Abstract
Enhancing the photocatalytic efficiency of oxide materials under Vis light remains a significant challenge within the scientific community. Natural zeolite–metal oxide composites exhibit enhanced properties, especially due to the zeolite’s large active surface area, which facilitates the incorporation of metal oxide nanoparticles into its structure, thereby significantly increasing photocatalytic efficiency. The present study presents the synthesis of Na-zeolite-decorated black-TiO2 by the impregnation method, in order to improve the structural characteristics to absorb into visible light. The experimental protocol involves two main steps: first, the synthesis of black-TiO2 and white-TiO2 nanocrystals using the sol-gel method, and second, the preparation of hybrid materials, consisting of Na-zeolite decorated with black-TiO2 and white-TiO2, through impregnation followed by thermal treatment. The morpho-structural and optical properties of the as-synthesized materials were investigated using XRD, SEM/EDX, FTIR, and DRUV-VIS analysis. The characterization results indicated that natural zeolite has a good thermal stabilization, the lamellar texture of natural zeolite and spherical form of anatase-TiO2 materials being highlighted by SEM. In the case of Na-zeolite-decorated black-TiO2, the adsorption edge is slightly shifted to the visible range, while Na-zeolite-decorated white-TiO2 absorbs only in the ultraviolet region. The above results showed that these hybrid materials are adequate for application in photocatalytic processes.
1. Introduction
Industrial development in recent decades has brought many benefits to increasing the quality of life, but also many disadvantages, especially for environmental pollution. Water contamination with multiple harmful chemical agents causes serious disruptions in the functioning of ecosystems. Water contamination has numerous and extremely severe effects on human health, as is widely known and proven. In this context, there are significant issues about promoting several water decontamination techniques. In the last years, there has been great interest regarding titanium dioxide (TiO2) since it has been considered the best semiconductor due to its exceptional photocatalytic activity, combined with its affordability, nontoxicity, and chemical stability. However, the wide electronic band gap of TiO2, between 3.0 and 3.2 eV, restricts its optical absorption to the ultraviolet spectrum. This limitation significantly reduces its efficiency in capitalizing solar energy, resulting in utilization rates of less than 5% [1,2]. Thereby, to improve TiO2’s optical absorption properties and photocatalytic activities, it is required to increase its surface area and number of active sites while also reducing its electron–hole recombination [3]. One of the most often used techniques for increasing the TiO2 optical response in the visible light range is metal and non-metal doping. Recently, researchers underlined that metal doping in TiO2 generated secondary impurities that decreased the crystallinity of TiO2 and reduced the efficiency of the material’s photoactivity. In addition, non-metal TiO2 doping exhibited minimal or no absorption in the solar spectrum’s infrared region [4]. Currently, there is significant interest in the hydrogen modification of TiO2 to create various defect states within the band gap. This process results in a significant color change, commonly referred to as “black-TiO2” [5]. Chen et al. reported for the first time, in 2011, that the hydrogenation of white-TiO2 (W-TiO2) to produce black-TiO2 (B-TiO2) could extend the light response into the visible and near-infrared regions. Further, this process enhanced visible light catalytic performance by introducing surface disorders in TiO2 [6]. Many synthesis processes, including hydrogenation, aluminum reduction, and chemical reduction, have been developed to obtain B-TiO2 nanomaterials. Thus, numerous researchers have used Al [7], Mg [8], Zn [9], H2 [10], NaBH4 [11], imidazole [12], and ascorbic acid [13] as reducing agents to convert W-TiO2 to B-TiO2. B-TiO2 has garnered significant interest across different applications, such as photocatalytic pollutant degradation [14,15,16], hydrogen generation via water splitting [17,18], photocatalytic CO2 reduction [19,20], supercapacitors [21,22], dye-sensitized solar cells [23], lithium-ion batteries [24,25], and various medical uses [26], achieving remarkable success. Research indicates that while W-TiO2 is a photocatalyst primarily active in the UV range, B-TiO2 exhibits a narrower band gap of approximately 3.0 eV, which is lower than that of W-TiO2. This distinction arises from an increased presence of Ti3+ species and oxygen vacancies, enhancing photon energy absorption (below the direct band gap). Additionally, studies reveal that oxygen vacancies (Ov) and Ti3+ surface defects effectively reduce the band gap of TiO2, facilitating charge carrier trapping and, consequently, extending the lifetime of electron–hole pairs [27].
