Zero-Dimensional Organic Amine-Copper Bromide Hybrid Crystal with Highly Efficient Yellow Emission
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of (C13H30N)2Cu5Br7 SCs
2.3. Characterization
2.4. Calculation Details
3. Results
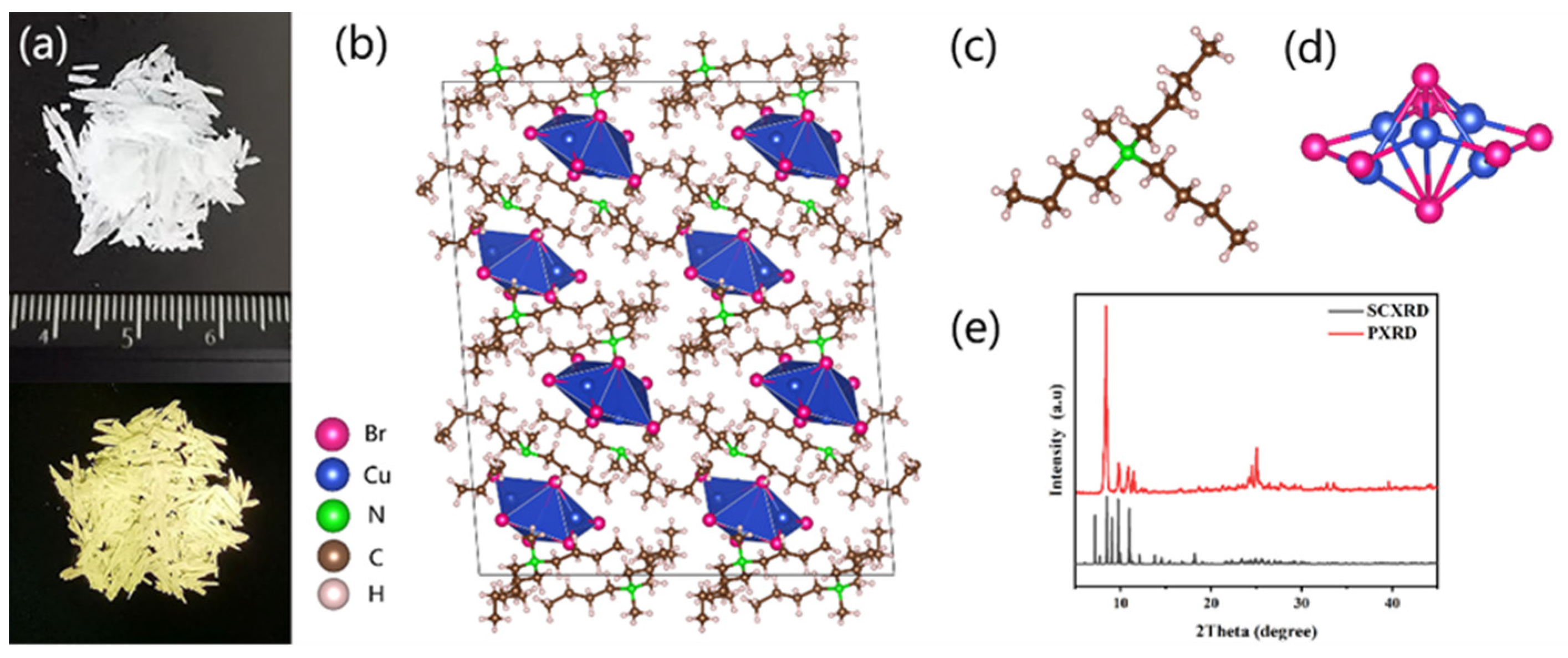
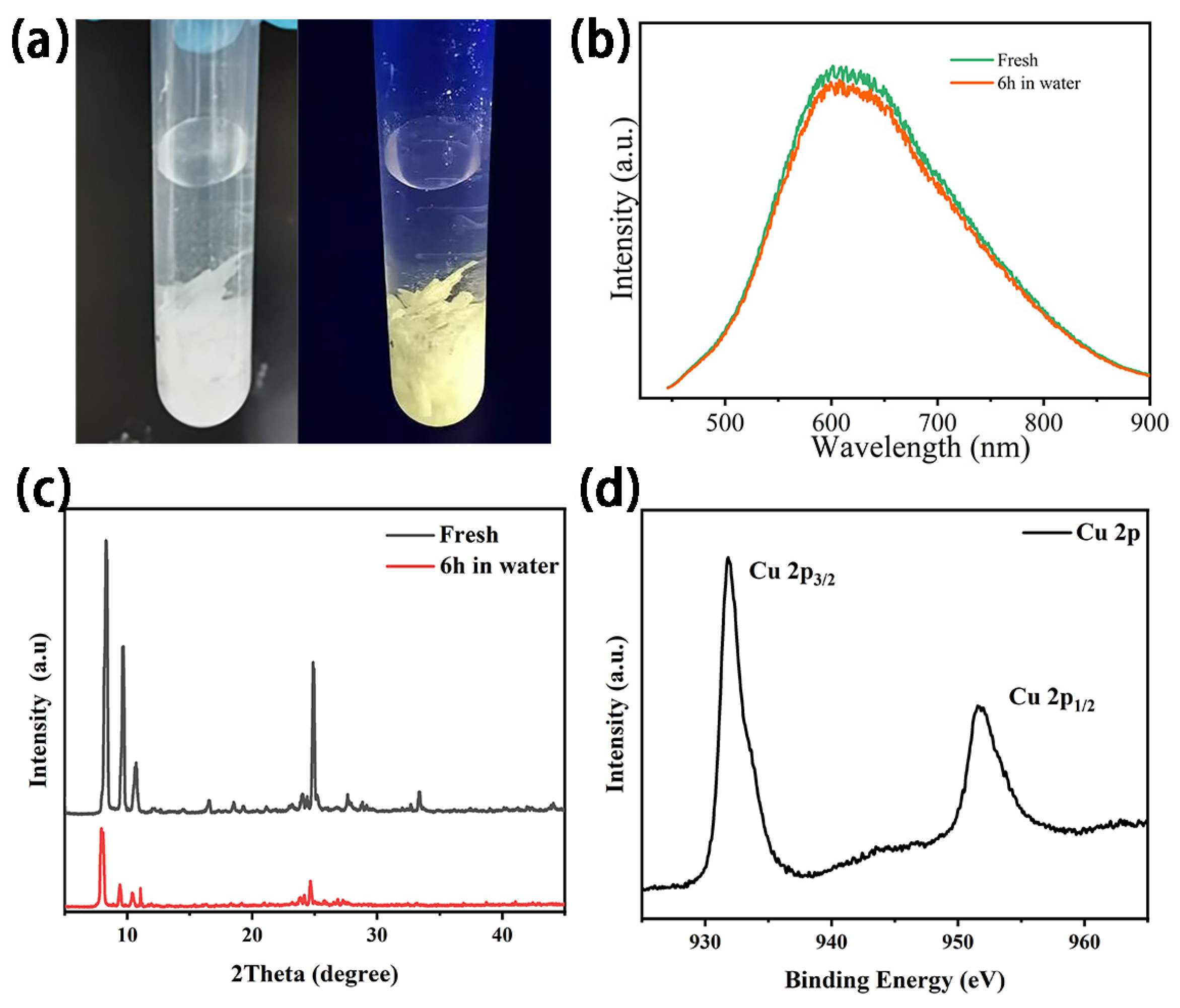
3.1. Structural Characterization
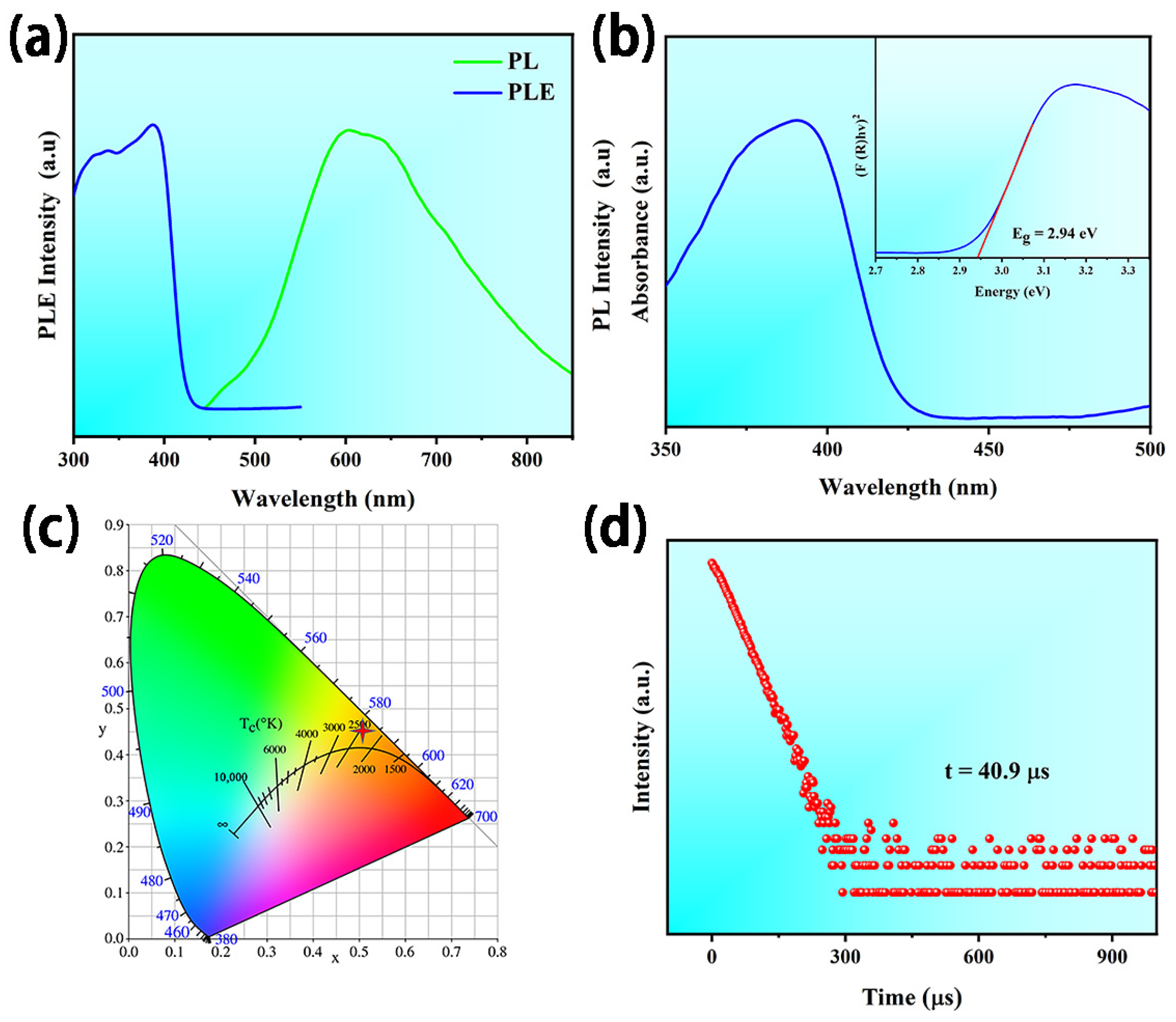
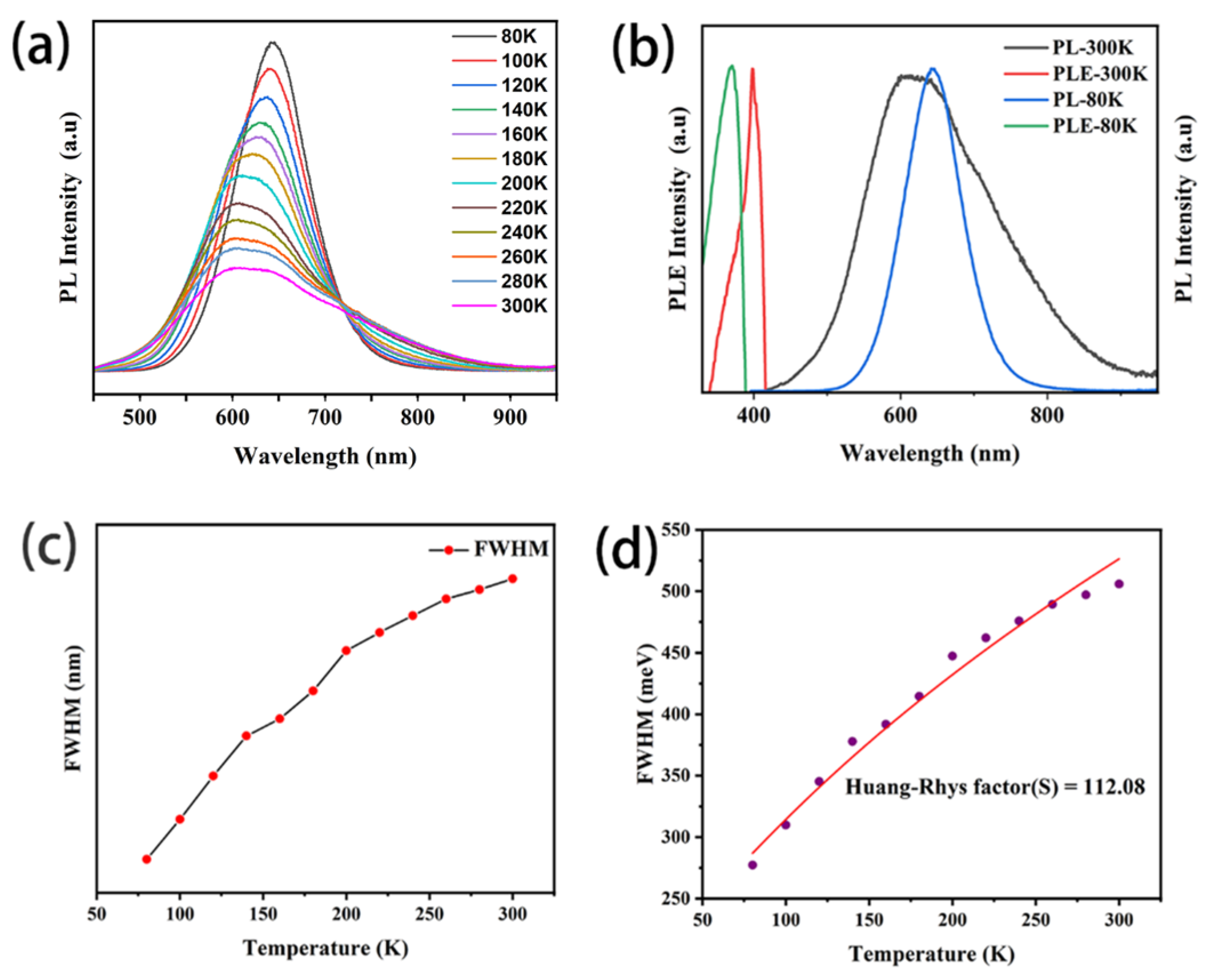
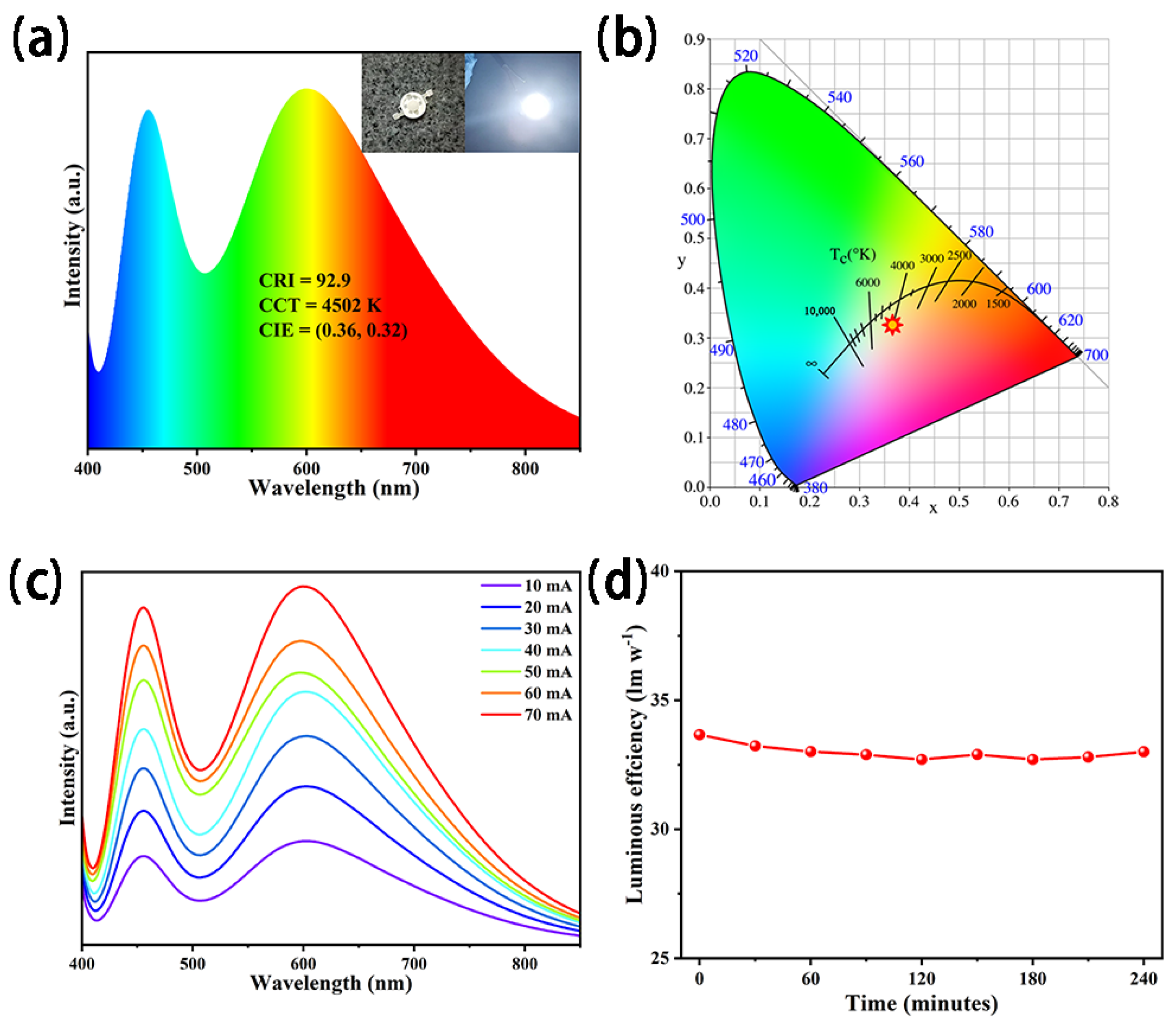
3.2. Optical Properties
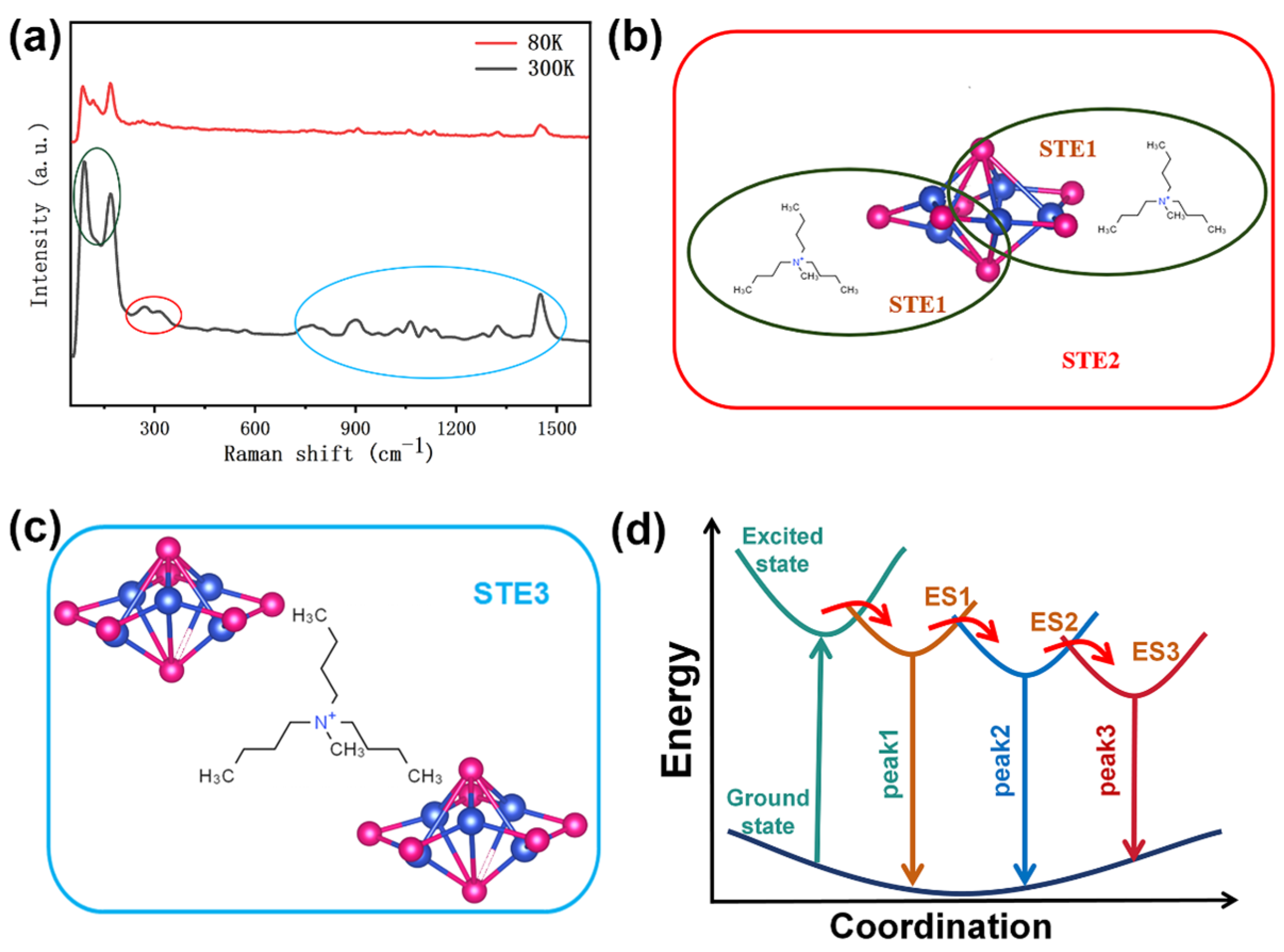
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, Z.; Chu, Y.; Chen, J.; Li, J.; Ji, G.; Niu, G.; Gao, L.; Xiao, Z.; Tang, J. Lead-free perovskite variant solid solutions Cs2Sn1-xTexCl6: Bright luminescence and high anti-water stability. Adv. Mater. 2020, 32, 2002443. [Google Scholar] [CrossRef] [PubMed]
- Yangui, A.; Roccanova, R.; Wu, Y.; Du, M.; Saparov, B. Highly Efficient Broad-Band Luminescence Involving Organic and Inorganic Molecules in a Zero-Dimensional Hybrid Lead Chloride. J. Phys. Chem. C 2019, 123, 22470–22477. [Google Scholar] [CrossRef]
- Saidaminov, M.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.; Bakr, O. Pure, Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, T.; Chen, J.; Zhao, M.; Zeng, H. Metal Halide, Perovskites for Optical