Abstract
The thermochemical interactions of Sc2O3 ceramics with CMAS at 1250 °C and 1300 °C were investigated in this paper. A continuously dense reaction layer (DRL) forms on the surface of the ceramic at the beginning of the reaction within 15 min, and temperature significantly affects the components of the DRL. The DRL is mainly composed of a diopside phase at 1250 °C, whose thickness decreases with reaction time, while it is composed of garnet and minor diopside phases at 1300 °C, and thickens in accordance with the parabolic law with exposure time. The DRL shows good effect on alleviating Mg2+ infiltration and some mitigating effect to Al3+, and relatively inferior resistance to Ca2+ and Si4+ penetration. The concentration of Sc3+ in the residual CMAS increases with reaction temperature and time, and the average contents are about 0.7 at% and 3.7 at% after reactions at 1250 °C and 1300 °C, respectively. The mechanism is discussed systematically.
1. Introduction
Thermal barrier coatings (TBCs) are widely applied to gas turbine engines, resulting from the advantages of effectively insulating and protecting hot components during service [1,2]. The 7–8 wt.% yttria-stabilized zirconia (YSZ) is one of the current commercial TBC materials for excellent mechanical properties [3,4]. However, it is difficult for YSZ to meet the operating temperature requirement with the development of the advanced gas turbine engine. Particularly, the CMAS glass formed by environmental deposits such as sand and dust in the air will melt, penetrate and damage the YSZ coating through dissolution-precipitation within a very short period, leading to phase transition, stress cracking and eventual failure of the TBC [5,6,7]. This significantly damages the service performance and safety of the TBC. It is urgent to propose a new strategy or develop new TBCs to be used against molten CMAS attack.
Extensive research has been conducted to reduce CMAS infiltration and corrosion, including modification of the compositions and microstructures of the coatings [8,9], the application of overlayered protective coatings [10,11] and the development of novel ceramic coating materials [12,13]. It is reported that co-doping Sc2O3–Y2O3 or co-doping Yb2O3–Y2O3 in ZrO2 exhibit superior phase stability and can significantly reduce CMAS corrosion and infiltration compared to YSZ [14,15]. The former is attributed to the low solubility of Sc3+ as well as its weak affinity with Ca2+, while the latter is a result of the low diffusion rate of Yb3+ in CMAS. The zirconates with a high concentration of RE2O3 (RE means the rare earth elements), such as La2Zr2O7 [16,17], Gd2Zr2O7 [18,19] and Y2Zr2O7 [20], also show exceptional CMAS infiltration owing to forming a dense reaction layer (DRL) composed of an RE-apatite phase (Ca4RE6(SiO4)6O or Ca2RE8(SiO6)6O2) after the reaction of RE2Zr2O7 and CMAS. The modification in microstructures of the coatings also show meaningful academic reference for the development of TBCs.
Overlayered protective coatings, such as Al2O3 [21,22], Y2O3 [23] and Pt [24,25], are also feasible methods to mitigate the attack of CMAS at high temperature. The overlayer can rapidly react with CMAS to form a continuous DRL composed of anorthite or an RE-apatite phase, which can greatly inhibit the CMAS infiltration and corrosion for weak wettability to the melt. Other materials such as RE-silicates [26,27], Ba2REAlO5 [28,29], BaLn2Ti3O10 [30], LaMgAl11O19 [31,32], LnPO4 (Ln refers to the rare earth lanthanides) [33], La2Ce2O7 [34], YAlO3 [35] and RE high-entropy ceramics [36,37] also can reduce the high-temperature penetration and corrosion of CMAS by forming an RE-apatite or anorthite DRL. In addition, the reaction of the coatings with CMAS leads to composition change and crystallization of the melts, which further inhibits CMAS infiltration [21,23]. The RE-apatite or anorthite DRL shows effective resistance to the molten CMAS attack, which is regarded as a promising method to protect against the penetration and corrosion of CMAS.
According to the present reports, zirconate doped with rich RE2O3 remains a valuable TBC material for its superior mechanical property and excellent resistance to infiltration and corrosion of CMAS [13,20]. Particularly, the addition of Sc2O3 can obviously enhance the phase stability and CMAS corrosion resistance of ZrO2-based materials. Wang [38,39] reported that the co-doping 2 mol.% Sc2O3–16 mol.% CeO2 in ZrO2 shows better tetragonal phase (t-phase) stability and resistance to infiltration and corrosion of CMAS compared to YSZ, resulting from the low solubility of Sc3+ and chemical inertness of Ce2+ in CMAS. Fan [40] and Liao [41] revealed that co-doping Sc2O3–Y2O3 in ZrO2 exhibits good CMAS infiltration and corrosion resistance owing to the low solubility of Sc3+, the difference in chemical potential and the poor affinity of Sc3+ with Ca2+. Sc2O3-based multi-R2O3 doping ZrO2 still is an effective design for the new TBC ceramics for excellent comprehensive performance.
The addition of Sc2O3 is a feasible method for improving the resistance to melt corrosion of ZrO2-based TBCs due to the special properties of Sc3+. However, research on the resistance of Sc2O3 to CMAS corrosion is relatively scarce, and its reaction products with CMAS, its effect against CMAS corrosion as well as its mechanism of corrosion resistance still need to be further investigated and improved. Therefore, it is of great significance to explore the thermochemical reaction of Sc2O3 with CMAS for the formation kinetics of the DRL and its inhibition on the infiltration of the melt. Aiming to reveal the thermochemical interactions of the Sc2O3 ceramic with the melt, the reaction products and penetration behavior of the CMAS at 1250 °C and 1300 °C are systematically investigated in this work. The growing of the DRL and its effect on inhibiting the melt infiltration also are analyzed. The interaction mechanism is discussed in detail.
