The Effect of Sulfur Concentration on the Crystallization and Electrochemical Behavior of Portland Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Crystallization Analysis
2.3. Crystalline Structure Analysis
2.4. Electrochemical Behavior Analysis
3. Results
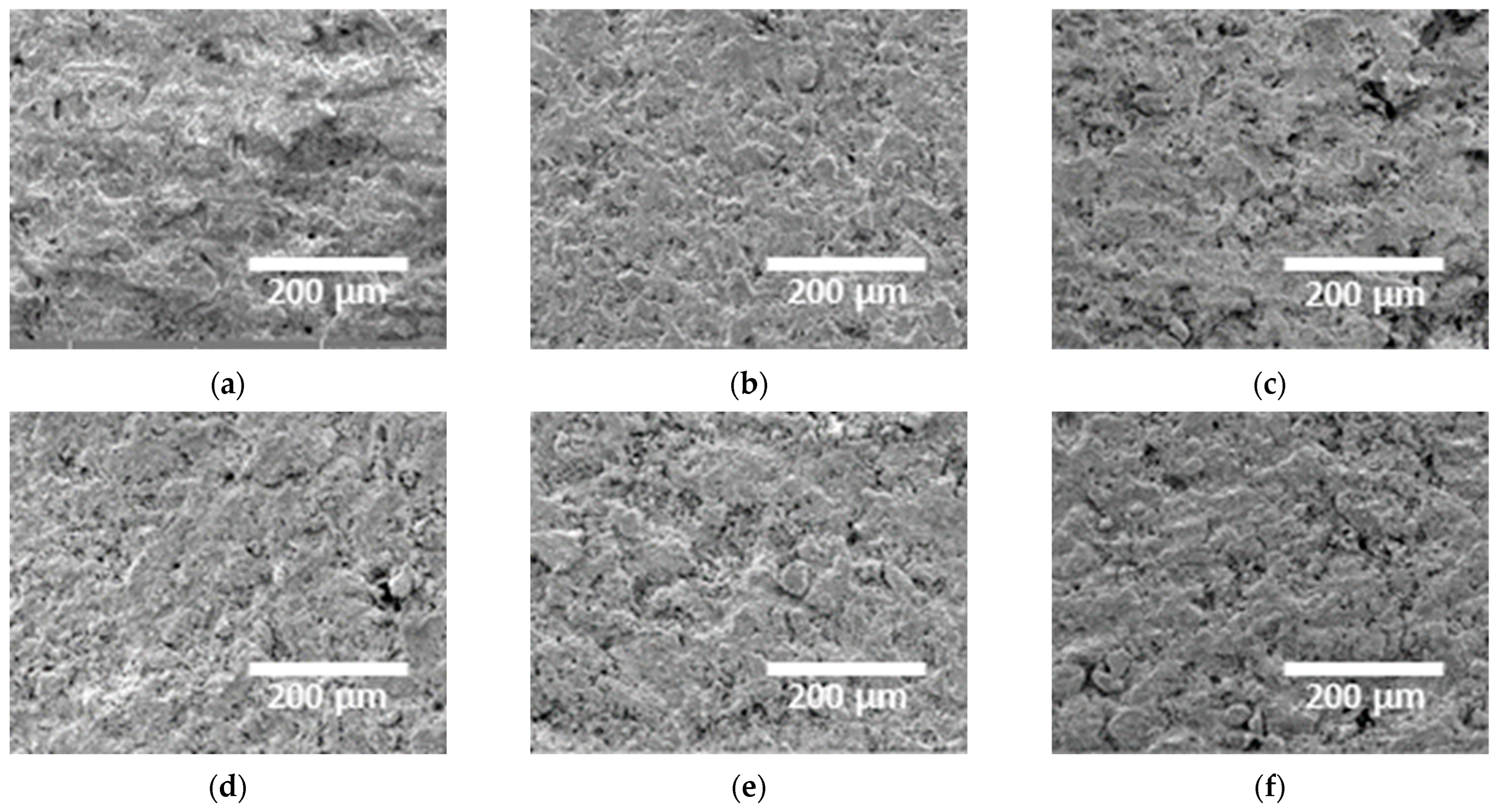
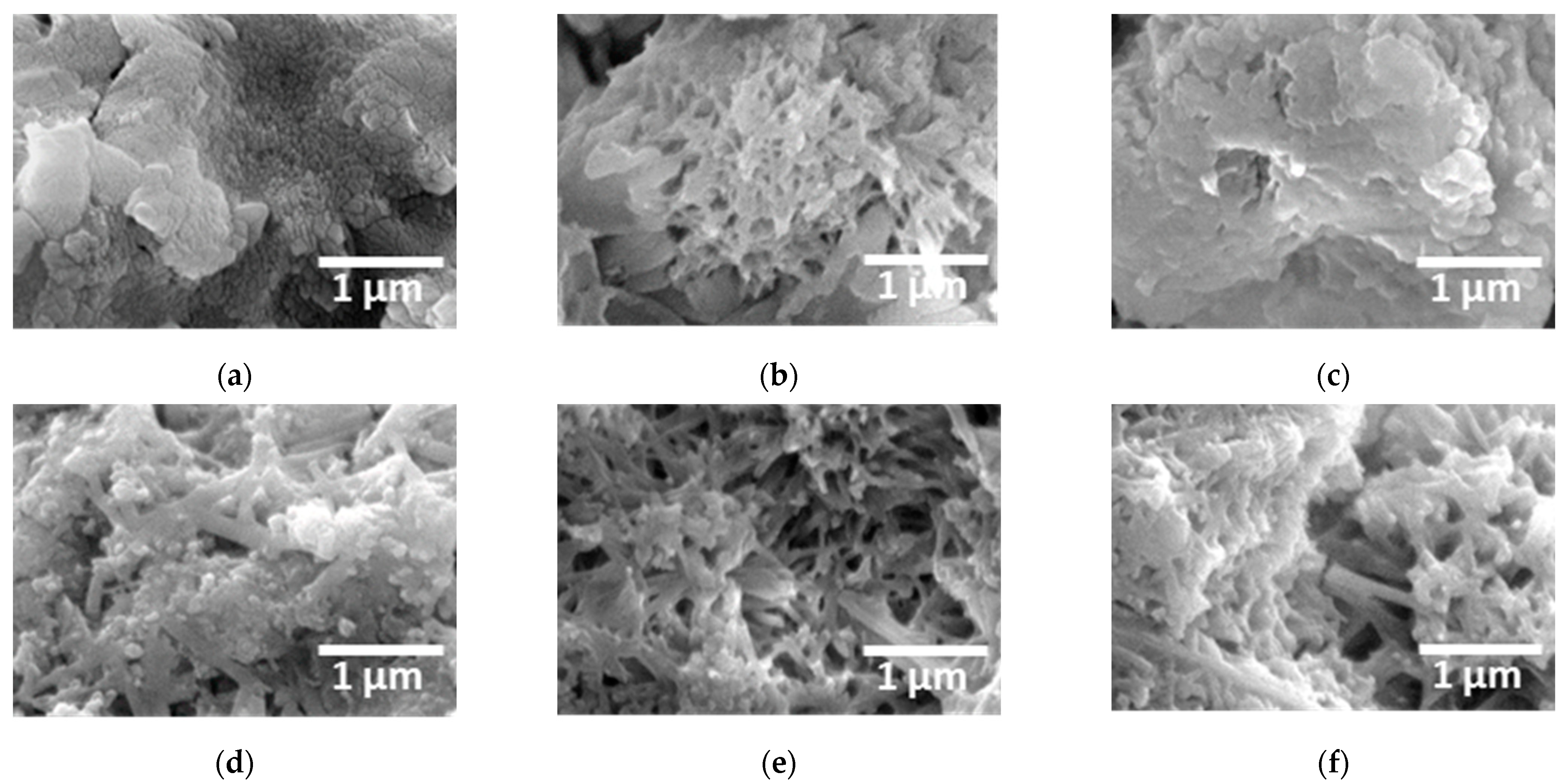
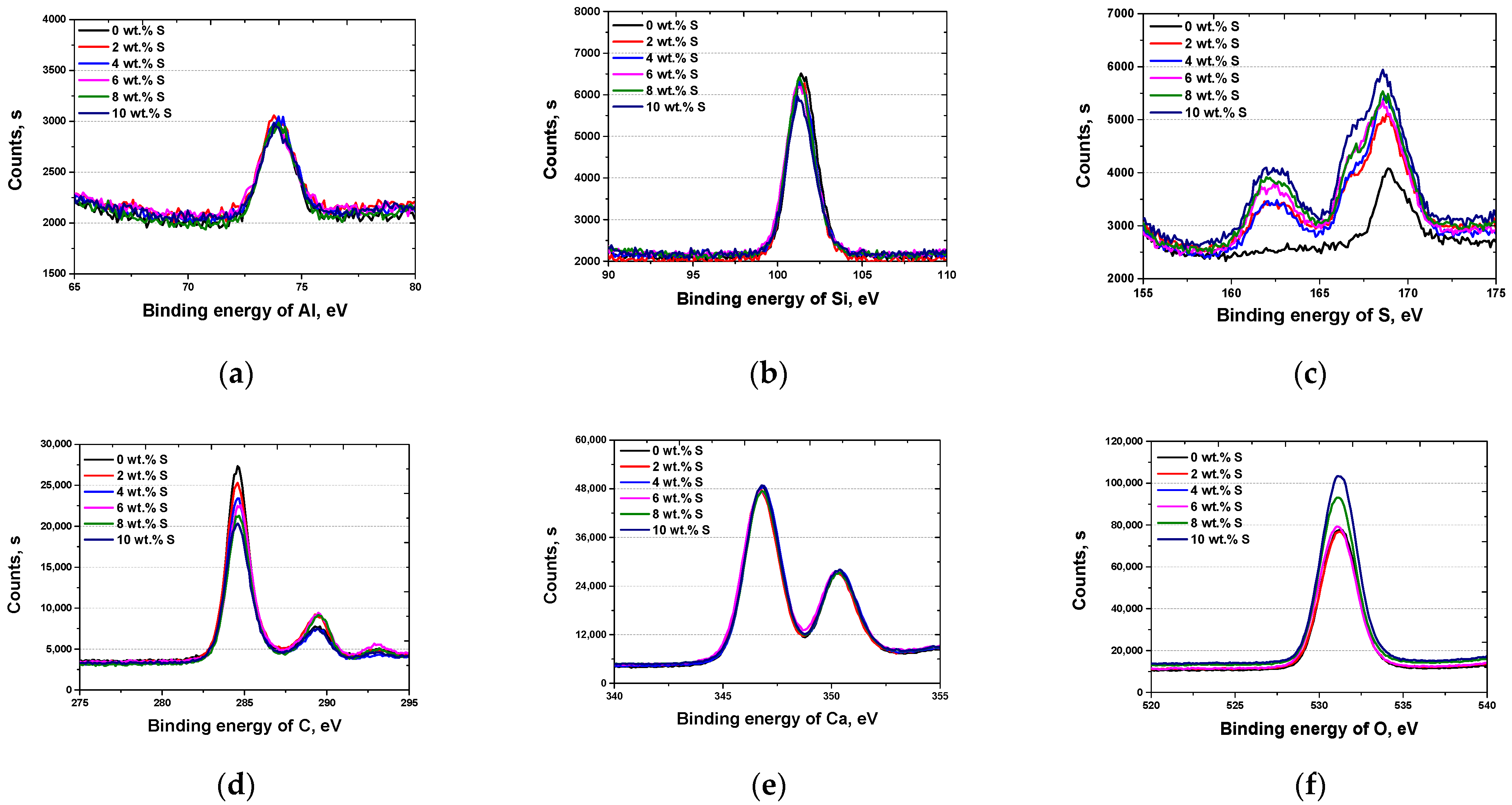
