Effects of Annealing on Hydrogen Storage Performance in TiZrCrMnFeNi High-Entropy Alloy
Abstract
1. Introduction
| Hydrogen Storage Alloy | Reversible Mass Hydrogen Storage Density (wt.%) | Hydrogen Absorption/Desorption Temperature (°C) | Initial Hydrogen Absorption Kinetics | Cyclic Stability | Ref. |
|---|---|---|---|---|---|
| LaNi5 | 1.2–1.4 | Room temperature | Excellent | Excellent | [20,21] |
| TiFe TiMn | 1.6–1.8 | Room temperature | Poor | Good | [22] |
| V-Ti-Cr V-Ti-Mn V-Ti-Fe | 2.0–2.4 | Room temperature | Moderately Poor | Poor | [23,24] |
| Mg2Si Mg2Fe | 3.6–7.6 | ≥300 | Moderately Poor | Good | [25,26] |
| TiZrCrMnFeNi | 1.6–1.7 | Room temperature | Excellent | Excellent | [16] |
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructural Characterization
2.3. Absorption/Desorption Tests
3. Results and Discussions
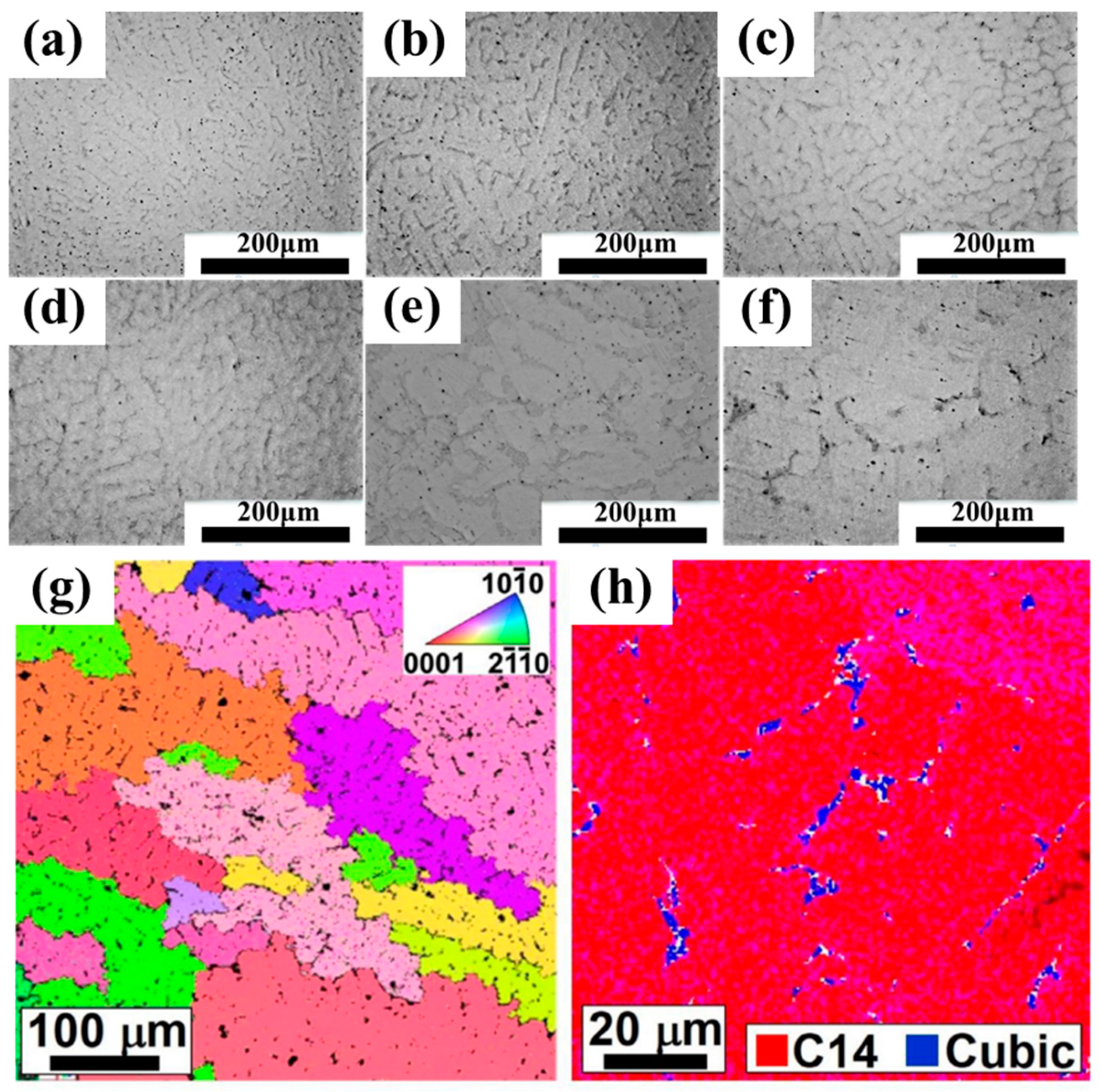
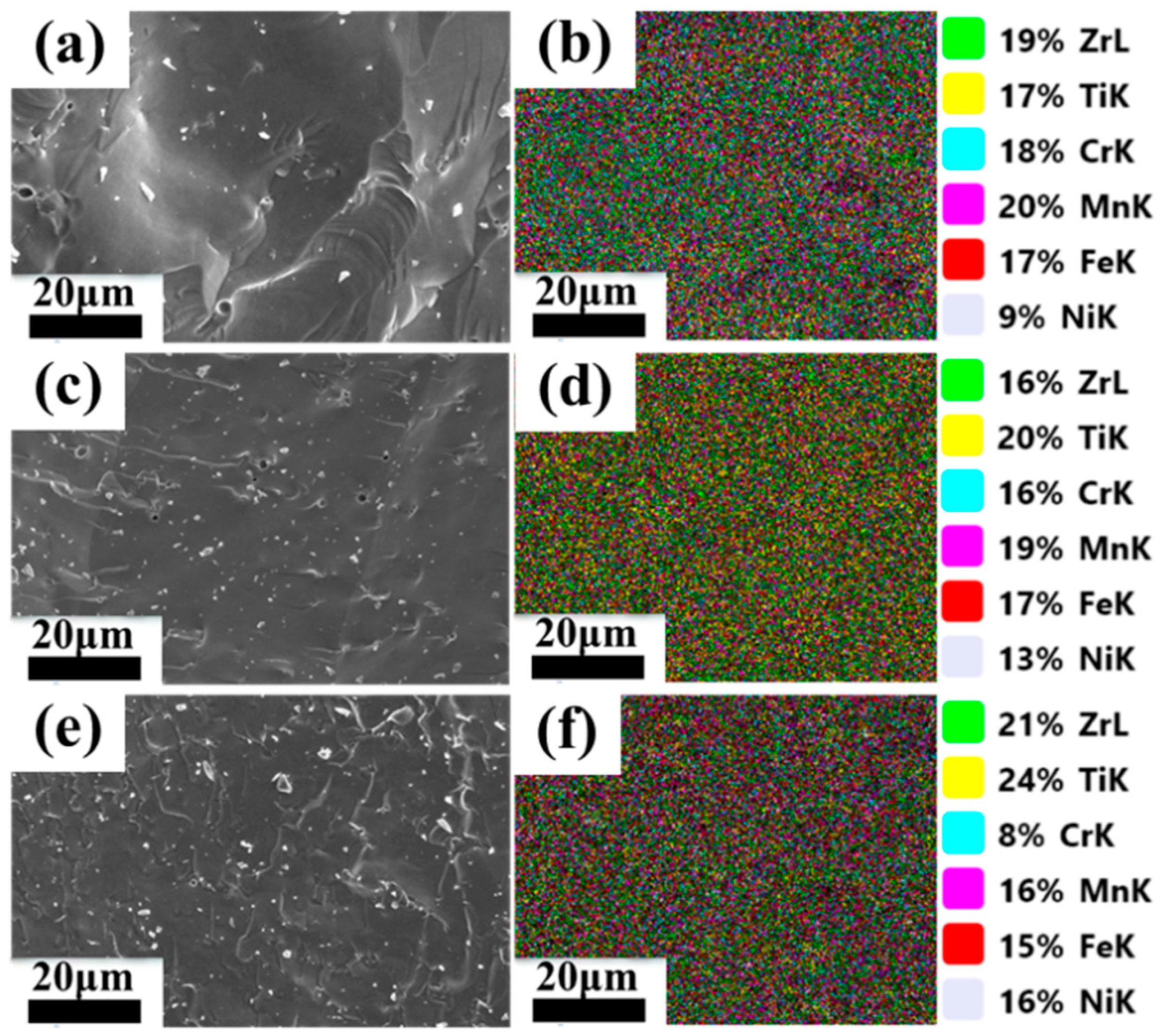
3.1. Microstructure and Phase Composition
3.2. Hydrogen Absorption KINETICS
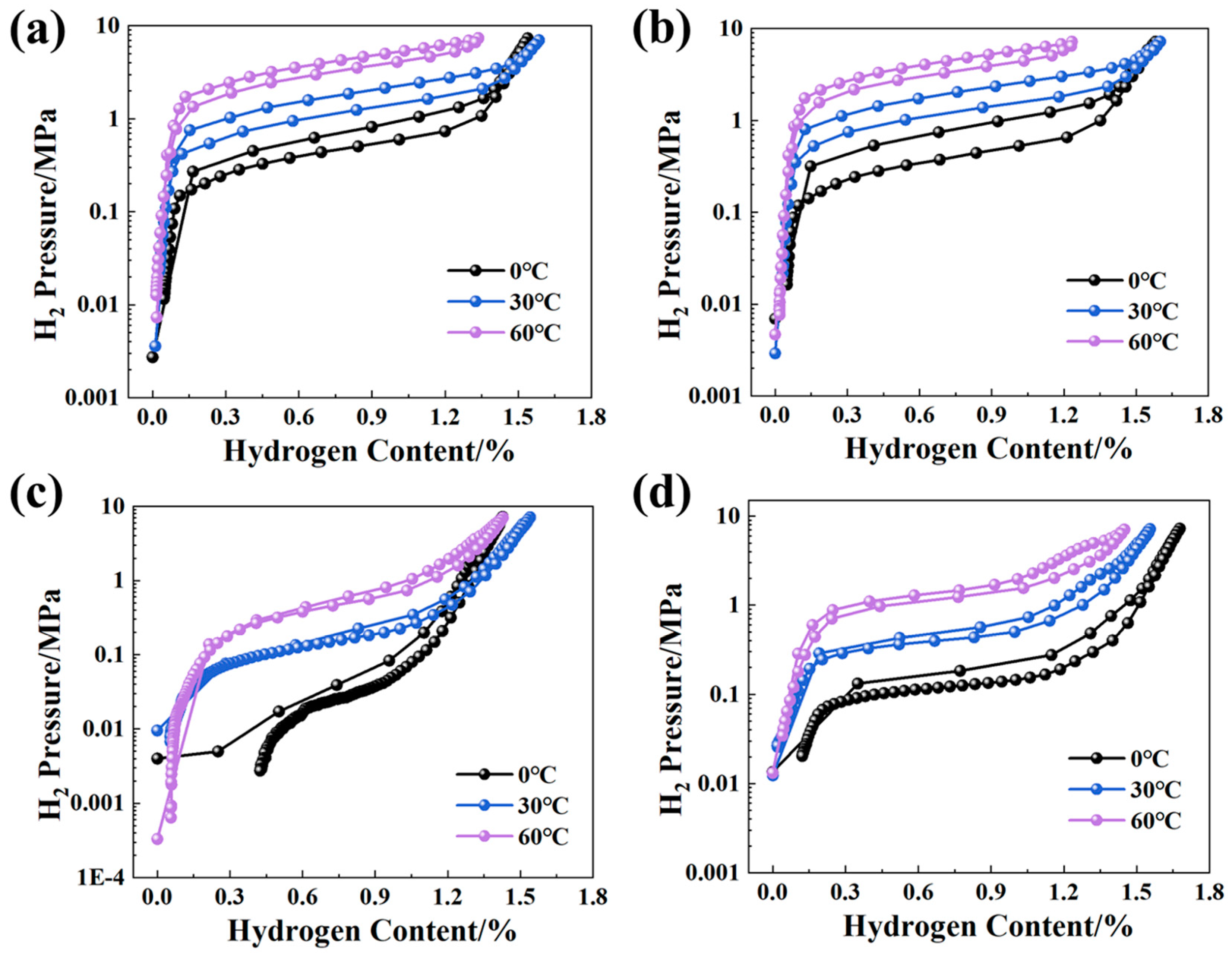
3.3. Curves and Effective Hydrogen Storage Capacity
3.4. Thermodynamic Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Bashir, M.F.; Pata, U.K.; Shahzad, L. Linking climate change, energy transition and renewable energy investments to combat energy security risks: Evidence from top energy consuming economies. Energy 2025, 314, 134175. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, J.; Choi, D.G.; Park, S.Y. Analysis of the role of hydrogen energy in achieving carbon neutrality by 2050: A case study of the Republic of Korea. Energy 2024, 304, 132023. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, C.; Zhang, Y.; Zhong, F.; Li, W. Tackling carbon peak and carbon neutrality challenges: A method with long-range energy alternatives planning system and Logarithmic Mean Divisia Index Integration. Energy 2024, 311, 133465. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, M.; Guo, S.; Akhtar, S.; Bhattacharya, S.; Dai, B.; Yu, J. Comprehensive review of development and applications of hydrogen energy technologies in China for carbon neutrality: Technology advances and challenges. Energy Convers. Manag. 2024, 315, 118776. [Google Scholar] [CrossRef]
- Terlouw, T.; Rosa, L.; Bauer, C.; McKenna, R. Future hydrogen economies imply environmental trade-offs and a supply-demand mismatch. Nat. Commun. 2024, 15, 7043. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 213, 375–377. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys-a review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Li, S.; Hu, H.; Zhang, X.; Li, C.; Liu, Y.; Liu, L.; Chen, Q. Hydrogen storage in a novel BCC-structured TiCrW alloys. Chem. Eng. J. 2025, 503, 157872. [Google Scholar] [CrossRef]