2. Experimental Design
2.1. Ceramic Samples and CMAS Preparation
The Sc2O3 powders (99.9%, Aladdin reagents Co., Ltd., Shanghai, China) were cold-pressed at 250 MPa for 10 min to prepare a disc with dimensions of around φ15 mm × 2 mm. Then, the Sc2O3 discs were sintered in a furnace at 1600 °C for 5 h to obtain a ceramic pellet. The chemical composition of glass CMAS in this work was 33CaO-9MgO-13AlO1.5-45SiO2 [42] (mol.%). The CaO, Si2, Al2O3 and MgO powders (Aladdin Reagents Ltd.) were slurry-mixed with appropriate molar ratios thoroughly in full ethyl alcohol, through ball-milling for 6 h at 300 rph, followed by drying for 6 h, then annealed at 1300 °C for 4 h to form a transparent glass block. After crushing, grinding and 200 mesh sieve filtering, glass CMAS powder was obtained.
The Sc2O3 ceramics were uniformly coated by CMAS, and the content was 20 mg/cm2. Then, the samples were heat-treated at various temperatures (1250 °C and 1300 °C) for 15 min, 4 h, 8 h and 24 h, respectively. To further understand the thermochemical behaviors and the reaction products between the Sc2O3 and CMAS, the mixture of the CMAS–Sc2O3 powders (1:1 wt.%) was heat-treated at 1250 °C and 1300 °C for different times (15 min and 4 h). Here, a furnace (KSL-1700X, MTI, Richmond, CA, USA) was used for the thermal reaction tests.
2.2. Characterizations
An X-ray diffractometer (XRD, Rigaku D/MAX2500V, Tokyo, Japan, Cu Kαradiation) with a scan rate of 6°/min was used to identify the phase constitution of the sample after the high-temperature reaction. The cross-sectional morphology of Sc2O3 ceramics sheets after reaction with CMAS was examined by a PhenomProx type integrated scanning electron microscope (FESEM, SU8020, HITACHI, Tokyo, Japan), and energy dispersive spectroscopy (EDS, OXFORD X-MAX 80, Birmingham, UK) was used to observe the elements’ distribution. Simultaneously, the reaction behavior of Sc2O3 with CMAS powder (1:1, wt.%) was carried out in air by differential scanning calorimetry (DSC, STA 449F3, NETZSCH, Selb, Germany) from room temperature to 1450 °C at a rate of 10 °C/min to further understand the thermochemical behaviors between Sc2O3 and CMAS.
3. Results and Discussion
3.1. Phase Analysis
The phase constituents of each set of the mixed powders of Sc2O3–CMAS (1:1 wt.%) under high temperatures for different times are shown in Figure 1. According to Figure 1a, the diopside (CaMgSi2O6, PDF#086-0932) and garnet (Ca3Sc2Si3O12, PDF#04-008-4631) phases and minor Sc2Si2O7 (PDF#031-1225) phase are observed in the Sc2O3–CMAS mixed powder after reaction for 15 min, and Sc2O3 (PDF#023-1402) is still detected after reaction with CMAS at elevated temperature. The phases of the mixture after exposure for 4 h is similar to that after reaction for 15 min (Figure 1b), showing that the reaction of the Sc2O3–CMAS mixed powder is not drastic [43]. Note that the amount of the diopside phase is more than that of the garnet according to the higher diffraction peaks of diopside around 25–35°. It is indicated that the reaction of the mixed powder mainly causes CMAS crystallization when below 1300 °C. Furthermore, Sc-apatite could not be detected in Figure 1, resulting from the low reactivity of Sc2O3 in the CMAS and the poor affinity between the Ca2+ and Sc3+ [41,44]. The whole reactions are summarized as:
Sc2O3 + CMAS → Ca3Sc2Si3O12 + CaMgSi2O6 + Sc2Si2O7.
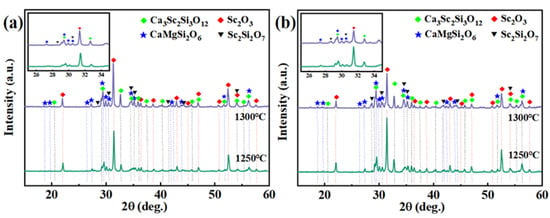
Figure 1.
XRD patterns of mixed powders with Sc2O3–CMAS (1:1 wt.%) after reaction at 1250 °C and 1300 °C for (a) 15 min and (b) 4 h.
Figure 2 shows the reaction products of the reactions of the Sc2O3 ceramics with CMAS for different times at 1250 °C and 1300 °C. The samples are of single Sc2O3 phase before reaction (Figure 2a). According to Figure 2b, the products are the main diopside, minor garnet and anorthite (CaAl2Si2O8, PDF#073-0264) phases on the surfaces of the ceramics after interactions at 1250 °C, while they are the diopside and garnet at 1300 °C (Figure 2c). Moreover, the reaction products of the ceramics with the melt are different to those of mixed powder, which is attributed to the different thermochemical reaction. For the Sc2O3 ceramics, the anorthite phase is formed owing to the composition change to the melts [44]. Additionally, it has been reported that Sc2Si2O7 will react with CMAS to form Ca3Sc2Si3O12 [45]. The formation of the DRL and the reduction in the grain boundary owing to the grain coarsening limit the diffusion of Sc3+, leading to a decrease in the diffusion flux of Sc3+. Combined with the low solubility of the Sc3+ in CMAS [40,41], the small amount of Sc2Si2O7 formed in CMAS subsequently disappeared by continuing to react with CMAS to form garnet. It can be seen that the amount of diopside phase decreases, when the garnet phase increases with higher temperature. This is related to the gradual dissolution of the diopside phase [46]. Furthermore, Sc2O3 also exists after reaction with CMAS. It is a result of the low reactivity of Sc3+ in CMAS [40]. The reactions are summarized as:
Sc2O3 + CMAS → Ca3Sc2Si3O12 + CaMgSi2O6 + Sc2Si2O7 + CaAl2Si2O8.
