On the Importance of Squaramide and Squarate Derivatives as Metal–Organic Framework Building Blocks
Abstract
1. Introduction
1.1. MOFs: Concept and General Applications
1.2. The Role of the Organic Ligand in MOF Structure and Functionality
1.3. MOFs: Current Applications
1.4. MOFs: Challenges and Future Perspectives
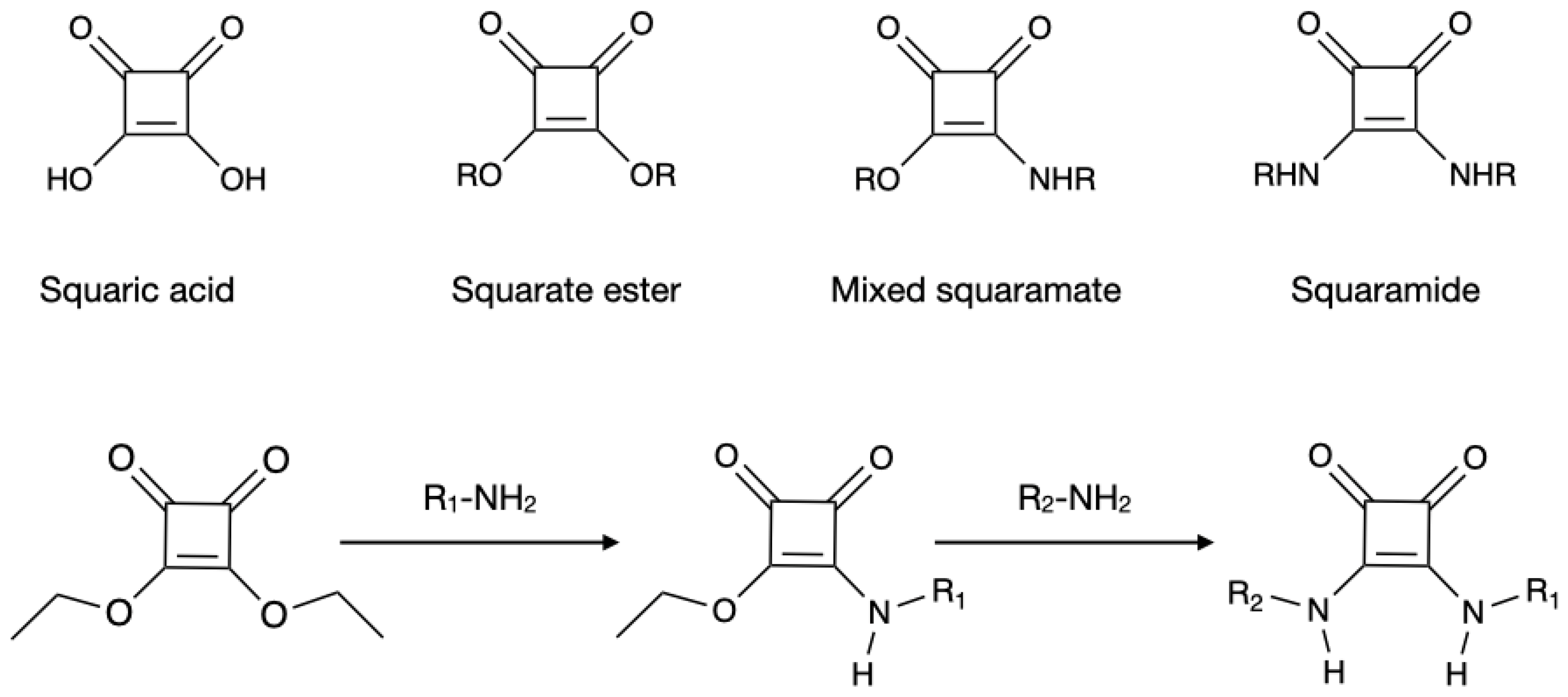
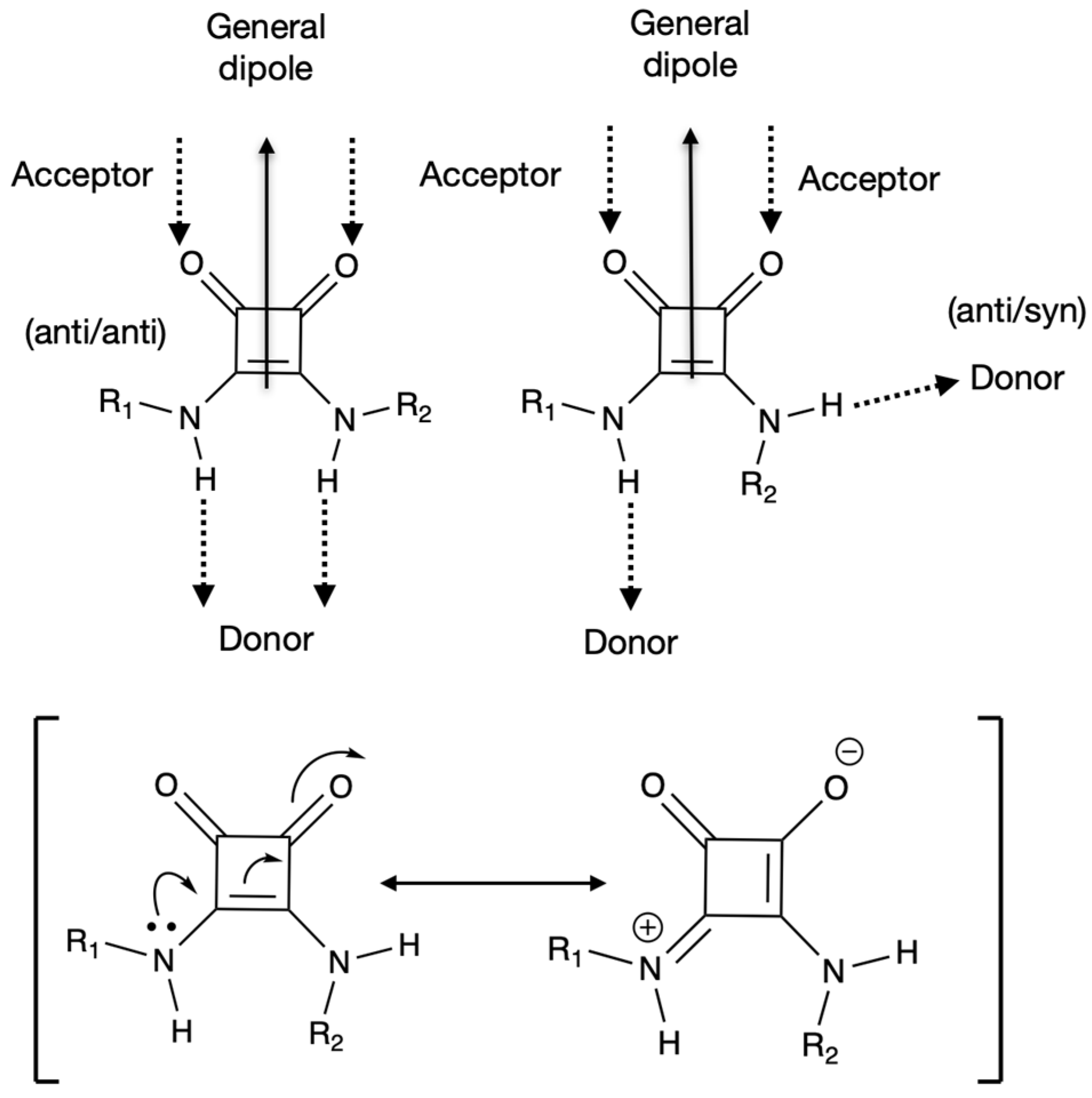
1.5. Squaric Acid and Squaramide as MOF Ligands: Main Features and Synthetic Pathways

2. Computational Details
3. Results and Discussion
3.1. Results from the Literature Review
| Organic ligand | Metal | Formula | Application |
|---|---|---|---|
| Amine fluoride | Zn, Co, Ni | {(NH(CH3)2)2[M4F4(SQU)3]}n (M = Zn, Co and Ni) | Separation of Acetylene from Ethylene [27] |
| Ca | [Ca(SQU)(H2O)] | Separation of Ethylene from Ethane [29] | |
| Ca | [Ca(SQU)(H2O)] | Separation of CO2 from fuel gas [81] | |
| Zr, Hf | MSQU (M = Zr and Hf) | Separation of Hydrogen from Nitrogen [78] | |
| Ca | [Ca(SQU)(H2O)] | Capture of trace propyne and propadiene from propylene [77] | |
| vim = 1-vinylimidazole | Co, Zn, Cd, Ni, Cu | [Co(SQU)(vim)2(H2O)2]n [Zn(SQU)(vim)2(H2O)2]n [Cd(SQU)(vim)2(H2O)2]n [M(sq)(vim)2(H2O)2]n (M = Ni and Cu) | Hydrogen storage [79,80] |
| Zn | SQUCALF-20 [Zn2(1,2,4-triazolate)2(SQU)] | Capture of CO2 [27] | |
| Co | [Co4(OH)4(SQU)3] | Separation CO2 of Nitrogen [26] | |
| Co | [Co4(OH)4(SQU)3] | Separation of Xenon from Krypton [25] | |
| Co | Co3C8H2O10·2.5H2O | Separation of ethanol from trace amount water [39] | |
| Co | [Co3(OH)2(SQU)2]·3H2O | Reversible ferromagnetic-antiferromagnetic [98] | |
| Ti | (Ti2O3)n USTC-700 [Ti2(μ2-O)(μ3-O)2(SQU)] | Separation of Deuterium (D2) from Hydrogen (H2) [76] | |
| bipy = 4,4′-bipyridin | Cd | {[Cd(SQU)(bipy)(H2O)2]·3H2O}n | Water Adsorption [24] |
| dpe = 1,2-bis(4-pyridyl)ethane) | Mn | [Mn(Hdpe)(SQU)0.5(H2O)3][Mn(SQU)2 (H2O)2] [Mn(Hdpe)2(H2O)4][Mn(SQU)2(H2O)2]2·8H2O | Water Adsorption and Magnetic applications [82] |
| Fe | [Fe3(OH)3(SQU)(SQU)0.5]n | CO2 and CH4 Adsorption [99] | |
| L1 = SQU-diisophthalic acid L2 = SQU-dibenzoic acid dpe = 1,2-di(pyridine-4-yl)ethane | Cd | Cd2(L1)(DMF)3 Cd(L2)(dpe) | Catalyst for the Michael Addition reaction [83] |
| Fe | [Fe3(OH)3(SQU)(SQU)0.5]n | Transformation of tetrazines to oxadiazole derivatives [85] | |
| zbr Topology | Ni, Co, Fe | Ni2Fe1 SQU-zbr | Electrocatalytic Oxygen Evolution Reaction [86,87] |
| Co | [Co3(SQU)2(OH)2]⋅3H2O | ||
| Ti | [Ti2O3(SQU)] | Photocatalyst Water Splitting Reaction [84] | |
| Tn = Tetrazole | Zn, Cd, Co | [M(TnSQU)(H2O)3]n (M= Zn, Cd and Co) | Photoluminescence and magnetic properties [100] |
| dpe = 1,2-bis(4-pyridyl)ethane | Zn | [Zn(dpe)(SQU)(H2O)2]n {[Zn(Hdpe)(SQU)0.5(H2O)3][Zn(SQU)2 (H2O)2]}n | Crystal engineering of 3D-dimensional networks [101,102,103,104,105] |
| Eu, Am, Cf, Sm, Dy, Ho, Er | M2(SQU)3(H2O)4 (M = Eu, Am and Cf) Sm(SQU)(C4O3OH)(H2O)2·0.5H2O [M4(SQU)5(H2O)12]Cl2·5H2O (M = Eu, Dy, Ho and Er) M2(SQU)2(SQU)(H2O)4 (M = Am and Cf) | ||
| Yb | [Yb5(OH)6(HCO2)3(CO3)2-(SQU)] 2.5 H2O | ||
| Co | [Co(SQU)4]⋅2H2O | ||
| btb = 1,4-bis(1,2,4-triazol-4-yl)butane | Cd | [Cd(C4O4)(btb)(H2O)2]n (btb = 1,4-bis(1,2,4-triazol-4-yl)butane) | |
| Nd | [(CH3)2NH2Nd(SQU)2] | Luminescense Sensing MnO4−, Cr2O72− [88] | |
| Co | [Co3(SQU)2O10]⋅3H2O | Electrochemical Sensing Dopamine [89] | |
| Nd | {(NH4)2[Nd2(H2O)10(SQU)3]SQU}n | Crystal engineering of 3D-dimensional networks [106,107,108,109,110,111,112,113] | |
| Cd | [Cd(2,2′-bpe)(SQU)(H2O)2] (2,2′-bpe = 1,2-bis(2-pyridyl)ethylene) | ||
| NNO = nicotinate N-oxide | Dy | [Dy(NNO)(SQU)(H2O)]n | |
| bpe = 1,2-bis(4-pyridyl)ethane. phen = 1,10-phenanthroline | Mn | {[Mn(H2O)2(bpe)(SQU)]·bpe·H2O}n [Mn2(H2O)4(phen)2(SQU2)]n [Mn2(H2O)2(phen)4(SQU)]·(SQU)·8(H2O) | |
| Cd | [Cd(SQU)(H2O)2]n | ||
| Co, Mn, Zn | Co(H2O)2(SQU) Mn(H2O)2(SQU) Zn(H2O)2(SQU) SODALITE | ||
| dpa = 2,2′-dipyridylamine | Co, Ni, Zn, Cu | [M(dpa)(SQU)H2O)] (M = Co, Ni, Zn) [Cu(dpa)(SQU)(H2O)]2·H2O | |
| dpa = 2,2′-dipyridylamine | Cd | [Cd2(SQU)2.5(H2O)4](dpaH)·1.5(H2O) [Cd(SQU)(dpa)(H2O)] (dpa= 2,2′-dipyridylamine) | |