Parametric Modulation. J. Phys. Chem. Lett. 2021, 12, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wei, Q.; Peng, H.; Yu, Z.; Yao, S.; Ke, B.; Li, Q.; Zou, B. A Zero-Dimensional Organic Lead Bromide of (TPA)2PbBr4 Single Crystal with Bright Blue Emission. Nanomaterials 2022, 12, 2222. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, X.; Molokeev, M.; Lin, Z.; Zhao, J.; Wang, J.; Xia, Z. Optically Modulated Ultra-Broad-Band Warm White Emission in Mn2+-Doped (C6H18N2O2)PbBr4 Hybrid Metal Halide Phosphor. Chem. Mater. 2019, 31, 5788–5795. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, Y.; Gong, X. Recent Advancements and Challenges for Low-Toxicity Perovskite Materials. ACS Appl. Mater. Interfaces 2020, 12, 26776–26811. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Liang, W.; Ma, J.; Chen, X.; Wu, D.; Tian, Y.; Li, X.; Shan, C.; Fang, X. Recent advances toward environment-friendly photodetectors based on lead-free metal halide perovskites and perovskite derivatives. Mater. Horiz. 2021, 8, 1367–1389. [Google Scholar] [CrossRef]
- Zhang, G.; Dang, P.; Xiao, H.; Lian, H.; Liang, S.; Yang, L.; Cheng, Z.; Li, G.; Lin, J. Antimony-Doped Lead-Free Zero-Dimensional Tin(IV)-Based Organic–Inorganic Metal Halide Hybrids with High Photoluminescence Quantum Yield and Remarkable Stability. Adv. Opt. Mater. 