- Elman, R.; Kudiiarov, V.; Sayadyan, A.; Pushilina, N.; Leng, H. Performance improvement of magnesium-based hydrogen storage tanks by using carbon nanotubes addition and finned heat exchanger: Numerical simulation and experimental verification. Int. J. Hydrogen Energy 2024, 92, 1375–1388. [Google Scholar] [CrossRef]
- Qureshi, T.; Khan, M.M.; Pali, H.S. The future of hydrogen economy: Role of high entropy alloys in hydrogen storage. J. Alloys Compd. 2024, 1004, 175668. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.T.; Zepon, G.; Li, H.W.; Edalati, K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387–390. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liu, B.; Zhang, S.B.; Ding, X.; Chen, R.R. Effect of heat treatment on microstructure and thermal stability of Ti19Hf4V40Mn35Cr2 hydrogen storage alloy. J. Alloy. Compd. 2022, 917, 165355. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, F.; Wang, J.; Wang, Z.M.; Yao, Q.R.; Deng, J.Q.; Tang, C.Y.; Rao, G.H. Hydrogen storage properties and thermal stability of V35Ti20Cr45 alloy by heat treatment. Int. J. Hydrogen Energy 2014, 39, 14887–14895. [Google Scholar] [CrossRef]
- Ma, P.; Wu, E.D.; Li, W.H.; Sun, K.; Chen, D.F. Microstructures and hydrogen storage properties of Ti0.7Zr0.3(Cr1-xVx)2 Alloys. Acta Metall. Sin. 2014, 50, 454–462. Available online: https://www.ams.org.cn/CN/10.3724/SP.J.1037.2013.00637 (accessed on 3 December 2024).
