Synthesis, Crystal Structure, DFT Analysis and Docking Studies of a Novel Spiro Compound Effecting on EGR-1-Regulated Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.2. Crystal Structure Determination
2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. High-Resolution Mass Spectrometry (HR/MS)
2.5. In Silico Docking
2.6. Density Functional Theory (DFT) Calculations
2.7. Cells and Cell Culture
2.8. TSLP Gene Promoter-Reporter Assay
2.9. Reverse Transcription-PCR (RT-PCR)
2.10. Quantitative Real-Time PCR (qPCR)
2.11. Electrophoretic Mobility Shift Assay (EMSA)
3. Results and Discussion
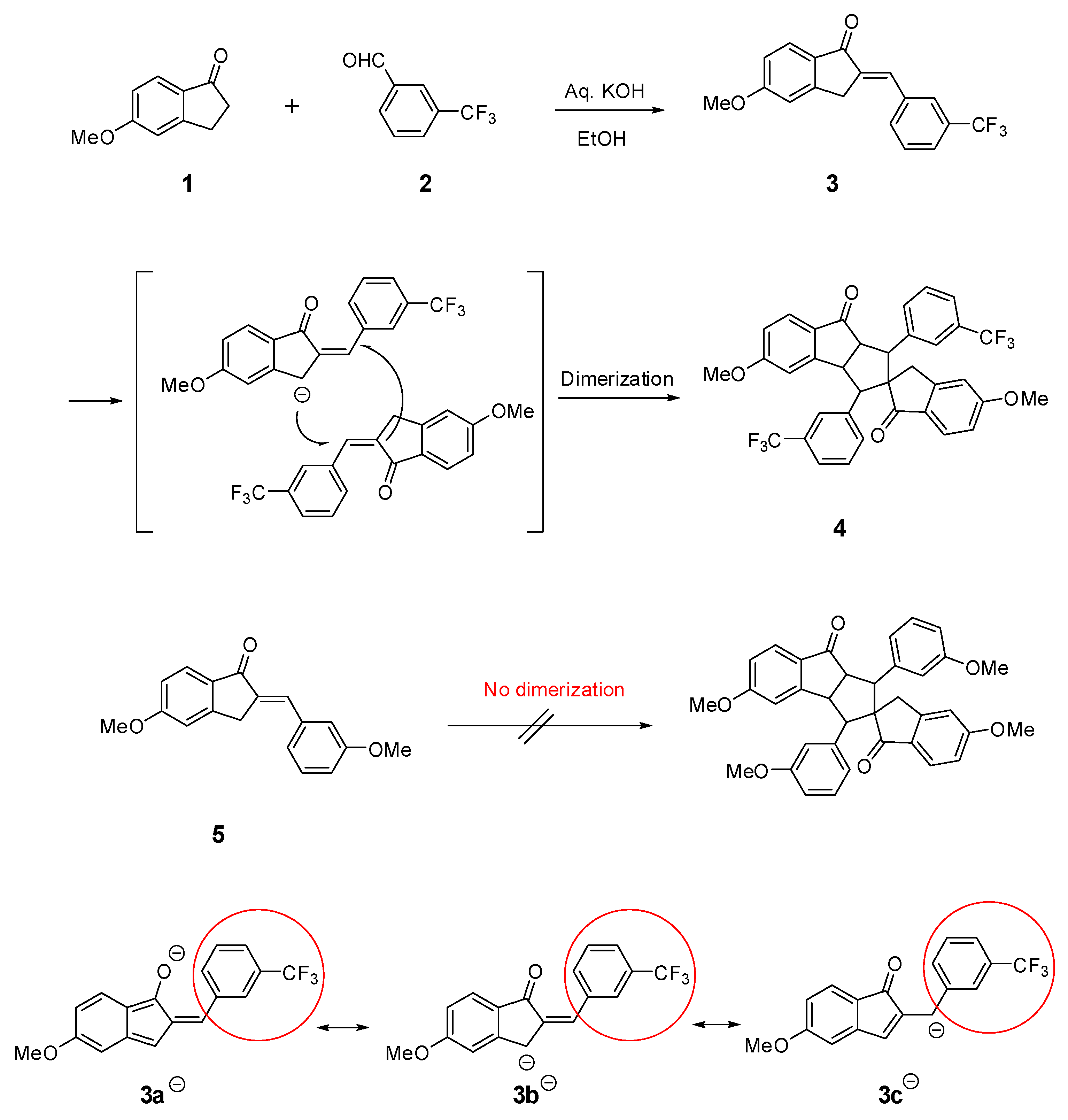
3.1. Synthesis
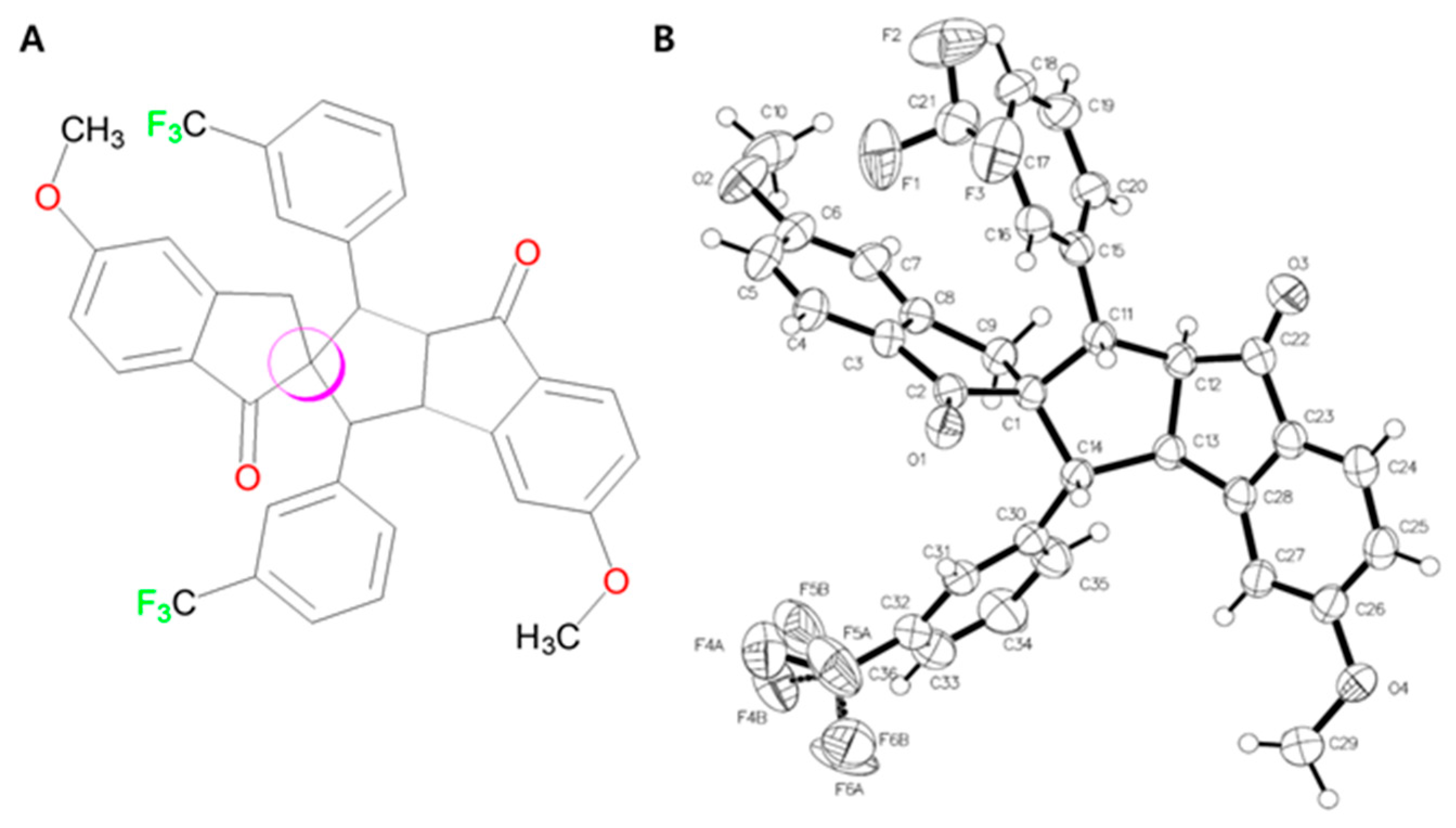
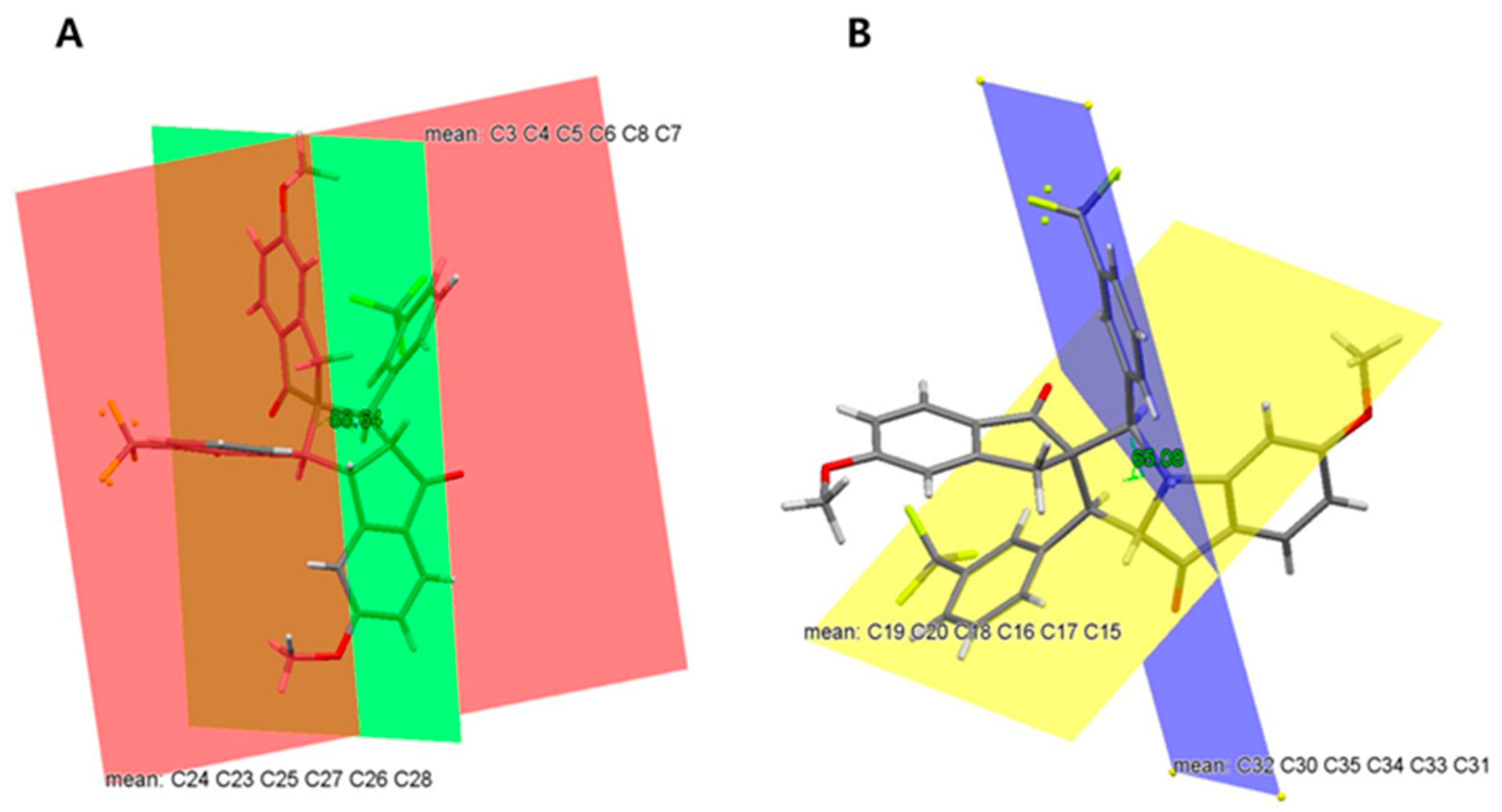
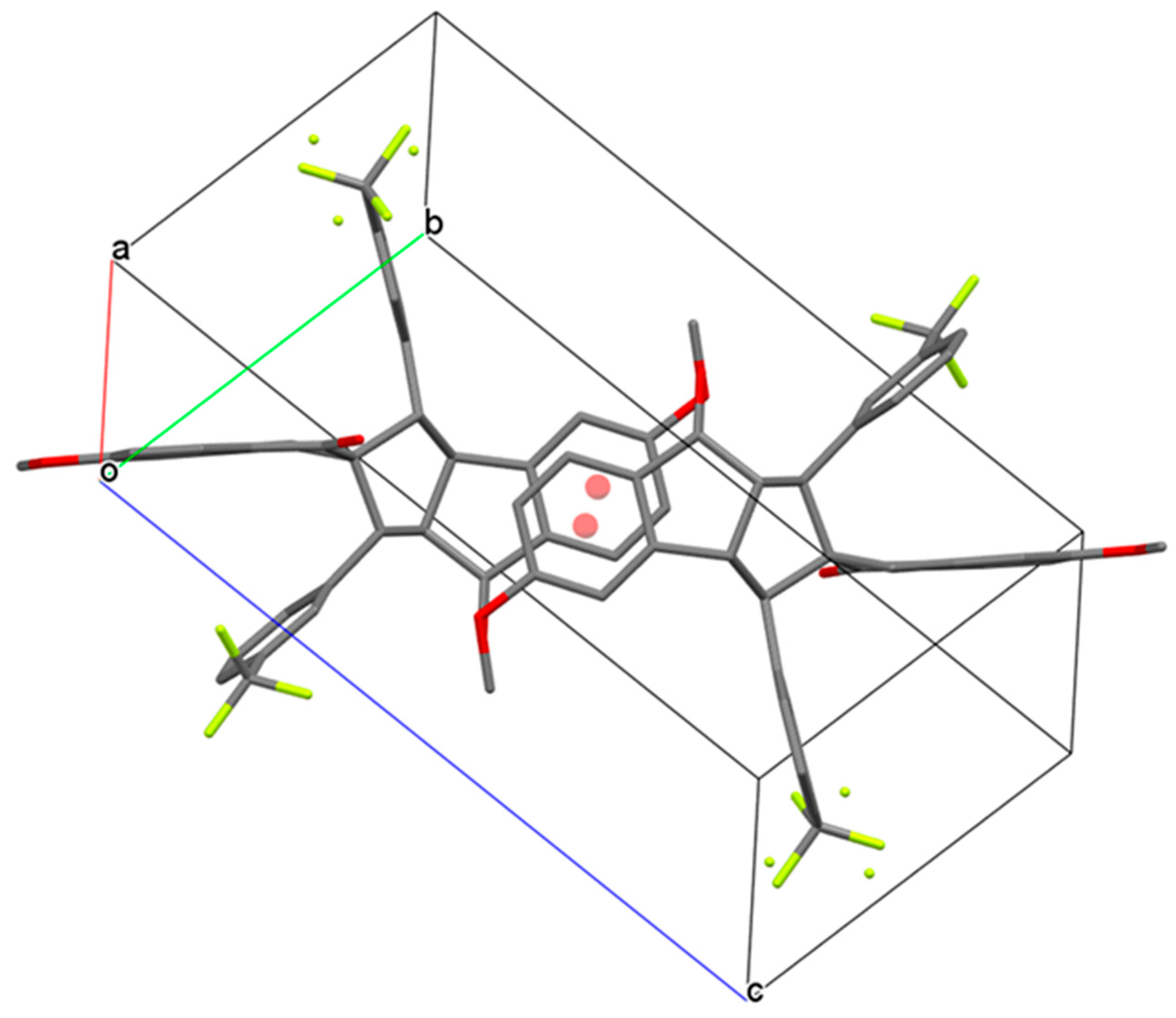
3.2. Crystal Structure of Spiro Compound 4
3.3. DFT Calculations of Compound 4
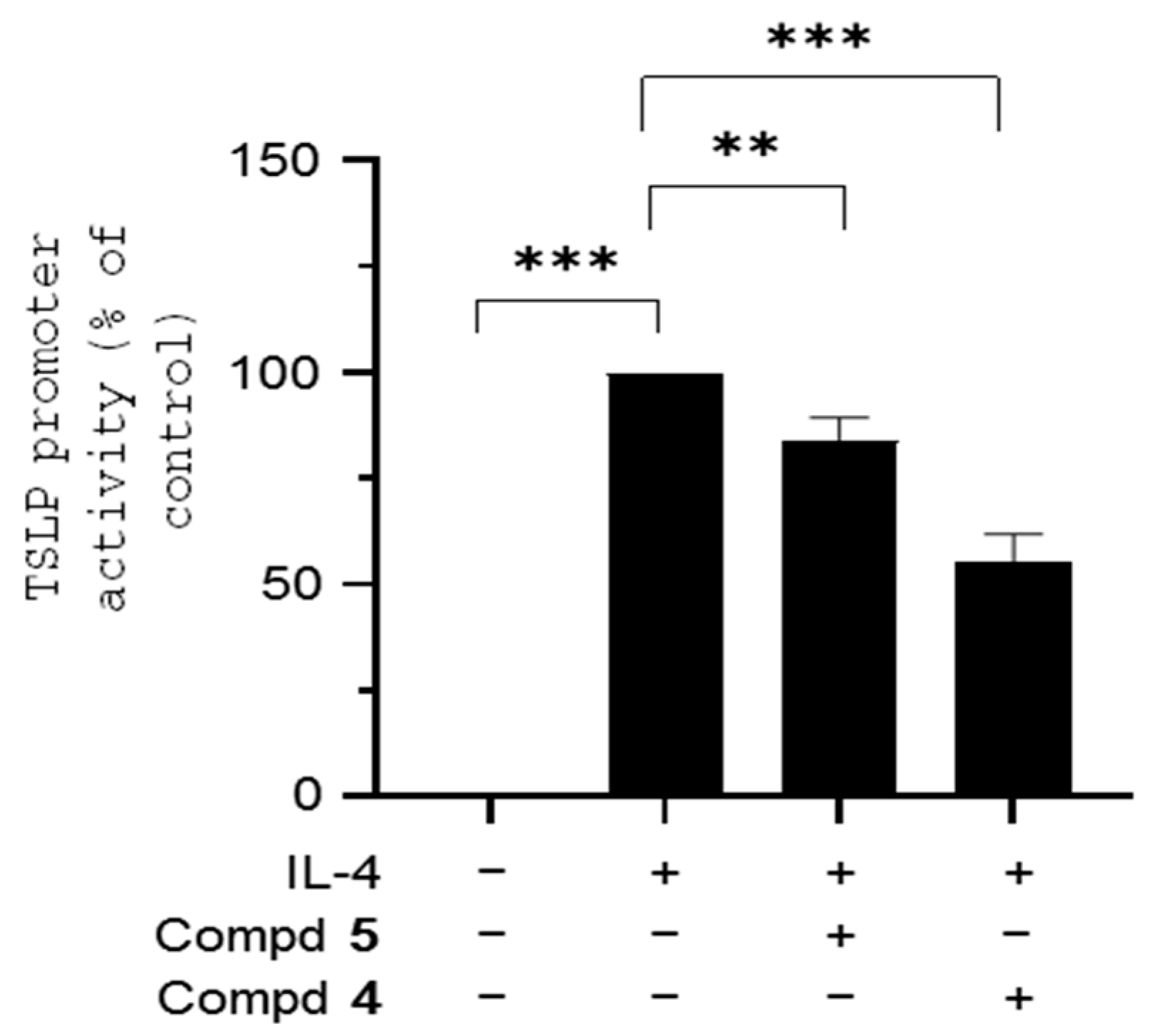
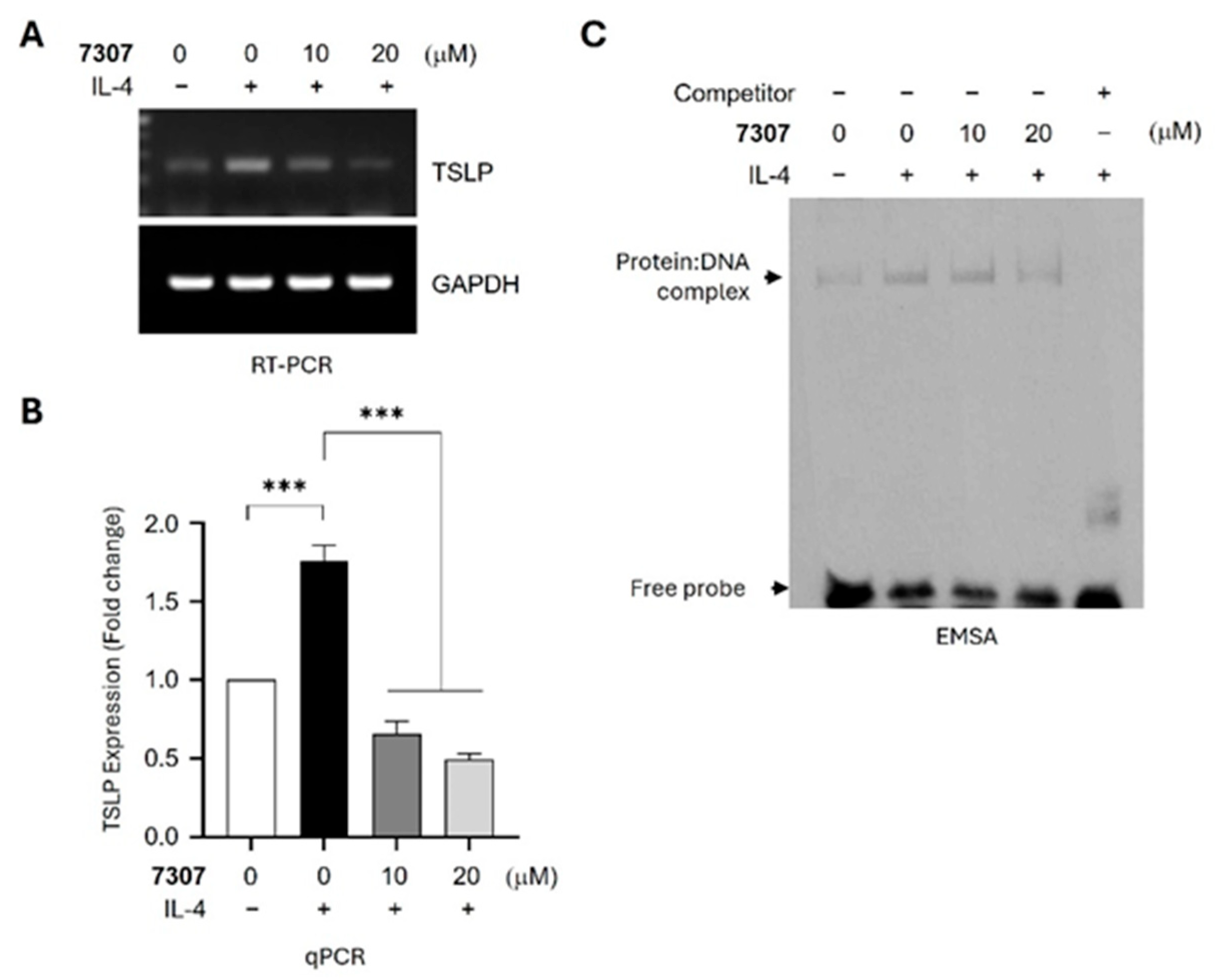
3.4. Effects on EGR-1-Regulated Gene Expression
3.5. In Silico Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, F. Chapter 1—Modeling Human Prostate Cancer in Genetically Engineered Mice. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 100, pp. 1–49. [Google Scholar]
- Zou, K.; Zeng, Z. Role of early growth response 1 in inflammation-associated lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L143–L154. [Google Scholar] [PubMed]
- Thiel, G.; Cibelli, G. Regulation of life and death by the zinc finger transcription factor Egr-1. J. Cell. Physiol. 2000, 193, 287–292. [Google Scholar]