Due to some issues such as TiO2’s adsorption capacity and high aggregation tendency, many researches have focused on supporting TiO2 nanoparticles on various materials such as activated carbon [28,29], clay [30,31], zeolite [32,33], and silica [34,35,36]. Numerous studies have demonstrated that these supports exhibit excellent pollutant adsorption and diffusion capabilities, along with consistent pore and channel sizes, leading to superior photocatalytic performance [37,38,39]. Natural zeolites with uniform pore size, unique topology, and high structural stability are widely employed in a variety of catalytic/photocatalytic processes, as well as separation and purification techniques. Zeolites are well-known for their remarkable catalytic properties, but they cannot perform every desired reaction on their own, which is why they are frequently used as substrates for metal nanoparticles to increase functionality. Therefore, zeolites are commonly used to support various metal particles, improving their functionality [40]. One of the most important characteristics of natural zeolite is the ion exchange property, which allows the resultant hybrid materials to be used in numerous applications such as catalytic/photocatalytic, medical, antibacterial, and so on. TiO2 supported on zeolite induces a synergetic effect because of the photocatalytic activity of TiO2 and the adsorption properties of zeolite, resulting in an enhanced photocatalytic efficiency. Natural minerals like zeolite and montmorillonite have long been utilized to manufacture adsorption–photocatalyst hybrid materials, owing to their large surface area, excellent thermal and chemical stability, enhanced active surface, and ability to prevent catalyst aggregation [41]. Many studies have demonstrated that zeolite’s unique physicochemical properties make them highly efficient supports for metal oxides, with a high potential in photocatalytic applications [42,43,44]. As shown in our previous studies [37,38,45,46,47], composite materials composed of natural zeolite and white-TiO2 display enhanced photocatalytic efficiency in degrading and mineralizing organic pollutants in water. Furthermore, in a recent study, M. R. Pour et al. obtained a functionalized commercial H-type zeolite with black-TiO2 using the sono-deposition method. The obtained hybrid material was applied in photocatalytic processes for the degradation of azo pollutants (methylene blue) under visible light. The valuable results of this study highlighted the advantage of using composite materials with significantly enhanced photocatalytic properties in the degradation of organic pollutants present in wastewater [48]. Our prior research work has focused on synthesizing hybrid materials based on natural zeolite and undoped/doped TiO2 using methods such as sol-gel [46], solid-state reactions under microwave-assisted hydrothermal conditions [37,45,47], and fast hydrothermal techniques [38].
Taking into account the previous results, the progress and novelty of this study consist in the development of new hybrid materials based on Romanian natural zeolite decorated with black-TiO2 by the impregnation method, followed by thermal treatment in a controlled atmosphere containing a gas mixture of 98% Ar and 2% O2. This research opens new paths for the synthesis of hybrid materials by an easy and reproducible impregnation approach, offering potential for the development of photocatalysts based on natural zeolite and TiO2, applicable in catalytic or photocatalytic degradation processes.
2. Materials and Methods
2.1. Regents
Commercial reagents, such as titanium (IV) isopropoxide (TTIP, 98%), polyethylene glycol 400 (PEG400), and nitric acid (HNO3), were purchased from the Sigma Aldrich Company (St. Louis, MO, USA) and used without any further treatment. The natural zeolite used for the synthesis of the hybrid material was Romanian Zeolitic Mineral from the Mirsid Area, supplied by the Cemacon Company, Salaj county, Romania. The mineral was powdered and sieved with a Multilab sieve shaker, with grain diameter sizes of about 315 and 500 µm and a mineralogical composition of clinoptilolite (SiO2 68.75%), volcanic glass (Al2O3 11.35%), plagioclase (Fe2O3 2.10%), SiO2 (CaO 2.86%), and other minerals (MgO 1.18%, Na2O+K2O 3.99%, P.C. 9.77%).
2.2. Synthesis of TiO2 Nanocrystals
TiO2 nanocrystals were synthesized by the sol-gel method, where 0.5 g of PEG400 was dispersed in 30 mL of ethanol, and afterward under continuous stirring at 40 °C, 6 mL of TTIP was added. The pH of the solution was adjusted with HNO3, and finally 1 mL of distilled water was added in drops to obtain the TiO2 gel. Before thermal treatment, the gel was washed with distilled water and dried at 60 °C, for 7 h. In order to obtain white-TiO2 (code name W-TiO2), the sample was treated in a furnace (Nabertherm GmbH, Lilienthal, Germany) in an air atmosphere at 550 °C for 5 h. Black-TiO2 (code name B-TiO2) was synthesized under an identical temperature and time conditions using an oven tube (GSL1100X Tube Furnace Vacuum System, MTI Corporation, Richmond, CA, USA) with a controlled atmosphere of a gas mixture of 98% Ar and 2% O2.
2.3. Synthesis of Hybrid Materials
Hybrid materials composed of natural zeolite and TiO2 nanocrystals were successfully synthesized using the impregnation method. The Romanian natural zeolite, for a more efficient ion exchange, was chemically modified to the sodium form (Na-zeolite), as described in our previous paper [37]. Therefore, an amount of Na-zeolite was mixed with B-TiO2, respectively, W-TiO2 nanocrystals with a mass ratio of 1:0.5, in a 40 mL ethanolic solution under continuous stirring for 4 h. After mixing, both types of the hybrid materials, Na-zeolite decorated with B-TiO2 (code name Na-zeolite@B-TiO2) and Na-zeolite decorated with W-TiO2 (code name Na-zeolite@W-TiO2), were washed with distilled water and dried at a temperature of 60 °C for 6 h. The final stage comprised treatment at 500 °C for 5 h in air atmosphere conditions in the case of Na-zeolite@W-TiO2, respectively, in a controlled atmosphere furnace of 98% Ar and 2% O2 for Na-zeolite@B-TiO2. A schematic representation of the synthesized materials is shown in Figure 1.
