In Situ Observation of Microwave Sintering-Induced Directional Pores in Lithium Cobalt Oxide for Vertical Microchannel Electrodes
Abstract
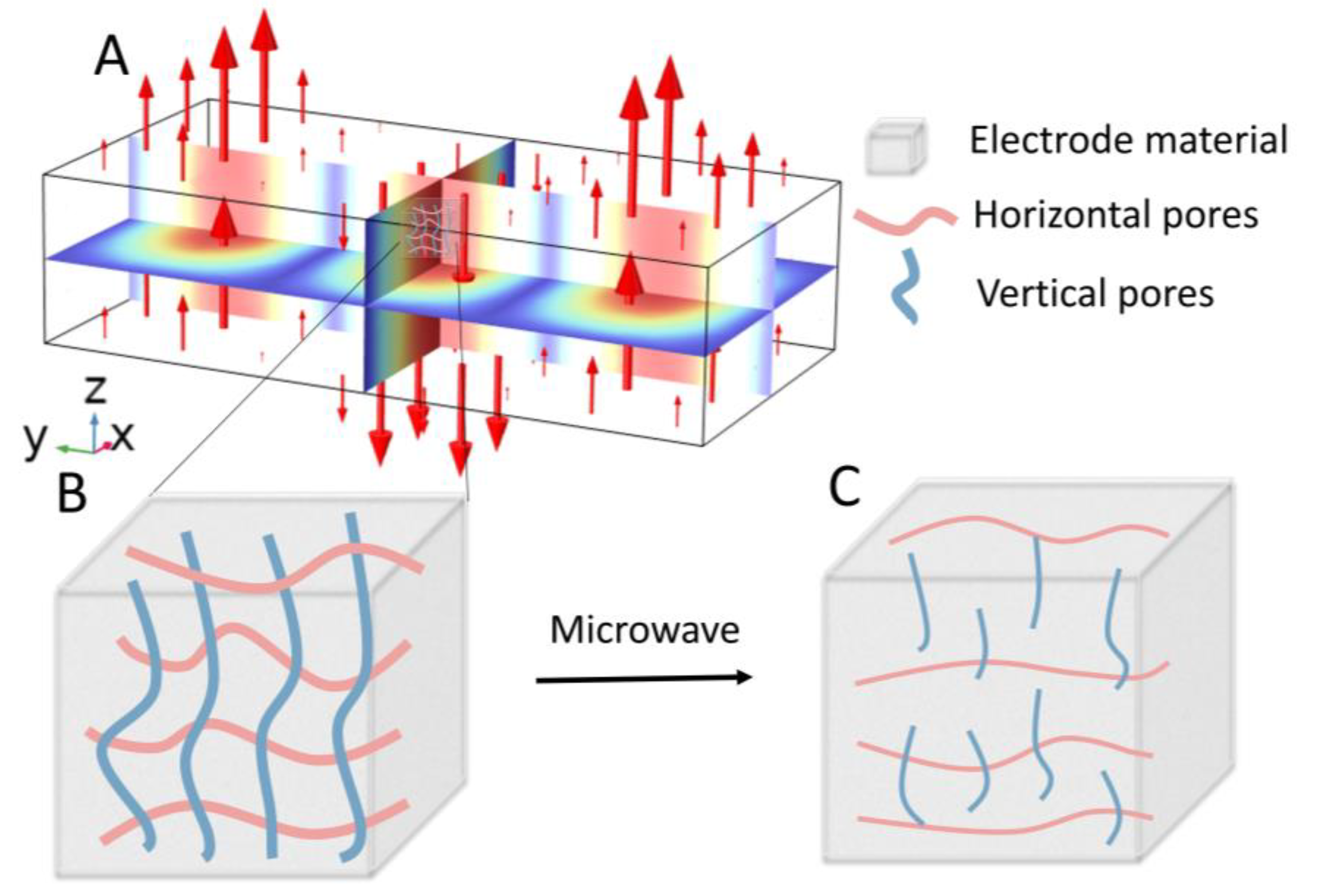
1. Introduction
2. Materials and Methods
2.1. Lithium Cobalt Oxide Materials
2.2. Sample Sintering
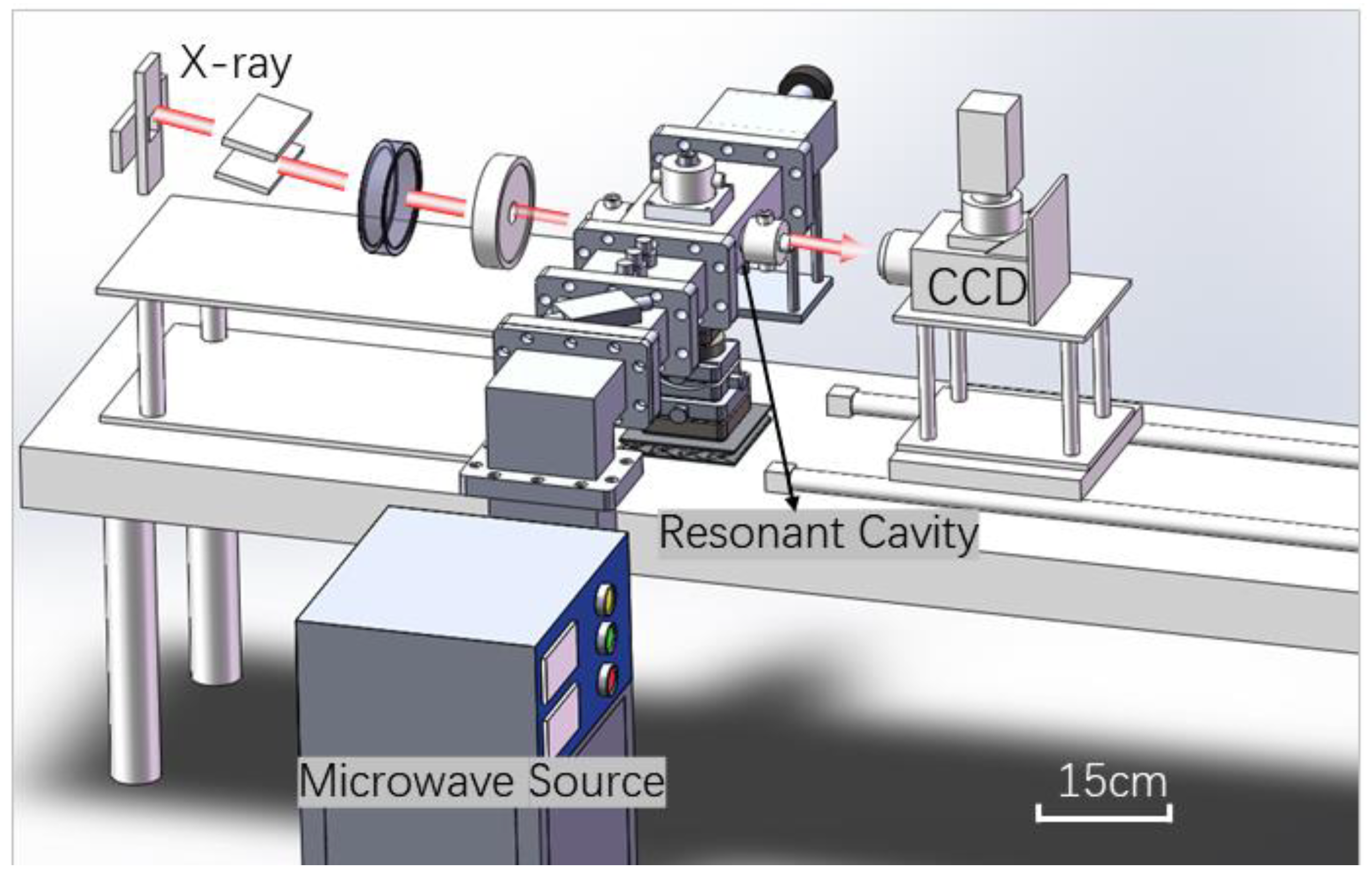
2.3. Synchrotron Radiation CT Imaging
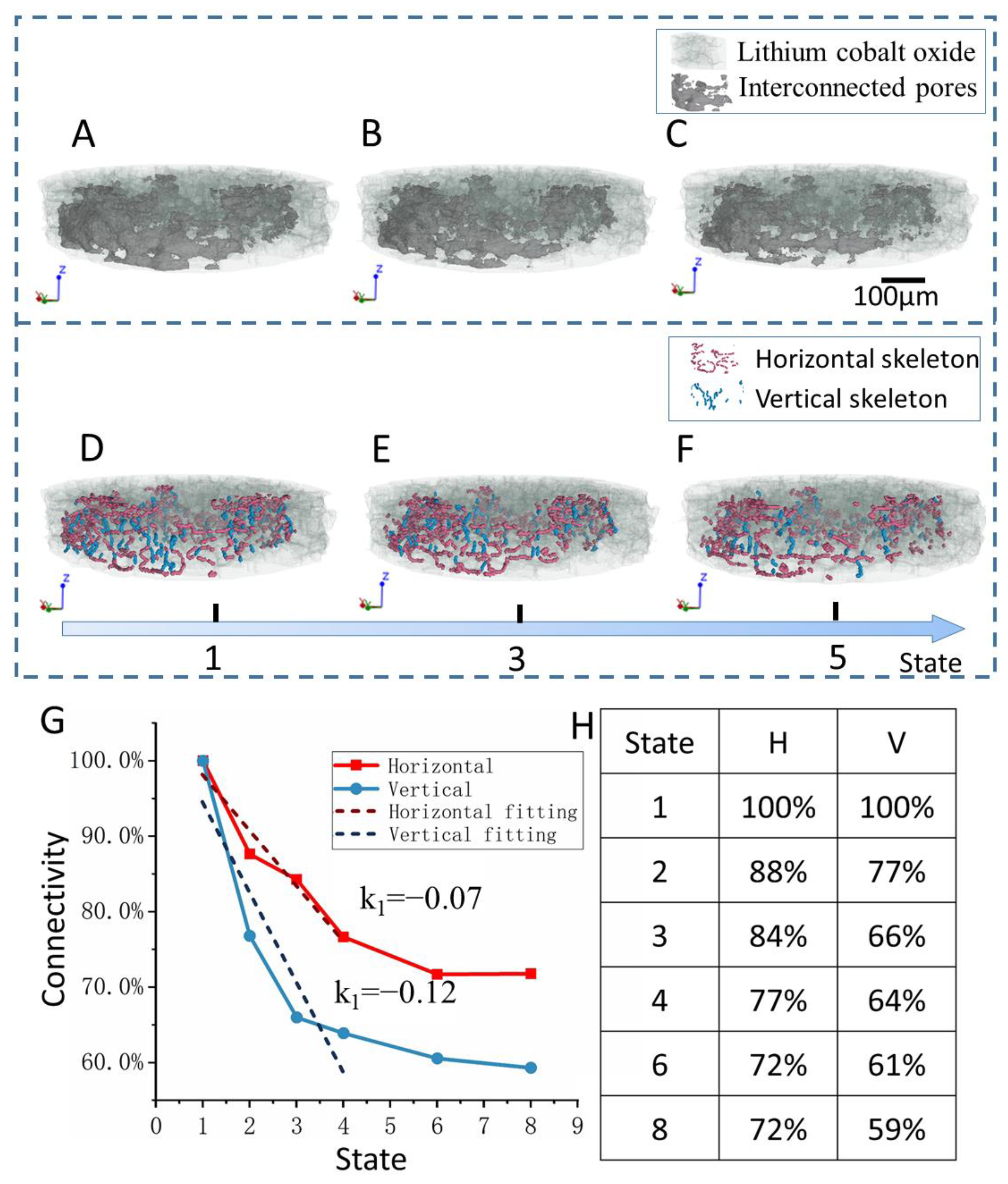
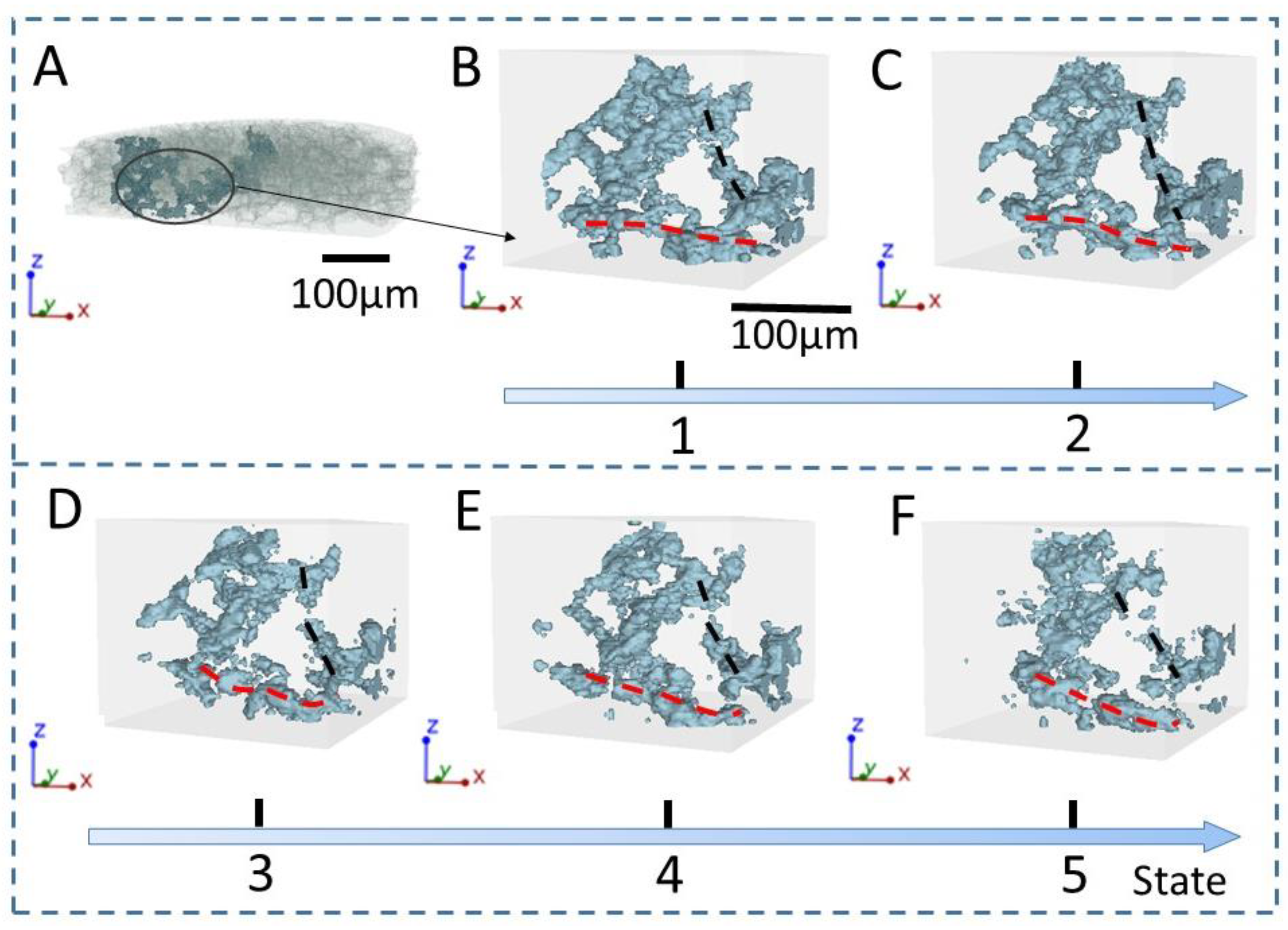
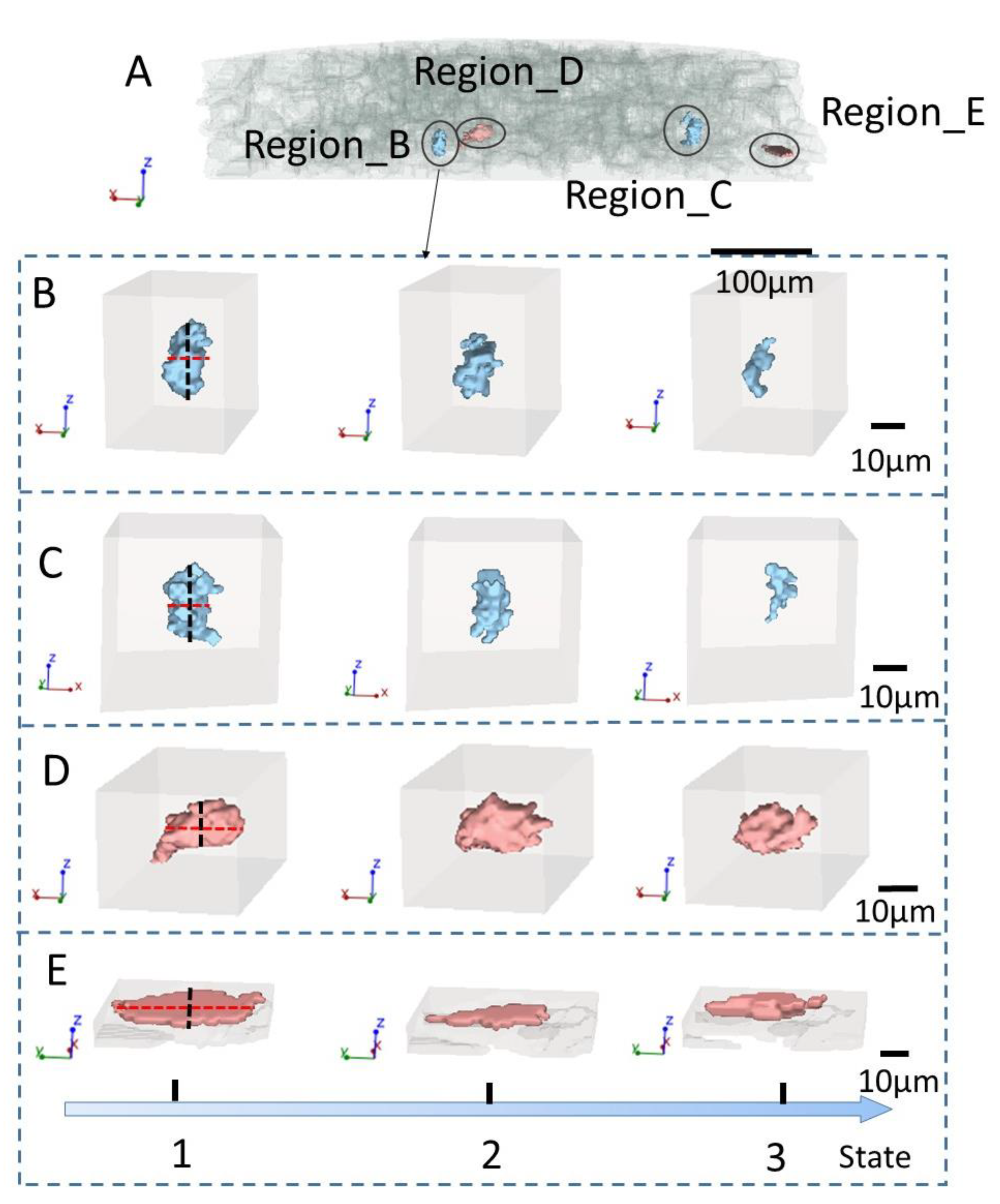
2.4. Pore Extraction and Skeletonization Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eng, A.Y.S.; Soni, C.B.; Lum, Y.; Khoo, E.; Yao, Z.; Vineeth, S.K.; Kumar, V.; Lu, J.; Johnson, C.S.; Wolverton, C.; et al. Theory-Guided Experimental Design in Battery Materials Research. Sci. Adv. 2022, 8, eabm2422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Rizwan, M.; Panwar, A.K. Advanced Intelligent Approach for State of Charge Estimation of Lithium-Ion Battery. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 10661–10681. [Google Scholar] [CrossRef]
- Nuhu, B.A.; Adun, H.; Bamisile, O.; Mukhtar, M. Ti-Fe-Si/C Composites as Anode Materials for High Energy Li-Ion Batteries. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 5154–5171. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Ju, Z.; Wang, L.; Hui, Z.; Mayilvahanan, K.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; et al. From Fundamental Understanding to Engineering Design of High-Performance Thick Electrodes for Scalable Energy-Storage Systems. Adv. Mater. 2021, 33, 2101275. [Google Scholar] [CrossRef]
- Arnot, D.J.; Mayilvahanan, K.S.; Hui, Z.; Takeuchi, K.J.; Marschilok, A.C.; Bock, D.C.; Wang, L.; West, A.C.; Takeuchi, E.S. Thick Electrode Design for Facile Electron and Ion Transport: Architectures, Advanced Characterization, and Modeling. Acc. Mater. Res. 2022, 3, 472–483. [Google Scholar] [CrossRef]
- Yang, X.; Doyle-Davis, K.; Gao, X.; Sun, X. Recent Progress and Perspectives on Designing High-Performance Thick Electrodes for All-Solid-State Lithium Batteries. eTransportation 2022, 11, 100152. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, B.; Yang, Y.; Cao, A. Toward High-Areal-Capacity Electrodes for Lithium and Sodium Ion Batteries. Adv. Energy Mater. 2022, 12, 2201834. [Google Scholar] [CrossRef]
- You, J.; Deng, L.; Wang, W.; Li, Y.; Meng, S.; Cao, Y.; Zhang, B.; Xu, Z. Cyclosulfate Additive Inhibits Li Dendrite Disorder Growth to Achieve Stable Cycles of Li Metal Battery. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 13125–13135. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, S.-J.; Chakravarthy, S.; Kim, K.H.; Lee, J.; Heo, J.S.; Park, H. Three-Dimensional Cathode with Periodically Aligned Microchannels for Improving Volumetric Energy Density of Lithium-Ion Batteries. J. Power Sources 2020, 451, 227764. [Google Scholar] [CrossRef]
- Tong, H.; Liu, J.; Qiao, Y.; Amardeep, A.; Song, X. Microstructure and Electrochemistry Performance of the Composite Electrode Prepared by Spark Plasma Sintering. J. Eur. Ceram. Soc. 2023, 43, 419–427. [Google Scholar] [CrossRef]
- Elango, R.; Nadeina, A.; Cadiou, F.; De Andrade, V.; Demortière, A.; Morcrette, M.; Seznec, V. Impact of Electrode Porosity Architecture on Electrochemical Performances of 1 Mm-Thick LiFePO4 Binder-Free Li-Ion Electrodes Fabricated by Spark Plasma Sintering. J. Power Sources 2021, 488, 229402. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, H.; Lee, H.C.; Shon, J.K.; Park, J.; Park, H.-Y. Stable Cycling of High-Density Three-Dimensional Sintered LiCoO2 Plate Cathodes. J. Power Sources 2022, 551, 232223. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, Q.; Yang, J.; Zhang, H.; Huo, W.; Xu, F. Spherical Mesophase Soft Carbon Materials with Micro-Nano Composite Structure and Their Applications in Lithium-Ion Batteries. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1675–1680. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Ma, J.; Sung, J.; Hong, J.; Chae, S.; Kim, N.; Choi, S.-H.; Nam, G.; Son, Y.; Kim, S.Y.; Ko, M.; et al. Towards Maximized Volumetric Capacity via Pore-Coordinated Design for Large-Volume-Change Lithium-Ion Battery Anodes. Nat. Commun. 2019, 10, 475. [Google Scholar] [CrossRef]
- Lu, L.-L.; Lu, Y.-Y.; Zhu, Z.-X.; Shao, J.-X.; Yao, H.-B.; Wang, S.; Zhang, T.-W.; Ni, Y.; Wang, X.-X.; Yu, S.-H. Extremely Fast-Charging Lithium Ion Battery Enabled by Dual-Gradient Structure Design. Sci. Adv. 2022, 8, eabm6624. [Google Scholar] [CrossRef]
- Cao, Q.; Gao, Y.; Pu, J.; Zhao, X.; Wang, Y.; Chen, J.; Guan, C. Gradient Design of Imprinted Anode for Stable Zn-Ion Batteries. Nat. Commun. 2023, 14, 641. [Google Scholar] [CrossRef]
- Mypati, O.; Pawar, R.M.A.; Sengupta, I.; Mani Sharma, V.; Majumder, S.B.; Pal, S.K.; Srirangam, P. The Synthesis of Novel Porous Graphene Anodes for Fast Charging and Improved Electrochemical Performance for Lithium-Ion Batteries. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 4349–4363. [Google Scholar] [CrossRef]