3.1. Microstructure with Added Sulfur
3(4CaO⋅Al2O3⋅SO3⋅12H2O) (monosulfate)
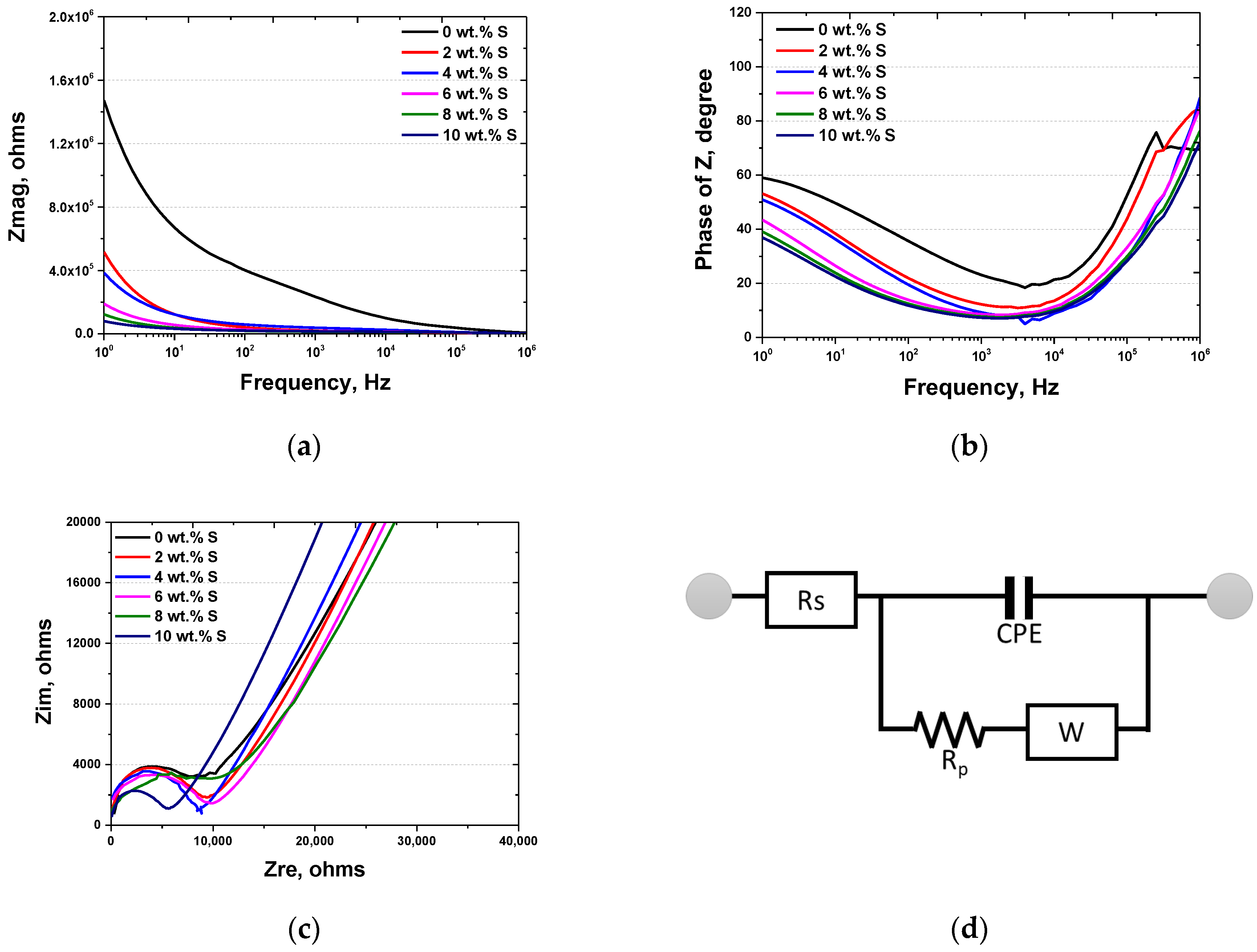
3.2. Results of Electrochemical Behavior Analysis
3.3. Discussion
4. Conclusions
- (1)
- This study investigated the effects of sulfur addition (0–10 wt.%) on the microstructure, electrochemical behavior, and corrosion resistance of cement. The results indicate that sulfur alters the phase composition, as confirmed by the XRD and XPS analyses, leading to a shift in the main peaks and crystallographic transformations. FE-SEM analysis revealed that increased sulfur content resulted in dendritic growth, increased porosity, and crack formation, which may negatively affect the mechanical strength and long-term durability of cement.