| Sr, Ca | [Sr0.88Ca 0.12(SQU)(H2O)3] | Antioxidant and Anticancer Activities [90] | |
| bpy = 4,4′-bipyridine bpydo = 4,4′-bipyridine-N,N′-dioxide phen= 1,10-phenanthroline OA= Oxalic acid. vidpy = 4,4′-vinylenedipyridine | U | [(UO2)(OH)(SQU)](Hbpy) (UO2)(H2O)(SQU)(bpydo)·2H2O (UO2)(H2O)(SQU)(phen)·H2O [(UO2)(SQU)(OA)0.5](Hvidpy) | Structure Regulation and Redox Activity [114] |
| OA= Oxalic acid | Ho | [Ho2(AO)(SQU)2(H2O)8]·4(H2O) [Ho(SQU)1.5(H2O)3] | Photo-Induced Color-Changing [115] |
| dbda = 3,3′-((3,4-dioxocyclobut-1-ene-1,2-diyl)bis(azanediyl))dibenzoic acid | Zn | {Zn1.5(OH)(dbda)·5DMF}n squaramide | Catalyst for the Michael Addition reaction [116] |
| bdpc = 4,4′-biphenyldicarboxylate | Zr | Zr6O4(OH)4(Squar)2(bpdc)2 | Catalyst for the Friedel–Crafts reaction [91,92,93,94] |
| dbda = N,N’-bis(3,5-dicarboxyphenyl)squaramide tptc = p-terphenyl-3,3″,5,5″-tetracarboxylic acid | Cu | [Cu2(dbda)] [Cu2(dbda)x(tptc)1-x] | |
| Sq_tpdc = 4,4′-((3,4-dioxocyclobut-1-ene-1,2- diyl)bis(azanediyl))dibenzoic acid | Zn, Zr | Sq_tpdc (Sq_IRMOF-16) (Sq_UiO-68) | |
| Sq_tpdc = 4,4′-((3,4-dioxocyclobut-1-ene-1,2- diyl)bis(azanediyl))dibenzoic acid 4,4′-bipyridine | Zn | Sq_SNU-8X | |
| Sq_tpdc = 4,4′-((3,4-dioxocyclobut-1-ene-1,2- diyl)bis(azanediyl))dibenzoic acid 1,2-bis(4-pyridyl) ethane | Zr | Sq_BptMOF | |
| 3,4-dioxocyclobut-1-ene-1,2-diyl)bis(azanedyil)-p-dibenzoic acid | Zn | Sq_IRMOF-16 | Catalyst for epoxide ring-opening reaction [95] |
| bpy = 4,4′-bipyridine dbda = 3,3′-((3,4-dioxocyclobut-1-ene-1,2-diyl)bis(azanediyl))dibenzoic acid | Co | Co(bpy)(dbda)·H2O | Luminescense Sensing [96] |
| SQ1 = 3,3′-((3,4-dioxocyclobut-1-ene-1,2-diyl)bis(phenylbenzoic) acid | Zr | UiO-68-SQ1 | Biomimetic Catalysts [97] |
3.2. Selected Squarate-Based MOF Examples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CV | Cyclic voltammetry |
| DFT | Density functional theory |
| LSV | Linear sweep voltammetry |
| MOF | Metal–organic framework |
| NCIs | Noncovalent interactions |
| OER | Oxygen evolution reaction |
| PXRD | Powder X-ray diffraction |
| SBU | Secondary building unit |
| VOC | Volatile organic compound |
References
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar]
- Gao, J.; Qian, X.; Lin, R.B.; Krishna, R.; Wu, H.; Zhou, W.; Chen, B. Mixed Metal–Organic Framework with Multiple Binding Sites for Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2020, 59, 4396–4400. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Yang, J. Spatially Nanoconfined Architectures: A Promising Design for Selective Catalytic Reduction of NOx. ChemCatChem 2020, 12, 5599–5610. [Google Scholar] [CrossRef]
- Guo, J.; Qin, Y.; Zhu, Y.; Zhang, X.; Long, C.; Zhao, M.; Tang, Z. Metal–organic frameworks as catalytic selectivity regulators for organic transformations. Chem. Soc. Rev. 2021, 50, 5366–5396. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar]
- Zheng, Y.; Sun, F.-Z.; Han, X.; Xu, J.; Bu, X.-H. Recent Progress in 2D Metal-Organic Frameworks for Optical Applications. Adv. Opt. Mater. 2020, 8, 2000110. [Google Scholar]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M. Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 2015, 39, 607–620. [Google Scholar]
- Ma, X.; Chai, Y.; Wang, B. Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar]
- Hu, M.-L.; Safarifard, V.; Doustkhah, E.; Rostamnia, S.; Morsali, A.; Nouruzi, N.; Beheshti, S.; Akhbari, K. Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Microporus Mesoporus Mater. 2018, 256, 111–127. [Google Scholar]
- Qian, Z.; Zhang, R.; Xiao, Y.; Huang, H.; Sun, Y.; Chen, Y.; Ma, T.; Sun, X. Trace to the Source: Self-Tuning of MOF Photocatalysts. Adv. Energy Mater. 2023, 13, 2300086. [Google Scholar]
- Liu, J.; Zhu, D.; Guo, C.; Vasileff, A.; Qiao, S.-Z. Design Strategies toward Advanced MOF-Derived Electrocatalysts for Energy-Conversion Reactions. Adv. Energy Mater. 2017, 7, 1700518. [Google Scholar]
- Pobłocki, K.; Drzeżdżon, J.; Gawdzik, B.; Jacewicz, D. Latest trends in the large-scale production of MOFs in accordance with the principles of green chemistry. Green Chem. 2022, 24, 9402–9427. [Google Scholar]