Sc2Si2O7 + CMAS → Ca3Sc2Si3O12.
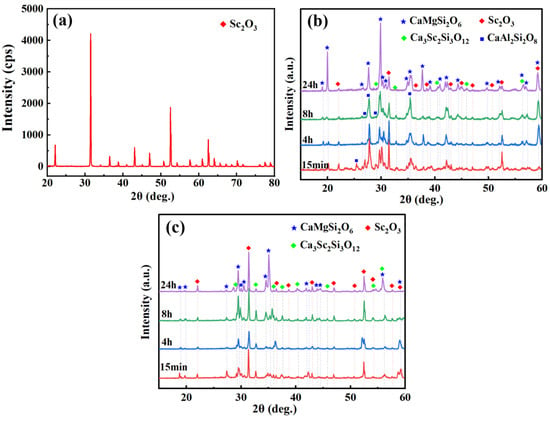
Figure 2.
XRD patterns of the Sc2O3 ceramics after reaction with the melt for different times. (a) Sc2O3 ceramic, (b) 1250 °C and (c) 1300 °C.
3.2. Interactions of CMAS/Sc2O3
The SEM graphs of the Sc2O3 ceramics after reaction with the melt at 1250 °C are presented in Figure 3 (the symbol “+” is used to highlight the zones, and the number is the name for the zones). It is clear that a distinct continuous DRL forms at the boundary of the Sc2O3 ceramic and CMAS. The DRL is mainly composed of the diopside phase (corresponding to regions #4, 8, 12 and 15 in Figure 3 and Table 1). The rod-like or block-like crystallization phases in CMAS mainly are diopside and a small amount of anorthite, which vertically grow into the CMAS. The diopside phase shows coarsening during the initial reaction stage, while it shows a short/small lumpy phase after long exposure. The diopside phase undergoes dissolution during reaction [46]. It is worth noting that no anorthite phase can be detected after reaction for 24 h, while it can be observed within 8 h (Figure 3a–c), indicating that the diopside and anorthite show poor thermostability in the molten CMAS.
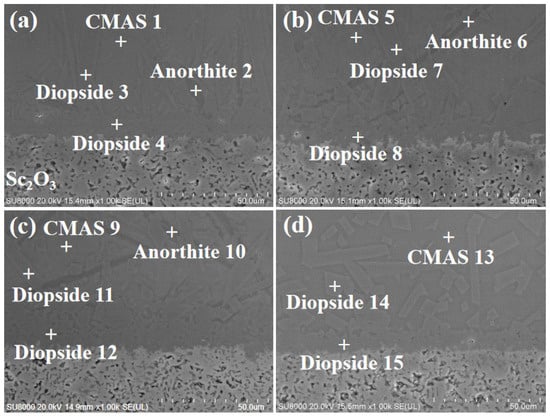
Figure 3.
SEM graphs of the Sc2O3 ceramics after reaction with the melt at 1250 °C for (a) 15 min, (b) 4 h, (c) 8 h and (d) 24 h.

Table 1.
Chemical compositions of the marked areas in Figure 3 (at%).
The reaction of the Sc2O3 ceramic with CMAS results in the crystallization of residual CMAS, resulting from the change in chemical composition [44]. According to Table 1, it can be seen that Ca2+ is one of the key ions from the CMAS. The concentration of Ca2+ in CMAS in region #1 (Figure 3a) is about 33.4 at% after reaction at 1250 °C for 15 min (Table 1), while it is 32.2, 32.0 and 25.7 (at%) for residual CMAS after reaction for 4 h, 8 h and 24 h (regions #5, 9 and 13 in Table 1), respectively. It decreases with reaction time, attributing to the formation of diopside and garnet phases. Furthermore, the content of Sc3+ in the remaining melt increases with time, resulting from the dissolution of the Sc2O3 ceramic when exposed to the molten CMAS, and the average content of Sc3+ is about 0.7 at% (except for that after 15 min). The contents of Si4+, Mg2+ and Al3+ also vary with exposure time. The change in composition leads to the crystallization of CMAS, which increases the melt viscosity and mitigates the infiltration [23].
Figure 4 shows the elemental mappings of the Sc2O3 ceramics after reaction with the melt at 1250 °C (the dotted lines refer to the thickness of the reaction layer). The DRL exhibits an excellent effect on mitigating Mg2+ infiltration, and some blocking effect on Al3+ penetration within 8 h. However, the resistance to Ca2+ and Si4+ infiltration is relatively poor compared to Mg2+ and Al3+. According to the Ca mapping, the infiltration depth of Ca2+ increases with reaction time, resulting in forming a Ca-rich zone beneath the DRL. The Ca2+ infiltration depths are 13.1 μm, 15.6 μm, 16.8 μm and 17.5 μm after reaction for 15 min, 4 h, 8 h and 24 h, respectively. As for the Al mapping, there is a localized enrichment of Al, showing the formation of the anorthite phase [21]. It is noted that the Sc content in CMAS is about 1.0 at% after 24 h (Table 1), suggesting that the amount of Sc3+ in the residual CMAS is very low at 1250 °C [40].
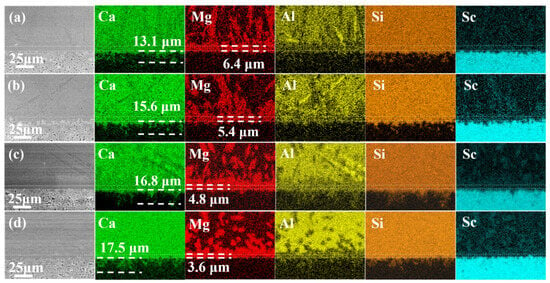
Figure 4.
