morandi, S.; Rytter, E.; Ghiotti, G.; Olsbye, U. Characterization of Pt, Sn/Mg(Al)O Catalysts for Light Alkane Dehydrogenation by FT-IR Spectroscopy and Catalytic Measurements. J. Phys. Chem. C 2007, 111, 14732–14742. [Google Scholar] [CrossRef]
- Kikuchi, I.; Haibara, Y.; Ohshima, M.; Kurokawa, H.; Miura, H. Dehydrogenation of N-Butane to Butadiene over Pt–Sn/MgO–Al2O3. J. Jpn. Petrol. Inst. 2012, 55, 33–39. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Zong, X.; Patra, A.; Caratzoulas, S.; Vlachos, D.G. Propane Dehydrogenation on PtxSny (x, y ≤ 4) Clusters on Al2O3(110). ACS Catal. 2023, 13, 2802–2812. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane Dehydrogenation: Catalyst Development, New Chemistry, and Emerging Technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Deng, G.-M.; Li, W.-C.; Miao, S.; Wang, Q.-N.; Zhang, W.-P.; Lu, A.-H. Al2O3 Nanosheets Rich in Pentacoordinate Al3+ Ions Stabilize Pt-Sn Clusters for Propane Dehydrogenation. Angew. Chem. Int. Ed. 2015, 54, 13994–13998. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, J.; Jeong, H.Y.; Kwak, J.H. Controlling the Acid-Base Properties of Alumina for Stable PtSn-Based Propane Dehydrogenation Catalysts. Appl. Catal. A 2019, 572, 1–8. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Almallahi, R.; Wortman, J.; Igenegbai, V.O.; Linic, S. Stable and Selective Catalysts for Propane Dehydrogenation Operating at Thermodynamic Limit. Science 2021, 373, 217–222. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium Oxide (MgO) Nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Chang, J.-R.; Koningsberger, D.C.; Gates, B.C. Structurally Simple Supported Platinum Clusters Prepared from [Pt15(CO)30]2- on Magnesium Oxide. J. Am. Chem. Soc. 1992, 114, 6460–6466. [Google Scholar] [CrossRef]
- Rivera Rocabado, D.S.; Koyama, M. Support-Dependent Modulation of Pt33 Nanoparticles: Insights into Oxygen Interaction, Stability, Electronic Properties, and Geometric Structure. Surf. Sci. 2025, 754, 122686. [Google Scholar] [CrossRef]
- Matczak, P. Theoretical Study of Sn Adsorbed on the MgO(100) Surface with Defects. C. R. Chim. 2018, 21, 669–675. [Google Scholar] [CrossRef]