- Sabzehmeidani, M.M.; Gafari, S.; Jamali, S.; Kazemzad, M. Concepts, fabrication and applications of MOF thin films in optoelectronics: A review. Appl. Mater. Today 2024, 38, 102153. [Google Scholar]
- Paz, F.A.A.; Klinowski, J.; Vilela, S.M.F.; Tomé, J.P.C.; Cavaleiro, J.A.S.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar]
- Chen, T.H.; Popov, I.; Kaveevivitchai, W.; Miljanić, O.Š. Metal-organic frameworks: Rise of the ligands. Chem. Mater. 2014, 26, 4322–4325. [Google Scholar]
- Lin, Z.J.; Lü, J.; Hong, M.; Cao, R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar]
- Zhang, L.P.; Yang, J.; Ma, J.F.; Jia, Z.F.; Xie, Y.P.; Wei, G.H. A series of 2D and 3D metal–organic frameworks based on different polycarboxylate anions and a flexible 2,2′-bis(1H-imidazolyl)ether ligand. CrystEngComm 2008, 10, 1410–1420. [Google Scholar]
- Wang, C.; Zhao, J.; Xia, L.; Wu, X.Q.; Wang, J.F.; Dong, W.W.; Wu, Y.P. Utilization of mixed ligands to construct diverse Ni(II)-coordination polymers based on terphenyl-2,2′,4,4′-tetracarboxylic acid and varied N-donor co-ligands. J. Solid State Chem. 2016, 238, 273–278. [Google Scholar]
- Ghassa, M.; Khorashe, F.; Hajjar, Z.; Soltanali, S. Comparative Study on Adsorptive Desulfurization of Thiophenic Compounds over Terephthalic Acid-Based and Trimesic Acid-Based Metal–Organic Frameworks. Energy Fuels 2023, 37, 6490–6502. [Google Scholar] [CrossRef]
- Zhao, Y.; Chai, Y.-H.; Ding, L.; Wang, S.; Wang, Y.-N.; Ma, L.-F.; Zhao, B.-T. A stable N-containing heterocyclic carboxylic acid ligand Co-MOF for photoelectric performance and anionic dyes adsorption. Arab. J. Chem. 2023, 16, 104878. [Google Scholar] [CrossRef]
- Pullen, S.; Clever, G.H. Mixed-Ligand Metal–Organic Frameworks and Heteroleptic Coordination Cages as Multifunctional Scaffolds—A Comparison. Acc. Chem. Res. 2018, 51, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Ke, S.-Y.; Chen, K.-T.; Hsieh, Y.-F.; Wang, T.-H.; Lee, G.-H.; Chuang, Y.-C. Reversible Single-Crystal-to-Single-Crystal Structural Transformation in a Mixed-Ligand 2D Layered Metal-Organic Framework: Structural Characterization and Sorption Study. Crystals 2017, 7, 364. [Google Scholar] [CrossRef]
- Li, L.; Guo, L.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q.; Li, J. A Robust Squarate-Based Metal-Organic Framework Demonstrates Record-High Affinity and Selectivity for Xenon over Krypton. J. Am. Chem. Soc. 2019, 141, 9358–9364. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.; Liu, Y.; You, J.; Lin, L.; Jia, J.; Chen, S.; Hua, N.; Ma, L.A.; Ye, X.; et al. A Robust Squarate-Cobalt Metal-Organic Framework for CO2/N2 Separation. ACS Appl. Mater. Interfaces 2023, 15, 30394–30401. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Fan, D.; Naskar, S.; Magnin, Y.; Maurin, G. Engineering of an Isoreticular Series of CALF-20 Metal–Organic Frameworks for CO2 Capture. ACS Appl. Eng. Mater. 2024, 2, 96–103. [Google Scholar] [CrossRef]
- Li, H.P.; Wang, J.W.; Zhai, Q.G. Development of MOF-5-like ultra-microporous metal-squarate frameworks for efficient acetylene storage and separation. J. Mater. Chem. A 2023, 11, 21203–21210. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, L.; Zhou, H.L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar]
- Arici, M.; Yesilel, O.Z.; Tas, M.; Demiral, H. Effect of Solvent Molecule in Pore for Flexible Porous Coordination Polymer upon Gas Adsorption and Iodine Encapsulation. Inorg. Chem. 2015, 54, 11283–11291. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Tian, H. Metal-Organic Framework (MOF)-Based Drug Delivery. Curr. Med. Chem. 2019, 27, 5949–5969. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232. [Google Scholar] [CrossRef]