- Sato, T.; Saitoh, H.; Utsumi, R.; Ito, J.; Nakahira, Y.; Obana, K.; Takagi, S.; Orimo, S.-I. Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules 2023, 28, 1256. [Google Scholar] [CrossRef]
- Liu, Y.; Chabane, D.; Elkedim, O. Optimization of LaNi5 hydrogen storage properties by the combination of mechanical alloying and element substitution. Int. J. Hydrogen Energy 2024, 53, 394–402. [Google Scholar] [CrossRef]
- Xu, R.; Cheng, T.; Li, C.; Yang, X.; Rong, J. Properties of Ti-Based Hydrogen Storage Alloy. J. Power Energy Eng. 2024, 12, 99–114. [Google Scholar] [CrossRef]
- Jiao, H.; Wu, Y.; Guo, X.; Wang, S.; Jiang, L.; Wang, S.; Hao, L.; Tan, G. Optimization of V-Ti-Fe hydrogen storage alloy based on orthogonal experiments. J. Alloys Compd. 2024, 1002, 175262. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Kong, H.; Xie, Q.; Wu, C.; Wang, Y.; Chen, Y.; Yan, Y. Development of Fe-containing BCC hydrogen storage alloys with high vanadium concentration. J. Alloys Compd. 2023, 958, 170294. [Google Scholar] [CrossRef]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Bowman, R.C.; Fultz, B. Altering Hydrogen Storage Properties by Hydride Destabilization through Alloy Formation: LiH and MgH2 Destabilized with Si. J. Phys. Chem. B 2004, 108, 13977–13983. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage in Mg2Fe(Ni)H6 nanowires synthesized from coarse-grained Mg and nano sized γ-Fe(Ni) precursors. Int. J. Hydrogen Energy 2016, 41, 14795–14806. [Google Scholar] [CrossRef]
- Liang, J.; Li, G.; Ding, X.; Wen, Z.; Zhang, T.; Qu, Y. The synergistic effect of Ni and C14 Laves phase on the hydrogen storage properties of TiVZrNbNi high entropy hydrogen storage alloy. Intermetallics 2024, 164, 108102. [Google Scholar] [CrossRef]
- Liang, J.; Li, G.; Ding, X.; Wen, Z.; Zhang, T.; Qu, Y. Effect of C14 Laves/BCC on microstructure and hydrogen storage properties of (Ti32.5V27.5Zr7.5Nb32.5) 1-xFex (x = 0.03, 0.06, 0.09) high entropy hydrogen storage alloys. J. Energy Storage 2023, 73, 108852. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liang, J.; Wen, Z.; Zhang, T.; Ding, X.; Qu, Y. Influence of C14 Laves phase and Zr-rich phase interactions on the hydrogen storage properties of Ti32.5V27.5Zr7.5Nb32.5 high entropy hydrogen storage alloy. Intermetallics 2024, 168, 108239. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Yang, X.S.; Xu, Z.L.; Tsui, G.C.; Zhou, Q.; Tang, R.H.; Xiao, F.M.; Chan, K.C. Development of AB2-type TiZrCrMnFeCoV intermetallic high-entropy alloy for reversible room-temperature hydrogen storage. J. Energy Storage 2024, 75, 109553. [Google Scholar] [CrossRef]
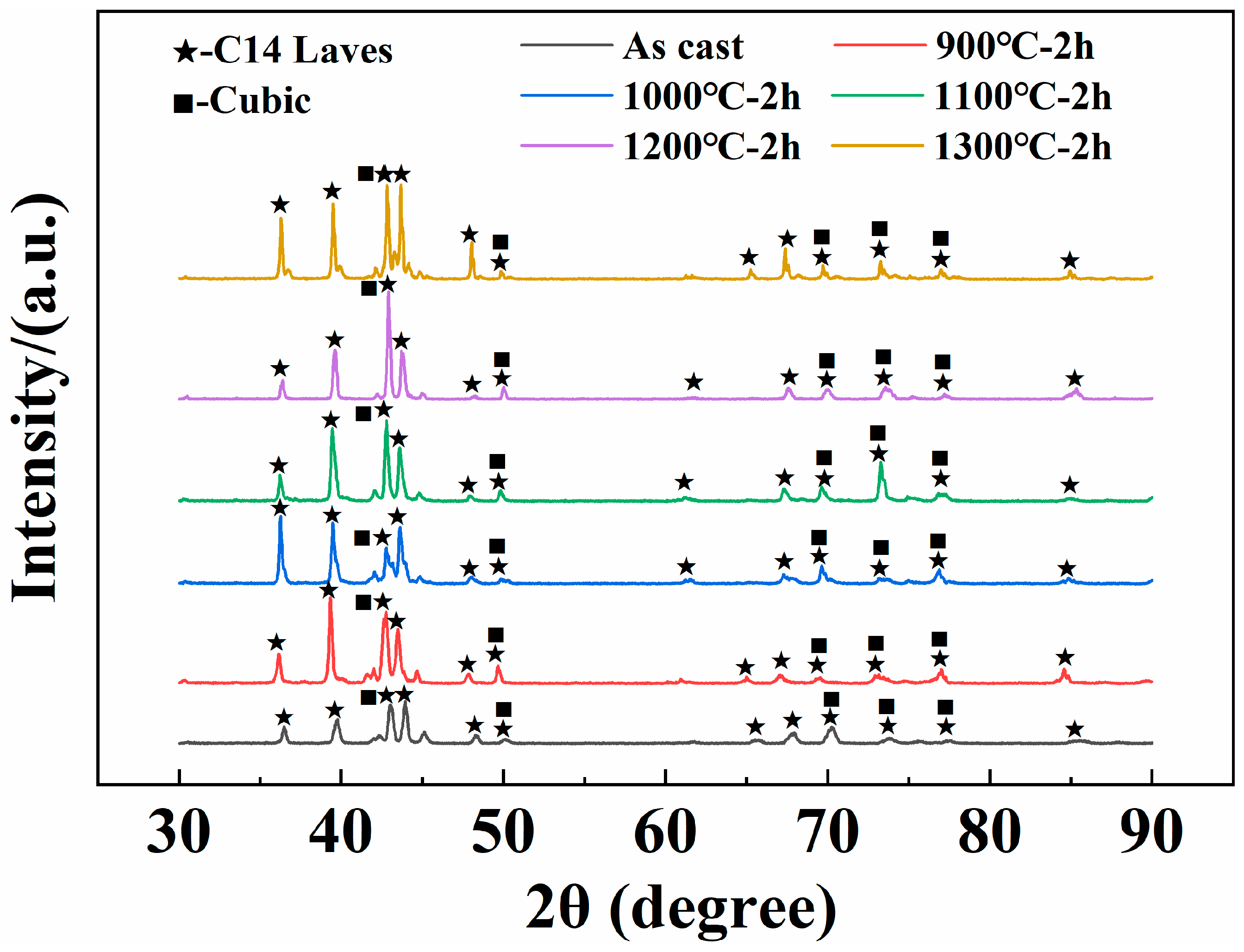

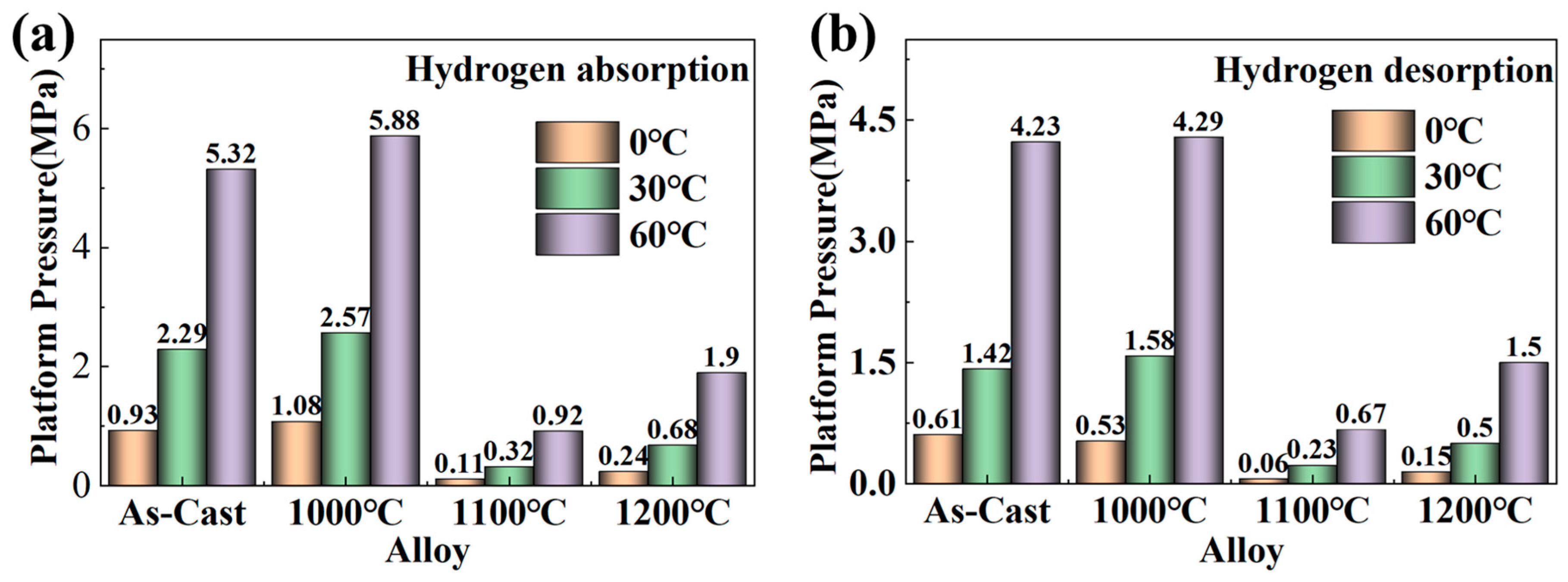
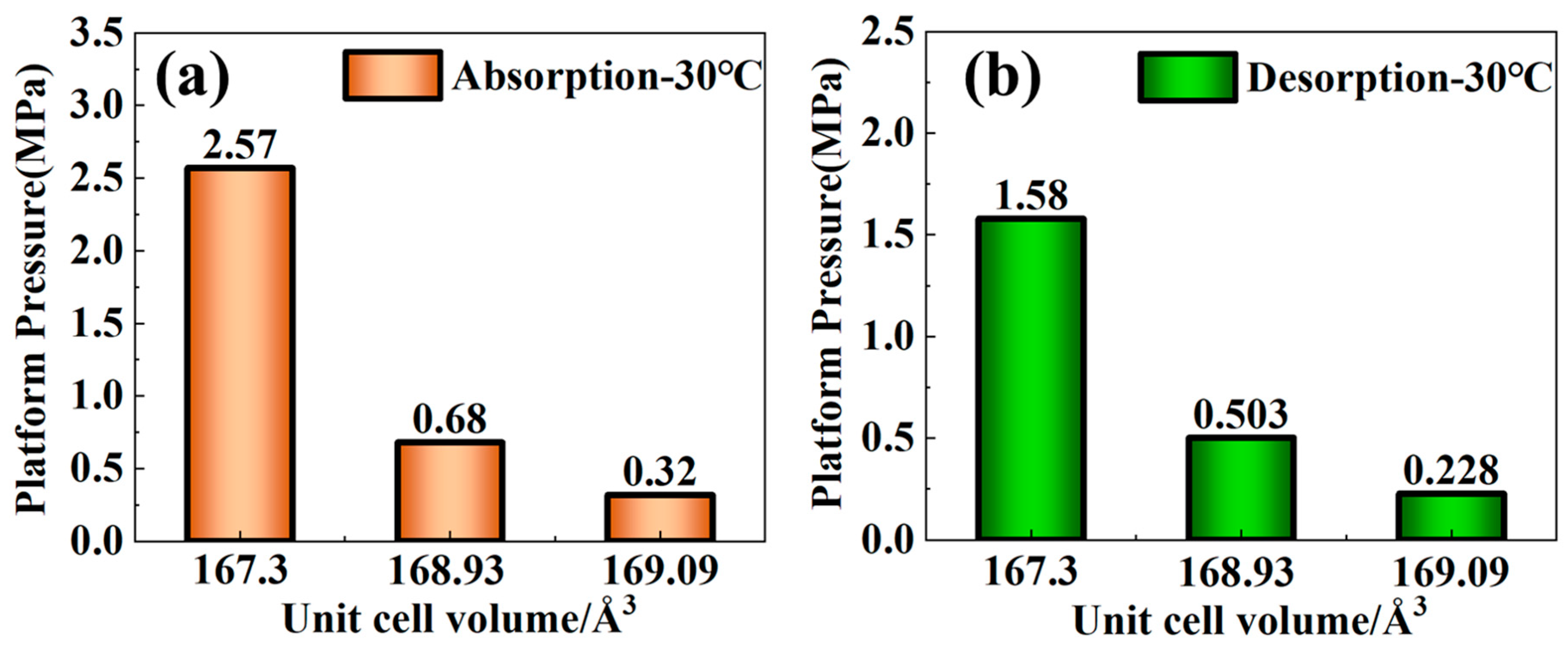
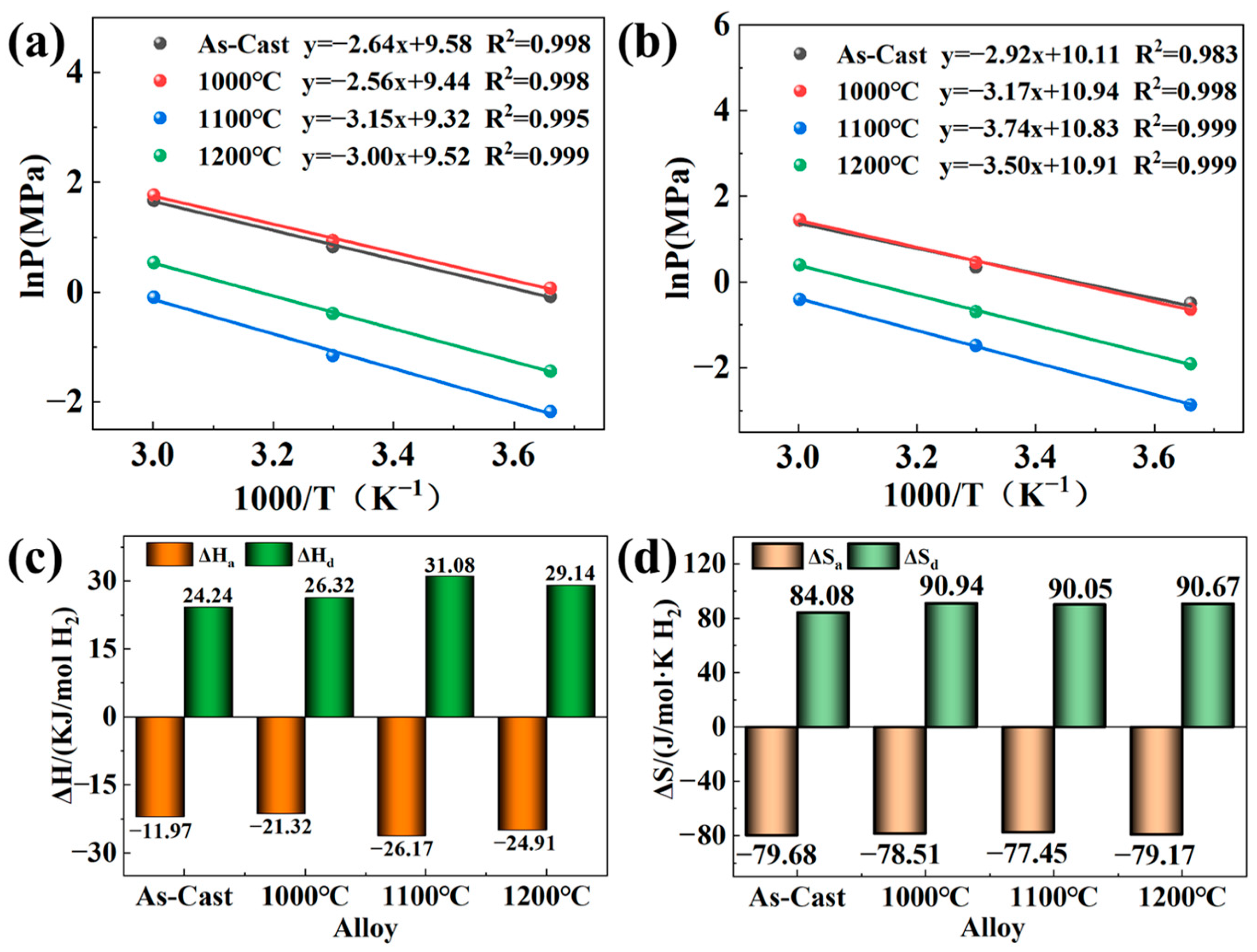
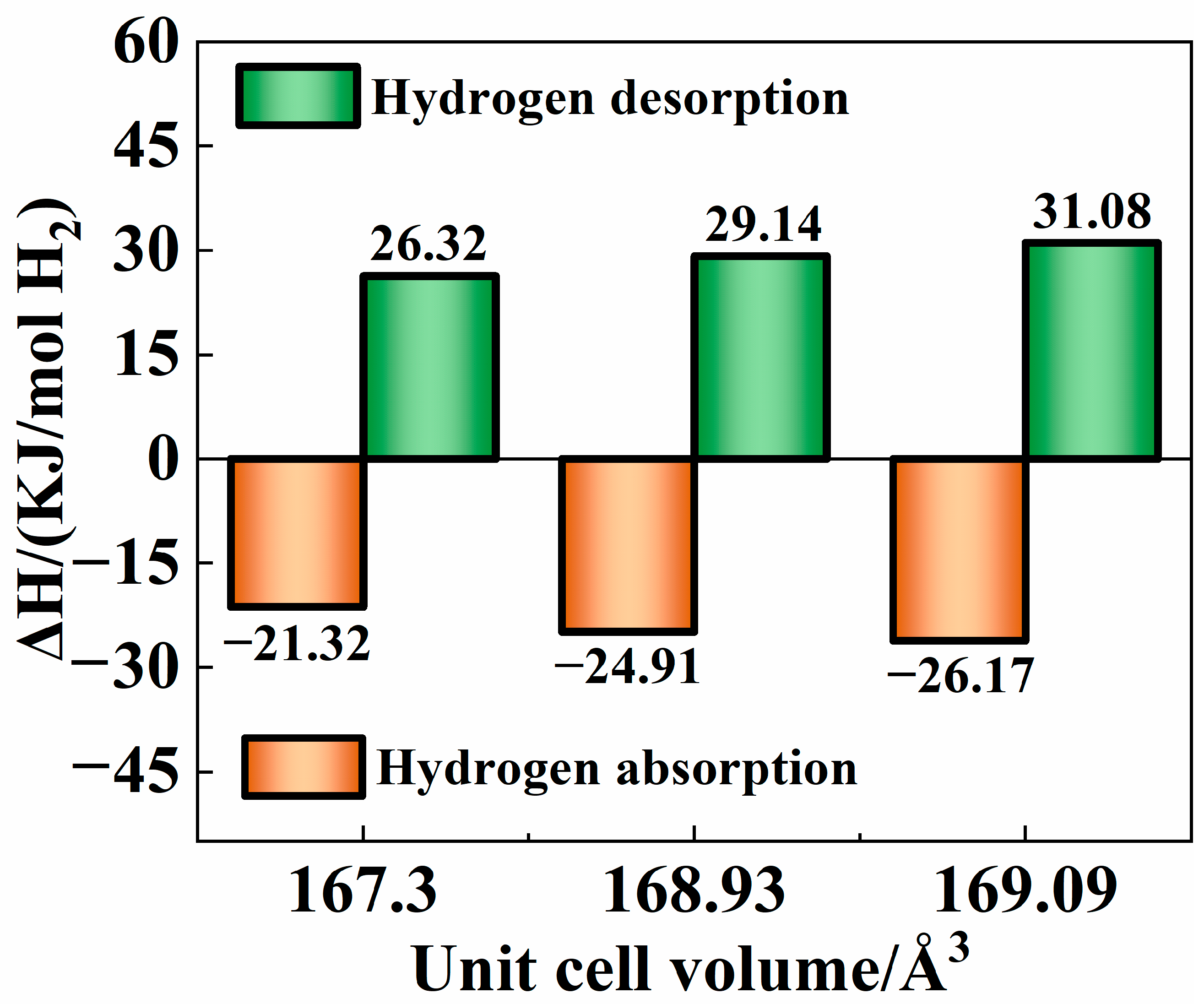
| Sample | Main Diffraction Peak Angle (2θ) | |||||
|---|---|---|---|---|---|---|
| As-Cast | 36.480 | 39.759 | 43.002 | 43.940 | 48.282 | 50.138 |