- (2)
- Electrochemical tests demonstrated that the addition of sulfur lowered the open circuit potential and reduced passive layer resistance, indicating an increased corrosion susceptibility to corrosion. While a moderate sulfur content (from 0 to 4 wt.%) maintained a relatively stable electrochemical response, higher sulfur levels significantly (to 6 wt.%) weakened the passive layer and increased the corrosion rates. The EIS results supported that the sulfur-rich cement exhibited a decrease in resistance, suggesting the degradation of the surface oxide layer, which is crucial for corrosion protection.
- (3)
- Overall, these findings highlight the importance of precisely controlling the sulfur content. Although small additions of sulfur may not pose significant risks, excessive sulfur disrupts crystal growth, reduces densification, and weakens passive film stability, leading to diminished strength and corrosion resistance. Future research should focus on enhancing cement properties by incorporating elements that promote crystal formation and strengthen the passive layer, ensuring improved durability and structural integrity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szczerba, J. Chemical Corrosion of Basic Refractories by Cement Kiln Materials. Ceram Int. 2010, 36, 1877–1885. [Google Scholar] [CrossRef]
- Marcotte, T.D.; Hansson, C.M. Corrosion Products That Form on Steel within Cement Paste. Mater. Struct. 2007, 40, 325–340. [Google Scholar] [CrossRef]
- Garcia, D.C.S.; de Soares, M.M.N.; da Bezerra, A.C.S.; Aguilar, M.T.P.; Figueiredo, R.B. Microstructure and Hardness of Cement Pastes with Mineral Admixture. Matéria 2017, 22, e11813. [Google Scholar] [CrossRef]
- Parrott, L.J. Some Effects of Cement and Curing upon Carbonation and Reinforcement Corrosion in Concrete. Mater. Struct. 1996, 29, 164–173. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z.; Wang, Y.; Yang, J.; Han, S.; Zhao, T. 3D Neutron Tomography of Steel Reinforcement Corrosion in Cement-Based Composites. Constr. Build. Mater. 2018, 162, 561–565. [Google Scholar] [CrossRef]
- Wittmann, F.H. Corrosion of Cement-Based Materials under the Influence of an Electric Field. Mater. Sci. Forum 1997, 247, 107–126. [Google Scholar] [CrossRef]
- Douglas, B.D.; Merrill, D.T.; Catlin, J.O. Water Quality Deterioration from Corrosion of Cement–Mortar Linings. J.-Am. Water Work. Assoc. 1996, 88, 99–107. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Liu, L.; Chen, Z.; Shi, C. A Review on Corrosion of Cement-Based Materials in CO2-Rich Karst Groundwater. Cem. Concr. Compos. 2024, 146, 105376. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J.A. Acid Corrosion Resistance of Different Cementing Materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Kamenskih, S.; Ulyasheva, N.; Buslaev, G.; Voronik, A.; Rudnitskiy, N. Research and Development of the Lightweight Corrosion-Resistant Cement Blend for Well Cementing in Complex Geological Conditions. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 15–17 October 2018; p. D023S010R009. [Google Scholar]
- Chaimongkhol, C.; Medepalli, S.; Zheng, Y.; Matsuda, T.; Ishida, T.; Wang, T. Investigating the Effects of Cracks and Low-Calcium Supplementary Cementitious Materials on Steel Fiber Corrosion in Cement Paste. Constr. Build. Mater. 2023, 399, 132554. [Google Scholar] [CrossRef]
- Li, W.; Dong, W.; Guo, Y.; Wang, K.; Shah, S.P. Advances in Multifunctional Cementitious Composites with Conductive Carbon Nanomaterials for Smart Infrastructure. Cem. Concr. Compos. 2022, 128, 104454. [Google Scholar] [CrossRef]