2021, 9, 2101637. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Li, H.; Shen, W.; Li, M.; He, R. Defect Passivation in Air-Stable Tin(IV)-Halide Single Crystal for Emissive Self-Trapped Excitons. Adv. Funct. Mater. 2021, 31, 202108561. [Google Scholar] [CrossRef]
- Jun, T.; Sim, K.; Iimura, S.; Sasase, M.; Kamioka, H.; Kim, J.; Hosono, H. Lead-Free Highly Efficient Blue-Emitting Cs3Cu2I5 with 0D Electronic Structure. Adv. Mater. 2018, 30, e1804547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Lou, Y.; Zhao, Y. All-inorganic lead-free perovskites for optoelectronic applications. Mater. Chem. Front. 2019, 3, 365–375. [Google Scholar] [CrossRef]
- Lin, R.; Guo, Q.; Zhu, Q.; Zhu, Y.; Zheng, W.; Huang, F. All-Inorganic CsCu2I3 Single Crystal with High-PLQY ( approximately 15.7%) Intrinsic White-Light Emission via Strongly Localized 1D Excitonic Recombination. Adv. Mater. 2019, 31, e1905079. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, L.; Niu, G.; Yuan, J.H.; Xue, K.H.; Tan, Z.; Miao, X.S.; Niu, M.; Du, X.; Song, H.; et al. Lead-Free Halide Rb2CuBr3 as Sensitive X-Ray Scintillator. Adv. Mater. 2019, 31, e1904711. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yin, L.; Yuan, J.-H.; Xue, K.-H.; Niu, G.; Yang, B.; Hu, Q.; Liu, X.; Tang, J. Lead-free violet-emitting K2CuCl3 single crystal with high photoluminescence quantum yield. Org. Electron. 2020, 86, 105903. [Google Scholar] [CrossRef]