- Zeng, L.W.; Hu, K.Q.; Mei, L.; Li, F.Z.; Huang, Z.W.; An, S.W.; Chai, Z.F.; Shi, W.Q. Structural Diversity of Bipyridinium-Based Uranyl Coordination Polymers: Synthesis, Characterization, and Ion-Exchange Application. Inorg. Chem. 2019, 58, 14075–14084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.M.; Li, H.H.; Wu, Y.; Zhang, S.Y.; Zhang, Z.J.; Shi, W.; Cheng, P.; Liao, D.Z.; Yan, S.P. Synthesis, Crystal Structures, and Magnetic Properties of Mn(II), Co(II), and Zn(II) Coordination Polymers Containing 1,2,4,5-Benzenetetracarboxylic Acid and 4,4’-Azobispyridine. Eur. J. Inorg. Chem. 2010, 2010, 1983–1990. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Zhou, Z.; Mukherjee, S.; Hou, S.; Li, W.; Elsner, M.; Fischer, R.A. Porphyrinic MOF Film for Multifaceted Electrochemical Sensing. Angew. Chem. Int. Ed. 2021, 60, 20551–20557. [Google Scholar] [CrossRef]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2015, 43, 5994–6010. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, F.; Hu, H.M.; Xu, B.; Xue, G.L. A luminescent coordination polymer with potential active site for the sensing of metal cation, anion and nitrobenzene explosive. Inorg. Chem. Commun. 2016, 71, 19–22. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Zhang, H.; Yao, Z.; Yang, Y.; Xiang, F.; Li, Y.; Xiang, S.; Zhang, Z.; Chen, B. Optimized Sieving Effect for Ethanol/Water Separation by Ultramicroporous MOFs. Angew. Chem. Int. Ed. 2023, 62, e202216710. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Zhou, Z.; Vázquez-González, M.; Willner, I. Stimuli-responsive metal-organic framework nanoparticles for controlled drug delivery and medical applications. Chem. Soc. Rev. 2021, 50, 4541–4563. [Google Scholar] [PubMed]
- Linnane, E.; Haddad, S.; Melle, F.; Mei, Z.; Fairen-Jimenez, D. The uptake of metal-organic frameworks: A journey into the cell. Chem. Soc. Rev. 2022, 51, 6065–6086. [Google Scholar] [PubMed]
- Horcajada, P.; Tamim, C.; Serre, C.; Gillet, B.; Sébrié, C.; Baati, T.; Eubank, J.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [PubMed]
- Rojas, S.; Arenas-Vivo, A.; Horcajada, P. Metal-organic frameworks: A novel platform for combined advanced therapies. Coord. Chem. Rev. 2019, 388, 202–226. [Google Scholar]
- Liu, J.; Huang, J.; Zhang, L.; Lei, J. Multifunctional metal–organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 1188–1218. [Google Scholar]
- Han, D.; Liu, X.; Wu, S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem. Soc. Rev. 2022, 51, 7138–7169. [Google Scholar]
- Huang, S.; Chen, G.; Ouyang, G. Confining enzymes in porous organic frameworks: From synthetic strategy and characterization to healthcare applications. Chem. Soc. Rev. 2022, 51, 6824–6863. [Google Scholar]
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The recent progress on metal–organic frameworks for phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125. [Google Scholar]
- Yao, H.; Cai, S.; Yang, B.; Han, L.; Wang, P.; Li, H.; Yan, T.; Shi, L.; Zhang, D. In situ decorated MOF-derived Mn–Fe oxides on Fe mesh as novel monolithic catalysts for NOx reduction. New J. Chem. 2020, 44, 2357–2366. [Google Scholar]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar]
- Suresh, K.; Kalenak, A.P.; Sotuyo, A.; Matzger, A.J. Metal-Organic Framework (MOF) Morphology Control by Design. Chem. Eur. J. 2022, 28, e202200334. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qian, S.; Wang, X.; Cui, X.; Chen, B.; Xing, H. Energy-efficient separation alternatives: Metal–organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in metal–organic framework-based membranes. Chem. Soc. Rev. 2022, 51, 8300–8350. [Google Scholar] [CrossRef]
- Zhang, X.; Maddock, J.; Nenoff, T.M.; Denecke, M.A.; Yang, S.; Schröder, M. Adsorption of iodine in metal–organic framework materials. Chem. Soc. Rev. 2022, 51, 3243–3262. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Adjal, C.; Guechtouli, N.; Timón, V.; Colmenero, F.; Hammoutène, D. Theoretical Study of Copper Squarate as a Promising Adsorbent for Small Gases Pollutants. Molecules 2024, 29, 3140. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Marshall, R.J.; Sastre, B.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Surface-Functionalization of Zr-Fumarate MOF for Selective Cytotoxicity and Immune System Compatibility in Nanoscale Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 31146–31157. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel Metal–Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th(IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S., Jr.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 16, 8267–8302. [Google Scholar] [CrossRef]