- Ju, Z.; King, S.T.; Xu, X.; Zhang, X.; Raigama, K.U.; Takeuchi, K.J.; Marschilok, A.C.; Wang, L.; Takeuchi, E.S.; Yu, G. Vertically Assembled Nanosheet Networks for High-Density Thick Battery Electrodes. Proc. Natl. Acad. Sci. USA 2022, 119, e2212777119. [Google Scholar] [CrossRef]
- Ju, Z.; Checko, S.; Xu, X.; Calderon, J.; Raigama, K.U.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Densified Vertically Lamellar Electrode Architectures for Compact Energy Storage. Proc. Natl. Acad. Sci. USA 2023, 120, e2308009120. [Google Scholar] [CrossRef]
- Chen, J.; Chen, M.; Chen, H.; Yang, M.; Han, X.; Ma, D.; Zhang, P.; Wong, C.-P. Wood-Inspired Anisotropic Hydrogel Electrolyte with Large Modulus and Low Tortuosity Realizing Durable Dendrite-Free Zinc-Ion Batteries. Proc. Natl. Acad. Sci. USA 2024, 121, e2322944121. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; de Jong, P.A.; Leiner, T.; de Heer, L.M.; Nievelstein, R.A.J.; Budde, R.P.J.; Schilham, A.M.R. Iterative Reconstruction Techniques for Computed Tomography Part 1: Technical Principles. Eur. Radiol. 2013, 23, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Kashyap, R.L.; Chu, C.N. Building Skeleton Models via 3-D Medial Surface Axis Thinning Algorithms. CVGIP Graph. Models Image Process. 1994, 56, 462–478. [Google Scholar] [CrossRef]
