3. Results
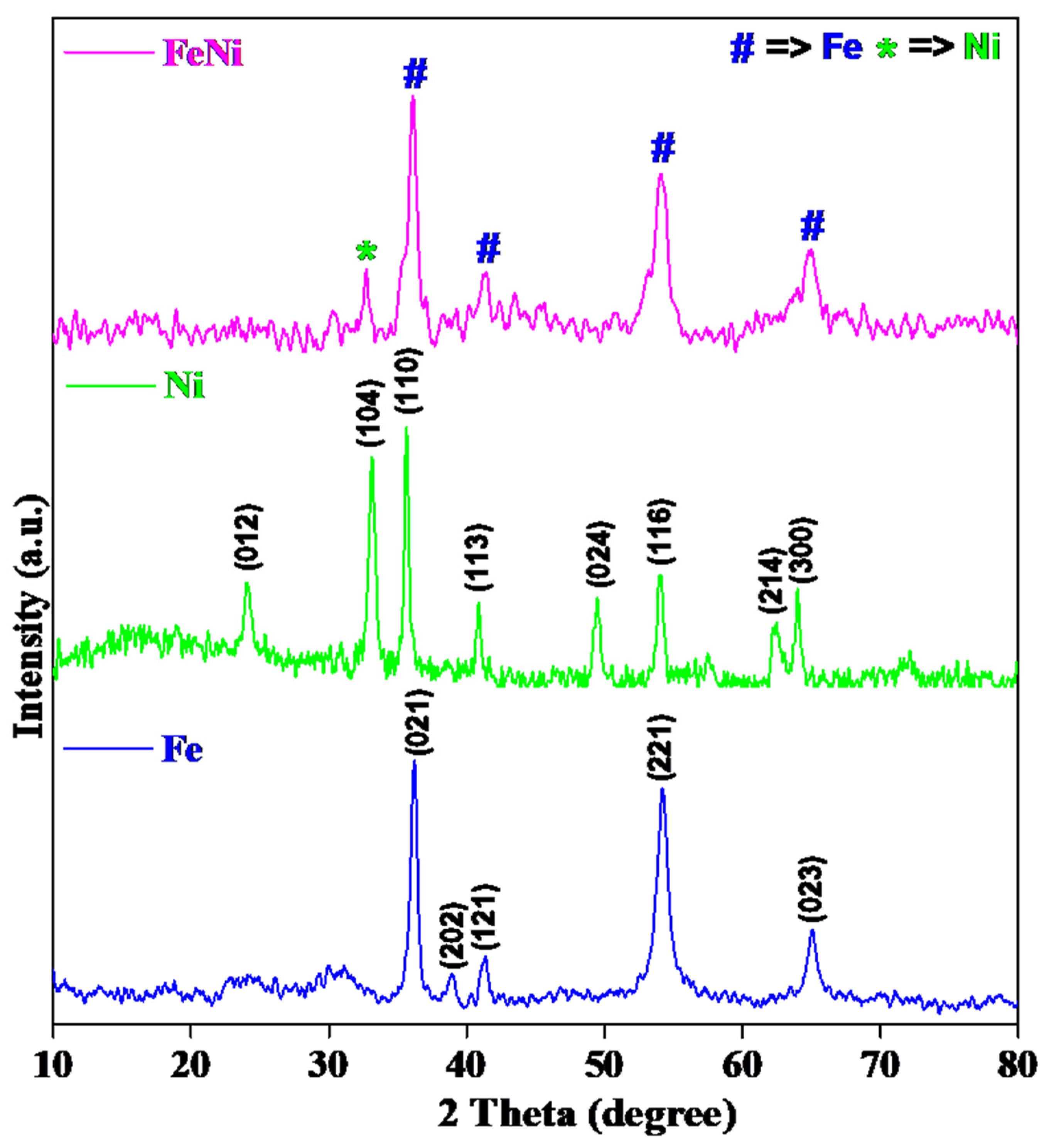
Figure 1 shows the diffraction peaks of FeWO
4 (Fe), NiFeOOH (Ni), and FeWO
4/NiFeOOH (FeNi). The peaks of FeWO
4 correspond to its monoclinic primitive structure (P2/c(13) space group) and unit cell parameters of a = 0.475 nm, b = 0.572 nm, c = 0.497 nm, and β = 90.17° (
Figure 1), consistent with the values reported in the literature (JCPDS card No. 71-2391). The diffraction peaks at 36.23°, 38.88°, 41.37°, 54.18°, and 65.05° correspond to the (021), (202), (121), (221), and (023) planes, respectively [
31]. The diffraction peaks of NiFeOOH were compared with the standard peaks of FeOOH (JCPDS No. 76-0182), which retains a rhombohedral crystal structure (R-3c space group) with the following unit cell parameters: a = 0.5031 nm, b = 0.5029 nm, c = 1.373 nm, and α = β = 90°, γ = 120° (
Figure 1). The diffraction peaks at 24.07°, 33.06°, 35.58°, 40.87°, 49.38°, 54.06°, 62.46°, and 64.02° correspond to the (012), (104), (110), (113), (024), (116), (214), and (300) planes, respectively. Ni incorporation causes the diffraction peaks to closely resemble those of FeOOH, suggesting that the crystal structure of FeOOH remains largely unchanged upon Ni doping [
32,
33].
Figure 1 shows that incorporating NiFeOOH into FeWO4 shifts the peaks to lower angles and decreases peak intensities compared to the Fe and Ni samples. Notably, peaks at 32.71° and a small bump at 35.47° from FeOOH appeared in the FeWO
4/NiFeOOH samples. Similarly, Chen et al. reported that NiFeOOH/Fe
2O
3 had no significant effect on its crystalline structure [
34]. The grain sizes of FeWO
4, NiFeOOH, and FeWO
4/NiFeOOH were approximately 59 nm, 44 nm, and 32 nm, respectively.
Figure 2a shows the SEM image of FeWO
4, revealing highly aggregated nanostructure spherical particles with an average size of 30–40 nm. Moreover, Zhou et al. recently reported that hierarchical plate-like FeWO
4 nanostructured materials exhibit excellent photocatalytic activity [
31]. The NiFeOOH sample consists of nanorods and sphere-like morphologies, forming aggregates of 50–60 nm (
Figure 2b). Lin et al. reported that incorporating NiFeOOH as a fluffy layer on WO
3 nanoflakes enhanced PEC photoelectrode performance [
35]. The maximum photocurrent density achieved was 2.58 mAcm
−2 at 1.8 V versus RHE, which is 4.3 and 2 times higher than that of pure WO
3 and WO
3/NiFeOOH, respectively.
Figure 2c shows significant agglomeration of interconnected spherical nanoparticles in the FeWO
4/NiFeOOH sample, likely caused by van der Waals forces and physicochemical attraction [
36]. The observed changes in surface roughness are attributed to the NiFeOOH nanostructure decoration, which may facilitate complete electrolyte infiltration [
37]. This modification improves structural stability and electron transfer within the electrode, thereby enhancing the electrochemical performance of FeWO
4. Furthermore, the results suggest that while FeWO
4/NiFeOOH has a minimal effect on surface morphology, adding hydrazine hydrate significantly influences surface roughness and nanoparticle agglomeration.