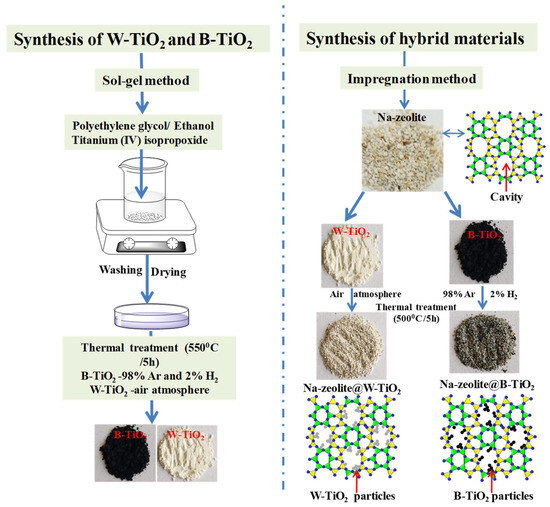
Figure 1.
The schematic diagram of the as-synthesized materials.
2.4. Characterization Methods
The structural properties of the as-synthesized materials were investigated using an X-ray diffraction spectrometer (XRD, PANalytical X’Pert PRO MPD Diffractometer, Almelo, The Netherlands) with Cu-Kα radiation (2theta = 20–60°). The Fourier transform infrared (FTIR) spectra were recorded in the range of 400–4000 cm−1 for all samples in KBr pellet form with a JASCO FT/IR 430 spectrometer, and the optical properties were studied through UV-VIS diffuse reflectance spectroscopy (PerkinElmer Lambda 950 UV/Vis, Shelton, WA, USA) in the range of 300–800 nm. Morphological and elemental characterizations were achieved with a scanning electron microscope (SEM), the Inspect S PANalytical model using an Everhart Thornley Detector (ETD), coupled with an energy dispersive X-ray analysis detector (EDX) with a 30 kV voltage.
3. Results
Figure 2 shows the XRD patterns of as-synthesized materials. For B-TiO2 and W-TiO2 (Figure 2a), the patterns reveal the pure anatase phase of TiO2 corresponding to specific peaks presented at 2theta: 25.3°, 37°, 37.8°, 38.6°, 48°, 54°, and 55.18° (JCPDS-01-078-2486) [49]. The X-ray patterns from Figure 2b show that the natural zeolite used in the experiment is mostly clinoptilolite, corresponding to peaks at 2theta: 10.00°, 22.5°, and 30.00° (JCPDS-00-025-1349) [49]. It can be seen that the main peak positions of natural zeolite (clinoptilolite) are unchanged, indicating that the structure of natural zeolite has a good thermal stabilization after TiO2 loading. The presence of anatase TiO2 peaks in the XRD spectra of the hybrid material certified that Na-zeolite was successfully functionalized with TiO2 nanocrystals.
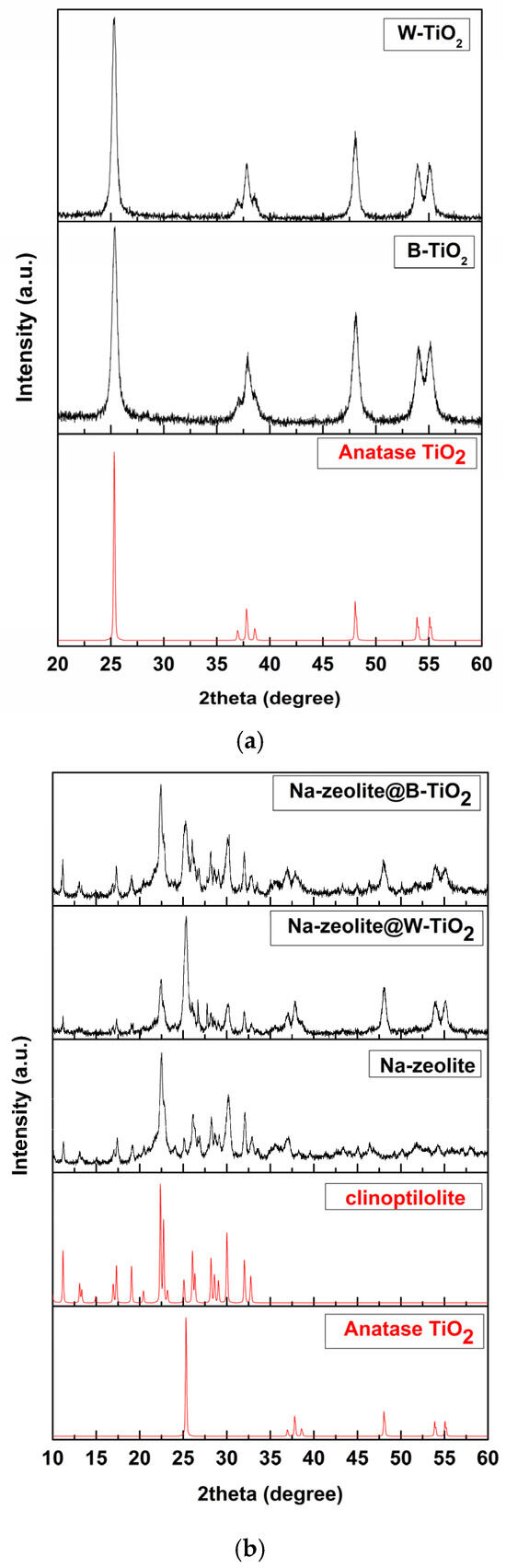
Figure 2.