- Pignatelli, M.; Luna-Medina, R.; Pérez-Rendón, A.; Santos, A.; Perez-Castillo, A. The transcription factor early growth response factor-1 (EGR-1) promotes apoptosis of neuroblastoma cells. Biochem. J. 2003, 373, 739–746. [Google Scholar]
- Khachigian, L.M. Early Growth Response-1, an Integrative Sensor in Cardiovascular and Inflammatory Disease. J. Am. Heart Assoc. 2021, 10, e023539. [Google Scholar]
- Wang, B.; Guo, H.; Yu, H.; Chen, Y.; Xu, H.; Zhao, G. The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 2021, 11, 642547. [Google Scholar]
- Bryant, M.; Drew, G.M.; Houston, P.; Hissey, P.; Campbell, C.J.; Braddock, M. Tissue repair with a therapeutic transcription factor. Hum. Gene Ther. 2000, 11, 2143–2158. [Google Scholar]
- Ryu, W.I.; Lee, H.; Kim, J.H.; Bae, H.C.; Ryu, H.J.; Son, S.W. IL-33 induces Egr-1-dependent TSLP expression via the MAPK pathways in human keratinocytes. Exp. Dermatol. 2015, 24, 857–863. [Google Scholar]
- Lohoff, M.; Giaisi, M.; Köhler, R.; Casper, B.; Krammer, P.H.; Li-Weber, M. Early growth response protein-1 (Egr-1) is preferentially expressed in T helper type 2 (Th2) cells and is involved in acute transcription of the Th2 cytokine interleukin-4. J. Biol. Chem. 2010, 285, 1643–1652. [Google Scholar]
- Jeong, S.H.; Kim, H.J.; Jang, Y.; Ryu, W.I.; Lee, H.; Kim, J.H.; Bae, H.C.; Choi, J.E.; Kye, Y.C.; Son, S.W. Egr-1 is a key regulator of IL-17A-induced psoriasin upregulation in psoriasis. Exp. Dermatol. 2014, 23, 890–895. [Google Scholar]
- Yeo, H.; Ahn, S.S.; Lee, J.Y.; Shin, S.Y. EGR-1 acts as a transcriptional activator of KLK7 under IL-13 stimulation. Biochem. Biophys. Res. Commun. 2021, 534, 303–309. [Google Scholar]