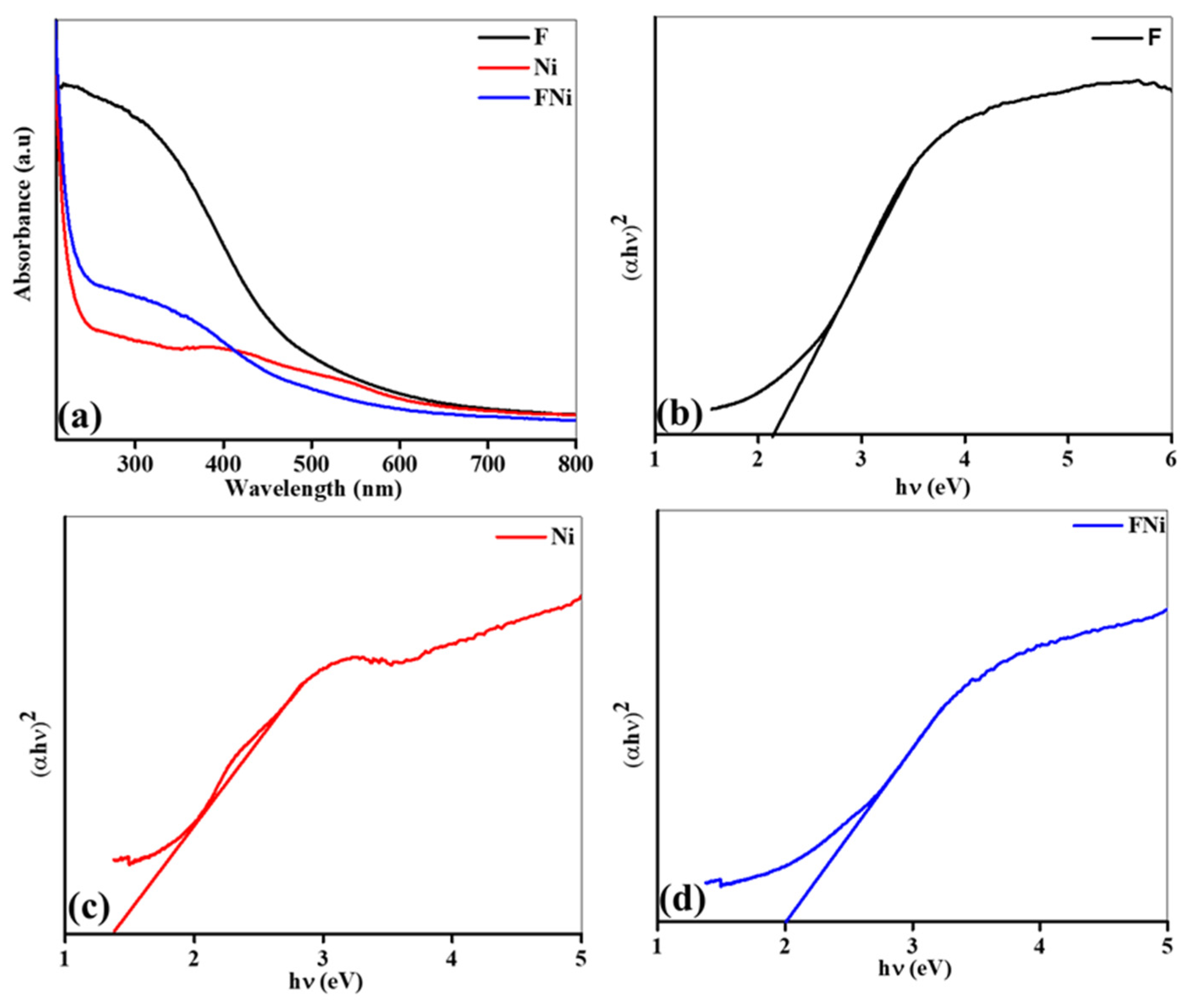
The UV–Vis absorption spectrum and bandgap analysis of Fe, Ni, and FeNi nanocomposites (
Figure 3) reveal crucial insights into their optical properties, highlighting unique behaviors across the 210–800 nm range. FeWO
4, characterized by its distinct electronic structure, demonstrates strong light absorption, particularly in the visible and ultraviolet regions. This characteristic is largely attributed to electronic states induced by oxygen vacancies in the material. NiFeOOH, in contrast, displays broad visible-light absorption, making it a promising material for photocatalytic and energy conversion applications. In the FeNi nanocomposite, FeWO
4 and NiFeOOH interact synergistically, enhancing absorption capacities. This occurs through the formation of additional electronic band levels within the composite, facilitated by their structural and electronic compatibility. The interaction between FeWO
4 and NiFeOOH couples their electronic states, introducing new energy levels within the bandgap of the material. These new levels modify optical transitions, extending the photon absorption range to bridge the gap between ultraviolet and visible light absorption, effectively broadening the absorption spectrum of the composite. This expansion enhances the light-harvesting efficiency of the composite, enabling additional light absorption and increasing its potential for energy conversion. Compared to their pristine samples, Fe and Ni, the FeNi composite demonstrates enhanced light-harvesting capabilities (
Figure 3a), highlighting the influence of nanoscale interactions and structural modifications in enhancing optical and electronic properties. Furthermore, the optical bandgaps of the materials—2.21 eV (FeWO
4), 1.42 eV (NiFeOOH), and 2.06 eV (FeNi composite)—highlight how the combination of these materials optimizes the light absorption spectrum, as shown in
Figure 3b–d. FeWO
4, with its wide bandgap, primarily absorbs UV light, limiting its effectiveness for visible-light-driven applications despite its stability. In contrast, NiFeOOH, with a narrower bandgap, excels in visible-light absorption, making it ideal for such applications. The FeNi composite, with a bandgap of 2.06 eV, achieves an optimal balance by integrating the strengths of both materials: the visible-light activity of NiFeOOH and the UV-light absorption and stability of FeWO
4. The optimized band alignment and interface engineering of the FeNi composite enable efficient light harvesting, charge transport, and stability, establishing it as a highly promising material for energy conversion applications, such as photocatalysis and photoelectrochemical (PEC) cells. This study highlights the importance of engineered nanostructures in enhancing material performance, offering significant potential for advancing sustainable energy technologies.
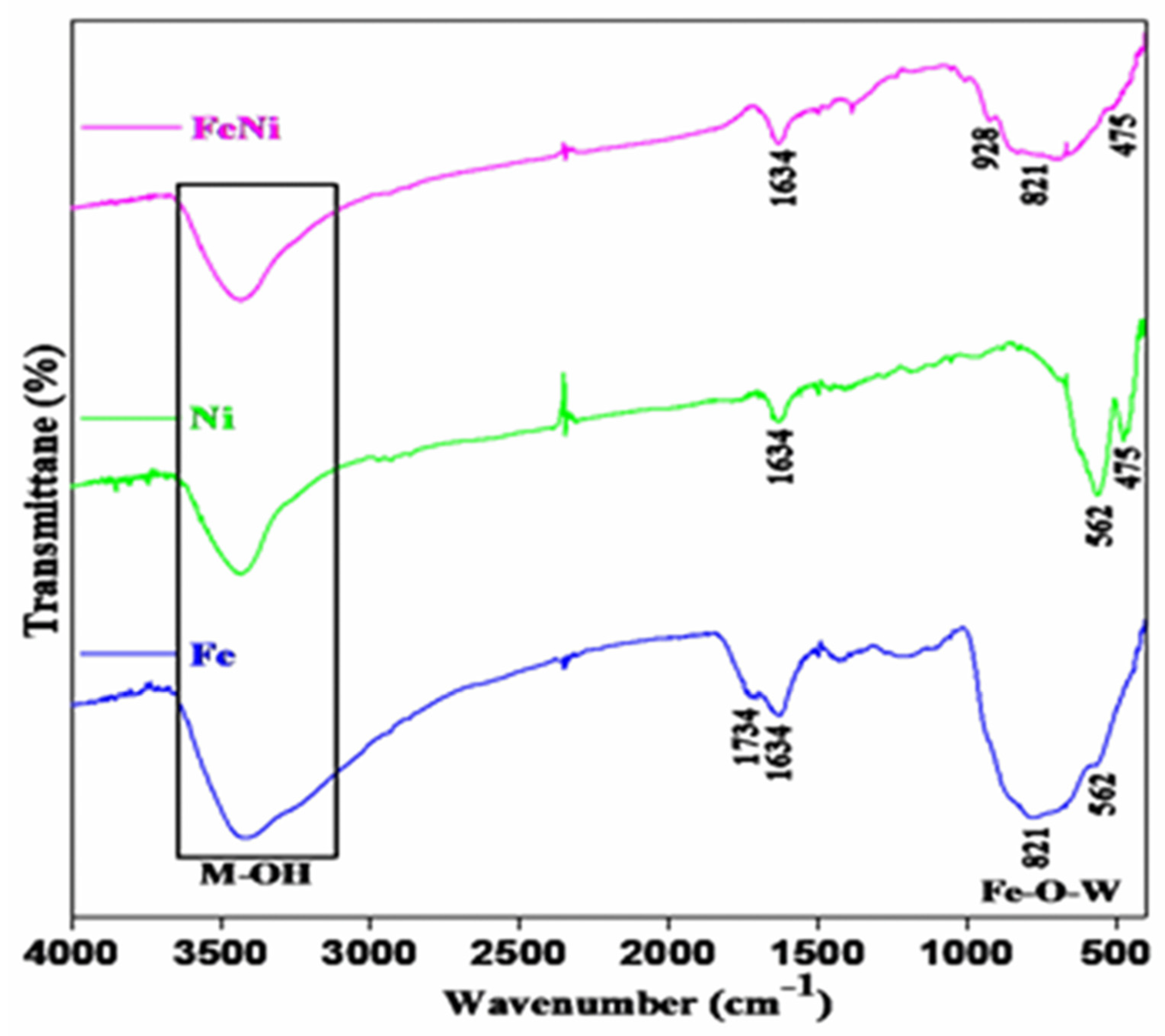
Figure 4 illustrates the chemical bonds of FeWO
4, NiFeOOH, and FeWO
4-NiFeOOH compounds. Broad bands spanning from 3000 to 3600 cm
−1 and 1600 to 1750 cm
−1 correspond to metal-conjugated O-H groups from water molecules within the FeWO
4 sample. The band at 562 cm
−1 corresponds to Fe-O stretching vibrations in hematite particles. Furthermore, the peaks at 887–637 cm
−1 correspond to W–O stretching vibrations in the WO
4 tetrahedra deformation mode for FeWO
4 samples (
Figure 4) [
38]. The broad band at 500–1000 cm
−1 corresponds to Fe-O-W stretching vibrations in the Fe and FeNi samples. The FTIR spectra of the Ni sample (
Figure 4) display peaks at a broad range from 3000 to 3600 cm
−1, attributed to water molecule stretching modes [
39]. Peaks at 1054 cm
−1 and 829 cm
−1 correspond to hydroxyl bending vibrations in Fe–OH, while bands at 670 cm
−1, 562 cm
−1, and 475 cm
−1 represent Fe-O bending vibrations [
40].