X-ray patterns for the as-prepared TiO2 materials (a); hybrid materials based on Na-zeolite@B-TiO2 and Na-zeolite@W-TiO2 (b).
The absorption spectra via DRUV-Vis analysis of the as-synthesized materials are presented in Figure 3. Because the band gap determination is a crucial characterization for photocatalytic materials, as it plays a significant role in the generation of electron–hole pairs and, respectively, in light absorption, in Figure 3a is presented the spectra for Na-zeolite, Na-zeolite@B-TiO2, and Na-zeolite@W-TiO2 in the range of 300–800 nm. The band gap energy values were calculated, and a slight shift was observed due to the decoration with TiO2 nanoparticles. Moreover, the absorbance spectra for B-TiO2 (Figure 3b) and W-TiO2 (Figure 3c) particles are presented, and based on Kubelka–Munk theory, the Eg optical band gap energy derived from the intersection of the straight line with the hν-axis was calculated to be about 1.80 eV for B-TiO2 (inset of Figure 3b) and 3.56 eV for W-TiO2 (inset of Figure 3c). It is well-known that W-TiO2 absorbs in the UV domain, an aspect certified also by the energy band gap of about 3.56 eV, while B-TiO2 clearly absorbs in the Vis region [38]. According to M. R. Pour et al., the increased visible light absorption of TiO2 can be attributed to the narrowing of the band gap caused by defect-induced energy states (Ov and Ti3+) near the top and bottom of the VBM and CBM, respectively [48].
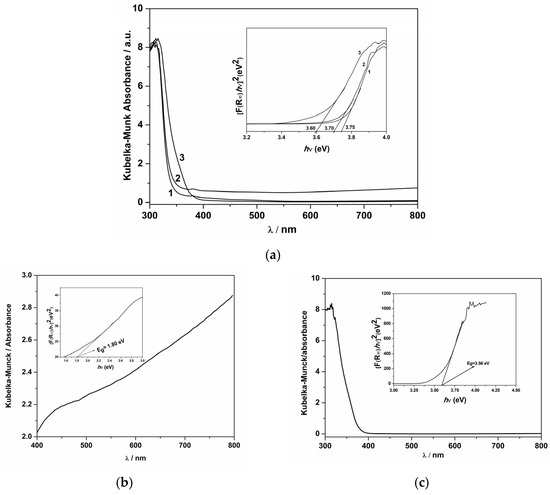
Figure 3.
UV-Vis diffuse reflectance spectra for 1—Na-zeolite, 2—Na-zeolite@W-TiO2, and 3—Na-zeolite@B-TiO2 (inset: band gap energy calculation) (a); B-TiO2 (inset: band gap energy calculation) (b), and W-TiO2 (inset: band gap energy calculation) (c).
Figure 4 shows the comparison of the FT-IR transmittance spectra of as-prepared TiO2 nanocrystals (Figure 4a) and, respectively, for the Na-zeolite@B-TiO2 and Na-zeolite@W-TiO2 hybrid materials (Figure 4b). From Figure 4a, it can be observed that both samples, B-TiO2 and W-TiO2, exhibited similar absorption bands from 400 to 1200 cm−1, the main characteristic features being the presence of a band at ~710 cm−1 specific for the stretching vibrations of the Ti-O-Ti, and the peak at ~3418 cm−1 attributed to hydroxyl and absorbed water [50]. Figure 4b elucidates that the mid-infrared spectrum, extended in the range of 400–2000 cm⁻1, reveals the characteristic chemical bond specific to zeolite. Notably, the infrared peaks around ~1060 cm⁻1 and ~1100 cm⁻1 are related to vibrations of the Al-O bond and Si-O-Si asymmetric stretching. Compared to Na-zeolite, a new weak band at 960 cm−1 was observed, which is generally attributed to Ti-O-Si vibration. Other researchers have reported that the band intensity is proportional to the amount of TiO2 particles present in the zeolite framework, which are fixed on the surface of Na-zeolite with the force of a chemical bond [51].
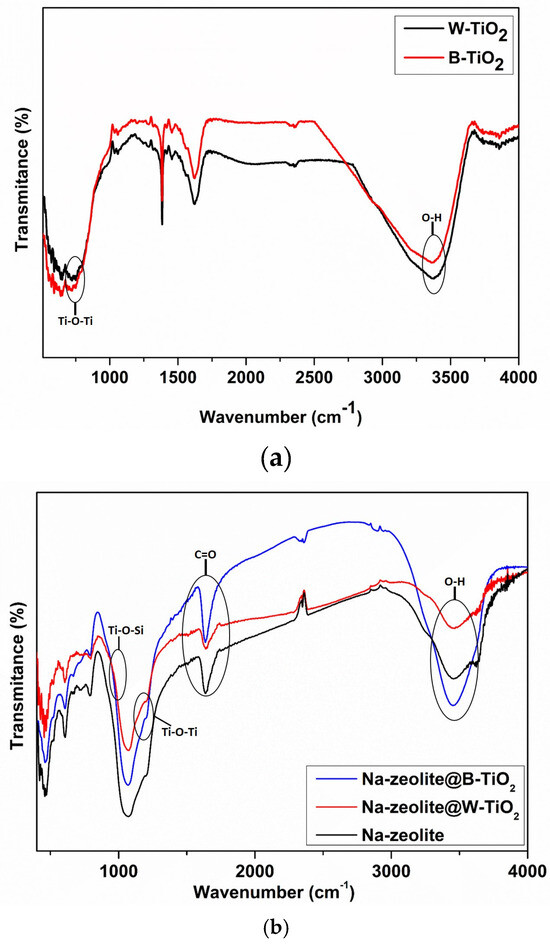
Figure 4.