Elemental mappings of Sc2O3 ceramics after reaction with the melt at 1250 °C for (a) 15 min, (b) 4 h, (c) 8 h and (d) 24 h.
Figure 5 shows the SEM graphs of the Sc2O3 ceramics reacting with CMAS at 1300 °C. A DRL forms on the surface of the ceramic after 15 min reaction, which is mainly composed of the garnet (Ca3Sc2Si3O12) and minor diopside phases according to the compositions of regions #3, 6, 9 and 13 in Figure 5 and Table 2. The garnet phase in the DRL and diopside phase in the residual CMAS increase and coarsen with prolonged exposure time [47]. No anorthite phase can be detected, consistent with the result in Figure 2c. According to Table 2, the concentration of Ca2+ in the melt as region #1 (Figure 5a) is about 30.2 at% after 15 min, and they are 30.1, 29.7 and 26.1 for the residual CMAS after reaction for 4 h, 8 h and 24 h (regions #4, 7 and 10), respectively. It decreases with time, resulting from the formation of more garnet and diopside phases. The content of Sc3+ in the remaining CMAS increases with time. The average content of Sc3+ is about 3.7 at% (except for that after 15 min), which is higher than that at 1250 °C. It is attributed to a more outward diffusion of Sc3+ from the substrate, as we know that the infiltration of the melt can greatly be improved due to the lower viscosity at higher temperature [23]. The penetrated melt reacts with the substrate Sc2O3 ceramic, resulting in generation and diffusion of Sc3+. This then leads to a concentration increase in Sc3+ in the CMAS even despite the formation of the various crystallization phases.
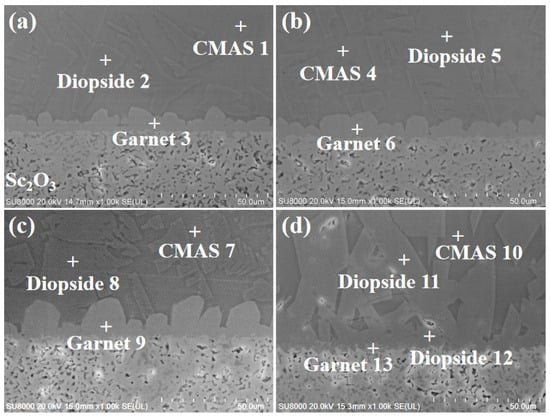
Figure 5.
SEM graphs of the Sc2O3 ceramics after reaction with the melt at 1300 °C for (a) 15 min, (b) 4 h, (c) 8 h and (d) 24 h.

Table 2.
Chemical compositions of the marked areas in Figure 5 (at%).
The elemental mappings of the Sc2O3 ceramics after reaction with the melt at 1300 °C are presented in Figure 6. The DRL shows a good effect on alleviating the Mg2+ infiltration and some mitigating effect on Al3+. An infiltration zone with some Ca forms beneath the DRL according to the Ca mapping. The penetration depth increases with reaction time, whose depths are 13.6 μm, 15.0 μm, 28.9 μm and 68.5 μm after reaction for 15 min, 4 h, 8 h and 24 h, respectively. The infiltration of Si is similar to that of Ca. More melt crystallization for more diopside phases is detected in the CMAS from the elemental mapping of Mg. It is a result of the composition change of the melt such as the decrease in Ca as well as the increase in Sc [21] (zones #1, 4, 7 and 10 in Table 2).
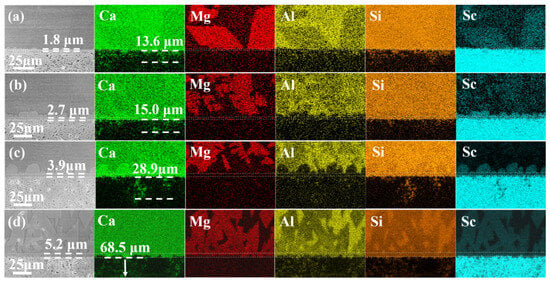
Figure 6.
Elemental mappings of the Sc2O3 ceramics after reaction with the melt at 1300 °C for (a) 15 min, (b) 4 h, (c) 8 h and (d) 24 h.
A DRL forms at the Sc2O3/CMAS interface after exposure to the molten CMAS for 15 min, and the changes of thickness of the DRL with reaction temperature and time are shown in Figure 7a. The growing of the thickness follows the parabolic law, and the corresponding equations are well fitted with y = Ax2 + Bx + C. It is obvious that the DRL decreases with time at 1250 °C, resulting from the dissolution of the diopside phase, while the DRL thickens with time at 1300 °C. This is a result of the lower viscosity of CMAS [23] and the increasing concentration of the Sc3+ in the residual CMAS (Table 2) [48]. The growing DRL becomes steady after reaction for 24 h, indicating that there is a dynamic balancing state of thickening and dissolution for the DRL during exposure to the melt.
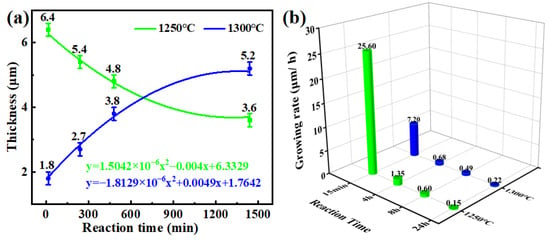
Figure 7.
The thicknesses (a) and the growing rates (b) of the DRLs with temperature and time.