Figure 4 depicts the incorporation of NiFeOOH into the FeWO
4 compound, showing the presence of Fe-O, W-O, and O-H vibrations from the FeWO
4 compound. However, the additional peaks at 1387 cm
−1 and 928 cm
−1 correspond to hydroxyl bending vibrations in Fe-OH. The vibrations corresponding to hydroxyl out-of-plane bending in Fe-OH at 1054 cm
−1 disappeared, probably owing to the dominance of W–O and Fe–O bands. Meanwhile, the peaks at 475 cm
−1, 670 cm
−1, and the broad band at 500–1000 cm
−1 remain intact, consistent with the NiFeOOH material composition. This indicates covalent bonding between the NiFeOOH and FeWO
4 materials.
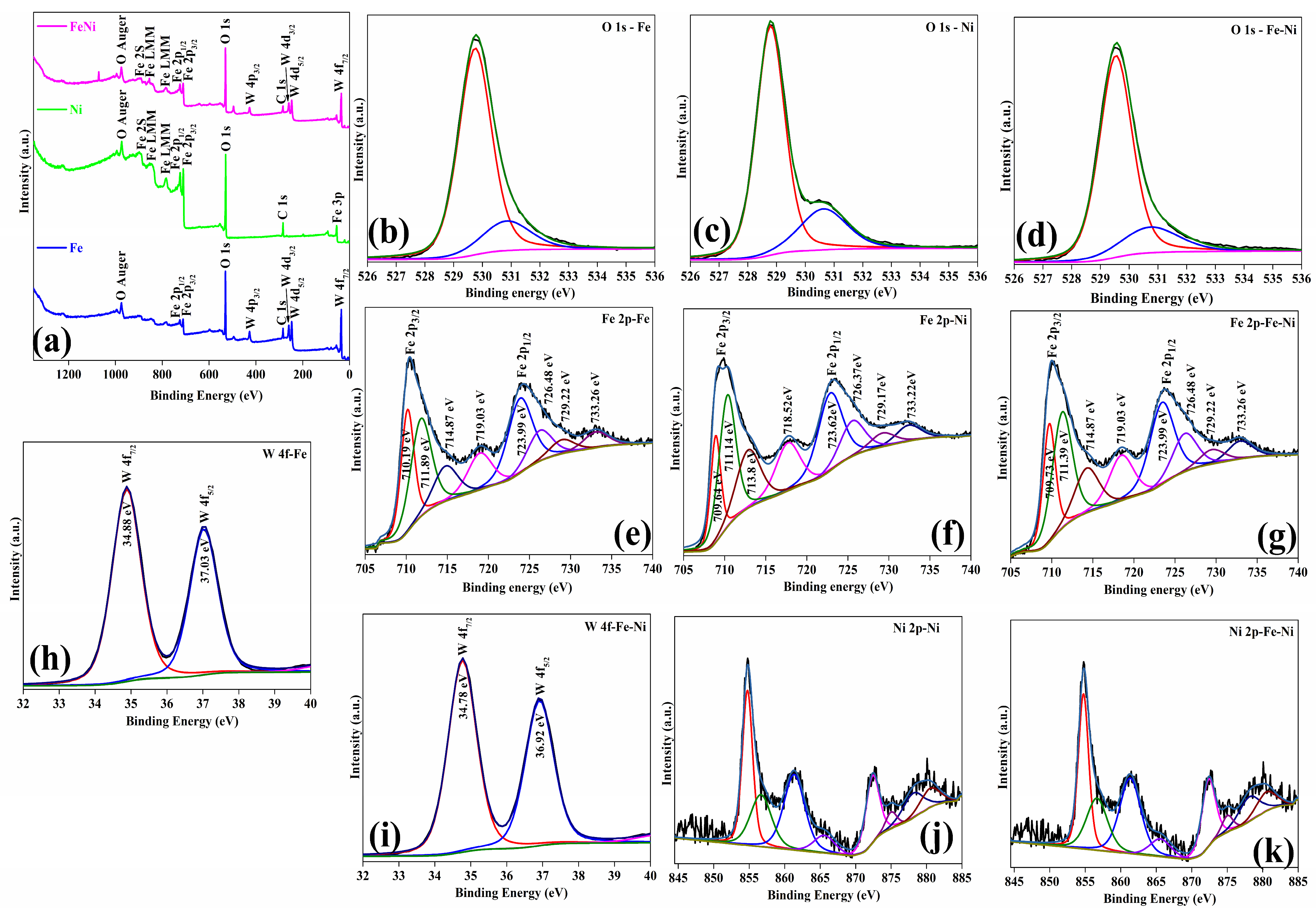
XPS analysis of the FeWO
4, NiFeOOH, and FeWO
4-NiFeOOH samples provided detailed insights into their elemental composition, chemical states, and structures. The survey spectra revealed distinct peaks for W, Fe, O, and Ni species, confirming their presence in the synthesized samples (
Figure 5a). High-resolution deconvoluted spectra (
Figure 5b–k) further elucidated the oxidation states and bonding environments of the elements. The O 1s core level spectra provide key insights into oxygen species in the FeWO
4, NiFeOOH, and FeWO
4/NiFeOOH samples. A peak at ~529.9 eV corresponds to lattice oxygen, while a higher-energy peak at ~529.6 eV represents adsorbed oxygen (
Figure 5b–d) [
41].
In the NiFeOOH sample, a peak at 528.8 eV indicates oxygen related to absorbed moisture, reflecting the surface chemistry and environmental interactions of the sample. The Fe 2p spectra of FeWO
4, NiFeOOH, and FeWO
4-NiFeOOH samples displayed binding energy peaks at 710.19 eV, 709.64 eV, and 709.73 eV (Fe 2p3/2) and 723.99 eV, 723.62 eV, and 723.99 eV (Fe 2p1/2), indicative of Fe in the +2 oxidation state (
Figure 5e–g) [
42]. Similarly, the W 4f core-level spectra of FeWO
4 and FeWO
4-NiFeOOH display peaks at approximately 34.88 eV and 34.78 eV (W 4f
7/
2) and 37.03 eV and 36.92 eV (W 4f
5/
2), indicating that W exists predominantly in the +6 oxidation state (
Figure 5h,i) [
43]. Furthermore, high-resolution scans of the Ni 2p spectra for the NiFeOOH and FeWO
4/NiFeOOH samples show peaks at 854.11 eV and 854.84 eV (Ni 2p
3/
2) and 875.65 eV and 872.49 eV (Ni 2p
1/
2), indicating the presence of Ni2+ and Ni3+, respectively [
44] (
Figure 5j,k). However, the presence of Ni ions in the Ni 2p spectra of the FeWO
4-NiFeOOH sample optimizes the electronic structures of FeOOH, weakens other binding energies, and potentially enhances the photocatalytic activity of the FeWO
4-NiFeOOH sample.