- Matus, C.E.; Ehrenfeld, P.E.; Figueroa, C.D. The family of kallikrein-related peptidases and kinin peptides as modulators of epidermal homeostasis. Am. J. Physiol. Cell Physiol. 2022, 323, C1070–C1087. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Ahn, S.S.; Lee, J.Y.; Jung, E.; Jeong, M.; Kang, G.S.; Ahn, S.; Lee, Y.; Koh, D.; Lee, Y.H.; et al. Disrupting the DNA Binding of EGR-1 with a Small-Molecule Inhibitor Ameliorates 2,4-Dinitrochlorobenzene-Induced Skin Inflammation. J. Investig. Dermatol. 2021, 141, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Ahn, S.S.; Ou, S.; Yun, S.J.; Lim, Y.; Koh, D.; Lee, Y.H.; Shin, S.Y. The EGR1-Artemin Axis in Keratinocytes Enhances the Innervation of Epidermal Sensory Neurons during Skin Inflammation Induced by House Dust Mite Extract from Dermatophagoidesfarinae. J. Investig. Dermatol. 2024, 144, 1817–1828.e17. [Google Scholar] [PubMed]
- Park, T.J.; Oh, H.; Kim, M.; Kim, J.; Kim, H.J.; Son, S.W. Urban particulate matters induce EGR-1 expression in keratinocytes which correlates with the severity of psoriasis. Mol. Cell. Toxicol. 2021, 17, 195–200. [Google Scholar] [CrossRef]
- Fania, L.; Moretta, G.; Antonelli, F.; Scala, E.; Abeni, D.; Albanesi, C.; Madonna, S. Multiple Roles for Cytokines in Atopic Dermatitis: From Pathogenic Mediators to Endotype-Specific Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 2684. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, S.; Jung, E.; Koh, D.; Lim, T.; Lee, Y.H.; Shin, S.Y. Design, synthesis, and biological evaluation of (E)-2-benzylidene-1-indanones derivatized by bioisosteric replacement of aurones. Appl. Biol. Chem. 2024, 67, 114. [Google Scholar] [CrossRef]