- Hui, Y.; Chen, S.; Lin, R.; Zheng, W.; Huang, F. Photophysics in Cs3Cu2I5 and CsCu2I3. Mater. Chem. Front. 2021, 5, 7088–7107. [Google Scholar]
- Liu, F.; Mondal, D.; Zhang, K.; Zhang, Y.; Huang, K.; Wang, D.; Yang, W.; Mahadevan, P.; Xie, R. Zero-dimensional plate-shaped copper halide crystals with green-yellow emissions. Mater. Adv. 2021, 2, 3744–3751. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, F.; Zhu, C.; Li, J.; Lv, X.; Xing, G.; Wei, Q.; Wang, G.; Dai, J.; Dong, H.; et al. Near-unity blue luminance from lead-free copper halides for light-emitting diodes. Nano Energy 2022, 91, 106664. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Y.; Wang, X.; Dong, T.; Yu, Z.; Xiao, Y.; Zhang, Z.; Wang, J.; Zou, B. Highly Efficient Broadband Green Emission of (TPA)CuCl2 Single Crystals: Understanding the Formation of Self-Trapped States. J. Phys. Chem. C 2022, 126, 8545–8552. [Google Scholar] [CrossRef]
- Peng, H.; Wang, X.; Tian, Y.; Zou, B.; Yang, F.; Huang, T.; Peng, C.; Yao, S.; Yu, Z.; Yao, Q.; et al. Highly Efficient Cool-White Photoluminescence of (Gua)3Cu2I5 Single Crystals: Formation and Optical Properties. ACS Appl. Mater. Interfaces 2021, 13, 13443–13451. [Google Scholar] [CrossRef]