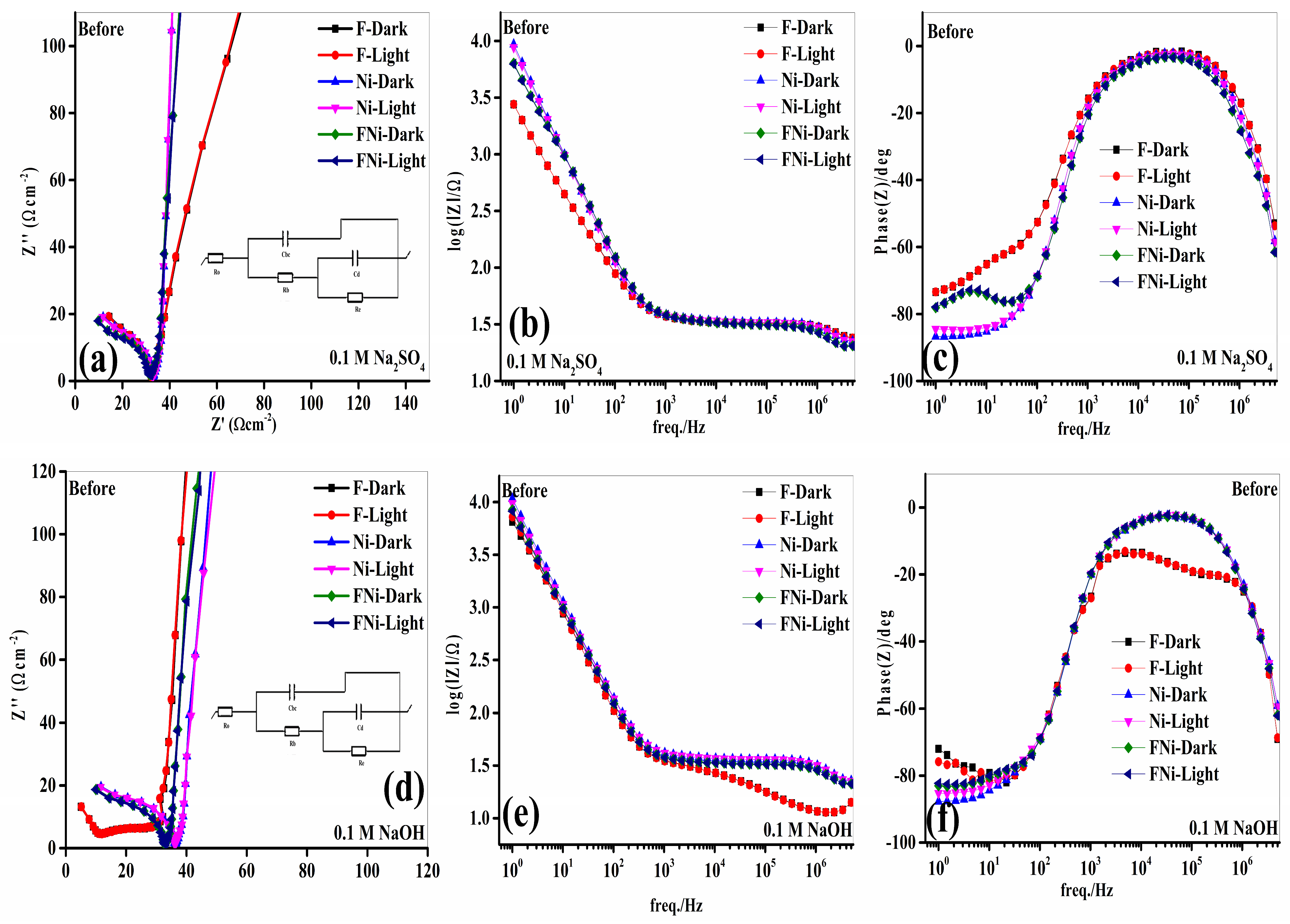
Electrochemical evaluation of FeWO
4 (Fe), NiFeOOH (Ni), and FeWO
4/NiFeOOH (FeNi) composite electrodes was conducted to assess their potential in energy conversion applications, focusing on their performance in 0.1 M Na
2SO
4 and NaOH electrolytes, under alternating illumination (
Figure 6).
Nyquist plots from impedance spectroscopy data were used to analyze charge transfer and ionic diffusion at the electrode–electrolyte interface. In these plots, the high-frequency semicircle represents the charge transfer resistance (Re), while the low-frequency slope indicates ionic diffusion resistance. Illumination significantly affected the electrochemical properties of the electrodes, particularly reducing the high-frequency semicircle radius, suggesting enhanced photocurrent generation. This phenomenon indicates that under light, the electrodes, particularly the FeNi composite, effectively utilize light energy to generate charge carriers (electrons and holes). The electrolyte plays a critical role in these electrochemical processes. In the Na
2SO
4 electrolyte, sulfate ions (SO
42−) enhanced the electrochemical performance by stabilizing the double-layer capacitance (Cd), which is crucial for efficient charge storage (
Figure 6a). SO
42− improves ionic mobility, reducing ionic impedance at the interface and facilitating more effective charge separation. Consequently, the FeNi composite, combining FeWO
4 and NiFeOOH, exhibited significantly lower Re than that in the individual components, indicating improved electrochemical kinetics. The results from the synergistic effects between FeWO
4 and NiFeOOH indicate enhanced light absorption and accelerated electron transfer during electrochemical reactions. Furthermore, the FeNi composite demonstrated superior capacitance, with values ranging from 72.91 × 10
−6 F to 32.56 × 10
−6 F, highlighting its effective charge storage in both light and dark conditions (
Table 1). This superior charge storage capability stems from the complementary properties of FeWO
4 and NiFeOOH, where FeWO
4 provides structural stability, and NiFeOOH enhances light absorption and charge separation. Using NaOH as the electrolyte distinctly improved electrochemical performance, primarily owing to the behavior of hydroxide ions (OH
−) (
Figure 6d). Hydroxide ions are smaller in size, with a higher diffusion coefficient than sulfate ions, enabling rapid movement through the electrolyte. This enhanced mobility of OH
− ions significantly reduces Re and improves charge separation and transport dynamics at the electrode–electrolyte interface. This promotes a robust Helmholtz double layer at the interface, boosting the electrochemical performance of the FeNi composite. Consequently, the FeNi composite exhibited impressive capacitance values ranging from 125.64 × 10
−6 F to 78.96 × 10
−6 F under various conditions, demonstrating its high charge storage capacity across different environments (
Table 1).
Bode plots and phase angle analyses were performed to validate these observations (
Figure 6b,c,e,f). These analyses showed that the FeNi composite in NaOH exhibited minimal impedance across a wide frequency range, indicating excellent ionic conductivity and rapid charge transfer kinetics. At low frequencies, NaOH electrolytes significantly enhanced ionic diffusion, promoting greater charge accommodation within the Helmholtz layer, which is crucial for efficient charge storage. At high frequencies, the electrolyte maintained rapid ionic mobility, which is crucial for high-frequency electrochemical applications. In contrast, Na
2SO
4 exhibited higher impedance across the frequency spectrum, indicating slower ionic diffusion and reduced charge transport efficiency. The larger ionic radius and lower diffusion coefficient of sulfate ions hindered rapid ion migration, slowing charge transfer and decreasing overall electrochemical performance. Additionally, the electron lifetime (τ
e), indicative of charge carrier stability, was estimated using impedance spectroscopy and phase analysis. The electron lifetime was longer in NaOH (4.72 × 10
−6 s) than in Na
2SO
4 (4.62 × 10
−6 s). This longer electron lifetime in NaOH suggests that the smaller ionic radius and faster diffusion coefficient of OH
− ions enhance charge transport and reduce recombination rates, improving overall electrochemical performance. In contrast, the shorter electron lifetime in Na
2SO
4 indicates increased recombination, as larger sulfate ions hinder ionic mobility and slow charge transfer. The FeWO
4/NiFeOOH composite combined with the optimal NaOH electrolyte demonstrates superior electrochemical performance. The enhanced ionic mobility, efficient charge separation, and long electron lifetime in NaOH highlight its suitability for energy conversion and storage applications. The synergistic effects of the FeWO
4/NiFeOOH composite and the advantageous properties of NaOH position the FeNi composite as a highly promising material for energy storage systems, where efficient charge transfer, rapid ionic diffusion, and long-term stability are crucial. These findings highlight the importance of electrolyte composition and its unique material properties in optimizing electrochemical device performance.
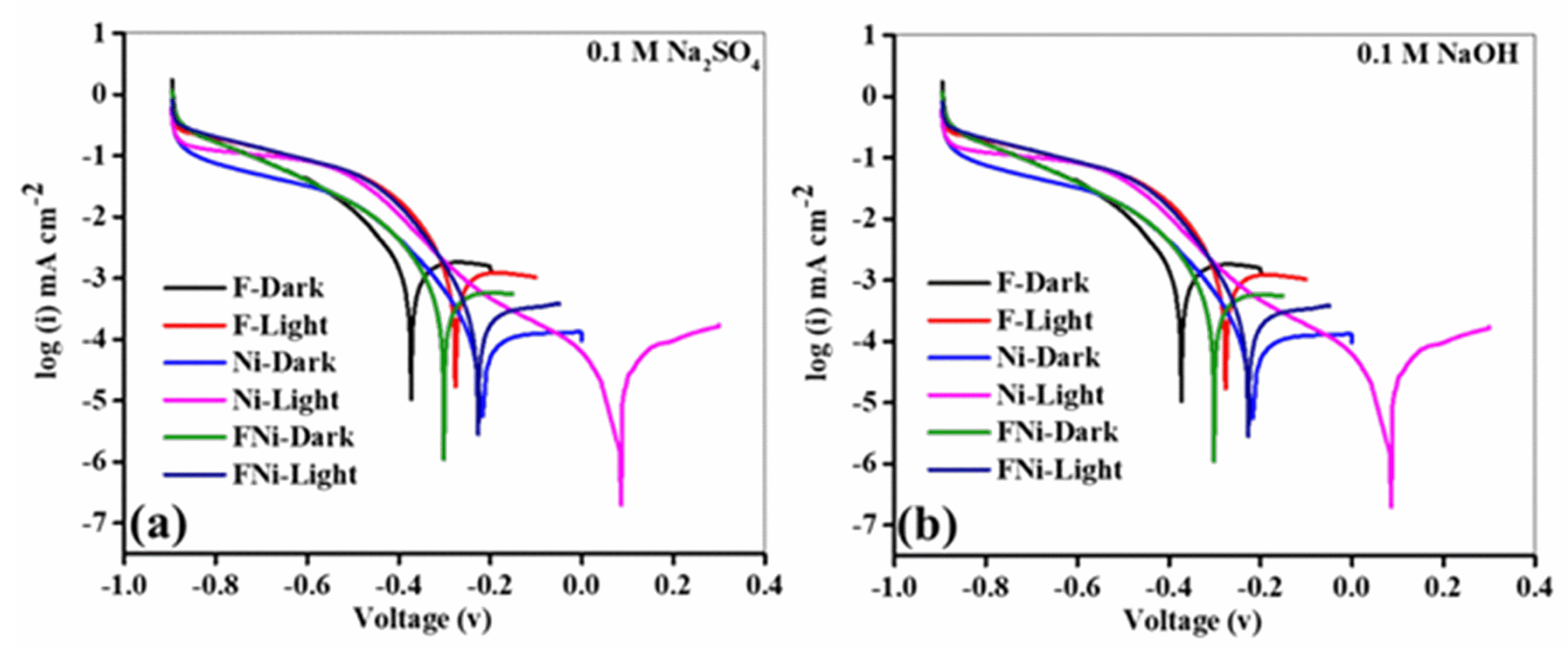
Tafel analysis of the Fe, Ni, and FeNi anodes revealed distinct electrochemical behavior when tested in Na
2SO
4 and NaOH electrolytes under both illuminated and dark conditions (
Figure 7).