- Nigam, S.; Majumder, C. Charge Reordering of MgO(100) Surface by Sn Cluster Deposition: Implications for Heterogeneous Catalysis. Appl. Surf. Sci. 2020, 506, 144963. [Google Scholar] [CrossRef]
- Nava, N.; Morales, M.A.; Vanoni, W.; Toledo, J.A.; Baggio-Saitovitch, E.; Viveros, T. Structural Behavior of Pt–Sn Supported on MgO. Hyperfine Interact. 2001, 134, 81–92. [Google Scholar] [CrossRef]
- Shashikala, V.; Jung, H.; Shin, C.-H.; Koh, H.-L.; Jung, K.-D. N-Butane Dehydrogenation on PtSn/Carbon Modified MgO Catalysts. Catal. Lett. 2013, 143, 651–656. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Zhang, N.; Song, J.; Fan, X.; Zhao, Z.; Kong, L.; Xiao, X.; Xie, Z. One-Pot Synthesis of MgAlO Support for PtSn Catalysts over Propane Dehydrogenation. ChemistrySelect 2022, 7, e202104367. [Google Scholar] [CrossRef]
- Lopez, N. Effect of the Basicity of the Support on the Properties of Deposited Metal Atoms. J. Chem. Phys. 2001, 114, 2355–2361. [Google Scholar] [CrossRef]
- Wang, Y.; Florez, E.; Mondragon, F.; Truong, T.N. Effects of Metal–Support Interactions on the Electronic Structures of Metal Atoms Adsorbed on the Perfect and Defective MgO(100) Surfaces. Surf. Sci. 2006, 600, 1703–1713. [Google Scholar] [CrossRef]
- Gao, J.; Ren, Y.; Han, Q.; Wen, H.; Jiang, Z. Influences of MgO(001) and TiO2(101) Supports on the Structures and Properties of Au Nanoclusters. Catalysts 2020, 10, 16. [Google Scholar] [CrossRef]
- Zinnatullin, A.L.; Gabbasov, B.F.; Lyadov, N.M.; Yusupov, R.V.; Khaibullin, R.I.; Vagizov, F.G. Endotaxial α-Fe Nanoparticles in the High-Fluence Iron-Implanted Single-Crystal MgO. Crystals 2022, 12, 1095. [Google Scholar] [CrossRef]
- Pacchioni, G. Theory of Metal Clusters on the MgO Surface: The Role of Point Defects. In Nanocatalysis. Nanoscience and Technology; Heiz, U., Landman, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 193–243. [Google Scholar]
- Sementa, L.; Stener, M.; Fortunelli, A. Optical Activity of Metal Nanoclusters Deposited on Regular and Doped Oxide Supports from First-Principles Simulations. Molecules 2021, 26, 6961. [Google Scholar] [CrossRef]
- Tasker, P.W. The Stability of Ionic Crystal Surfaces. J. Phys. C Solid State Phys. 1979, 12, 4977–4984. [Google Scholar] [CrossRef]