- Shi, C. Corrosion Resistance of Alkali-Activated Slag Cement. Adv. Cem. Res. 2003, 15, 77–81. [Google Scholar] [CrossRef]
- Arachchige, A.D.M. Influence of Cement Content on Corrosion Resistance. Proc. Inst. Civ. Eng.-Constr. Mater. 2008, 161, 31–39. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Acidic Corrosion of Hydrated Cement Based Materials. Ceram. Silikáty 2000, 44, 152–160. [Google Scholar]
- Dumont, A.; Patin, J.-B.; Le Floch, G. A Single Tool for Corrosion and Cement Evaluation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 16–19 September 1984; p. SPE-13140. [Google Scholar]
- Batis, G.; Pantazopoulou, P.; Tsivilis, S.; Badogiannis, E. The Effect of Metakaolin on the Corrosion Behavior of Cement Mortars. Cem. Concr. Compos. 2005, 27, 125–130. [Google Scholar] [CrossRef]
- Lu, D.; Zhong, J. Carbon-Based Nanomaterials Engineered Cement Composites: A Review. J. Infrastruct. Preserv. Resil. 2022, 3, 2. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Rocha, F.C.; Medeiros-Junior, R.A.; Helene, P. Corrosion Potential: Influence of Moisture, Water-Cement Ratio, Chloride Content and Concrete Cover. Rev. IBRACON Estrut. E Mater. 2017, 10, 864–885. [Google Scholar] [CrossRef]
- Zunino, F.; Boehm-Courjault, E.; Scrivener, K. The Impact of Calcite Impurities in Clays Containing Kaolinite on Their Reactivity in Cement after Calcination. Mater. Struct. 2020, 53, 1–15. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Lee, H.-S.; Karthick, S.; Saraswathy, V.; Yang, H.-M. Long-Term Corrosion Performance of Blended Cement Concrete in the Marine Environment–A Real-Time Study. Constr. Build. Mater. 2017, 154, 349–360. [Google Scholar] [CrossRef]
- Manzano, H.; Durgun, E.; Abdolhosseine Qomi, M.J.; Ulm, F.-J.; Pellenq, R.J.M.; Grossman, J.C. Impact of Chemical Impurities on the Crystalline Cement Clinker Phases Determined by Atomistic Simulations. Cryst. Growth. Des. 2011, 11, 2964–2972. [Google Scholar] [CrossRef]
- Pavlik, V. Corrosion of Hardened Cement Paste by Acetic and Nitric Acids Part I: Calculation of Corrosion Depth. Cem. Concr. Res. 1994, 24, 551–562. [Google Scholar] [CrossRef]
- Khedaywi, T.; Haddad, M.; Mujalli, R.; Shareef, S. Effect of Sulfur on the Asphalt Cement and Asphalt Concrete Mixture: State of the Art. Innov. Infrastruct. Solut. 2023, 8, 286. [Google Scholar] [CrossRef]
- Khademi, A.G.; Sar, H.I.K. Comparison of Sulfur Concrete, Cement Concrete and Cement-Sulfur Concrete and Their Properties and Application. Curr. World Environ. 2015, 10, 201–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlangen, E.; Çopuroğlu, O. Effect of Slags of Different Origins and the Role of Sulfur in Slag on the Hydration Characteristics of Cement-Slag Systems. Constr. Build. Mater. 2022, 316, 125266. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Corrosion of Reinforcement Steel Embedded in High Water-Cement Ratio Concrete Contaminated with Chloride. Cem. Concr. Compo.S 1998, 20, 263–281. [Google Scholar] [CrossRef]
- Hussain, S.E.; Al-Saadoun, S.S. Effect of Cement Composition on Chloride Binding and Corrosion of Reinforcing Steel in Concrete. Cem. Concr. Res. 1991, 21, 777–794. [Google Scholar]
- Kurdowski, W. The Protective Layer and Decalcification of CSH in the Mechanism of Chloride Corrosion of Cement Paste. Cem. Concr. Res. 2004, 34, 1555–1559. [Google Scholar] [CrossRef]
- Gay, H.; Meynet, T.; Colombani, J. Local Study of the Corrosion Kinetics of Hardened Portland Cement under Acid Attack. Cem. Concr. Res. 2016, 90, 36–42. [Google Scholar] [CrossRef]