Across all experiments, NaOH consistently outperformed Na
2SO
4 (
Figure 7a,b). Illumination caused an anodic shift for all anodes in both electrolytes, indicating enhanced charge carrier generation. This shift refers to a move of the electrode potential towards more positive values, typically induced by illumination or conditions that increase charge carrier activity. The anodic shift for the FeNi anode in Na
2SO
4 between dark and illuminated states was 80 mV, slightly higher than the 70 mV shift in NaOH. This indicates that while Na
2SO
4 enhances charge carrier generation and oxidation under illumination, it also causes higher overpotentials and slower charge transfer kinetics. This is attributed to the lower ionic conductivity of Na
2SO
4 and weaker electrode interactions, restricting its electrochemical efficiency. Conversely, NaOH promotes more efficient charge transfer owing to its superior ionic conductivity, reduced overpotentials, and enhanced stabilization of reaction intermediates despite a smaller anodic shift. The reduced shift in NaOH is attributed to its favorable electrochemical environment, where rapid OH
− ion mobility improves charge transport and lowers energy barriers. Under illumination, the Tafel slope of the FeNi anode was 53.11 mV·dec
−1 in Na
2SO
4, significantly higher than the 28.91 mV·dec
−1 in NaOH (
Figure 7a). This difference highlights the superior charge transfer efficiency in the alkaline medium, reducing energy losses and enhancing reaction kinetics (
Table 2). Photocurrent density values for a diffusion-limited current (J1) and charge exchange current (J2) were lower in NaOH than in Na
2SO
4. This indicates that the high reactivity and ionic mobility of NaOH improved charge transport dynamics and interfacial interactions. Hydroxyl ions in NaOH enhance adsorption at anode active sites, stabilize catalytic intermediates, and reduce recombination losses, enhancing charge generation and transfer (
Table 2). In contrast, Na
2SO
4 is hindered by its lower ionic conductivity and weaker electrode interactions, resulting in slower charge dynamics and higher energy losses. Under both illuminated and dark conditions, the FeNi anode demonstrated optimal performance with NaOH (
Figure 7b). This is evident in its lower Tafel slopes and higher photocurrent values (J1 and J2). The synergy between the FeNi anode and the alkaline medium facilitated reduced overpotentials, accelerated charge transfer, and sustained electrochemical activity. High hydroxyl ion mobility and strong electrode–electrolyte interactions in NaOH contributed to efficient ionic transport, intermediate stabilization, and minimizing resistive losses. The comparison between Na
2SO
4 and NaOH highlights the superiority of NaOH as an electrolyte for the FeNi anode. Its ability to optimize electrochemical processes through enhanced charge kinetics, reduced energy barriers, and improved ionic conductivity establishes it as the preferred medium for high-efficiency and stable applications.
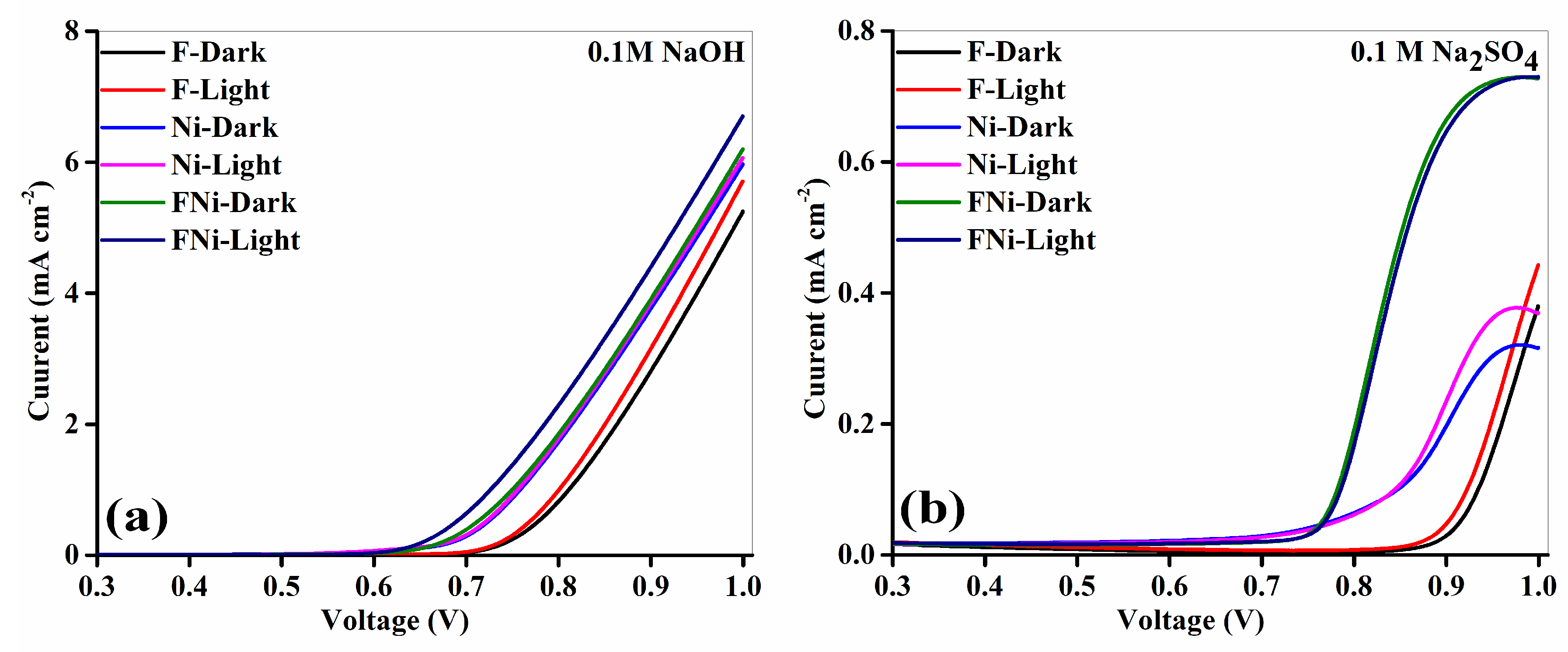
Linear voltammetry analysis was performed on the synthesized samples in different electrolytes under illuminated and non-illuminated conditions (
Figure 8).