- Wander, A.; Bush, I.J.; Harrison, N.M. Stability of Rocksalt Polar Surfaces: An Ab Initio Study of MgO(111) and NiO(111). Phys. Rev. B 2003, 68, 233405. [Google Scholar] [CrossRef]
- Kramer, J.; Ernst, W.; Tegenkamp, C.; Pfnur, H. Mechanism and Kinetics of Color Center Formation on Epitaxial Thin Films of MgO. Surf. Sci. 2002, 517, 87–97. [Google Scholar] [CrossRef]
- Matveev, A.V.; Neyman, K.M.; Yudanov, I.V.; Rosch, N. Adsorption of Transition Metal Atoms on Oxygen Vacancies and Regular Sites of the MgO(001) Surface. Surf. Sci. 1999, 426, 123–139. [Google Scholar] [CrossRef]
- Fuente, S.A.; Belelli, P.G.; Ferullo, R.M.; Castellani, N.J. Adsorption of NO on Au Atoms and Dimers Supported on MgO(100): DFT Studies. Surf. Sci. 2008, 602, 1669–1676. [Google Scholar] [CrossRef]
- Peterka, D.; Tegenkamp, C.; Schröder, K.-M.; Ernst, W.; Pfnür, H. Oxygen Surplus and Oxygen Vacancies on the Surface of Epitaxial MgO Layers Grown on Ag(100). Surf. Sci. 1999, 431, 146–155. [Google Scholar] [CrossRef]
- Sterrer, M.; Fischbach, E.; Heyde, M.; Nilius, N.; Rust, H.-P.; Risse, T.; Freund, H.-J. Electron Paramagnetic Resonance and Scanning Tunneling Microscopy Investigations on the Formation of F+ and F0 Color Centers on the Surface of Thin MgO(001) Films. J. Phys. Chem. B 2006, 110, 8665–8669. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pacchioni, G. Electronic Structure of F and V Centers on the MgO Surface. J. Phys. Chem. 1995, 99, 17010–17018. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley: Weinheim, Germany, 2001. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon: Oxford, UK, 1990. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Misra, N. Ab Initio Investigations on Planar (MgO)n Clusters (n = 1–5) and Their Hydrogen Adsorption Behaviour. Mol. Simul. 2016, 42, 208–214. [Google Scholar] [CrossRef]
- Reckien, W.; Janetzko, F.; Peintinger, M.F.; Bredow, T. Implementation of Empirical Dispersion Corrections to Density Functional Theory for Periodic Systems. J. Comput. Chem. 2012, 33, 2023–2031. [Google Scholar] [CrossRef]