Figure 7b exhibits the growing speeds of the DRLs at 1250 °C and 1300 °C. The thickening rates of the DRL are about 25.6 and 7.20 μm/h for the Sc2O3 ceramics within the first 15 min of the reaction at two temperatures (1250 °C, 1300 °C), respectively, and the growing speeds are only around 0.15 and 0.22 μm/h after reaction for 24 h. It is obvious that the growing speed of the DRL decreases with time at high temperature. As for 1250 °C, it is attributed to the dissolution of the diopside phase [46]. At 1300 °C, it is related to the increase in DRL thickness and garnet grain coarsening, resulting in an increase in the diffusion path and fewer diffusion channels. Furthermore, the growing rate of the DRL at 1300 °C is slower compared to that at 1250 °C during the initial 8 h. It may be related to the growing behavior of the DRL and thermochemical stability of the diopside and garnet phases in the molten CMAS. While the growing speed of the DRL at 1300 °C is relatively higher after more than 8 h, it may be due to the decreasing viscosity and the severe infiltration of the melt.
3.3. Mechanism Analysis
To further understand the reactions of the Sc2O3 ceramic with the CMAS at high temperature, the DSC tests were conducted on the CMAS and the mixed powders with Sc2O3–CMAS (1:1 wt.%) during heating from room temperature to 1450 °C, and the corresponding patterns are exhibited in Figure 8. A small peak exists at around 800 °C, representing the glass transition (GT) of CMAS [45]. Moreover, the CMAS begins to melt at about 1207.6 °C, and the endothermic peak P1 of the CMAS curve is around 1229.5 °C, close to that in Ref. [49]. The peak P1 of the mixed powder is around 1225.3 °C, indicating that Sc2O3 shows a small effect for the melting of CMAS. The reaction of Sc2O3 with CMAS causes peak P2, forming garnet, diopside and Sc2Si2O7 phases. The Sc2Si2O7 will react with CMAS to form Ca3Sc2Si3O12 [45], suggesting that Sc2Si2O7 is a transition phase during thermochemical reactions. Peak P3 (1350.7 °C) indicates the melting of diopside [50]. Additionally, peak P4 may be related to the melting behavior of garnet. The whole thermochemical reaction processes are summarized as:
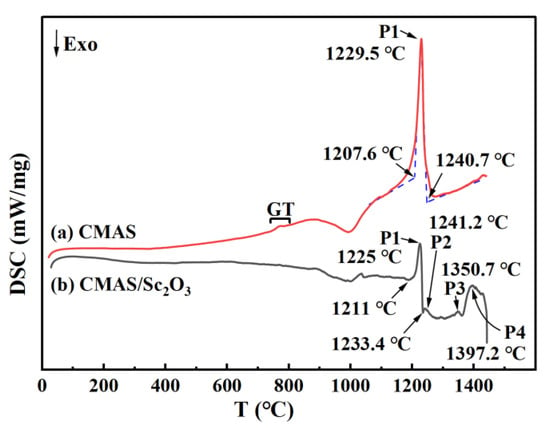
Figure 8.
DSC curves of (a) CMAS and (b) mixture of CMAS–Sc2O3 (1:1 wt.%).
GT: CMAS undergoes glass transition;
P1: Sc2O3 and CMAS powders melt;
P2:
Sc2O3 + CMAS → Ca3Sc2Si3O12 + CaMgSi2O6 + Sc2Si2O7.
Sc2Si2O7 + CMAS → Ca3Sc2Si3O12;
P3: The melting of CaMgSi2O6;
P4: The melting of Ca3Sc2Si3O12.
Figure 9 illustrates the thermochemical reaction mechanisms of the Sc2O3 ceramic and the CMAS at high temperature. At the beginning of the reaction at 1250 °C, the Sc2O3 ceramic reacts with the melt to form a DRL within the initial 15 min, and it is mainly the diopside phase. Then, the anorthite phase precipitates in the residual CMAS owing to the composition change in the CMAS (Figure 9b). The DRL becomes thin with reaction time, attributed to the dissolution of the diopside [46], and the anorthite phase disappears after 24 h. As for 1300 °C, the DRL is composed of the dominating garnet and minor diopside phases. The DRL thickens with reaction time, resulting from the good stability of the garnet phase and the increasing Sc3+ infiltration from the substrate [48]. The DRL shows good effect on inhibiting Mg2+ penetration and some blocking effect on Al3+, and relatively inferior resistance to Ca2+ and Si4+ penetration.
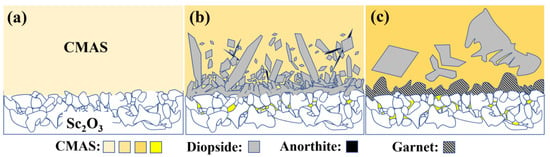
Figure 9.
Schematics of the thermochemical interactions of the CMAS and Sc2O3 ceramic at high temperature (a) before interaction, (b) 1250 °C and (c) 1300 °C.
4. Conclusions
The thermochemical interactions of the Sc2O3 ceramic with CMAS at 1250 °C and 1300 °C were investigated in this work. Different reaction products and the CMAS crystallization phases form at various temperatures, resulting in distinct phase components for the dense reaction layer (DRL). The DRL is mainly composed of the diopside phase (CaMgSi2O6) at 1250 °C, decreasing with reaction time, while it is of the dominant garnet phase (Ca3Sc2Si3O12) at 1300 °C, increasing with exposure time. It is attributed to the difference in stability between the garnet and diopside phases in the molten CMAS. The thickening rate of the DRL decreases and following the parabolic law with duration time. They are about 25.6 and 7.20 (μm/h) after reaction for the initial 15 min, and only 0.15 and 0.22 (μm/h) after 24 h reaction at 1250 °C and 1300 °C, respectively. The DRL shows good effect on inhibiting Mg2+ and some blocking effect on Al3+ penetration, and relatively inferior resistance to Ca2+ and Si4+ infiltration. It is indicated that a DRL composed of diopside and/or garnet phases is a potential strategy for protective coating against molten CMAS attack.