| 900 °C | 36.160 | 39.323 | 42.761 | 43.500 | 47.841 | 49.660 |
| 1000 °C | 36.258 | 39.479 | 42.779 | 43.620 | 48.079 | 49.841 |
| 1100 °C | 36.223 | 39.441 | 42.799 | 43.600 | 47.979 | 49.821 |
| 1200 °C | 36.397 | 39.620 | 42.920 | 43.721 | 48.291 | 50.020 |
| 1300 °C | 36.283 | 39.499 | 42.821 | 43.679 | 48.036 | 49.861 |
| Alloys | Phase | Lattice Parameter/Å (C14 Laves Phase) | Unit Cell Volume/Å3 (C14 Laves Phase) |
|---|---|---|---|
| As-Cast | C14 Laves (95.5%) + Cubic (4.5%) | a = 4.93259; c = 8.03742 | 169.36 |
| 900 °C | C14 Laves (81.3%) + Cubic (18.7%) | a = 4.95507; c = 7.97617 | 169.60 |
| 1000 °C | C14 Laves (96.3%) + Cubic (3.7%) | a = 4.92544; c = 7.96313 | 167.30 |
| 1100 °C | C14 Laves (96.9%) + Cubic (3.1%) | a = 4.95462; c = 7.95379 | 169.09 |
| 1200 °C | C14 Laves (98.9%) + Cubic (1.1%) | a = 4.93250; c = 8.01737 | 168.93 |