- Varjovi, M.J.; Tosoni, S. DFT Investigation of X55 (X = Ni, Pd, and Pt) Clusters on Ultrathin Supported MgO Films: Evidence of Oxygen Spillover and Relevance for Catalytic Model Studies. J. Phys. Chem. C 2024, 128, 21331–21342. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Wang, F.-F.; Chen, D.-L.; Zhang, F.; Zhu, W. Theoretical Insights on the Synergistic Effect of Dual Metal Sites Supported on MgO(100) Promoting the Hydrogenation Reaction. J. Phys. Chem. C 2025, 129, 359–368. [Google Scholar] [CrossRef]
- Meier, L.A.; Castellani, N.J. Theoretical Study of Sn Adsorbed on the Au(111) Surface. Comput. Mater. Sci. 2017, 127, 48–59. [Google Scholar] [CrossRef]
- Shen, D.; Liu, Y.; Li, M.; Sun, W.; Tang, S.; Dong, W.; Yang, S. Structural Stability and Lithium Storage Property of Snx Clusters (x ≤ 6) Deposited on Graphene Based on First-Principles Calculation. Diamond Relat. Mater. 2023, 131, 109567. [Google Scholar] [CrossRef]
- Sousa, C.; de Graaf, C.; Lopez, N.; Harrison, N.M.; Illas, F. Ab Initio Theory of Magnetic Interactions at Surfaces. J. Phys. Condens. Matter 2004, 16, S2557. [Google Scholar] [CrossRef]
- Valero, R.; Gomes, J.R.B.; Truhlar, D.G.; Illas, F. Good Performance of the M06 Family of Hybrid Meta Generalized Gradient Approximation Density Functionals on a Difficult Case: CO Adsorption on MgO(001). J. Chem. Phys. 2008, 129, 124710. [Google Scholar] [CrossRef]
- Di Valentin, C.; Ferullo, R.; Binda, R.; Pacchioni, G. Oxygen Vacancies and Peroxo Groups on Regular and Low-Coordinated Sites of MgO, CaO, SrO, and BaO Surfaces. Surf. Sci. 2006, 600, 1147–1154. [Google Scholar] [CrossRef]
- Reveles, J.U.; Köster, A.M.; Khanna, S.N.; Quintanar, C. Surface Oxygen Diffusion into Neutral, Cationic, and Dicationic Oxygen Vacancies on MgO(100) Surfaces. J. Phys. Chem. C 2010, 114, 12265–12270. [Google Scholar] [CrossRef]
- Zamora, A.Y.; Reveles, J.U.; Mejia-Olvera, R.; Baruah, T.; Zope, R.R. FeO2/MgO(100) Supported Cluster: Computational Pursual for a Low-Cost and Low-Temperature CO Nanocatalyst. Chem. Phys. Lett. 2014, 612, 117–123. [Google Scholar] [CrossRef]
- Matczak, P. Effect of Surface Vacancies on the Adsorption of Pd and Pb on MgO(100). Monatsh. Chem. 2018, 149, 1009–1015. [Google Scholar] [CrossRef]