- Chen, X. 3D-Printed Hierarchical Pillar Array Electrodes for High-Performance Semi-Artificial Photosynthesis. Nat. Mater. 2022, 21, 811–818. [Google Scholar] [CrossRef]
- Yeon, J.S.; Gupta, N.; Bhattacharya, P.; Park, H.S. A New Era of Integrative Ice Frozen Assembly into Multiscale Architecturing of Energy Materials. Adv. Funct. Mater. 2022, 32, 2112509. [Google Scholar] [CrossRef]
- Lacava, F. Classical Electrodynamics: From Image Charges to the Photon Mass and Magnetic Monopoles; Undergraduate Lecture Notes in Physics; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Masin, B.; Ashok, K.; Sreemoolanadhan, H. Microwave Hybrid Sintering of 0.95(Mg0.95Zn0.05)TiO3-0.05CaTiO3 Ceramics and Its Microwave Dielectric Properties. J. Eur. Ceram. Soc. 2022, 42, 4974–4979. [Google Scholar] [CrossRef]
- Ramarao, S.D.; Kiran, S.R.; Murthy, V.R.K. Structural, Morphological and Microwave Dielectric Studies on Microwave Sintered ZnWO4 Ceramic Compounds. Ceram. Int. 2023, 49, 23075–23081. [Google Scholar] [CrossRef]