- Lüttge, A. Crystal Dissolution Kinetics and Gibbs Free Energy. J. Electron Spectros Relat. Phenom. 2006, 150, 248–259. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lee, S.H. Review of the Potential of the Ni/Cu Plating Technique for Crystalline Silicon Solar Cells. Materials 2014, 7, 1318–1341. [Google Scholar] [CrossRef]
- Rajesh, D.; Sunandana, C.S. Briefly Brominated Ag Thin Films: XRD, FESEM, and Optical Studies of Surface Modification. Appl. Surf. Sci. 2012, 259, 276–282. [Google Scholar] [CrossRef]
- Sen, S.K.; Paul, T.C.; Dutta, S.; Hossain, M.N.; Mia, M.N.H. XRD Peak Profile and Optical Properties Analysis of Ag-Doped h-MoO3 Nanorods Synthesized via Hydrothermal Method. J. Mater. Sci. Mater. Electron. 2020, 31, 1768–1786. [Google Scholar] [CrossRef]
- Fredriksson, W.; Edström, K. XPS Study of Duplex Stainless Steel as a Possible Current Collector in a Li-Ion Battery. Electrochim Acta 2012, 79, 82–94. [Google Scholar] [CrossRef]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Electrochemical Behavior of Stainless Steel in Aerated NaCl Solutions by Electrochemical Impedance and Rotating Disk Electrode Methods. Russ. J. Electrochem. 2006, 42, 370–374. [Google Scholar] [CrossRef]
- Guerrini, E.; Cristiani, P.; Grattieri, M.; Santoro, C.; Li, B.; Trasatti, S. Electrochemical Behavior of Stainless Steel Anodes in Membraneless Microbial Fuel Cells. J. Electrochem. Soc. 2013, 161, H62. [Google Scholar] [CrossRef]
- Shin, B.-H.; Park, J.; Kim, S.; Ok, J.-W.; Kim, D.-I.; Yoon, J.-H. Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time. Metals 2024, 14, 307. [Google Scholar] [CrossRef]
- Sung, C.; Kim, K.; Chung, W.; Shin, B.-H. Electrochemical Properties of UNS S 32750 and UNS S 32760 Annealed Super Duplex Stainless Steels. Int. J. Electrochem. Sci. 2022, 17, 220526. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Lee, Y.-S.; Park, J.; Ok, J.-W.; Kim, S.; Shin, B.-H.; Yoon, J.-H. Study of Effects of Post-Weld Heat Treatment Time on Corrosion Behavior and Manufacturing Processes of Super Duplex Stainless SAF 2507 for Advanced Li-Ion Battery Cases. Materials 2024, 17, 4107. [Google Scholar] [CrossRef]
- Han, Z.; Ren, W.; Yang, J.; Du, Y.; Wei, R.; Zhang, C.; Chen, Y.; Zhang, G. The Deformation Behavior and Strain Rate Sensitivity of Ultra-Fine Grained CoNiFeCrMn High-Entropy Alloys at Temperatures Ranging from 77 K to 573 K. J. Alloys Compd. 2019, 791, 962–970. [Google Scholar] [CrossRef]
- Jo, H.; Ok, J.-W.; Lee, Y.-S.; Lee, S.; Je, Y.; Kim, S.; Kim, S.; Park, J.; Hong, J.; Lee, T. Impact of Ag Coating Thickness on the Electrochemical Behavior of Super Duplex Stainless Steel SAF2507 for Enhanced Li-Ion Battery Cases. Crystals 2025, 15, 62. [Google Scholar] [CrossRef]
- Cho, J.; Shin, B.-H.; You, M.; Kim, S.; Park, J.; Ok, J.-W.; Hong, J.; Lee, T.; Bae, J.-S.; Song, P. The Effect of Carbon on the Crystallization and Electrochemical Behavior of Portland Cement. Crystals 2025, 15, 189. [Google Scholar] [CrossRef]
- Shin, B.-H.; Kim, D.; Yoon, J.-H. Crystallization of Secondary Phase on Super-Duplex Stainless Steel SAF2507: Advanced Li-Ion Battery Case Materials. Crystals 2024, 14, 378. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the Influences of Alloying Elements on the Microstructure and Mechanical Properties of Aluminum Alloys and Aluminum Alloy Composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Rajesh, D.; Sunandana, C.S. XRD, Optical and AFM Studies on Pristine and Partially Iodized Ag Thin Film. Results Phys. 2012, 2, 22–25. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Ahmad, A.; Kamran, M.; Abid, K.; Haider, W. Microstructural and Electrochemical Behavior of 2205 Duplex Stainless Steel Weldments. Surf. Interfaces 2017, 9, 189–195. [Google Scholar] [CrossRef]