The results show significant variations in current density and electrochemical behavior, influenced by the electrolyte type and lighting conditions. In the Na
2SO
4 electrolyte (
Figure 8a), all anodes displayed increased current density with rising voltage, indicating effective catalytic activity and charge transfer. Illumination further improved performance, highlighting the photo-responsive nature of the anodes. Among the materials tested, the FeNi anode exhibited the highest current density, attributed to the complementary properties of its components. The superior electron transport from Fe, coupled with the photoactivity of Ni, facilitated efficient electron–hole pair generation and minimized recombination. This synergy increased active site availability for electrochemical reactions. The FeNi anode achieved a peak current density of approximately 6.70 mA·cm
−2, 17.75% higher than that of the Fe anode alone, demonstrating significantly improved catalytic performance under illumination. The onset voltages in Na
2SO
4 for Fe (0.82 V), Ni (0.68 V), and FeNi (0.69 V) showed varying electrochemical efficiencies. The Fe anode exhibited the highest onset voltage, requiring higher energy to initiate reactions and lower charge transfer efficiency. The reduced onset voltage of the Ni anode enhanced charge separation and suppressed electron–hole recombination. The FeNi anode showed a 0.13 V lower onset potential than that in Fe, demonstrating more efficient charge transfer and enhanced reaction kinetics. It exhibited the lowest onset voltage, indicating synergy between Fe and Ni, which optimized active site availability, improved ionic conductivity, and facilitated efficient charge transfer. The FeNi anode demonstrated superior catalytic activity in the neutral Na
2SO
4 electrolyte, making it ideal for applications with moderate electrochemical conditions. In the NaOH electrolyte (
Figure 8b), its performance improved significantly, achieving a photocurrent density of approximately 6.70 mA·cm
−2 under illumination—nearly 830.56% higher than that in Na
2SO
4. This improvement is attributed to the alkaline NaOH environment, which enhances ionic conductivity, reduces resistive losses, and accelerates electron transport. The conductive properties of Fe and the photoactivity of Ni synergistically optimize charge separation. OH
− ions present in NaOH are crucial in enhancing electrochemical reactivity at the anode–electrolyte interface, further improving reaction kinetics and overall performance. The onset voltages in NaOH for Fe (0.68 V), Ni (0.58 V), and FeNi (0.55 V) confirmed significant improvements in electrochemical performance owing to the favorable properties of the alkaline medium. Compared with Fe, the FeNi anode showed a 0.13 V lower onset potential, indicating that FeNi can achieve advanced charge transfer efficiency and improved reaction kinetics. This superior performance is attributed to the synergistic interaction between Fe and Ni, which maximized active site utilization, improved electron transport, and reduced energy barriers for reactions. The use of NaOH amplified these benefits. Its high ionic conductivity enhanced OH
− mobility, minimized resistive losses, and stabilized charge carriers. Additionally, the alkaline environment accelerated reaction kinetics, facilitating faster electron transfer and higher catalytic efficiency. The significant increase in photocurrent density observed in NaOH compared to that in Na
2SO
4 underscores the advantages of an alkaline medium. NaOH promotes faster charge transport, enhances charge separation, and mitigates recombination losses, creating an ideal environment for electrochemical reactions. These effects stem from enhanced electron mobility, stabilized charge carriers, and increased electrochemical reactivity, all of which contribute to the higher efficiency of the system. The FeNi anode demonstrated outstanding photoelectrochemical performance, particularly in the NaOH electrolyte, establishing it as a promising candidate for renewable energy applications, such as photoelectrochemical water splitting. Its superior efficiency and operational stability highlight its potential for driving sustainable energy solutions.
The photocatalytic performance of the synthesized photoanodes was systematically evaluated under diverse applied bias potentials in two different electrolytes: 0.1 M Na
2SO
4 and 0.1 M NaOH (
Figure 9a–f). In the Na
2SO
4 electrolyte, the photocurrent density increased steadily as the applied bias potential rose, peaking at +0.6 V. This increase, observed from 0.4 to 0.6 V, can be attributed to the enhanced electric field, which facilitated the efficient separation of photogenerated electron–hole pairs. The stable Na
2SO
4 environment supported moderate charge transport, minimizing surface instability and enabling consistent photocurrent generation within this potential range. At lower bias potentials, the electric field was insufficient to drive effective charge carrier movement, leading to higher recombination rates. As the bias potential increased, the stronger electric field directed electrons towards the external circuit and holes to the photoanode surface, lowering recombination and promoting oxidation reactions. At higher potentials, the activation of favorable surface states further enhanced photocurrent generation. However, beyond 0.5 V, the photocurrent density began to decline, possibly due to the inability of the electrolyte to effectively suppress recombination processes, resulting in a reduction in the driving force for charge separation. In contrast, in the NaOH electrolyte, the photocurrent density increased up to 0.5 V, driven by the enhanced electric field at moderate bias potentials, which facilitated efficient separation and transport of photogenerated charge carriers (