- Evjen, H.M. On the Stability of Certain Heteropolar Crystals. Phys. Rev. 1932, 39, 675–687. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for Main Group Elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XXIII. A Polarization-Type Basis Set for Second-Row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Pietro, W.J.; Francl, M.M.; Hehre, W.J.; DeFrees, D.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods. 24. Supplemented Small Split-Valence Basis Sets for Second-Row Elements. J. Am. Chem. Soc. 1982, 104, 5039–5048. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Matczak, P. Assessment of B3LYP Combined with Various ECP Basis Sets for Systems Containing Pd, Sn, and Pb. Comput. Theoret. Chem. 2012, 983, 25–30. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Del Vitto, A.; Pacchioni, G.; Delbecq, F.; Sautet, P. Au Atoms and Dimers on the MgO(100) Surface: A DFT Study of Nucleation at Defects. J. Phys. Chem. B 2005, 109, 8040–8048. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Becke, A.D. A Multicenter Numerical Integration Scheme for Polyatomic Molecules. J. Chem. Phys. 1988, 88, 2547–2553. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Calculation of Molecular Orbital Composition. Acta Chim. Sin. 2011, 69, 2393–2406. [Google Scholar]
- Keith, T.A. AIMAll 19.10.12; TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
- Li, S.; Zee, R.J.V.; Weltner, W., Jr. Magneto-Infrared Spectra of the Si2, Ge2, and Sn2 Molecules in Rare-Gas Matrices. J. Chem. Phys. 1994, 100, 7079–7086. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Xiao, C.; Neyman, K.M.; Pacchioni, G.; Rösch, N. Pd and Ag Dimers and Tetramers Adsorbed at the MgO(001) Surface: A Density Functional Study. Phys. Chem. Chem. Phys. 1999, 1, 4655–4661. [Google Scholar] [CrossRef]
- Sterrer, M.; Risse, T.; Giordano, L.; Heyde, M.; Nilius, N.; Rust, H.-P.; Pacchioni, G.; Freund, H.-J. Palladium Monomers, Dimers, and Trimers on the MgO(001) Surface Viewed Individually. Angew. Chem. Int. Ed. 2007, 46, 8703–8706. [Google Scholar] [CrossRef]
- Simic-Milosevic, V.; Heyde, M.; Nilius, N.; König, T.; Rust, H.-P.; Sterrer, M.; Risse, T.; Freund, H.-J.; Giordano, L.; Pacchioni, G. Au Dimers on Thin MgO(001) Films: Flat and Charged or Upright and Neutral? J. Am. Chem. Soc. 2008, 130, 7814–7815. [Google Scholar] [CrossRef]