| 1300 °C | C14 Laves (83.7%) + Cubic (16.3%) | a = 4.94705; c = 7.94097 | 168.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.; Huang, J.; Fang, W.; He, L.; Duan, X.; Zou, G.; Li, X.; Ren, X. Effects of Annealing on Hydrogen Storage Performance in TiZrCrMnFeNi High-Entropy Alloy. Crystals 2025, 15, 297. https://doi.org/10.3390/cryst15040297
Cheng T, Huang J, Fang W, He L, Duan X, Zou G, Li X, Ren X. Effects of Annealing on Hydrogen Storage Performance in TiZrCrMnFeNi High-Entropy Alloy. Crystals. 2025; 15(4):297. https://doi.org/10.3390/cryst15040297
Chicago/Turabian StyleCheng, Tengfei, Jing Huang, Wanggang Fang, Liqing He, Xiangqun Duan, Guotong Zou, Xiao Li, and Xinghai Ren. 2025. "Effects of Annealing on Hydrogen Storage Performance in TiZrCrMnFeNi High-Entropy Alloy" Crystals 15, no. 4: 297. https://doi.org/10.3390/cryst15040297
APA StyleCheng, T., Huang, J., Fang, W., He, L., Duan, X., Zou, G., Li, X., & Ren, X. (2025). Effects of Annealing on Hydrogen Storage Performance in TiZrCrMnFeNi High-Entropy Alloy. Crystals, 15(4), 297. https://doi.org/10.3390/cryst15040297