Figure 9e).
The alkaline nature of NaOH played a critical role in suppressing recombination, stabilizing surface states, and minimizing charge trapping at defect sites. However, beyond 0.5 V, the photocurrent density decreased owing to increased electron–hole recombination and surface instability. The stronger electric field at higher potentials exacerbated recombination by inducing charge trapping at grain boundaries or triggering undesirable surface reactions, such as excessive oxidation of the photoanode material. These findings indicate the need to optimize the bias potential to balance charge separation efficiency and photoanode stability. The FeNi composite exhibited a higher photocatalytic performance than that of the pristine Fe and Ni samples in both the Na2SO4 and NaOH electrolytes across all applied voltages. This enhanced performance is attributed to the synergistic effects of the composite structure, which integrates the advantageous properties of both components. The FeNi composite exhibited enhanced light absorption, efficient charge carrier generation, and improved separation and transport dynamics, superior to those of the individual components. Furthermore, the formation of heterojunctions between the Fe and Ni phases reduced recombination rates by directing electrons and holes to distinct regions within the composite. Additionally, the FeNi structure, characterized by reduced grain boundary resistance and increased surface area, significantly enhances photocatalytic activity. In the NaOH electrolyte, the alkaline environment further amplifies these advantages by stabilizing surface states and minimizing charge trapping. At an applied voltage of 0.5 V, the electric field effectively separates charge carriers without inducing significant recombination or surface instability, leading to the highest observed photocurrent density. The superior ionic conductivity of NaOH also facilitates oxidation reactions at the photoanode surface, thus improving the overall PEC performance of the FeNi composite. The FeNi photoanode demonstrated outstanding stability during extended testing, maintaining a consistent photocurrent output for over 300 s under applied bias conditions. In the NaOH electrolyte, the photoanode exhibited stable photocurrent without significant degradation, indicating strong interaction with the electrolyte and effective suppression of recombination. The absence of photocurrent overshoots upon switching to the light ON state highlights the rapid and efficient utilization of photogenerated charge carriers. This responsiveness is essential for practical applications requiring stable and reproducible performance under dynamic light conditions. A comparative analysis of the two electrolytes revealed that NaOH offers a more favorable environment for PEC activity than Na2SO4. The alkaline medium enhances photocurrent generation, stability, and charge transport efficiency, establishing it as the preferred choice for solar-driven water-splitting applications. The significant performance of the FeNi composite in NaOH underscores its potential as a high-efficiency, durable photoanode material for renewable energy applications.
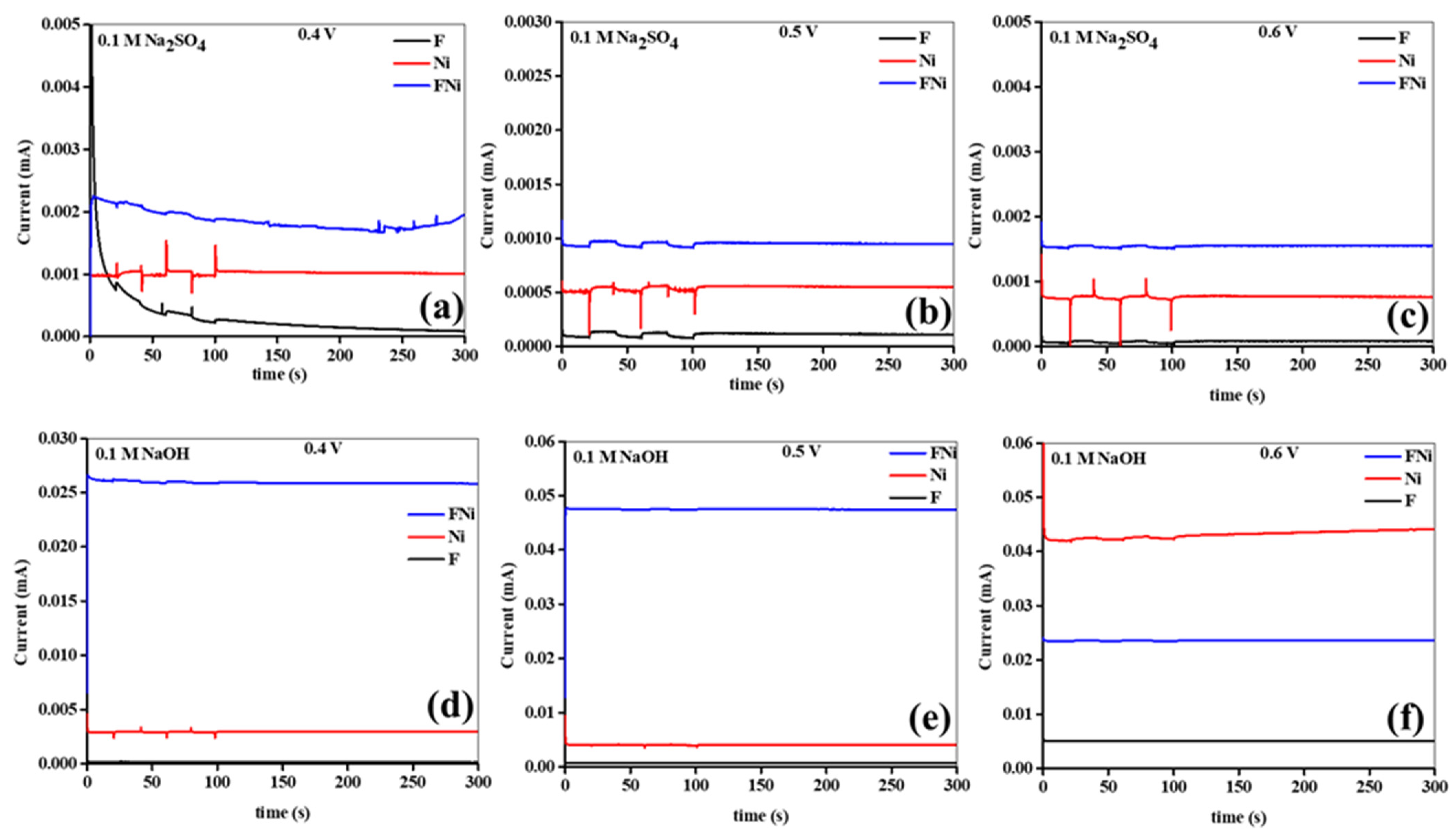
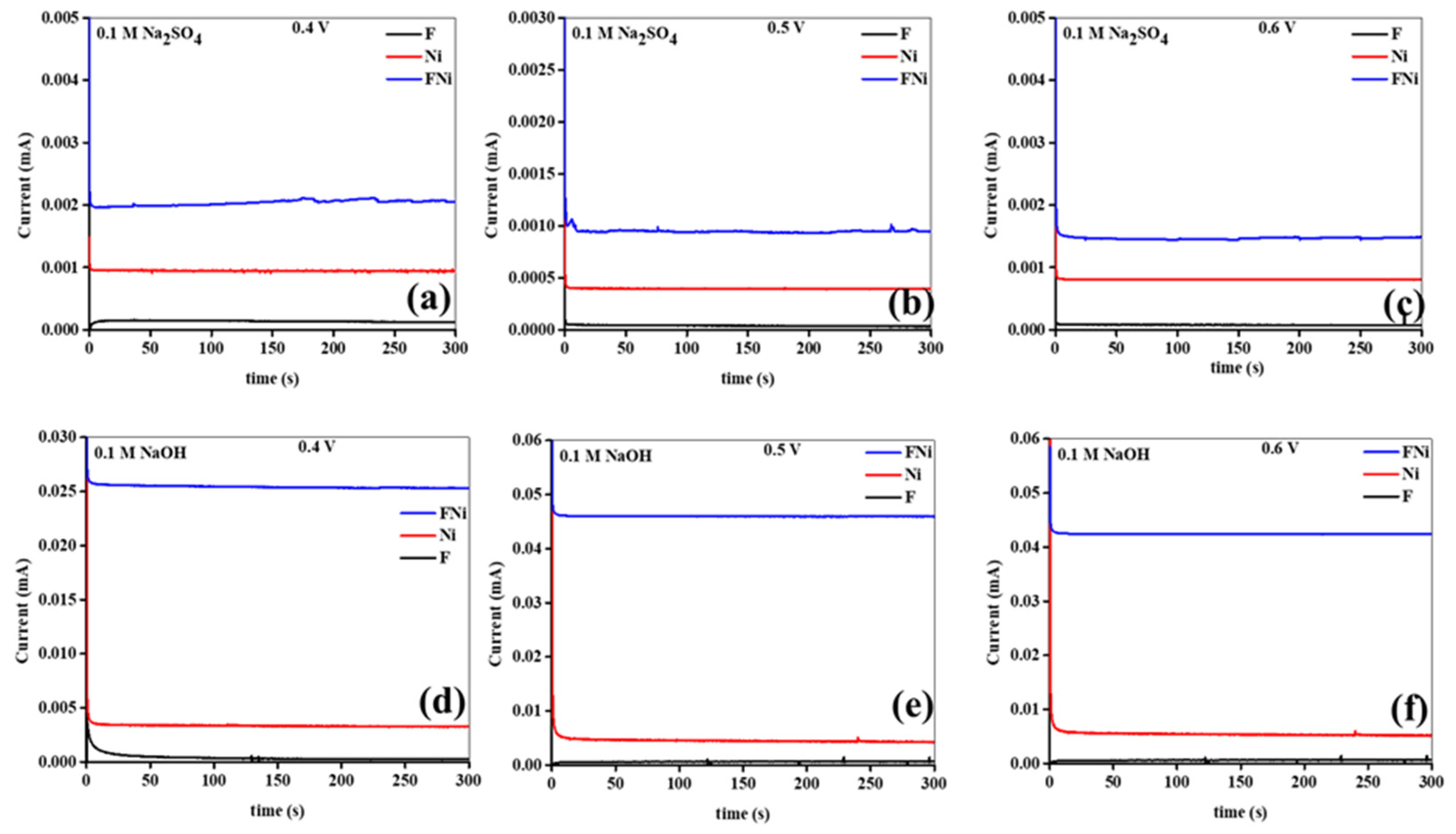
Additionally, the photocurrents were monitored over 300 s in the Na
2SO
4 and NaOH electrolytes, with no switching behavior observed (
Figure 10a–f). The results showed that the induced photocurrents were consistently generated under steady-state conditions without significant fluctuations or overshoots, emphasizing the photoanode material stability during prolonged illumination in both electrolytes. An increase in the applied bias potential resulted in higher photocurrent density, indicating that elevated potentials facilitate more efficient separation and transport of the photogenerated charge carriers in both electrolytes. This behavior aligns with the expected photoanode response to the applied electric field, where higher potentials drive electrons and holes to the external circuit and photoanode surface, respectively, enhancing overall photocatalytic performance. Photocurrent generation is significantly influenced by the applied bias potential, demonstrating that photocatalytic efficiency depends on how well the applied electric field can promote charge separation and transport in the photoanode material. However, the photoanodes in NaOH exhibited more stable and higher photocurrent generation than those in Na
2SO
4 (
Figure 10a–f). As mentioned earlier, this may be attributed to the enhanced ionic conductivity, lower resistance, and superior capacitance of the photoanodes in NaOH. These findings underscore the importance of optimizing the applied potential and the electrolyte composition to maximize photocurrent generation and ensure stable, high-performance operation of the photoanode materials in practical photoelectrochemical applications.
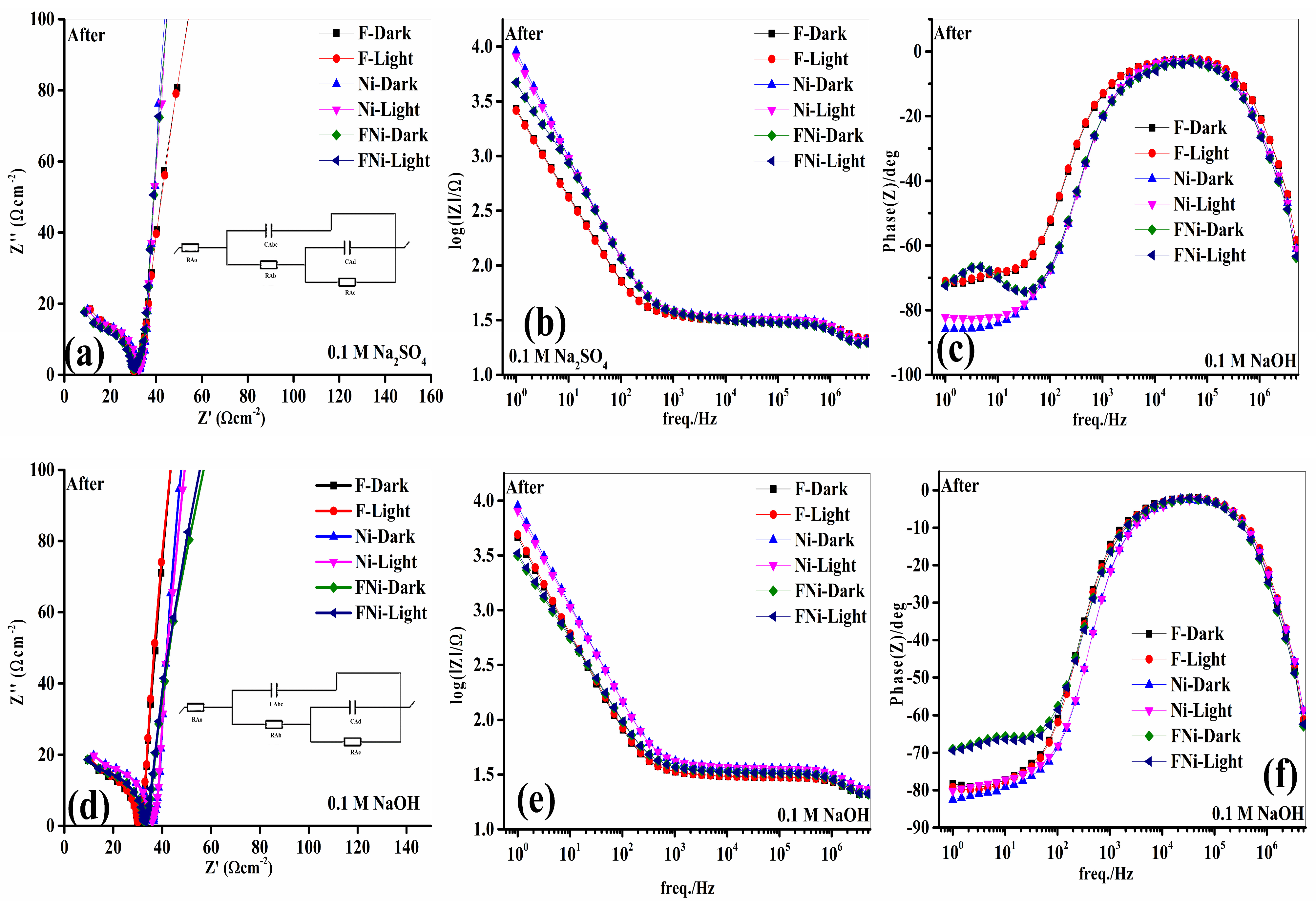
A comprehensive post-analysis of the electrochemical behavior of the anodes was performed in the Na
2SO
4 and NaOH electrolytes under similar experimental conditions to ensure a valid comparison. Nyquist plots were recorded for both dark and illuminated states (
Figure 11a–f), revealing two key features: a semicircular arc in the high-frequency region, representing charge transfer resistance, and a sloped line in the low-frequency region, indicating ionic diffusion at the photoelectrode–electrolyte interface.