- Beniwal, S.; Chai, W.; Metavarayuth, K.; Maddumapatabandi, T.D.; Shakya, D.M.; Henkelman, G.; Chen, D.A. Oxidation of Sn at the Cluster–Support Interface: Sn and Pt–Sn Clusters on TiO2(110). J. Phys. Chem. C 2021, 125, 17671–17683. [Google Scholar] [CrossRef]
- Yu, J.; Su, N.Q.; Yang, W. Describing Chemical Reactivity with Frontier Molecular Orbitalets. JACS Au 2022, 2, 1383–1394. [Google Scholar] [CrossRef]
- Macchi, P.; Sironi, A. Chemical Bonding in Transition Metal Carbonyl Clusters: Complementary Analysis of Theoretical and Experimental Electron Densities. Coord. Chem. Rev. 2003, 238–239, 383–412. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]

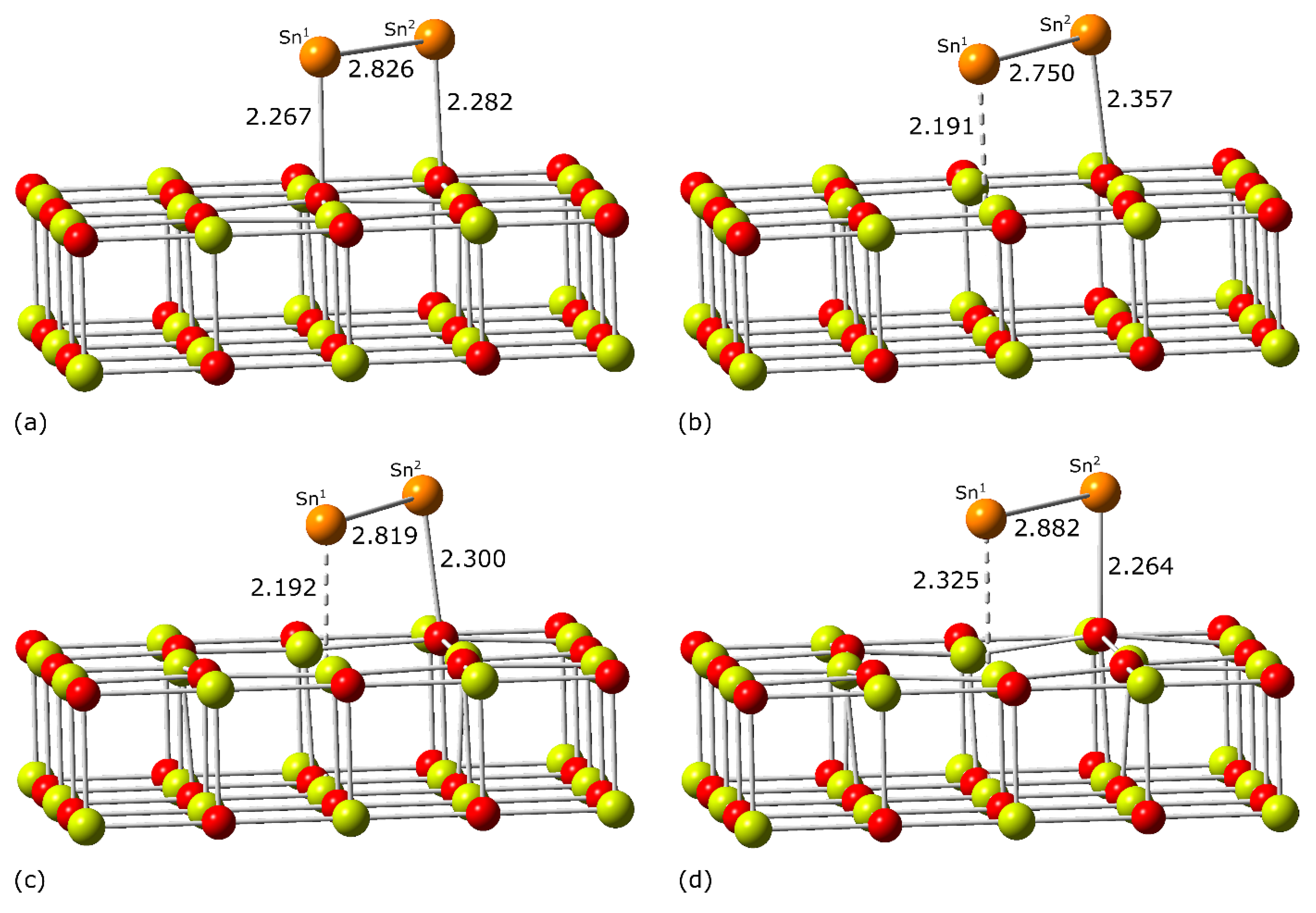
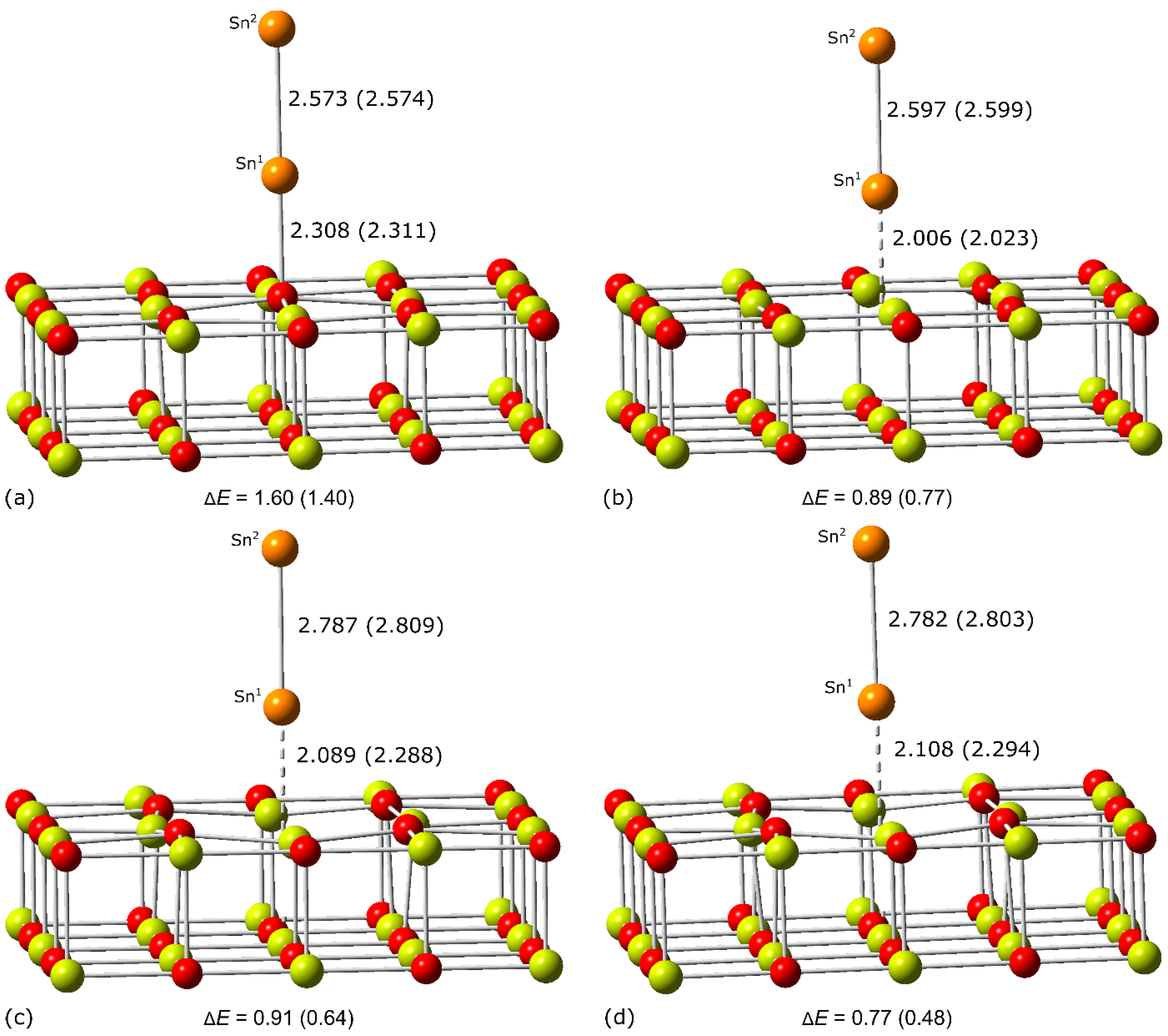
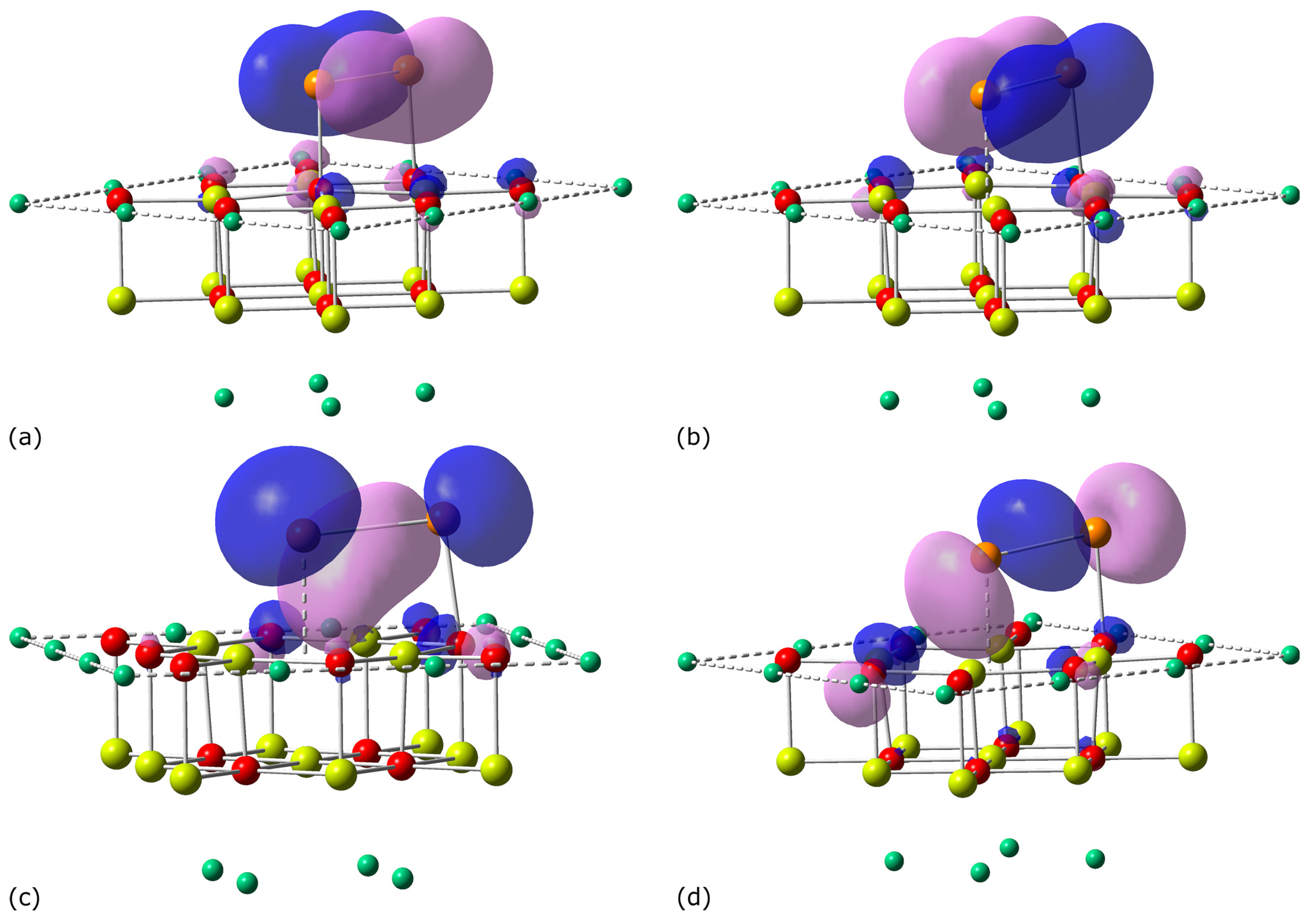
| Adsorption Center | 2S + 1 | Distance | ΔE | Eads | Eadh | ||
|---|---|---|---|---|---|---|---|
| Sn1-Surface a | Sn2-Surface b | Sn1-Sn2 | |||||
| O5c | 1 | 2.267 (2.233) | 2.282 (2.355) | 2.826 (2.808) | 0.00 (0.00) | −2.86 (−2.85) | −3.46 (−3.44) |
| 3 | 2.318 (2.259) | 2.802 (2.954) | 2.879 (2.857) | 0.92 (0.85) | −1.93 (−2.01) | −1.80 (−1.88) | |
| 5 | 2.242 (2.195) | 2.887 (3.256) | 3.074 (3.053) | 1.83 (1.71) | −1.03 (−1.15) | −1.96 (−2.10) | |
| 1 | 2.191 (2.195) | 2.357 (2.447) | 2.750 (2.724) | 0.00 (0.00) | −4.24 (−4.28) | −4.80 (−4.84) | |
| 3 | 2.193 (2.195) | 2.332 (2.426) | 3.014 (2.970) | 0.96 (1.03) | −3.28 (−3.25) | −3.18 (−3.11) | |
| 5 | 2.171 (2.170) | 3.129 (3.413) | 3.115 (3.062) | 2.09 (2.07) | −2.15 (−2.21) | −3.04 (−3.04) | |
| 2 | 2.192 (2.169) | 2.300 (2.390) | 2.819 (2.796) | 0.00 (0.00) | −3.08 (−2.92) | −3.55 (−3.41) | |
| 4 | 2.108 (2.039) | 2.305 (2.434) | 3.023 (2.987) | 0.82 (0.94) | −2.26 (−1.98) | −2.17 (−1.85) | |
| 6 | 2.315 (2.294) | 3.062 (3.342) | 3.229 (3.118) | 2.27 (2.27) | −0.81 (−0.64) | −1.65 (−1.45) | |
| 1 | 2.327 (2.118) | 2.273 (2.364) | 2.893 (2.869) | 0.11 (0.17) | −2.30 (−2.28) | −2.96 (−2.95) | |
| 3 | 2.325 (2.105) | 2.264 (2.366) | 2.882 (2.865) | 0.00 (0.00) | −2.41 (−2.45) | −2.40 (−2.39) | |
| 5 | 2.244 (2.244) | 2.299 (2.345) | 3.163 (3.121) | 0.90 (0.98) | −1.51 (−1.47) | −2.45 (−2.37) | |