- Cohen, S.; Cohen, S.G. Preparation and Reactions of Derivatives of Squaric Acid. Alkoxy-, Hydroxy-, and Aminocyclobutenediones1. J. Am. Chem. Soc. 1966, 88, 1533–1536. [Google Scholar] [CrossRef]
- Ito, M.; West, R. New Aromatic Anions. IV. Vibrational Spectra and Force Constants for C4O4−2 and C5O5−2. J. Am. Chem. Soc. 1963, 85, 2580–2584. [Google Scholar] [CrossRef]
- Quiñonero, D.; Frontera, A.; Ballester, P.; Deyà, P.M. A theoretical study of aromaticity in squaramide and oxocarbons. Tetrahedron Lett. 2000, 41, 2001–2005. [Google Scholar] [CrossRef]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef]
- Frontera, A.; Orell, M.; Garau, C.; Quiñonero, D.; Molins, E.; Mata, I.; Morey, J. Preparation, solid-state characterization, and computational study of a crown ether attached to a squaramide. Org. Lett. 2005, 7, 1437–1440. [Google Scholar] [CrossRef]
- Rostami, A.; Colin, A.; Li, X.Y.; Chudzinski, M.G.; Lough, A.J.; Taylor, M.S. N,N′-Diarylsquaramides: General, high-yielding synthesis and applications in colorimetric anion sensing. J. Org. Chem. 2010, 75, 3983–3992. [Google Scholar] [CrossRef]
- López, C.; Vega, M.; Sanna, E.; Rotger, C.; Costa, A. Efficient microwave-assisted preparation of squaric acid monoamides in water. RSC Adv. 2013, 3, 7249–7253. [Google Scholar] [CrossRef]
- Larm, N.E.; Essner, J.B.; Pokpas, K.; Canon, J.A.; Jahed, N.; Iwuoha, E.I.; Baker, G.A. Room-Temperature Turkevich Method: Formation of Gold Nanoparticles at the Speed of Mixing Using Cyclic Oxocarbon Reducing Agents. J. Phy. Chem. C 2018, 122, 5105–5118. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571. [Google Scholar] [CrossRef]
- Balasubramani, S.G.; Chen, G.P.; Coriani, S.; Diedenhofen, M.; Frank, M.S.; Franzke, Y.J.; Furche, F.; Grotjahn, R.; Harding, M.E.; Hättig, C.; et al. TURBOMOLE: Modular program suite for ab initio quantum-chemical and condensed-matter simulations. J. Chem. Phys. 2020, 152, 184107. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Squaramide-Immobilized Carbon Nanoparticles for Rapid and High-Efficiency Elimination of Anthropogenic Mercury Ions from Aquatic Systems. ACS Appl. Mater. Interfaces 2022, 14, 35789–35801. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, J.; Zhang, L.; Liu, J.; Wahiduzzaman, M.; Yan, N.; Yu, L.; Dupuis, R.; Wang, H.; Maurin, G.; et al. A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation. Nat. Commun. 2023, 14, 4189. [Google Scholar] [CrossRef]
- Li, L.; Guo, L.; Zheng, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Calcium-Based Metal-Organic Framework for Simultaneous Capture of Trace Propyne and Propadiene from Propylene. ACS Appl. Mater. Interfaces 2020, 12, 17147–17154. [Google Scholar] [CrossRef]
- Bueken, B.; Reinsch, H.; Reimer, N.; Stassen, I.; Vermoortele, F.; Ameloot, R.; Stock, N.; Kirschhock, C.E.A.; De Vos, D. A zirconium squarate metal–organic framework with modulator-dependent molecular sieving properties. Chem. Commun. 2014, 50, 10055–10058. [Google Scholar] [CrossRef]
- Yilmaz, H.; Andac, O.; Gorduk, S. Synthesis, characterization, and hydrogen storage capacities of polymeric squaric acid complexes containing 1-vinylimidazole. Polyhedron 2017, 133, 16–23. [Google Scholar] [CrossRef]
- Yilmaz, H.; Gorduk, S.; Andac, O. Polymeric Ni(II) and Cu(II) complexes based on squaric acid and 1-vinylimidazole: Structural studies and hydrogen adsorption properties. Inorganica Chim. Acta 2018, 469, 154–163. [Google Scholar] [CrossRef]
- Tu, R.; Zhang, W.; Zhang, J.; Wang, M.; Zhang, F.; Yang, K.; Li, J.; Pan, H.; Bernards, M.T.; Xie, P.; et al. Squarate-Calcium Metal-Organic Framework for Molecular Sieving of CO2 from Flue Gas with High Water Vapor Resistance. Energy Fuels 2021, 35, 13900–13907. [Google Scholar] [CrossRef]