FT-IR transmittance spectra of TiO2 nanocrystals (a) and hybrid materials (b).
The morphology of the synthesized materials, TiO2 nanocrystals, and hybrid materials, Na-zeolite@B-TiO2 and Na-zeolite@W-TiO2, highlighted by SEM analysis and EDX spectra are depicted in Figure 5. As can be observed in Figure 5a, the representative morphology of clinoptilolite, a stacked lamellar structure, is observed for both the hybrid materials, Na-zeolite@W-TiO2 and Na-zeolite@B-TiO2. The surface morphology of the W-TiO2 and B-TiO2 samples synthesized by the sol-gel method (Figure 5b,c) reveals that the particles have a spherical shape with a good dimensional dispersion. Spherical TiO2 nanoparticles attached to the surface of Na-zeolite and exhibited obvious agglomerates due to the high-temperature treatment. Using the Debye–Scherrer formula [52], the average crystallite size was calculated to be about 17.91 nm for W-TiO2, with a slightly lower value of 12.87 nm for B-TiO2 materials. From the images presented in Figure 5d,e, it can be seen that the W-TiO2 and B-TiO2 particles were successfully incorporated into the natural zeolite support, obtaining the Na-zeolite@W-TiO2 and Na-zeolite@B-TiO2 hybrid materials. The morphology of zeolite remains unchanged, and the TiO2 particles were distributed over a large surface of the natural zeolite forming conglomerates; the place of TiO2 on the zeolite surface being marked within the images. These aspects were also demonstrated by M. R. Pour et al. [48], who observed the same agglomeration of the TiO2 particles on the surface of zeolite.
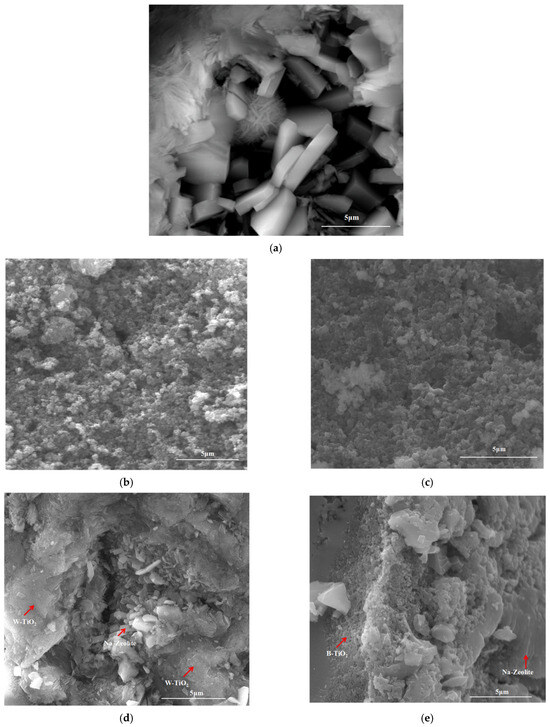
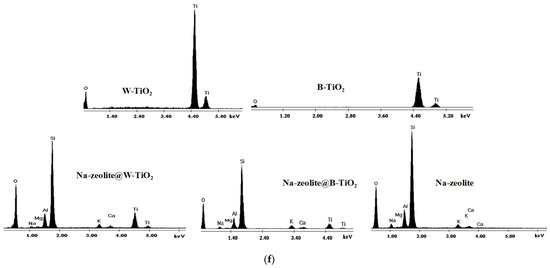
Figure 5.
SEM morphologies for Na-zeolite (a), W-TiO2 (b), B-TiO2 (c), Na-zeolite@W-TiO2 (d), and Na-zeolite@B-TiO2 (e); EDX analysis for the as-synthesized materials (f).
In addition, the heating treatment applied to the hybrid materials did not affect the structure of the Na-zeolite or the TiO2 nanocrystals. To confirm the purity of the as-synthesized materials, EDX analysis was performed. From the spectra, only the specific elements Na, Si, Al, Ca, K, Mg, Ti, and O corresponding to Na-zeolite and TiO2 particles were observed, this aspect confirming the presence of the TiO2 on the hybrid material surface (Figure 5f). The EDX quantifications are presented in the Supplementary Materials as Figure S1. Moreover, the major peak of Ti at ~4.5 keV seen on the EDX spectrum for TiO2 nanocrystals represents the binding energy for the Ti4+ oxidation present in the anatase-TiO2 phases, which confirms the XRD analysis for TiO2.