Author Contributions
Conceptualization, T.L.; formal analysis, Z.M. (Zupeng Mo) and Z.Y.; investigation, Z.M. (Zupeng Mo), Z.M. (Zijian Mo), Z.Y., Y.C. and Y.M.; data curation, Z.M. (Zupeng Mo) and Z.M. (Zijian Mo); writing—initial draft preparation, Z.M. (Zupeng Mo); writing—review and editing, Z.M. (Zupeng Mo) and T.L.; supervision, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the National Natural Science Foundation of China, grant number 52061004.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fang, H.J.; Wang, W.Z.; Deng, S.J.; Yang, T.; Zhu, H.; Huang, J.B.; Ye, D.D.; Guo, X.P. Interaction between Yb2O3-Y2O3 co-stabilized ZrO2 ceramic powder and molten silicate deposition, and its implication on thermal barrier coating application. Mater. Charact. 2021, 180, 111418. [Google Scholar] [CrossRef]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Dai, J.W.; Huang, B.; He, L.M.; Mu, R.D.; Tian, H.; Xu, Z.H. Thermal cycling behavior and failure mechanism of Yb2O3-doped yttria-stabilized zirconia thermal barrier coatings. Mater. Today Commun. 2023, 34, 105–409. [Google Scholar] [CrossRef]
- Rai, A.K.; Schmitt, M.P.; Bhattacharya, R.S.; Zhu, D.M.; Wolfe, D.E. Thermal conductivity and stability of multilayered thermal barrier coatings under high temperature annealing conditions. J. Eur. Ceram. Soc. 2015, 35, 1605–1612. [Google Scholar] [CrossRef]
- Jackson, R.W.; Zaleski, E.M.; Poerschke, D.L.; Hazel, B.T.; Begley, M.R.; Levi, C.G. Interaction of molten silicates with thermal barrier coatings under temperature gradients. Acta. Mater. 2015, 89, 396–407. [Google Scholar] [CrossRef]
- Naraparaju, R.; Chavez, J.J.G.; Schulz, U.; Ramana, C.V. Interaction and infiltration behavior of Eyjafjallajőkull, Sakurajima volcanic ashes and a synthetic CMAS containing FeO with/in EB-PVD ZrO2-65 wt% Y2O3 coating at high temperature. Acta. Mater. 2017, 136, 164–180. [Google Scholar] [CrossRef]
- Holgate, C.S.; Seward, G.G.E.; Ericks, A.R.; Poerschke, D.L.; Levi, C.G. Dissolution and diffusion kinetics of yttria-stabilized zirconia into molten silicates. J. Eur. Ceram. Soc. 2021, 41, 1984–1994. [Google Scholar] [CrossRef]
- Zhang, B.P.; Song, W.J.; Wei, L.L.; Xiu, Y.X.; Xu, H.B.; Dingwell, D.B.; Guo, H.B. Novel thermal barrier coatings repel and resist molten silicate deposits. Scr. Mater. 2019, 163, 71–76. [Google Scholar] [CrossRef]
- He, Y.X.; Xiao, G.Z.; Wang, C.; Lu, X.F.; Li, L.Y.; Liu, S.Y.; Wu, Y.S.; Wang, Z.J. Improved thermal properties and CMAS corrosion resistance of rare-earth monosilicates by adjusting the configuration entropy with RE-doping. Corros. Sci. 2024, 226, 111664. [Google Scholar] [CrossRef]
- Rai, A.K.; Bhattacharya, R.S.; Wolfe, D.E.; Eden, T.J. CMAS-resistant thermal barrier coatings (TBC). Int. J. Appl. Ceram. Technol. 2010, 7, 662–674. [Google Scholar] [CrossRef]
- Fang, H.J.; Wang, W.Z.; Huang, J.B.; Ye, D.D. Investigation of CMAS resistance of sacrificial plasma-sprayed mullite-YSZ protective layer on 8YSZ thermal barrier coating. Corros. Sci. 2020, 173, 108764. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, H.; Shan, X.; Yang, F.; Guo, F.W.; Xiao, P.; Gong, S.K. A novel CMAS-resistant material based on thermodynamic equilibrium design: Apatite-type Gd10(SiO4)6O3. J. Am. Ceram. Soc. 2020, 103, 3401–3415. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ma, Z.; Liu, L.; Liu, Y.B. Reaction products of Sm2Zr2O7 with calcium–magnesium–aluminum–silicate (CMAS) and their evolution. J. Adv. Ceram. 2021, 10, 1389–1397. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Z.Z.; Bai, Y.; Che, J.W.; Wang, R.J.; Ma, F.; Tao, W.Z.; Liang, G.Y. Improved properties of scandia and yttria co-doped zirconia as a potential thermal barrier material for high temperature applications. J. Eur. Ceram. Soc. 2018, 38, 4502–4511. [Google Scholar] [CrossRef]
- Fang, H.J.; Wang, W.Z.; Huang, J.B.; Li, Y.J.; Ye, D.D. Corrosion behavior and thermos-physical properties of a promising Yb2O3 and Y2O3 co-stabilized ZrO2 ceramic for thermal barrier coatings subject to calciummagnesium-aluminum-silicate (CMAS) deposition: Experiments and first-principles calculation. Corros. Sci. 2021, 128, 111418. [Google Scholar] [CrossRef]