- Kitta, M.; Kataoka, R. Ability of Li4Ti5O12 to Suppress Li Metal Deposition under Overpotential Conditions Confirmed by Electrochemical Surface Plasmon Resonance Spectroscopy. Int. J. Electrochem. Sci. 2023, 18, 100223. [Google Scholar] [CrossRef]
- Masarapu, C.; Subramanian, V.; Zhu, H.; Wei, B. Long-Cycle Electrochemical Behavior of Multiwall Carbon Nanotubes Synthesized on Stainless Steel in Li Ion Batteries. Adv. Funct. Mater. 2009, 19, 1008–1014. [Google Scholar] [CrossRef]
- Kim, M.; Ha, J.; Kim, Y.-T.; Choi, J. Stainless Steel: A High Potential Material for Green Electrochemical Energy Storage and Conversion. Chem. Eng. J. 2022, 440, 135459. [Google Scholar] [CrossRef]
- Acharyya, S.G.; Khandelwal, A.; Kain, V.; Kumar, A.; Samajdar, I. Surface Working of 304L Stainless Steel: Impact on Microstructure, Electrochemical Behavior and SCC Resistance. Mater Charact 2012, 72, 68–76. [Google Scholar] [CrossRef]
- Paulraj, P.; Garg, R. Effect of Intermetallic Phases on Corrosion Behavior and Mechanical Properties of Duplex Stainless Steel and Super-Duplex Stainless Steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Amatsuka, S.; Nishimoto, M.; Muto, I.; Kawamori, M.; Takara, Y.; Sugawara, Y. Micro-Electrochemical Insights into Pit Initiation Site on Aged UNS S32750 Super Duplex Stainless Steel. Npj Mater. Degrad. 2023, 7, 15. [Google Scholar] [CrossRef]
- Vignal, V.; Delrue, O.; Heintz, O.; Peultier, J. Influence of the Passive Film Properties and Residual Stresses on the Micro-Electrochemical Behavior of Duplex Stainless Steels. Electrochim Acta 2010, 55, 7118–7125. [Google Scholar] [CrossRef]
- Lisowski, M.; Skopec, A. Effective Area of Thin Guarded Electrode in Determining of Permittivity and Volume Resistivity. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 24–31. [Google Scholar] [CrossRef]
- Skrotzki, W.; Pukenas, A.; Odor, E.; Joni, B.; Ungar, T.; Völker, B.; Hohenwarter, A.; Pippan, R.; George, E.P. Microstructure, Texture, and Strength Development during High-Pressure Torsion of CrMnFeCoNi High-Entropy Alloy. Crystals 2020, 10, 336. [Google Scholar] [CrossRef]
- Garrault-Gauffinet, S.; Nonat, A. Experimental Investigation of Calcium Silicate Hydrate (CSH) Nucleation. J. Cryst. Growth 1999, 200, 565–574. [Google Scholar] [CrossRef]
- Yuanhua, L.; Dajiang, Z.; Dezhi, Z.; Yuanguang, Y.; Taihe, S.; Kuanhai, D.; Chengqiang, R.; Deping, Z.; Feng, W. Experimental Studies on Corrosion of Cement in CO2 Injection Wells under Supercritical Conditions. Corros. Sci. 2013, 74, 13–21. [Google Scholar] [CrossRef]
- Faraji, H.; Yıldız, Ç.; Arshad, A.; Arıcı, M.; Choukairy, K.; El Alami, M. Passive Thermal Management Strategy for Cooling Multiple Portable Electronic Components: Hybrid Nanoparticles Enhanced Phase Change Materials as an Innovative Solution. J. Energy Storage 2023, 70, 108087. [Google Scholar] [CrossRef]

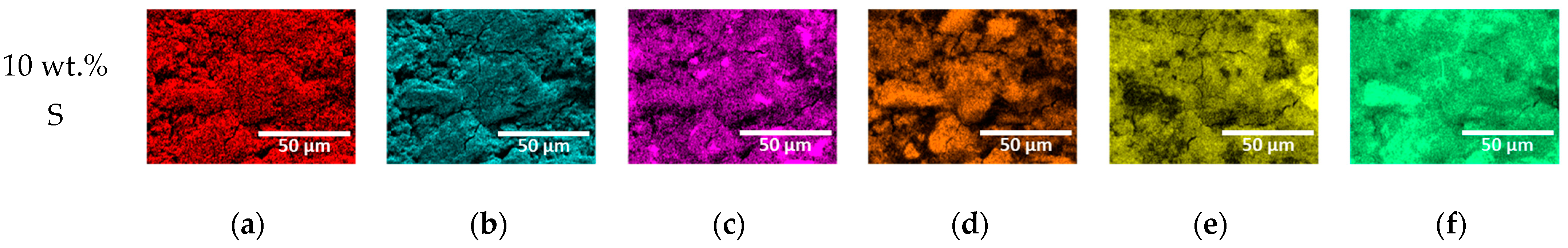



| (a) | (b) | (c) | (d) | (e) | (f) | |
|---|---|---|---|---|---|---|
| S | 0.6 | 2.5 | 4.5 | 6.5 | 8.6 | 10.5 |
| C | 0.7 | 0.7 | 0.7 | 0.6 | 0.6 | 0.6 |
| O | 45.3 | 44.5 | 43.7 | 42.9 | 42.1 | 41.3 |
| Si | 5.3 | 5.1 | 4.9 | 4.7 | 4.5 | 4.3 |
| Ca | 47.1 | 46.2 | 45.3 | 44.4 | 43.5 | 42.6 |
| Al | 1.1 | 1.0 | 0.9 | 0.8 | 0.7 | 0.7 |
| No. | Degree | Shifted Degree | Mineralogical Phase | Chemical Formula | Abbreviation | Crystal Structure | Miller Index |
|---|---|---|---|---|---|---|---|
| 1 | 23.8 | Ettringite | CaO⋅Al2O3⋅SO3⋅12H2O | AFt | Hexagonal | (114) | |
| 2 | 26.6 | Silica | SiO2 | Silicon dioxide | Hexagonal | (101) | |