- Moss, G.P. Extension and revision of the nomenclature for spiro compounds. Pure Appl. Chem. 1999, 71, 531–558. [Google Scholar] [CrossRef]
- Talele, T.T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 2020, 63, 13291–13315. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, J. Synthesis and Crystal Structure of a New Hydrated Benzimidazolium Salt Containing Spiro Structure. Crystals 2017, 7, 303. [Google Scholar] [CrossRef]
- Romo, P.E.; Quiroga, J.; Cobo, J.; Glidewell, C. Synthesis and spectroscopic and structural characterization of spiro-[indoline-3,3′-indolizine]s formed by 1,3-dipolar cyclo-additions between isatins, pipecolic acid and an electron-deficient alkene. Acta Cryst. 2021, C77, 496–504. [Google Scholar]
- Bruña, S.; Cuadrado, I.; Perles, J. Unexpected formation of a silicon-centered spirocyclic oligosiloxane bearing eight pendant ferrocene units. Crystals 2022, 12, 1122. [Google Scholar] [CrossRef]
- Zheng, Y.; Tic, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar]
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic scaffolds in medicinal chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar]
- Romero-Hernández, L.L.; Ahuja-Casarín, A.I.; Merino-Monti, P.; Montiel-Smith, S.; Vega-Baez, J.L.; Sandoval-Ramirez, J. Syntheses and medicinal chemistry of spiro heterocyclic steroids. Beilstein J. Org. Chem. 2024, 20, 1713–1745. [Google Scholar]