- Perdew, J.P.; Levy, M. Physical Content of the Exact Kohn-Sham Orbital Energies: Band Gaps and Derivative Discontinuities. Phys. Rev. Lett. 1983, 51, 1884–1887. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Tian, Y.; Dong, T.; Xiao, Y.; Huang, T.; Guo, Y.; Wang, J.; Zou, B. Water-Stable Zero-Dimensional (C4H9)4NCuCl2 Single Crystal with Highly Efficient Broadband Green Emission. J. Phys. Chem. Lett. 2021, 12, 6639–6647. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Chen, H.Y.; Kuang, D.B. Intrinsic Self-Trapped Emission in 0D Lead-Free (C4H14N2)2In2Br10 Single Crystal. Angew. Chem. Int. Ed. 2019, 58, 15435–15440. [Google Scholar] [CrossRef]
- Li, J.; Inoshita, T.; Ying, T.; Ooishi, A.; Kim, J.; Hosono, H. A Highly Efficient and Stable Blue-Emitting Cs5Cu3Cl6I2 with a 1D Chain Structure. Adv. Mater. 2020, 32, e2002945. [Google Scholar] [CrossRef]
- Wang, L.-T.; Ma, Z.-Z.; Zhang, F.; Wang, M.; Chen, X.; Wu, D.; Tian, Y.-T.; Li, X.-J.; Shi, Z.-F. Stable down-conversion white light-emitting devices based on highly luminescent copper halides synthesized at room temperature. J. Mater. Chem. C 2021, 9, 6151–6159. [Google Scholar] [CrossRef]