- Wang, L.; Shen, L.; Li, Y.; Wang, Y.; Xiao, Y.; Zhang, X.; Xu, F.; Hu, X. In Situ SR-CT Experimental Study on the Directional Sintering of High-Temperature Superconductor YBCO Materials in the Microwave Fields. Acta Metall. Sin. (Engl. Lett.) 2022, 35, 67–77. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric Loss Mechanism in Electromagnetic Wave Absorbing Materials. Adv. Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xiao, Y.; Lu, Y.; Wang, X. In Situ Observation of Microwave Sintering-Induced Directional Pores in Lithium Cobalt Oxide for Vertical Microchannel Electrodes. Crystals 2025, 15, 368. https://doi.org/10.3390/cryst15040368
Wang L, Xiao Y, Lu Y, Wang X. In Situ Observation of Microwave Sintering-Induced Directional Pores in Lithium Cobalt Oxide for Vertical Microchannel Electrodes. Crystals. 2025; 15(4):368. https://doi.org/10.3390/cryst15040368
Chicago/Turabian StyleWang, Liangyuan, Yu Xiao, Yilin Lu, and Xiao Wang. 2025. "In Situ Observation of Microwave Sintering-Induced Directional Pores in Lithium Cobalt Oxide for Vertical Microchannel Electrodes" Crystals 15, no. 4: 368. https://doi.org/10.3390/cryst15040368
APA StyleWang, L., Xiao, Y., Lu, Y., & Wang, X. (2025). In Situ Observation of Microwave Sintering-Induced Directional Pores in Lithium Cobalt Oxide for Vertical Microchannel Electrodes. Crystals, 15(4), 368. https://doi.org/10.3390/cryst15040368