- Ramachandran, C.S.; Balasubramaniana, V.; Ananthapadmanabhan, P.V. Thermal cycling behavior of plasma sprayed lanthanum zirconate based coatings under concurrent infiltration by a molten glass concoction. Ceram. Int. 2013, 39, 1413–1431. [Google Scholar]
- Gok, M.G.; Karabas, M. Production of Re doped La2Zr2O7 based TBCs and numerical analysis of their use on IC engine piston surface. Ceram. Int. 2022, 48, 11173–11180. [Google Scholar] [CrossRef]
- Dolmaire, A.; Béchade, E.; Geffroy, P.M.; Goutier, S.; Vardelle, M.; Vilasi, M.; Joulia, A. Reaction mechanisms of Gd2Zr2O7 in silicate melts derived from CAS. J. Eur. Ceram. Soc. 2022, 42, 7247–7257. [Google Scholar] [CrossRef]
- Bahamirian, M. Nanostructured Gd2Zr2O7: A promising thermal barrier coating with high resistance to CaO-MgO-Al2O3-SiO2 corrosion. J. Aust. Ceram. Soc. 2023, 59, 165–177. [Google Scholar] [CrossRef]
- Drexler, J.M.; Ortiz, A.L.; Padture, N.P. Composition effects of thermal barrier coating ceramics on their interaction with molten Ca–Mg–Al–silicate (CMAS) glass. Acta. Mater. 2012, 60, 5437–5447. [Google Scholar] [CrossRef]
- Naraparaju, R.; Pubbysetty, R.P.; Mechnich, P.; Schulz, U. EB-PVD alumina (Al2O3) as a top coat on 7YSZ TBCs against CMAS/VA infiltration: Deposition and reaction mechanisms. J. Eur. Ceram. Soc. 2018, 38, 3333–3346. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Wei, L.L.; He, Q.; Deng, Y.P.; He, W.T.; Guo, H.B. PS–PVD alumina overlayer on thermal barrier coatings against CMAS attack. J. Therm. Spray Technol. 2021, 30, 864–872. [Google Scholar] [CrossRef]
- Liang, T.Q.; Huang, Z.H.; Li, M.H.; Xiao, W.T.; He, H.; He, A.P.; Chen, X.Y.; Luo, N.N. Thermochemical reaction behavior between Y2O3 and calcium-magnesium-aluminosilicate (CMAS) at elevated temperature. Corros Sci. 2022, 208, 110631. [Google Scholar] [CrossRef]
- Liu, H.; Cai, J.; Zhu, J.H. CMAS (CaO-MgO-Al2O3-SiO2) resistance of Y2O3-stabilized ZrO2 thermal barrier coatings with Pt layers. Ceram. Int. 2018, 44, 452–458. [Google Scholar] [CrossRef]
- Zhao, H.B.; Levi, C.G.; Wadley, H.N.G. Molten silicate interactions with thermal barrier coatings. Int. J. Appl. Ceram. Technol. 2014, 251, 74–86. [Google Scholar] [CrossRef]
- Tian, Z.L.; Zhang, J.; Zheng, L.Y.; Hu, W.P.; Ren, X.M.; Lei, Y.M. General trend on the phase stability and corrosion resistance of rare earth monosilicates to molten calcium–magnesium–aluminosilicate at 1300 °C. Corros. Sci. 2019, 148, 281–292. [Google Scholar] [CrossRef]
- Tian, Z.L.; Ren, X.M.; Lei, Y.M.; Zheng, L.Y.; Geng, W.R.; Zhang, J. Corrosion of RE2Si2O7 (RE = Y, Yb, and Lu) environmental barrier coating materials by molten calcium-magnesium-alumino-silicate glass at high temperatures. J. Eur. Ceram. Soc. 2019, 39, 4245–4254. [Google Scholar] [CrossRef]
- Wei, L.L.; Guo, L.; Li, M.Z.; Guo, H.B. Calcium-magnesium-alumina-silicate (CMAS) resistant Ba2REAlO5 (RE = Yb, Er, Dy) ceramics for thermal barrier coatings. J. Eur. Ceram. Soc. 2018, 37, 4991–5000. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.L.; He, Q.; Yu, J.X.; Yan, Z.; Ye, F.X.; Dan, C.Y.; Ji, V. Microstructure evolution and hot corrosion mechanisms of Ba2REAlO5 (RE = Yb, Er, Dy) exposed to V2O5 + Na2SO4 molten salt. J. Eur. Ceram. Soc. 2018, 38, 3555–3563. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.Z.; Yang, C.X.; Zhang, C.L.; Xu, L.M.; Ye, F.X.; Dan, C.Y.; Ji, V. Calcium-magnesium-alumina-silicate (CMAS) resistance property of BaLn2Ti3O10 (Ln=La, Nd) for thermal barrier coating applications. Ceram. Int. 2017, 43, 10521–10527. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Sun, J.B.; Zhang, H.; Yang, X.; Qiu, F.Y.; Zhou, P.F.; Niu, W.B.; Dong, S.J.; Zhou, X.; Cao, X.Q. Lanthanum magnesium hexaluminate thermal barrier coatings with pre-implanted vertical microcracks: Thermal cycling lifetime and CMAS corrosion behavior. Ceram. Int. 2018, 44, 11472–11485. [Google Scholar] [CrossRef]
- Song, C.X.; Qi, X.; Huang, L.; Sun, J.B.; Li, L.Z.; Li, C.G.; Lu, W.H. CaO-MgO-Al2O3-SiO2 (CMAS) corrosion behaviour of LaMgAl11O19/GdPO4 thermal barrier coating materials. Ceram. Int. 2023, 49, 26578–26588. [Google Scholar] [CrossRef]
- Guo, L.; Yan, Z.; Yu, Y.; Yang, J.; Li, M.Z. CMAS resistance characteristics of LaPO4/YSZ thermal barrier coatings at 1250 °C–1350 °C. Corros. Sci. 2019, 154, 111–122. [Google Scholar] [CrossRef]