- Roccanova, R.; Yangui, A.; Nhalil, H.; Shi, H.; Du, M.-H.; Saparov, B. Near-Unity Photoluminescence Quantum Yield in Blue-Emitting Cs3Cu2Br5–xIx (0 ≤ x ≤ 5). ACS Appl. Electron. Mater. 2019, 1, 269–274. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, X.; Zheng, D.; Yang, Y.; Yang, S.; Han, K. A Lead-Free All-Inorganic Metal Halide with Near-Unity Green Luminescence. Laser Photonics Rev. 2020, 14, 2000027. [Google Scholar] [CrossRef]
- Creason, T.D.; Yangui, A.; Roccanova, R.; Strom, A.; Du, M.; Saparov, B. Rb2CuX3 (X = Cl, Br): 1D all-inorganic Copper halides with ultrabright blue emission and up-conversion photoluminescence. Adv. Opt. Mater. 2020, 8, 1901338. [Google Scholar] [CrossRef]
- Creason, T.D.; McWhorter, T.M.; Bell, Z.; Du, M.-H.; Saparov, B. K2CuX3 (X = Cl, Br): All-Inorganic Lead-Free Blue Emitters with Near-Unity Photoluminescence Quantum Yield. Chem. Mater. 2020, 32, 6197–6205. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Sun, C.; Xu, D.; Tao, J.; Wei, T.; Zhang, Z.H.; Zhang, Y.; Wang, Z.; Bi, W. Lead-free, stable orange-red-emitting hybrid copper based organic-inorganic compounds. J. Phys. Chem. Lett. 2021, 50, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tian, Y.; Zhang, Z.; Wang, X.; Huang, T.; Dong, T.; Xiao, Y.; Wang, J.; Zou, B. Bulk Assembly of Zero-Dimensional Organic Copper Bromide Hybrid with Bright Self-Trapped Exciton Emission and High Antiwater Stability. J. Phys. Chem. C 2021, 125, 20014–20021. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Chang, J.; Li, Y.; Huangfu, C.; Meng, H.; Wang, Y.; Xia, G.; Feng, L. Family of Highly Luminescent Pure Ionic Copper(I) Bromide Based Hybrid Materials. ACS Appl. Mater. Interfaces 2019, 11, 17513–17520. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, C.; Xu, C.; Fan, P.; Qin, F.; Gowri Manohari, A.; Lu, J.; Shi, Z.; Xu, Q.; Pan, A. Crystal structure and electron transition underlying photoluminescence of methylammonium lead bromide perovskites. J. Mater. Chem. C 2017, 5, 7739–7745. [Google Scholar] [CrossRef]

| Components | PL (nm) | PLE (nm) | Stokes Shift (nm) | FWHM (nm) | PLQY (%) | Color | Ref. |
|---|---|---|---|---|---|---|---|
| (C13H30N)2Cu5Br7 | 600/635 | 390 | 210/245 | 118/214 | 92.3 | yellow | This work |
| Cs3Cu2I5 | 445 | 290 | 155 | 75 | 90 | blue | [27] |
| Cs5Cu3Cl6I2 | 462 | 271 | 191 | 95 | 95 | blue | [28] |
| CsCu2I3 | 558 | 330 | 228 | 115 | 15 | yellow | [29] |
| Cs3Cu2Br5 | 455 | 298 | 157 | 75 | 50.1 | blue | [30] |
| Cs3Cu2Cl5 | 510 | 310 | 200 | 92 | 100 | green | [31] |
| Rb2CuCl3 | 400 | 300 | 100 | 52 | 100 | blue | [32] |
| Rb2CuBr3 | 385 | 300 | 85 | 54 | 98.6 | blue | [14] |
| K2CuCl3 | 392 | 291 | 101 | 54 | 96.58 | blue | [14] |
| K2CuBr3 | 388 | 296 | 92 | 54 | 55 | blue | [14] |