| 3 | 27.0 | Silica | SiO2 | Silicon dioxide | Hexagonal | (100) | |
| 4 | 27.5 | Silica | SiO2 | Silicon dioxide | Hexagonal | (102) | |
| 5 | 28.5 | Calcite | CaCO3 | Calcium carbonate | Trigonal | (104) | |
| 6 | 28.7 | 29.4 | Aragonite | CaCO3 | Calcium carbonate | Trigonal | (104) |
| 7 | 29.4 | 30.1 | Dolomite | CaCO3 | Calcium carbonate | Trigonal | (104) |
| 8 | 29.7 | Aluminite | 3CaO·Al2O3 | C3A | Tetragonal | (110) | |
| 9 | 29.7 | Calcite | CaCO3 | Calcium carbonate | Trigonal | (104) | |
| 10 | 32.2 | Alite | 3CaO·SiO2 | C-S-H, calcium silicate hydrate | Monoclinic | (200) | |
| 11 | 32.9 | Belite | 2CaO·SiO2 | C2S, dicalcium silicate | Monoclinic | (200) | |
| 12 | 33.3 | Belite | 2CaO·SiO2 | C2S, dicalcium silicate | Monoclinic | (201) | |
| 13 | 34.1 | 34.8 | Belite | 2CaO·SiO2 | C2S, dicalcium silicate | Monoclinic | (001) |
| 14 | 34.1 | 34.8 | Slaked Lime | Ca(OH)2 | Calcium hydroxide | Hexagonal | (201) |
| 15 | 41.9 | Aluminite | 3CaO·Al2O3 | C4AF | Tetragonal | (202) | |
| 16 | 43.4 | Aluminite | 3CaO·Al2O3 | C3A | Tetragonal | (211) | |
| 17 | 47.1 | 47.8 | Slaked Lime | Ca(OH) 2 | Calcium hydroxide | Hexagonal | (211) |
| 18 | 47.1 | 47.8 | Aluminite | 3CaO·Al2O3 | C4AF | Tetragonal | (001) |
| 19 | 50.8 | 51.5 | Slaked Lime | Ca(OH)2 | Calcium hydroxide | Hexagonal | (211) |
| 20 | 50.8 | 51.5 | Aluminite | 3CaO·Al2O3 | C3A | Tetragonal | (001) |
| 21 | 51.6 | 52.0 | Aluminite | 3CaO·Al2O3 | C4AF | Tetragonal | (212) |
| 22 | 52.3 | Aluminite | 3CaO·Al2O3 | C3A | Tetragonal | (213) | |
| 23 | 53.8 | Aluminite | 3CaO·Al2O3 | C4AF | Tetragonal | (213) | |
| 24 | 53.3 | 55.0 | Aluminite | 3CaO·Al2O3 | C3A | Tetragonal | (214) |
| 25 | 57.0 | Aluminite | 3CaO·Al2O3 | C4AF | Tetragonal | (216) |
| 0 wt.% | 2 wt.% | 4 wt.% | 6 wt.% | 8 wt.% | 10 wt.% | |
|---|---|---|---|---|---|---|
| Ecorr, vs. SCE, V | −0.21 | −0.30 | −0.31 | −0.32 | −0.33 | −0.39 |
| Icorr, A/cm2 | 5 × 10−7 | 2 × 10−6 | 5 × 10−6 | 6 × 10−6 | 7 × 10−6 | 1 × 10−6 |
| Epit, vs. SCE, V | 0.71 | 0.63 | 0.60 | 0.60 | 0.51 | 0.06, 0.62 |
| 0 wt.% | 2 wt.% | 4 wt.% | 6 wt.% | 8 wt.% | 10 wt.% | |
|---|---|---|---|---|---|---|
| Rs, ohms | 6.1 | 6.1 | 6.1 | 6.2 | 6.2 | 6.1 |
| n of CPE | 13,125 | 12,250 | 10,500 | 10,000 | 9500 | 6750 |
| P of CPE | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Rp, ohms | 10,500 | 9800 | 8400 | 8000 | 7600 | 5400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-H.; Park, J.; Cho, J.; You, M.; Kim, S.; Ok, J.-W.; Hong, J.; Lee, T.; Bae, J.-S.; Song, P.; et al. The Effect of Sulfur Concentration on the Crystallization and Electrochemical Behavior of Portland Cement. Crystals 2025, 15, 358. https://doi.org/10.3390/cryst15040358
Shin B-H, Park J, Cho J, You M, Kim S, Ok J-W, Hong J, Lee T, Bae J-S, Song P, et al. The Effect of Sulfur Concentration on the Crystallization and Electrochemical Behavior of Portland Cement. Crystals. 2025; 15(4):358. https://doi.org/10.3390/cryst15040358
Chicago/Turabian StyleShin, Byung-Hyun, Jinyong Park, Jeunghyeuon Cho, Miyoung You, Seongjun Kim, Jung-Woo Ok, Jonggi Hong, Taekyu Lee, Jong-Seung Bae, Pungkeun Song, and et al. 2025. "The Effect of Sulfur Concentration on the Crystallization and Electrochemical Behavior of Portland Cement" Crystals 15, no. 4: 358. https://doi.org/10.3390/cryst15040358
APA StyleShin, B.-H., Park, J., Cho, J., You, M., Kim, S., Ok, J.-W., Hong, J., Lee, T., Bae, J.-S., Song, P., & Yoon, J.-H. (2025). The Effect of Sulfur Concentration on the Crystallization and Electrochemical Behavior of Portland Cement. Crystals, 15(4), 358. https://doi.org/10.3390/cryst15040358