- Zhou, L.M.; Qu, R.Y.; Yang, G.F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar]
- Qu, Y.-K.; Qi, Z.; Jian, F.; Liao, L.S.; Jiang, Z.Q. Spiro Compounds for organic light-emitting diodes. Acc. Mater. Res. 2021, 2, 1261–1271. [Google Scholar]
- Saragi, T.P.I.; Spehr, T.; Siebert, A.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro compounds for organic optoelectronics. Chem. Rev. 2007, 107, 1011–1065. [Google Scholar]
- Yu, L.; Dai, A.; Zhang, W.; Liao, A.; Guo, S.; Wu, J. Spiro derivatives in the discovery of new pesticides: A Research review. J. Agric. Food Chem. 2022, 70, 10693–10707. [Google Scholar]
- Rios, R. Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 2012, 41, 1060–1074. [Google Scholar] [PubMed]
- Babar, K.; Zahoor, A.F.; Ahmad, S.; Akhtar, R. Recent synthetic strategies toward the synthesis of spirocyclic compounds comprising six-membered carbocyclic/heterocyclic ring systems. Mol. Divers. 2021, 25, 2487–2532. [Google Scholar] [PubMed]
- Patel, G.; Patel, A.R.; Kheti, S.; Sao, P.K.; Rathore, G.; Banerjee, S. Review on the Synthesis of Bio-Active Spiro-Fused Heterocyclic Molecules. Curr. Organocatal. 2023, 10, 180–208. [Google Scholar]
- Singh, R.; Bhardwaj, D.; Saini, M.R. Recent advancement in the synthesis of diverse spiro-indeno [1,2-b]quinoxalines: A review. RSC Adv. 2021, 11, 4760–4804. [Google Scholar]
- Gilles, L.; Antoniotti, S. Spirocyclic compounds in fragrance Chemistry: Synthesis and olfactory properties. ChemPlusChem 2022, 87, e202200227. [Google Scholar]
- Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Re-finement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar]
- Bax, A. Two-dimensional heteronuclear relayed coherence transfer spectroscopy. J. Magn. Reson. 1983, 53, 149–153. [Google Scholar]
- Clore, G.M.; Gronenborn, A.M. Multidimensional heteronuclear nuclear magnetic resonance of proteins. Methods Enzymol. 1994, 239, 349–363. [Google Scholar]
- Reynolds, W.F.; Burns, D.C. Getting the Most out of HSQC and HMBC Spectra. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012; Volume 76, pp. 1–21. [Google Scholar]
- Charisiadis, P.; Venianakis, T.; Papaemmanouil, C.D.; Primikyri, A.; Tzakos, A.G.; Siskos, M.G.; Gerothanassis, I.P. On the Use of Strong Proton Donors as a Tool for Overcoming Line Broadening in NMR: A Comment. Magn. Reason. Chem. 2025, 63, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qin, Y.; Li, Z.; Lan, J.; Zhang, T.; Ding, Y. Comparative Pharmacokinetics of Cinobufacini Capsule and Injection by UPLC-MS/MS. Front. Pharmacol. 2022, 13, 944041. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Kramer, B.; Rarey, M.; Lengauer, T. Evaluation of the FLEXX incremental construction algorithm for protein–ligand docking. Proteins Struct. Funct. Genet. 1999, 37, 228–241. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar]
- Knizia, G.; Klein, J.E.M.N. Electron Flow in Reaction Mechanisms—Revealed from First Principles. Angew. Chem. Int. Ed. 2015, 54, 5518–5522. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, Y.H.; Ahn, S.S.; Jung, E.; Lim, Y.; Shin, S.Y. Chrysin Inhibits TNF?-Induced TSLP Expression through Downregulation of EGR1 Expression in Keratinocytes. Int. J. Mol. Sci. 2021, 22, 4350. [Google Scholar] [CrossRef]