| (PEA)4Cu4I4 | 598 | 370 | 228 | 112 | 68 | orange-red | [33] |
| (DTA)2Cu2I4 | 540 | 330 | 210 | 180 | 60 | green-yellow | [17] |
| TEA2Cu2Br4 | 463 | 280 | 183 | 90 | 94.73 | blue | [18] |
| (TBA)CuCl2 | 508 | 282 | 226 | 86 | 82 | green | [19] |
| (Gua)3Cu2I5 | 481 | 324 | 157 | 125 | 96 | bluish-white | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tian, Y.; Huang, T.; Yao, S.; Peng, H.; Zou, B. Zero-Dimensional Organic Amine-Copper Bromide Hybrid Crystal with Highly Efficient Yellow Emission. Crystals 2025, 15, 312. https://doi.org/10.3390/cryst15040312
Chen Y, Tian Y, Huang T, Yao S, Peng H, Zou B. Zero-Dimensional Organic Amine-Copper Bromide Hybrid Crystal with Highly Efficient Yellow Emission. Crystals. 2025; 15(4):312. https://doi.org/10.3390/cryst15040312
Chicago/Turabian StyleChen, Yanxi, Ye Tian, Tao Huang, Shangfei Yao, Hui Peng, and Bingsuo Zou. 2025. "Zero-Dimensional Organic Amine-Copper Bromide Hybrid Crystal with Highly Efficient Yellow Emission" Crystals 15, no. 4: 312. https://doi.org/10.3390/cryst15040312
APA StyleChen, Y., Tian, Y., Huang, T., Yao, S., Peng, H., & Zou, B. (2025). Zero-Dimensional Organic Amine-Copper Bromide Hybrid Crystal with Highly Efficient Yellow Emission. Crystals, 15(4), 312. https://doi.org/10.3390/cryst15040312