- Wang, C.C.; Tsai, J.H.; Ke, S.Y.; Lee, G.H.; Chuang, Y.C.; Yang, E.C. Structural Characterization, Water Adsorption, and Magnetic Properties of Two Composite Mn(II)-Squarate-dpe Supramolecular Architectures. Cryst. Growth Des. 2020, 20, 5395–5405. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, P.; Zheng, J.J.; Tian, X.; Jiang, M.; He, Y.; Dong, H.; Wang, J. A nanoporous metal–organic framework as a renewable size-selective hydrogen-bonding catalyst in water. Dalton Trans. 2019, 48, 11855–11861. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Abraira, P.; Babaryk, A.A.; Montero-Lanzuela, E.; Contreras-Almengor, O.R.; Cabrero-Antonino, M.; Grape, E.S.; Willhammar, T.; Navalón, S.; Elkäim, E.; García, H.; et al. A Novel Porous Ti-Squarate as Efficient Photocatalyst in the Overall Water Splitting Reaction under Simulated Sunlight Irradiation. Adv. Mater. 2021, 33, 2106627. [Google Scholar] [CrossRef]
- Goswami, S.; Jena, H.S.; Konar, S. Study of heterogeneous catalysis by iron-squarate based 3d metal organic framework for the transformation of tetrazines to oxadiazole derivatives. Inorg. Chem. 2014, 53, 7071–7073. [Google Scholar] [CrossRef]
- Kandambeth, S.; Kale, V.S.; Fan, D.; Bau, J.A.; Bhatt, P.M.; Zhou, S.; Shkurenko, A.; Rueping, M.; Maurin, G.; Shekhah, O.; et al. Unveiling Chemically Robust Bimetallic Squarate-Based Metal–Organic Frameworks for Electrocatalytic Oxygen Evolution Reaction. Adv. Energy Mater. 2023, 13, 2202964. [Google Scholar] [CrossRef]
- Zheng, X.; Jia, G.; Fan, G.; Luo, W.; Li, Z.; Zou, Z. Modulation of Disordered Coordination Degree Based on Surface Defective Metal–Organic Framework Derivatives toward Boosting Oxygen Evolution Electrocatalysis. Small 2020, 16, 2003630. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Xie, C.; Wang, R.; Sun, D. Rare-earth squarate frameworks with scu topology. Dalton Trans. 2022, 51, 18378–18382. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, X.; Jia, G.; Chi, H.; Lin, B.; Qin, H.; Xie, H.; Yuan, Y.; Fu, D. Squarate-Based Metal-Organic Frameworks for Highly Selective and Sensitive Electrochemical Sensing of Dopamine. J. Electrochem. Soc. 2022, 169, 116504. [Google Scholar] [CrossRef]
- Priya Vadhana, K.T.; Vairam, S.; Ushadevi, B.; Parveen, S. New Mg(II) and Ca(II) Mixed Strontium Squarates: Structural Characterization, DNA/BSA Interaction, Antioxidant and Anticancer Activities. J. Clust. Sci. 2021, 33, 867–885. [Google Scholar] [CrossRef]
- Malerich, P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416. [Google Scholar] [CrossRef] [PubMed]
- McGuirk, C.M.; Katz, M.J.; Stern, C.L.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Turning On Catalysis: Incorporation of a Hydrogen-Bond-Donating Squaramide into a Zr Metal-Organic Framework. J. Am. Chem. Soc. 2015, 137, 919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Boissonnault, J.; Cohen, S.M. Design and Synthesis of Squaramide-based MOFs as Efficient MOF-supported Hydrogen Organocatalysts. Chem. Comm. 2016, 52, 8585. [Google Scholar] [CrossRef]
- Broto-Ribas, A.; Vignatti, C.; Jimenez-Almarza, A.; Luis-Barrera, J.; Dolatkhah, Z.; Gándara, F.; Imaz, I.; Mas-Ballesté, R.; Alemán, J.; Maspoch, D. Heterogeneous Catalysts with Programmable Topologies Generated by Reticulation of Organocatalysts into Metal-Organic Frameworks: The Case of Squaramide. Nano Res. 2021, 14, 458. [Google Scholar] [CrossRef]
- Vignatti, C.; Luis-Barrera, J.; Guillerm, V.; Imaz, I.; Mas-Ballesté. Squaramide-IRMOF-16 Analogue for Catalysis of Solvent-Free, Epoxide Ring-Opening Tandem and Multicomponent Reactions. ChemCatChem 2018, 10, 3995. [Google Scholar] [CrossRef]
- Jiang, M.; Li, P.; Wu, P.; Zhang, F.; Tian, X.; Deng, C.; Wang, J. A Squaramide-Based Metal Organic Framework as a Luminescent Sensor for Detection of Lactose in Aqueous Solution and in Milk. Chem. Comm. 2018, 54, 9131. [Google Scholar] [CrossRef]
- Cohen, S.M.; Zhang, Z.; Boissonnault, J.A. Toward “MetalloMOFzymes”: Metal-Organic Frameworks with Single-Site Metal Catalysts for Small-Molecules Transformations. Inorg. Chem. 2016, 55, 7281. [Google Scholar] [CrossRef]