Under illumination, the anodes consistently exhibited a smaller semicircle radius than that under the dark state, confirming that light exposure enhances charge generation. However, the arc radius in Na
2SO
4 was significantly larger than in NaOH, reflecting the distinct properties of the electrolytes. Sulfate-based electrolytes typically exhibit lower ionic conductivity than that of alkaline electrolytes, resulting in increased charge transfer resistance. Additionally, the SO
42− ions in Na
2SO
4 interact more strongly with the electrode surface than the OH
− ions in NaOH, potentially causing sulfate ion adsorption or structural modifications. These interactions hinder charge carrier mobility and impede electrochemical reactions, contributing to the higher resistance observed in Na
2SO
4, as reflected in the larger arc radius in the Nyquist plot. To gain deeper insight, the Nyquist plots were analyzed using an equivalent circuit model (inset of
Figure 11a,d), and
Table 3 summarizes the extracted fitted values. In this model, RAo, RAb, RAe, film capacitance (CAbc), and double-layer capacitance (Cad) represent the solution resistance, internal electrode resistance, charge transfer resistance at the electrode–electrolyte interface, film capacitance, and double-layer capacitance, respectively. The RAo values for the anodes in dark and illuminated states in Na
2SO
4 and NaOH were consistently higher than the corresponding Ro values, likely owing to variations in the electrode surface state. Among the tested anodes, the FeNi anode exhibited the lowest resistance values in both electrolytes, outperforming the Fe and Ni anodes. Moreover, the FeNi anode exhibited lower resistance in NaOH than in Na
2SO
4. The RAo values for the FeNi anode under dark and illuminated conditions were 26.89, 5.23, 23.51, and 4.77 Ω (
Table 3). These values were higher than the corresponding Ro values (26.67, 4.86, 22.53, and 3.64 Ω, respectively,
Table 1), indicating a reduction in the energetic states of the electrode surface in both electrolytes. The RAb values, representing the path resistance within the electrode, were consistently higher than the corresponding Rb values for all anodes under both dark and illuminated states. This increase may be attributed to the adsorption and desorption of ions or molecules that influence the conductive pathways within the anodes. Ion accumulation or depletion of ions during repeated electrochemical reactions can alter these pathways, leading to variations in internal resistance. Among all the anodes, FeNi exhibited the lowest path resistance in both electrolytes, particularly in NaOH, highlighting its superior ionic transport. The RAb values for FNi were 27.76, 12.09, 25.11, and 9.14 Ω under dark and illuminated conditions (
Table 3), slightly exceeding the corresponding Ro values of 24.53, 11.57, 26.12, and 8.94 Ω (
Table 1). The RAe values, corresponding to the charge transfer resistance at the electrode–electrolyte interface, were higher than the Re values in both dark and illuminated states. This finding suggests that the accumulation of reaction byproducts or oxide layer formation during electrochemical cycling may impede electron transfer. Additionally, the depletion or redistribution of reactive species near the electrode surface could limit ion availability, thereby lowering the charge transfer efficiency. FeNi demonstrated the lowest charge transfer resistance in the NaOH and Na
2SO
4 electrolytes, with lower resistance in NaOH. This improved performance is likely attributed to the enhanced ionic conductivity of OH
− in NaOH, which facilitates more efficient ionic transport at the electrode–electrolyte interface. Under dark and illuminated conditions, the RAe values for FeNi were 4.10, 3.99, 4.01, and 3.18 kΩ (
Table 3), while the corresponding Re values were 3.98, 3.31, 3.62, and 2.98 kΩ (
Table 1). The slight increase in resistance during cycling may result from surface modifications or localized ion depletion near the electrode. The CAbc and Cad values were generally lower in both electrolytes than the corresponding Cbc and Cd values. This reduction in capacitance may arise from surface modifications during electrochemical cycling, which alters the ion adsorption capacity of the electrode. Furthermore, changes in electrolyte composition near the electrode–electrolyte interface—such as ion depletion or shifts in ionic species—could disrupt the stability of the electrical double layer, leading to diminished capacitance. Accumulation of reaction products may further impede ion diffusion, and interface polarization, which becomes more pronounced during cycling, could lower the effective charge storage capacity of the electrode. FeNi exhibited increased CAbc and CAd values compared to those of Fe and Ni, likely due to its optimized material composition, which enhances electron transfer and reduces internal resistance. This results in a more stable electrochemical network. Moreover, FeNi showed higher CAbc and CAd values in NaOH than those in Na
2SO
4, likely owing to the higher ionic conductivity of OH
− in NaOH, which promotes improved ion interactions and electron flow. The CAbc and CAd values for the FeNi anode ranged from 31.27 to 79.78 μF and 62.37 to 1119.78 µF under dark and illuminated conditions, respectively, which were lower than the corresponding Cbc and Cd values of 32.56–82.65 μF and 65.35–125.64 µF (
Table 1 and
Table 3). The differences in electrochemical performance between NaOH and Na
2SO
4 can be attributed to the specific ionic properties of their electrolytes—OH
− in NaOH and SO
42− in Na
2SO
4. The OH
− ions in NaOH have smaller ionic radii and higher ionic mobility than those of the SO
42− ions in Na
2SO
4. This leads to more efficient ionic diffusion and enhanced ionic conductivity in NaOH, facilitating faster charge transfer at the electrode–electrolyte interface. This is evidenced by the sharper impedance drop with increasing frequency in NaOH, suggesting more rapid charge transfer kinetics. Additionally, OH
− ions exhibit a lower tendency to form strong bonds with the electrode surface, reducing surface passivation and preserving active sites for charge transfer. Consequently, NaOH shows lower impedance values and smaller phase angles across the frequency spectrum, further confirming its superior electrochemical performance. In contrast, SO
42− ions in Na
2SO
4, with their larger ionic radii and lower mobility, hinder ionic diffusion and increase resistance to charge transfer. This is reflected in the higher impedance and phase angle values observed across the frequency spectrum in Na
2SO
4. The stronger interactions of SO
42− ions with the electrode surface may lead to adsorption or the formation of surface layers, increasing charge transfer resistance and impeding ionic movement. These factors contribute to the higher impedance and phase angles observed in Na
2SO
4, indicating less efficient charge transfer and slower electrochemical dynamics. The τ
e values in NaOH and Na
2SO
4 are slightly lower in the updated analysis than in the initial values, likely due to electrochemical stability factors and ionic interactions. In NaOH, the electron lifetime decreased from 4.72 × 10
−6 s to approximately 4.55 × 10
−6 s. This reduction in electron lifetime may result from prolonged cycling or the gradual accumulation of surface passivation layers, which impede electron transport over time. Although NaOH maintains relatively stable charge dynamics, this decrease in electron lifetime suggests minor performance degradation as cycling progresses. In contrast, Na
2SO
4 shows a slightly higher electron lifetime of 4.57 × 10
−6 s than that of NaOH, but this is still lower than its initial value of 4.62 × 10
−6 s. This reduction reflects the combined effects of slower ionic diffusion and increased surface interactions involving the larger SO
42− ions, which accelerate electron recombination and reduce charge carrier stability over time. Initially, the higher ionic mobility of OH
− in NaOH supported a more stable electron lifetime. However, as cycling-induced degradation affects both electrolytes, the reductions in electron lifetime underscore the effects of the ionic environment and surface interactions on the long-term electrochemical performance. In overextended cycling, the performance of both electrolytes deteriorates. However, NaOH outperforms Na
2SO
4 owing to its higher ionic conductivity, lower impedance, and reduced surface passivation effects. The superior mobility of OH
− ions in NaOH facilitates efficient charge transfer, minimizes recombination losses, and prevents significant impedance buildup. In contrast, Na
2SO
4 experiences a more pronounced increase in impedance and phase angles, suggesting a greater electrochemical performance decline over time. Thus, NaOH emerges as the more favorable electrolyte for high-performance electrochemical applications due to its ability to support faster ionic movement, mitigate passivation effects, and maintain lower impedance and phase angles.