- Yoon, H.; Koh, D.; Lim, Y.; Lee, Y.H.; Lee, J.K.; Shin, S.Y. Pyrazolines inhibiting the activity of the early growth response-1 DNA-binding domain. Bioorg. Med. Chem. Lett. 2024, 113, 129952. [Google Scholar] [CrossRef]



| Empirical Formula | C36H26F6O4 |
|---|---|
| Formula weight | 636.57 |
| Temperature | 223(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 8.8669(5) Å b = 10.5298(8) Å c = 17.0135(11) Å α = 91.396(2)°. β = 90.490(2)°. γ = 109.235(2)°. |
| Volume | 1499.14(17) Å3 |
| Z | 2 |
| Density (calculated) | 1.410 Mg/m3 |
| Absorption coefficient | 0.116 mm−1 |
| F(000) | 656 |
| Crystal size | 0.226 × 0.204 × 0.056 mm3 |
| Theta range for data collection | 2.049 to 28.2492°. |
| Index ranges | −1–11 ≤ h ≤ 11, −14 ≤ k ≤ 14, −22 ≤ l ≤ 22 |
| Reflections collected | 44768 |
| Independent reflections | 7389 [R(int) = 0.0426] |
| Completeness to theta = 25.242° | 99.9% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 7389/30/445 |
| Goodness-of-fit on F2 | 1.036 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0548, wR2 = 0.1309 |
| R indices (all data) | R1 = 0.0814, wR2 = 0.1481 |
| Largest diff. peak and hole | 0.531 and −0.420 e·Å−3 |
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| C(12)-H(12)…O(3)#1 | 0.99 | 2.57 | 3.444(3) | 146.4 |
| C(34)-H(34)…O(1)#2 | 0.94 | 2.53 | 3.413(3) | 157.4 |
| C(25)-H(25)…O(1)#3 | 0.94 | 2.43 | 3.356(2) | 169.2 |
| 4 | 5 | |