4. Conclusions
The new as-synthesized hybrid materials, Na-zeolite@B-TiO2 and Na-zeolite@W-TiO2,were successfully prepared by decorating B-TiO2 and W-TiO2 on a Na-zeolite surface via the impregnation method. Firstly, B-TiO2 and W-TiO2 were synthesized by the sol-gel method in this study, and according to the XRD and EDX analysis, only the pure anatase form was presented as the major crystalline phase. Moreover, the Debye–Scherrer formula was used to calculated the average crystallite size, and the obtained dimensions were about 17.91 nm for W-TiO2 and 12.87 nm for the B-TiO2 materials. FT-IR spectroscopy was used to demonstrate the chemical bond between TiO2 and natural zeolite through the detection of a weak absorption peak near 960 cm−1, which was assigned to the asymmetrical stretching vibration of the Ti-O-Si bond. Also, the absorption edge of Na-zeolite@B-TiO2 was slightly shifted to the visible range, with the optical band gap energy calculated to be about 1.80 eV for B-TiO2 and 3.56 eV for W-TiO2. The morphology investigations presented a spherical shape with a good dimensional dispersion of the TiO2 samples, highly agglomerated, embedded on the natural zeolite support. Based on the morphological, structural, and optical characterization results, these hybrid materials based on natural zeolite decorated with black-TiO2 offer new perspectives for applications as photocatalysts in catalytic or photocatalytic processes for water treatment.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15040319/s1, Figure S1: EDX quantifications for as-synthesized materials.
Author Contributions
C.O., C.L. and C.B.—conceptualization; C.O., M.N., M.-I.M., C.B. and C.L.—methodology; C.L. and C.B.—validation; C.O., M.N. and M.-I.M.—investigation; C.O., C.L. and C.B.—writing—original draft preparation; C.O., C.L. and C.B.—writing—review and editing; C.L. and C.B.—visualization; C.L. and C.B.—supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of the project granted by the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-IV-P8-8.3-ROMD-2023-0227 within PNCDI IV and partial project number PN 23 27 01 02 INOMAT, 23-27 29N/2023.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lijun, L.; Mingtao, W.; Zhenzi, L.; Xuepeng, W.; Wei, Z. Recent Advances in Black TiO2 Nanomaterials for Solar Energy Conversion. Nanomaterials 2023, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black hydroxylated titanium dioxide prepared via ultrasonication with enhanced photocatalytic activity. Sci. Rep. 2015, 5, 11712. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, Y.; Xia, T. Black titanium dioxide nanomaterials in photocatalysis. Int. J. Photoenergy 2017, 2017, 8529851. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 nanomaterials: A review of recent advances. J. Chem. Eng. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Balog, Á.; Samu, G.F.; Pető, S.; Janáky, C. The mystery of black TiO2: Insights from combined surface science and in situ electrochemical methods. ACS Mater. Au 2021, 1, 157–168. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Li, J.; Wu, E.H.; Hou, J.; Huang, P.; Xu, Z.; Jiang, Y.; Liu, Q.S.; Zhong, Y.Q. A facile method for the preparation of black TiO2 by Al reduction of TiO2 and their visible light photocatalytic activity. RSC Adv. 2020, 10, 34775. [Google Scholar] [CrossRef]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O’Brien, P.G.; Sun, W.; Dong, Y.; Ozin, G.A. Synthesis of black TiOx nanoparticles by Mg reduction of TiO2 nanocrystals and their application for solar water evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H.; Lv, Y.; Zhou, L.J.; Song, Y.; Sun, Z. Reduced TiO2 rutile nanorods with well-defined facets and their visible-light photocatalytic activity. Chem. Commun. 2014, 50, 2755–2757. [Google Scholar] [CrossRef]
- Wang, C.C.; Chou, P.H. Effects of various hydrogenated treatments on formation and photocatalytic activity of black TiO2 nanowire arrays. Nanotechnology 2016, 27, 325401. [Google Scholar] [CrossRef]