- Kurmoo, M.; Kumagai, H.; Chapman, K.W.; Kepert, C.J. Reversible ferromagnetic–antiferromagnetic transformation upon dehydration–hydration of the nanoporous coordination framework, [Co3(OH)2(C4O4)2]·3H2O. Chem. Commun. 2005, 24, 3012–3014. [Google Scholar] [CrossRef]
- Goswami, S.; Adhikary, A.; Jena, H.S.; Biswas, S.; Konar, S. A 3D iron(II)-based MOF with squashed cuboctahedral nanoscopic cages showing spin-canted long-range antiferromagnetic ordering. Inorg. Chem. 2013, 52, 12064–12069. [Google Scholar] [CrossRef]
- Seco, J.M.; Calahorro, A.J.; Sebastian, E.S.; Salinas-Castillo, A.; Colacio, E.; Rodríguez-Diéguez, A. Experimental and theoretical study of photoluminescence and magnetic properties of metal–organic polymers based on squarate and tetrazolate moieties containing linkers. New J. Chem. 2015, 39, 9926–9930. [Google Scholar] [CrossRef]
- Wang, C.C.; Chung, W.C.; Lin, H.W.; Dai, S.C.; Shiu, J.S.; Lee, G.H.; Sheu, H.S.; Lee, W. Assembly of two Zinc(II)-squarate coordination polymers with noncovalent and covalent bonds derived from flexible ligands, 1,2-bis(4-pyridyl)ethane (dpe). CrystEngComm 2011, 13, 2130–2136. [Google Scholar] [CrossRef]
- Brenner, N.; Sperling, J.M.; Poe, T.N.; Celis-Barros, C.; Brittain, K.; Villa, E.M.; Albrecht-Schmitt, T.E.; Polinski, M.J. Trivalent f-Element Squarates, Squarate-Oxalates, and Cationic Materials, and the Determination of the Nine-Coordinate Ionic Radius of Cf(III). Inorg. Chem. 2020, 59, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Lai, Y.C.; Wang, S.L. Intrinsic Green Phosphor Containing a {Y5O22} Pentamer Unit and a Carbonate Ligand Generated In Situ from Squaric Acid. Chem. Eur. J. 2012, 18, 8614–8616. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Wang, Y.; Ma, R.; Sun, J.; Deng, J.; Chen, J.; Sun, D.; Xing, X. Facile Synthesis of Dicelike Cobalt Squarate Cages through a Spontaneous Dissolution-Regrowth Process. Chem. Mater. 2020, 32, 6765–6771. [Google Scholar] [CrossRef]
- Ke, X.J.; Li, D.S.; Zhao, J.; Bai, L.; Yang, J.J.; Duan, Y.P. Unique entangling CdII-framework featuring 2D → 3D inclined polycatenate motif and (4,6)-connected self-catenated H-bonding topology. Inorg. Chem. Commun. 2012, 21, 129–132. [Google Scholar] [CrossRef]
- Bensaddek, A.; Akkari, H.; Kinzhybalo, V. A Novel Layered Neodymium Squarate MOF Intercalating Free Ammonium and Squarate Ions {(NH4)2[Nd2(H2O)10(C4O4)3]C4O4}n: Synthesis, Crystal Structure and Thermal Decomposition. J. Inorg. Organomet. Polym. 2019, 29, 302–307. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, Y.-F.; Ke, S.-Y.; Xiu, Y.; Lee, G.-H.; Chen, B.-H.; Chuang, Y.-C.; Wang, C.-C.; Wang, Y.-F.; Ke, S.-Y.; et al. Synthesis, structural characterization and thermal stability of a 2D layered Cd(II) coordination polymer constructed from squarate (C4O42−) and 2,2’-bis(2-pyridyl)ethylene (2,2’-bpe) ligands. AIMS Mater. Sci. 2018, 5, 145–155. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhang, D.; Hao, X.; Zhu, D.-B. Field-Induced Relaxation of Magnetization in a Three-Dimensional LnMOF with the Second Bridging Ligand Squarate. ACS Omega 2016, 1, 286–292. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Ghoshal, D.; Zangrando, E.; Ribas, J.; Ray Chaudhuri, N. Structural diversity in manganese squarate frameworks using N,N-donor chelating/bridging ligands: Syntheses, crystal structures and magnetic properties. Dalton Trans. 2006, 1554–1563. [Google Scholar] [CrossRef]
- Maji, T.K.; Mostafa, G.; Sain, S.; Prasad, J.S.; Chaudhuri, N.R. Construction of a 3D array of cadmium (II) using squarate as a building block. CrystEngComm 2001, 3, 155–158. [Google Scholar] [CrossRef]
- Neeraj, S.; Noy, M.L.; Rao, C.N.R.; Cheetham, A.K. Sodalite networks formed by metal squarates. Solid State Sci. 2002, 4, 1231–1236. [Google Scholar]
- Wang, C.C.; Yang, C.H.; Lee, G.H.; Tsai, H.L. Syntheses, Structures, and Magnetic Properties of Two 1D, Mixed-Ligand, Metal Coordination Polymers, [M(C4O4)(dpa)(OH2)] (M = CoII, NiII, and ZnII; dpa = 2,2’-dipyridylamine) and [Cu(C4O4)(dpa)(H2O)]2·(H2O). Eur. J. Inorg. Chem. 2005, 2005, 1334–1342. [Google Scholar]