- Kang, Y.X.; Bai, Y.; Fan, W.; Yuan, T.; Gao, Y.; Bao, C.G.; Li, B.Q. Thermal cycling performance of La2Ce2O7/50 vol.% YSZ composite thermal barrier coating with CMAS corrosion. J. Eur. Ceram. Soc. 2018, 38, 2851–2862. [Google Scholar] [CrossRef]
- Turcer, L.R.; Krause, A.R.; Garces, H.F.; Zhang, L.; Padture, N.P. Environmental barrier coating ceramics for resistance against attack by molten calcia-magnesia-aluminosilicate (CMAS) glass: Part I, YAlO3 and γ-Y2Si2O7. J. Eur. Ceram. Soc. 2018, 38, 3905–3913. [Google Scholar] [CrossRef]
- Yan, R.X.; Liang, W.P.; Miao, Q.; Zhao, H.; Liu, R.X.; Li, J.L.; Zang, K.; Dong, M.J.; He, X.P.; Gao, X.G.; et al. Mechanical, thermal and CMAS resistance properties of high-entropy (Gd0.2Y0.2Er0.2Tm0.2Yb0.2)2Zr2O7 ceramics. Ceram. Int. 2023, 49, 20729–220741. [Google Scholar] [CrossRef]
- Deng, S.X.; He, G.; Yang, Z.C.; Wang, J.X.; Li, J.T.; Jiang, L. Calcium-magnesium-alumina-silicate (CMAS) resistant high entropy ceramic (Y0.2Gd0.2Er0.2Yb0.2Lu0.2)2Zr2O7 for thermal barrier coatings. J. Mater. Sci. Technol. 2022, 107, 259–265. [Google Scholar] [CrossRef]
- Wang, J.S.; Chen, M.D.; Li, C.Z.; Chen, L.Y.; Yu, Y.S.; Wang, Y.H.; Liu, B.; Jing, Q.S. Comparison of corrosion behaviors of Sc2O3-CeO2 co-stabilized ZrO2 and YSZ ceramics exposed to CMAS at 1250 °C. Surf. Coat. Technol. 2021, 428, 127879. [Google Scholar] [CrossRef]
- Wang, J.S.; Lu, X.J.; Hu, M.Q.; Chen, M.D.; Sun, J.R.; Wang, Y.H.; Shu, C.X.; Zhang, H.; Liu, B.; Sun, J.B.; et al. Phase stability, thermophysical properties, thermal shock behavior and CMAS resistance of Sc2O3-CeO2 co-stabilized ZrO2 TBCs. Surf. Coat. Technol. 2023, 467, 129679. [Google Scholar] [CrossRef]
- Fan, W.; Bai, Y.; Liu, Y.F.; Kang, Y.X.; Wang, Y.; Wang, Z.Z.; Tao, W.Z. Corrosion behavior of Sc2O3-Y2O3 co-stabilized ZrO2 thermal barrier coatings with CMAS attack. Ceram. Int. 2019, 45, 15763–15767. [Google Scholar] [CrossRef]
- Liao, Y.X.; Dai, Y.F.; Zhai, Y.F.; He, A.P.; He, H.; Liang, T.Q. The corrosion behavior of Sc2O3-Y2O3 co-doped ZrO2 influenced by Sc2O3 content in CMAS at 1300 °C. J. Eur. Ceram. Soc. 2024, 44, 1179–1187. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, L.; Li, Z.; Yu, Y.; He, Q. Effects of laser glazing on CMAS corrosion behavior of Y2O3 stabilized ZrO2 thermal barrier coatings. Corros. Sci. 2019, 157, 450–461. [Google Scholar] [CrossRef]
- Ye, F.X.; Yuan, Y.H.; Yan, S.; Guo, L.; Yu, J.X. High-temperature corrosion mechanism of a promising scandium tantalate ceramic for next generation thermal barrier coating under molten calcium–magnesium-aluminosilicate (CMAS). Mater. Chem. Phys. 2020, 256, 123679. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Barth, T.L.; Levi, C.G. Equilibrium relationships between thermal barrier oxides and silicate melts. Act. Mater. 2016, 120, 302–314. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.T.; Liu, Q.M.; Cheng, L.F.; Wang, Y.G. Calcium–magnesium–aluminosilicate corrosion behaviors of rare-earth disilicates at 1400 °C. J. Eur. Ceram. Soc. 2013, 33, 3419–3428. [Google Scholar] [CrossRef]
- Guo, L.; Xin, H.; Li, Y.Y.; Yu, Y.; Yan, Z.; Hu, C.W.; Ye, F.X. Self-crystallization characteristics of calcium-magnesium-alumina- silicate (CMAS) glass under simulated conditions for thermal barrier coating applications. J. Eur. Ceram. Soc. 2020, 40, 5683–5691. [Google Scholar] [CrossRef]
- Zhou, B.Y.; Wu, Y.; Ke, X.J.; Zhou, Q.J.; Cui, Y.J.; Wang, C.L. Resistance of ytterbium silicate environmental barrier coatings against molten calcium-magnesium-aluminosilicate (CMAS): A comprehensive study. Surf. Coat. Technol. 2024, 479, 130540. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhou, M.F.; Li, M.Y.H.; Robinson, P.T.; Harlov, D.E. Kinetic controls on Sc distribution in diopside and geochemical behavior of Sc in magmatic systems. Geochim. Cosmochim. Acta 2022, 325, 316–332. [Google Scholar] [CrossRef]
- Zaleski, E.M.; Ensslen, C.; Levi, C.G. Melting and Crystallization of Silicate Systems Relevant to Thermal Barrier Coating Damage. J. Am. Ceram. Soc. 2015, 98, 1642–1649. [Google Scholar] [CrossRef]
- Reinsch, S.; Nascimento, M.L.F.; Müller, R.; Zanotto, E.D. Crystal growth kinetics in cordierite and diopside glasses in wide temperature ranges. J. Non-Cryst. Solids 2008, 354, 5386–5394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).