- Ariyanti, D.; Mills, L.; Dong, J.; Yao, Y.; Gao, W. NaBH4 modified TiO2: Defect site enhancement related to its photocatalytic activity. Mater. Chem. Phys. 2017, 199, 571–576. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Azab, N.A.; Bayoumy, W.A.A.; El-Sharkawy, A.A.M.; Omran, Z.A. Novel syntheses of modified black TiO2/C3N4 and their efficient behavior toward water splitting under neutral conditions. J. Environ. Chem. Eng. 2022, 10, 107418. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. Sec. Catal. Photocatal. 2020, 8, 565489. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Nawaz, R.; Haider, S.; Anjum, M.; Haneef, T.; Oad, K.V.; Aqif, M.; Haider, A.; Khan, R. Photodegrading hazardous pollutants using black TiO2 materials with different morphology and estimation of energy requirement. Mater. Chem. Phys. 2023, 309, 128401. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, G.; Xin, X.; Yao, Y.; Li, Y.; Yin, J.; Li, X.; Wu, Y.; Gao, S. Black titanium dioxide nanomaterials for photocatalytic removal of pollutants: A review. J. Mater. Sci. Technol. 2022, 112, 239–262. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuk, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, H.; Yang, C.; Cui, H.; Wang, Z.; Xu, J.; Lin, T.; Huang, F. Black titania for superior photocatalytic hydrogen production and photoelectrochemical water splitting. ChemCatChem 2015, 7, 2614–2619. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Gao, X.; An, C.; Wang, Z.; Zuo, C.; Dionysiou, D.D.; He, H.; Jiang, Z. Enhancing photocatalytic CO2 reduction with TiO2-based materials: Strategies, mechanisms, challenges, and perspectives. Environ. Sci. Ecotechnol. 2024, 20, 100368. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Bilal, M.; Hou, J.; Butt, F.K.; Ahmad, J.; Ali, S.; Hussain, A. Photocatalytic CO2 reduction using TiO2-based photocatalysts and TiO2 Z-scheme heterojunction composites: A Review. Molecules 2022, 27, 2069. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Dong, W.; Chen, I.W.; Wang, Z.; Xu, J.; Lin, T.; Gu, H.; Huang, F. Nitrogen-doped black titania for high performance supercapacitors. Sci. China Mater. 2020, 63, 1227–1234. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Rychagov, A.Y.; Sosenkin, V.E.; Baskakov, S.A.; Kabachkov, E.N.; Shulga, Y.M. Supercapacitor properties of rGO-TiO2 nanocomposite in two-component acidic electrolyte. Materials 2022, 15, 7856. [Google Scholar] [CrossRef] [PubMed]
- Ullattil, S.G.; Thelappurath, A.V.; Tadka, S.N.; Kavil, J.; Vijayan, B.K.; Periyat, P. A Sol-solvothermal Processed ‘Black TiO2’ as Photoanode Material in Dye Sensitized Solar Cells. Sol. Energy 2017, 155, 490–495. [Google Scholar] [CrossRef]
- Patil, S.B.; Phattepur, H.; Kishore, B.; Viswanatha, R.; Nagaraju, G. Robust electrochemistry of black TiO2 as stable and high-rate negative electrode for lithium-ion batteries. Mater. Renew. Sustain. Energy 2019, 8, 10. [Google Scholar] [CrossRef]
- Ge, J.; Du, G.; Kalam, A.; Bi, X.; Ding, S.; Su, Q.; Xu, B.; Al-Sehemi, A.G. Oxygen vacancy-rich black TiO2 nanoparticles as a highly efficient catalyst for Li–O2 batteries. Ceram. Int. 2021, 47, 6965–6971. [Google Scholar] [CrossRef]
- Dai, T.; Ren, W.; Wu, A. Chapter 6. Cancer Theranostics of Black TiO2 Nanoparticles. In TiO2 Nanoparticles: Applications in Nanobiotechnology and Nanomedicine Book; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 185–215. [Google Scholar]
- Tuntithavornwat, S.; Saisawang, C.; Ratvijitvech, T.; Watthanaphanit, A.; Hunsom, M.; Kannan, A.M. Recent development of black TiO2 nanoparticles for photocatalytic H2 production: An extensive review. Int. J. Hydrogen Energy 2024, 55, 1559–1593. [Google Scholar] [CrossRef]
- Gloria, D.C.S.; Brito, C.H.V.; Mendonça, T.A.P.; Brazil, T.R.; Domingues, R.A.; Vieira, N.C.S.; Santos, E.B.; Gonçalves, M. Preparation of TiO2/activated carbon nanomaterials with enhanced photocatalytic activity in paracetamol degradation. Mater. Chem. Phys. 2023, 305, 127947. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Raza, S.M.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Dlamini, M.C.; Maubane-Nkadimeng, M.S.; Moma, J.A. The use of TiO2/clay heterostructures in the photocatalytic remediation of water containing organic pollutants: A review. J. Environ. Chem. Eng. 2021, 9, 106546. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Duraczynska, D.; Czerwenka, J.K.; Nowak, P.; Serwicka, E.M. Clay Minerals/TiO2 Composites—Characterization and Application in Photocatalytic Degradation of Water Pollutants. Molecules 2024, 29, 4852. [Google Scholar] [CrossRef]
- Huayna, G.; Antonio, L.; Churata, R.; Lazo, L.; Guzmán, R.; Ramos, P.G.; Rodriguez, J.M. Synthesis and Characterization of a Photocatalytic Material from TiO2 Nanoparticles Supported on Zeolite Obtained from Ignimbrite Residue Used in Decolorization of Methyl Orange. Appl. Sci. 2024, 14, 3146. [Google Scholar] [CrossRef]
- Rahman, A.; Nurjayadi, M.; Wartilah, R.; Kusrini, E.; Prasetyanto, E.A.; Degermenci, V. Enhanced activity of TiO2/natural zeolite composite for degradation of methyl orange under visible light irradiation. Int. J. Technol. 2018, 6, 1159–1167. [Google Scholar] [CrossRef]