- Wang, C.C.; Yang, C.H.; Lee, G.H. Hydrothermal Synthesis and Structural Characterization of Two pH-Controlled Cd–Squarate Coordination Frameworks, [Cd2(C4O4)2.5(H2O)4]·(dpaH)·1.5(H2O) and [Cd(C4O4)(dpa)(OH2)] (dpa = 2,2′-dipyridylamine). Eur. J. Inorg. Chem. 2006, 2006, 820–826. [Google Scholar]
- Meng, L.; Liang, Y.Y.; Mei, L.; Geng, J.S.; Hu, K.Q.; Yu, J.P.; Wang, X.P.; Fujita, T.; Chai, Z.F.; Shi, W.Q. Mixed-Ligand Uranyl Squarate Coordination Polymers: Structure Regulation and Redox Activity. Inorg. Chem. 2022, 61, 302–316. [Google Scholar]
- Wang, C.-C.; Ke, S.-Y.; Feng, Y.; Ho, M.-L.; Chang, C.-K.; Chuang, Y.-C.; Lee, G.-H. Synthesis, Structural Characterization and Ligand-Enhanced Photo-Induced Color-Changing Behavior of Two Hydrogen-Bonded Ho(III)-Squarate Supramolecular Compounds. Polymers 2019, 11, 1369. [Google Scholar] [CrossRef]
- Zhan, D.; Xu, N.; Du, S.; Ju, Z.; Yuan, D. Structures and Catalytic Properties of two New Squaramide-decorated Cd-MOFs. Z. Anorg. Allg. Chem. 2022, 648, e202200064. [Google Scholar]
- Lenz, A.; Ojamäe, L. A theoretical study of water clusters: The relation between hydrogen-bond topology and interaction energy from quantum-chemical computations for clusters with up to 22 molecules. Phys. Chem. Chem. Phys. 2005, 7, 1905. [Google Scholar]
- Matar, S.; Hatch, L.F. Chemistry of Petrochemical Processes, 2nd ed.; Elsevier Science & Technology: Centro Rio de Janeiro, Brazil, 2001. [Google Scholar]
- Friedrich, M.F.; Lucas, M.; Claus, P. Selective Hydrogenation of Propyne on a Solid Pd/Al2O3 Catalyst Modified with Ionic Liquid Layer (SCILL). Catal. Commun. 2017, 88, 73–76. [Google Scholar] [CrossRef]
- Prohens, R.; Portell, A.; Font-Bardia, M.; Bauzá, A.; Frontera, A. A combined crystallographic and theoretical study of weak intermolecular interactions in crystalline squaric acid esters and amides. CrystEngComm 2017, 19, 3071. [Google Scholar]
- Ran, J.; Wong, M.W. Saturated Hydrocarbon−Benzene Complexes: Theoretical Study of Cooperative CH/π Interactions. J. Phys. Chem. A 2006, 110, 9702. [Google Scholar]
- Chen, Y.; Lu, W.; Schröder, M.; Yang, S. Analysis and Refinement of Host–Guest Interactions in Metal–Organic Frameworks. Acc. Chem. Res. 2023, 56, 2569. [Google Scholar] [CrossRef]




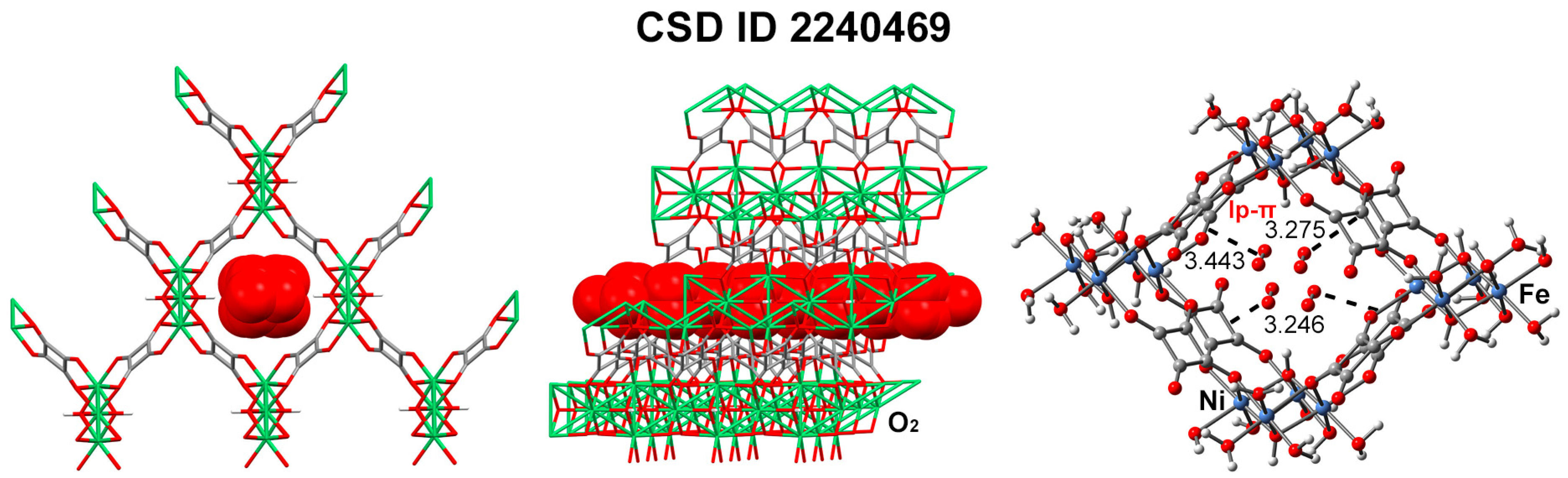
| MOF Formula | NCIs | EMOF | EFrag | Multiplicity a | ΔEBSSE |
|---|---|---|---|---|---|
| [Ti2O3(SQU)] CSD ID: 2104929 | O···H hydrogen bond | −11,974.556 | −11,898.280/ –76.276 | 1 | −0.9 |
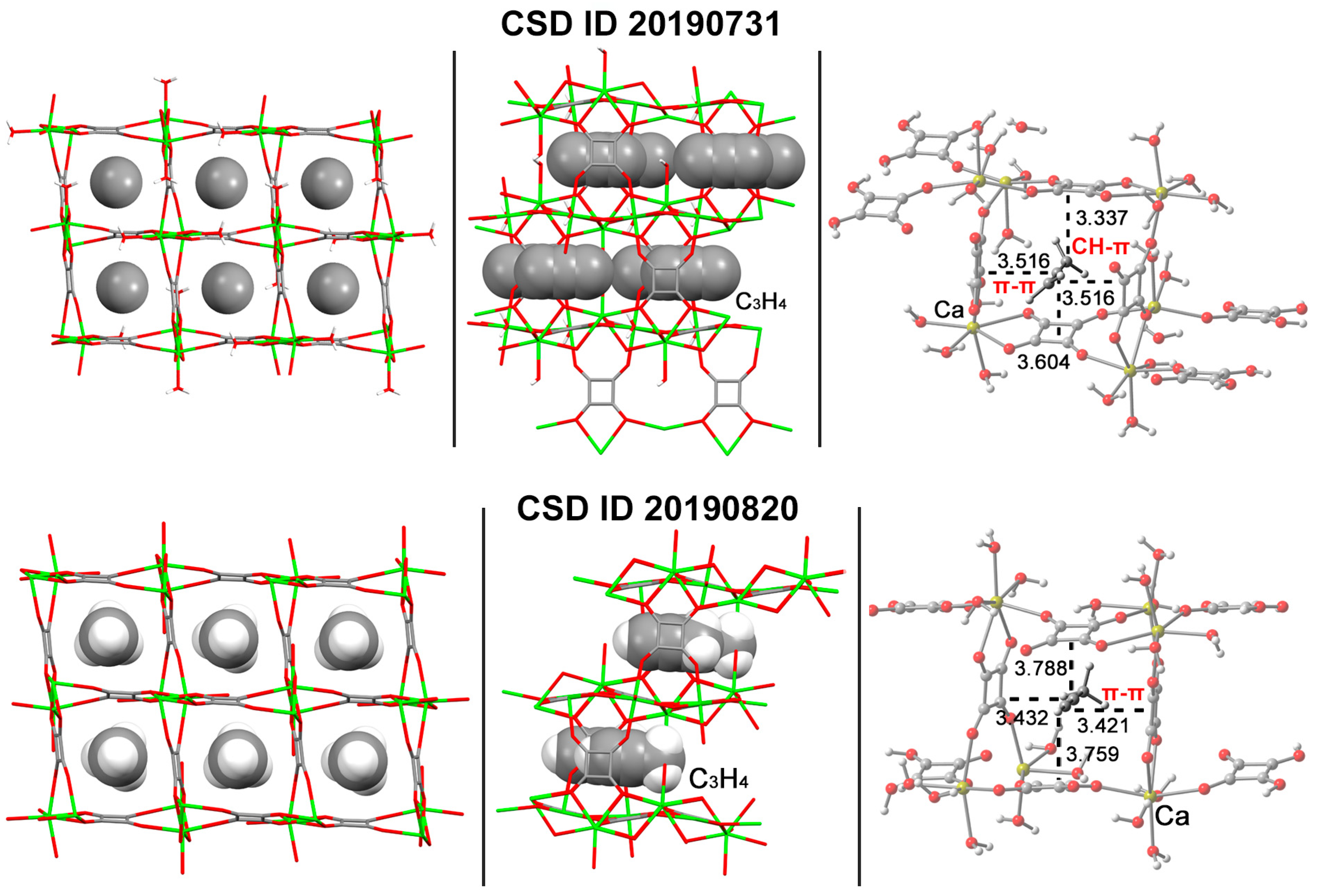
| [Ca(SQU)(H2O)] CSD ID: 20190731 | CH–π and π–π stacking interactions | −9329.507 | −9213.071/ –116.415 | 1 | −12.9 |
| [Ca(SQU)(H2O)] CSD ID: 20190820 | CH–π and π–π stacking interactions | −9177.019 | −9060.578/ −116.422 | 1 | −11.9 |
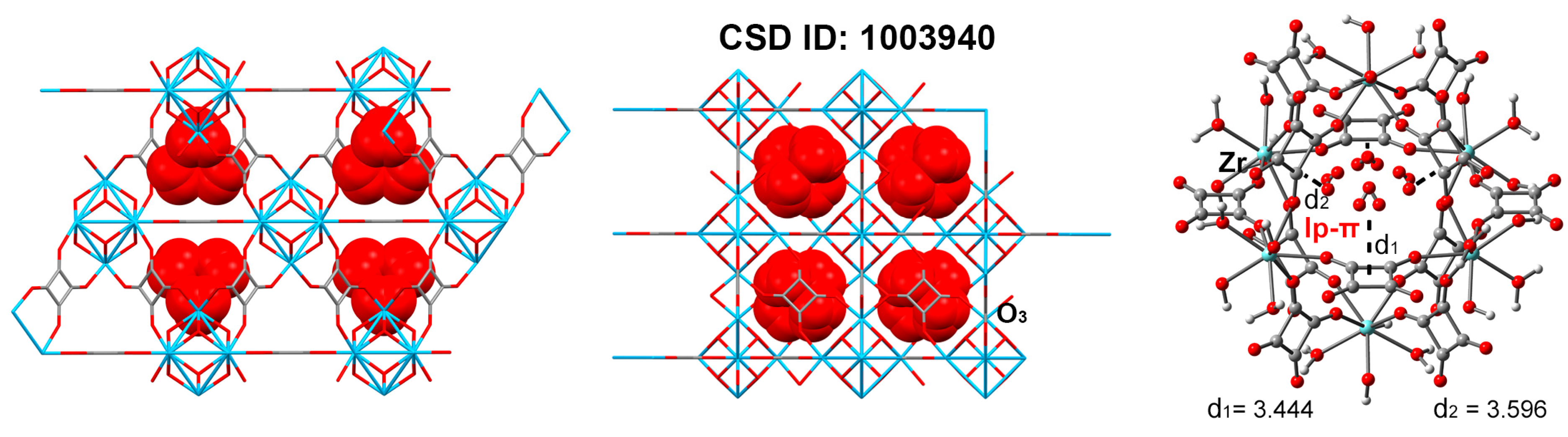
| ZrSQU CSD ID: 1003940 | O···C lone pair–π interactions | −8427.827 | −7544.976/ −882.642 | 1 | −32.9 |
| Ni2Fe1 SQU-zbr CSD ID: 2240469 | O···C lone pair–π interactions | −25,924.487 | −25,325.035/ −598.955 | Ni(3)/Fe(1) | −77.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolau, C.; Piña, M.d.l.N.; Morey, J.; Bauzá, A. On the Importance of Squaramide and Squarate Derivatives as Metal–Organic Framework Building Blocks. Crystals 2025, 15, 294. https://doi.org/10.3390/cryst15040294
Nicolau C, Piña MdlN, Morey J, Bauzá A. On the Importance of Squaramide and Squarate Derivatives as Metal–Organic Framework Building Blocks. Crystals. 2025; 15(4):294. https://doi.org/10.3390/cryst15040294
Chicago/Turabian StyleNicolau, Catalina, María de las Nieves Piña, Jeroni Morey, and Antonio Bauzá. 2025. "On the Importance of Squaramide and Squarate Derivatives as Metal–Organic Framework Building Blocks" Crystals 15, no. 4: 294. https://doi.org/10.3390/cryst15040294
APA StyleNicolau, C., Piña, M. d. l. N., Morey, J., & Bauzá, A. (2025). On the Importance of Squaramide and Squarate Derivatives as Metal–Organic Framework Building Blocks. Crystals, 15(4), 294. https://doi.org/10.3390/cryst15040294