| Adsorption Center | 2S + 1 | Sn-Surface a | ΔE | Eads | Eadh | q(Sn) | Nspin(Sn) |
|---|---|---|---|---|---|---|---|
| O5c | 1 | 2.270 (2.235) | 0.17 (0.18) | −1.64 (−1.83) | −2.80 (−3.00) | −0.114 (−0.131) | 0.000 (0.000) |
| 3 | 2.276 (2.241) | 0.00 (0.00) | −1.81 (−2.01) | −1.86 (−2.05) | −0.117 (−0.135) | 1.694 (1.691) | |
| 1 | 2.229 (2.230) | 0.14 (0.14) | −2.82 (−2.94) | −3.85 (−3.96) | −1.369 (−1.381) | 0.000 (0.000) | |
| 3 | 2.244 (2.246) | 0.00 (0.00) | −2.96 (−3.08) | −2.86 (−2.98) | −1.371 (−1.384) | 1.639 (1.657) | |
| 2 | 2.204 (2.344) | 0.13 (0.22) | −1.98 (−1.93) | −2.96 (−2.93) | −0.719 (−0.671) | 0.837 (0.866) | |
| 4 | 2.188 (2.152) | 0.00 (0.00) | −2.11 (−2.15) | −2.00 (−2.05) | −0.697 (−0.699) | 2.300 (2.332) | |
| 1 | 2.080 (2.300) | 0.14 (0.20) | −1.26 (−1.29) | −2.41 (−2.42) | −0.087 (−0.056) | 0.000 (0.000) | |
| 3 | 2.326 (2.316) | 0.00 (0.00) | −1.40 (−1.49) | −1.32 (−1.42) | −0.031 (−0.021) | 1.542 (1.573) |
| Adsorption Center | 2S + 1 | q(Sn2) | Nspin(Sn1) | Nspin(Sn2) |
|---|---|---|---|---|
| O5c | 1 | −0.242 (−0.299) | 0.000 (0.000) | 0.000 (0.000) |
| 3 | −0.167 (−0.207) | 0.639 (0.575) | 1.001 (1.095) | |
| 5 | −0.149 (−0.194) | 1.457 (1.393) | 1.982 (2.151) | |
| 1 | −1.462 (−1.508) | 0.000 (0.000) | 0.000 (0.000) | |
| 3 | −1.470 (−1.515) | 0.809 (0.825) | 0.845 (0.871) | |
| 5 | −1.437 (−1.486) | 1.537 (1.558) | 1.834 (1.929) | |
| 2 | −0.753 (−0.779) | 0.473 (0.483) | 0.323 (0.313) | |
| 4 | −0.768 (−0.806) | 1.499 (1.537) | 0.961 (0.938) | |
| 6 | −0.688 (−0.698) | 2.071 (2.082) | 2.041 (2.144) | |
| 1 | −0.032 (−0.077) | 0.000 (0.000) | 0.000 (0.000) | |
| 3 | −0.025 (−0.075) | 1.126 (1.169) | 0.430 (0.369) | |
| 5 | −0.054 (−0.064) | 1.890 (1.894) | 1.297 (1.345) |
| Adsorption Center | 2S + 1 | ||
|---|---|---|---|
| O5c | 1 | −3.63 (−3.43) | −1.81 (−1.42) |
| 3 | −2.70 (−2.58) | −0.89 (−0.57) | |
| 5 | −1.80 (−1.72) | 0.02 (0.29) | |
| 1 | −3.87 (−3.78) | −1.86 (−1.77) | |
| 3 | −2.91 (−2.76) | −0.90 (−0.74) | |
| 5 | −1.78 (−1.71) | 0.23 (0.30) | |
| 2 | −3.55 (−3.35) | −1.54 (−1.34) | |
| 4 | −2.73 (−2.41) | −0.72 (−0.40) | |
| 6 | −1.28 (−1.08) | 0.73 (0.93) | |
| 1 | −3.48 (−3.37) | −1.47 (−1.36) | |
| 3 | −3.60 (−3.54) | −1.59 (−1.53) | |