- Negi, G.S.; Anirbid, S.; Sivakumar, P. Applications of silica and titanium dioxide nanoparticles in enhanced oil recovery: Promises and challenges. Pet. Res. 2021, 6, 224–246. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kuwahara, Y.; Sumida, Y.; Yamashita, H. Fabrication of Photocatalytic Paper Using TiO2 Nanoparticles Confined in Hollow Silica Capsules. Langmuir 2017, 33, 288–295. [Google Scholar] [CrossRef]
- Parra-Ortiz, E.; Caselli, L.; Agnoletti, M.; Skoda MW, A.; Li, X.; Zhao, D.; Malmsten, M. Mesoporous silica as a matrix for photocatalytic titanium dioxide nanoparticles: Lipid membrane interactions. Nanoscale 2022, 14, 12297–1231241. [Google Scholar] [CrossRef]
- Lazau, C.; Ratiu, C.; Orha, C.; Pode, R.; Manea, F. Photocatalytic activity of undoped and Ag-doped TiO2-supported zeolite for humic acid degradation and mineralization. Mater. Res. Bull. 2011, 46, 1916–1921. [Google Scholar] [CrossRef]
- Bandas, C.; Orha, C.; Misca, C.; Lazau, C.; Sfirloaga, P.; Olariu, S. Photocatalytical inactivation of Enterococcus faecalis from water using functional materials based on natural zeolite and titanium dioxide. Chin. J. Chem. Eng. 2014, 22, 38–43. [Google Scholar] [CrossRef]
- Orha, C.; Pop, A.; Lazau, C.; Grozescu, I.; Tiponut, V.; Manea, F. Silver doped natural and synthetic zeolites for removal of humic acid from water. Environ. Eng. Manag. J. 2012, 11, 641–649. [Google Scholar] [CrossRef]
- Juneau, M.; Liu, R.; Peng, Y.; Malge, A.; Ma, Z.; Porosoff, M.D. Characterization of Metal-zeolite Composite Catalysts: Determining the Environment of the Active Phase. ChemCatChem 2020, 12, 1826–1852. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Abitayev, Z.; Batyrbayeva, M.; Vakros, J.; Mantzavinos, D.; Atabaev, T.S.; Poulopoulos, S.G. TiO2/Zeolite composites for SMX degradation under UV Irradiation. Catalysts 2024, 14, 147. [Google Scholar] [CrossRef]
- Batistela, V.R.; Fogaça, L.Z.; Favaro, S.L.; Caetano, W.; Fernandes-Machado, N.R.C.; Hioka, N. ZnO supported on zeolites: Photocatalyst design, microporosity and properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 20–27. [Google Scholar] [CrossRef]
- Rubab, M.; Bhatti, I.A.; Nadeem, N.; Shah, S.A.R.; Yaseen, M.; Naz, M.Y.; Zahid, M. Synthesis and photocatalytic degradation of rhodamine B using ternary zeolite/WO3/Fe3O4 composite. Nanotechnology 2021, 32, 345705. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, L.; Wang, X.; Meng, X. Photocatalytic degradation of wastewater by molecularly imprinted Ag2S-TiO2 with high-selectively. Sci. Rep. 2020, 10, 1192–1210. [Google Scholar] [CrossRef]
- Ilinoiu, E.C.; Pode, R.; Manea, F.; Colar, L.A.; Jakab, A.; Orha, C.; Ratiu, C.; Lazau, C.; Sfarloaga, P. Photocatalytic activity of a nitrogen-doped TiO2 modified zeolite in the degradation of Reactive Yellow 125 azo dye. J. Taiwan Inst. Chem. Eng. 2013, 44, 270–278. [Google Scholar] [CrossRef]
- Ratiu, C.; Manea, F.; Lazau, C.; Orha, C.; Burtica, G.; Grozescu, I.; Schoonman, J. Photocatalytically-assisted electrochemical degradation of p-aminophenol in aqueous solutions using zeolite-supported TiO2 catalyst. Chem. Pap. 2011, 65, 289–298. [Google Scholar] [CrossRef]
- Ratiu, C.; Manea, F.; Lazau, C.; Grozescu, I.; Radovan, C.; Schoonman, J. Electrochemical oxidation of p-aminophenol from water with boron-doped diamond anodes and assisted photocatalytically by TiO2-supported zeolite. Desalination 2010, 260, 51–56. [Google Scholar] [CrossRef]
- Pour, M.R.; Fereidooni, M.; Praserthdam, S.; Praserthdam, P.; Marquez, V.; Uesaka, K.; Yamakata, A.; Paz, C.V.; Kamjam, N.; Kanjanaboos, P. Zeolite-decorated black TiOx quantum dots for photocatalytic degradation of organic cationic dyes under LED light irradiation. J. Alloys Compd. 2024, 1008, 176797. [Google Scholar] [CrossRef]
- Ratiu, C.; Lazau, C.; Orha, C.; Sfirloaga, P.; Manea, F.; Burtica, G.; Iovi, A.; Grozescu, I. Synthesis of hybrid zeolitic materials with TiO2 nanocrystals using solid-solid method. J. Optoelectron. Adv. Mater. 2009, 11, 838–844. [Google Scholar]
- Chen, S.; Xiao, Y.; Wang, Y.; Hu, Z.; Zhao, H.; Xie, W. A Facile Approach to Prepare Black TiO2 with Oxygen Vacancy for Enhancing Photocatalytic Activity. Nanomaterials 2018, 8, 245. [Google Scholar] [CrossRef]
- Gang, L.; Wei, H.; Yuming, H. Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO2 Composite. Catalysts 2019, 9, 502. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison-Wesley Pub. Co.: Reading, MA, USA, 1978. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).