|---|---|---|
| FMO | Energy/Hartree(Eh) | Energy/Hartree(Eh) |
| 3rd LUMO | 0.1022 | 0.1233 |
| 2nd LUMO | 0.1016 | 0.1163 |
| 1st LUMO | 0.0919 | 0.0648 |
| 1st HOMO | −0.1635 | −0.3183 |
| 2nd HOMO | −0.1994 | −0.3236 |
| 3rd HOMO | −0.2541 | −0.3369 |
| 1st ΔE | 0.2554 | 0.3831 |
| 2nd ΔE | 0.3010 | 0.4399 |
| 3rd ΔE | 0.3563 | 0.4602 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.Y.; Jung, E.; Lee, Y.; Lee, H.-J.; Lee, H.; Yoo, J.; Ahn, S.; Koh, D. Synthesis, Crystal Structure, DFT Analysis and Docking Studies of a Novel Spiro Compound Effecting on EGR-1-Regulated Gene Expression. Crystals 2025, 15, 338. https://doi.org/10.3390/cryst15040338
Shin SY, Jung E, Lee Y, Lee H-J, Lee H, Yoo J, Ahn S, Koh D. Synthesis, Crystal Structure, DFT Analysis and Docking Studies of a Novel Spiro Compound Effecting on EGR-1-Regulated Gene Expression. Crystals. 2025; 15(4):338. https://doi.org/10.3390/cryst15040338
Chicago/Turabian StyleShin, Soon Young, Euitaek Jung, Youngshim Lee, Ha-Jin Lee, Hyeonhwa Lee, Jinju Yoo, Seunghyun Ahn, and Dongsoo Koh. 2025. "Synthesis, Crystal Structure, DFT Analysis and Docking Studies of a Novel Spiro Compound Effecting on EGR-1-Regulated Gene Expression" Crystals 15, no. 4: 338. https://doi.org/10.3390/cryst15040338
APA StyleShin, S. Y., Jung, E., Lee, Y., Lee, H.-J., Lee, H., Yoo, J., Ahn, S., & Koh, D. (2025). Synthesis, Crystal Structure, DFT Analysis and Docking Studies of a Novel Spiro Compound Effecting on EGR-1-Regulated Gene Expression. Crystals, 15(4), 338. https://doi.org/10.3390/cryst15040338