| 5 | −2.70 (−2.56) | −0.69 (−0.55) |
| 2S + 1 | Bond Length | Ebind | ρb | ∇2ρb | Gb/ρb | Hb/ρb | δ |
|---|---|---|---|---|---|---|---|
| 1 | 2.830 | −2.41 | 0.0547 | 0.0050 | 0.1511 | −0.3405 | 2.05 |
| 3 | 2.823 | −2.58 | 0.0553 | 0.0054 | 0.1516 | −0.3432 | 2.05 |
| 5 | 2.996 | −1.63 | 0.0350 | 0.0201 | 0.1994 | −0.2427 | 1.35 |
| Adsorption Center | 2S + 1 | ρb | ∇2ρb | Gb/ρb | Hb/ρb | δ |
|---|---|---|---|---|---|---|
| O5c | 1 | 0.0532 (0.0543) | 0.0088 (0.0099) | 0.1511 (0.1498) | −0.3320 (−0.3363) | 1.61 (1.65) |
| 3 | 0.0505 (0.0518) | 0.0050 (0.0055) | 0.1484 (0.1476) | −0.3180 (−0.3251) | 1.47 (1.51) | |
| 5 | 0.0320 (0.0330) | (0.0167) (0.0163) | 0.1930 (0.1877) | −0.2203 (−0.2279) | 0.95 (0.94) | |
| 1 | 0.0550 (0.0566) | 0.0238 (0.0269) | 0.1717 (0.1710) | −0.3446 (−0.3519) | 1.77 (1.86) | |
| 3 | 0.0414 (0.0434) | 0.0056 (0.0067) | 0.1501 (0.1513) | −0.2651 (−0.2779) | 1.10 (1.19) | |
| 5 | 0.0323 (0.0347) | 0.0128 (0.0133) | 0.1724 (0.1702) | −0.2156 (−0.2327) | 0.94 (1.01) | |
| 2 | 0.0527 (0.0543) | 0.0115 (0.0133) | 0.1585 (0.1593) | −0.3326 (−0.3402) | 1.51 (1.58) | |
| 4 | 0.0393 (0.0424) | 0.0070 (0.0050) | 0.1506 (0.1448) | −0.2584 (−0.2753) | 0.96 (1.04) | |
| 6 | 0.0250 (0.0293) | 0.0155 (0.0169) | 0.2026 (0.1983) | −0.1695 (−0.2037) | 0.74 (0.84) | |
| 1 | 0.0501 (0.0514) | 0.0026 (0.0037) | 0.1448 (0.1468) | −0.3180 (−0.3259) | 1.30 (1.33) | |
| 3 | 0.0503 (0.0521) | 0.0036 (0.0032) | 0.1461 (0.1457) | −0.3202 (−0.3285) | 1.30 (1.32) | |
| 5 | 0.0293 (0.0310) | 0.0131 (0.0144) | 0.1838 (0.1871) | −0.1993 (−0.2099) | 0.73 (0.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matczak, P. A First-Principles Study of Sn Dimer Adsorbed on MgO Surface. Crystals 2025, 15, 410. https://doi.org/10.3390/cryst15050410
Matczak P. A First-Principles Study of Sn Dimer Adsorbed on MgO Surface. Crystals. 2025; 15(5):410. https://doi.org/10.3390/cryst15050410
Chicago/Turabian StyleMatczak, Piotr. 2025. "A First-Principles Study of Sn Dimer Adsorbed on MgO Surface" Crystals 15, no. 5: 410. https://doi.org/10.3390/cryst15050410
APA StyleMatczak, P. (2025). A First-Principles Study of Sn Dimer Adsorbed on MgO Surface. Crystals, 15(5), 410. https://doi.org/10.3390/cryst15050410






