Regulating the Mechanical and Corrosion Properties of Mg-2Zn-0.1Y Alloy by Trace SiC with Different Morphologies
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials Preparation
2.2. Microstructure Characterization
2.3. Tensile Test
2.4. Immersion Test
2.5. Electrochemical Test
3. Results
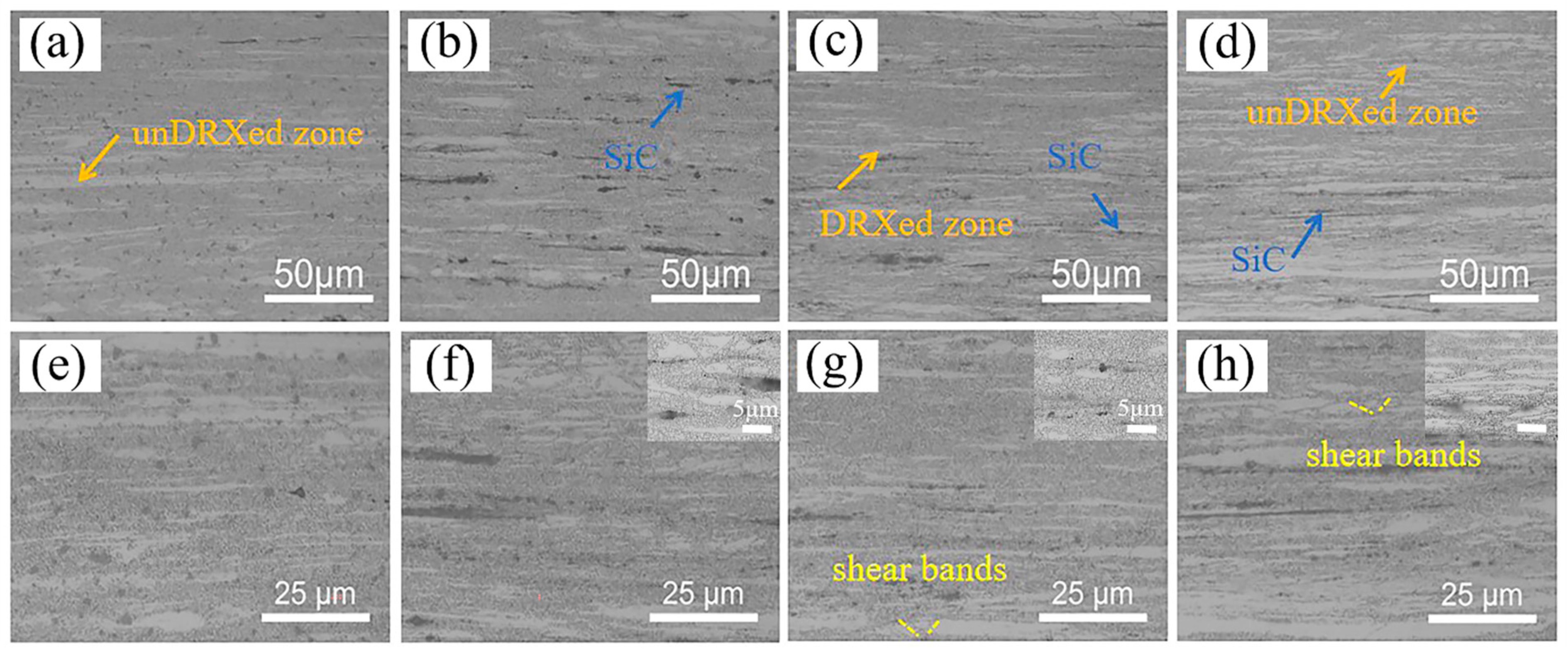
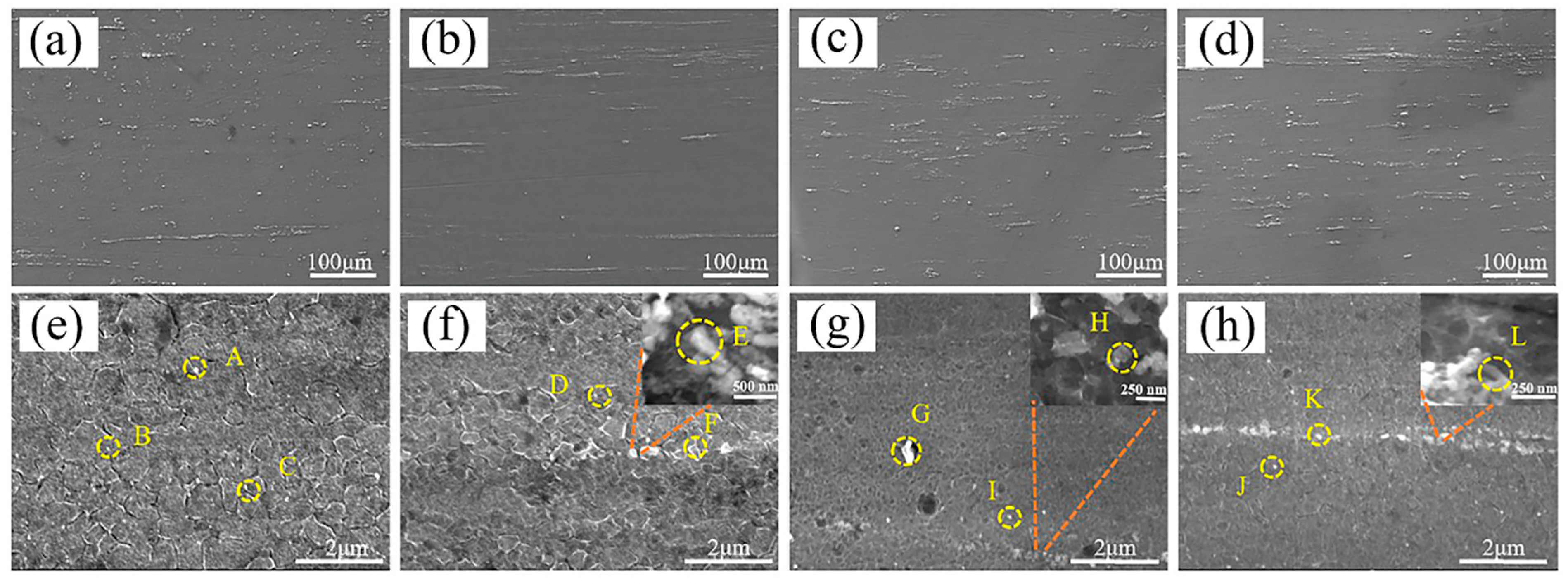
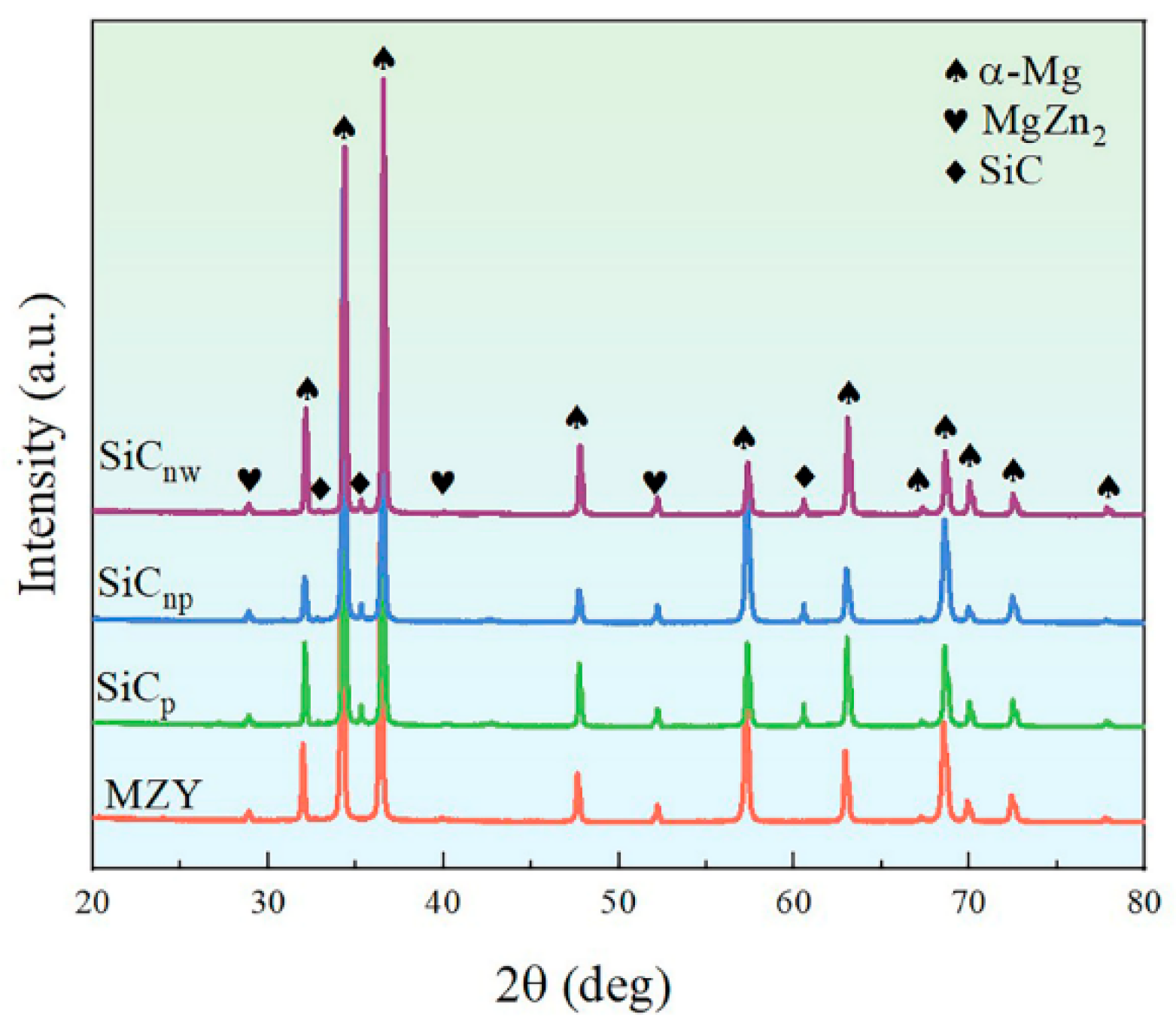
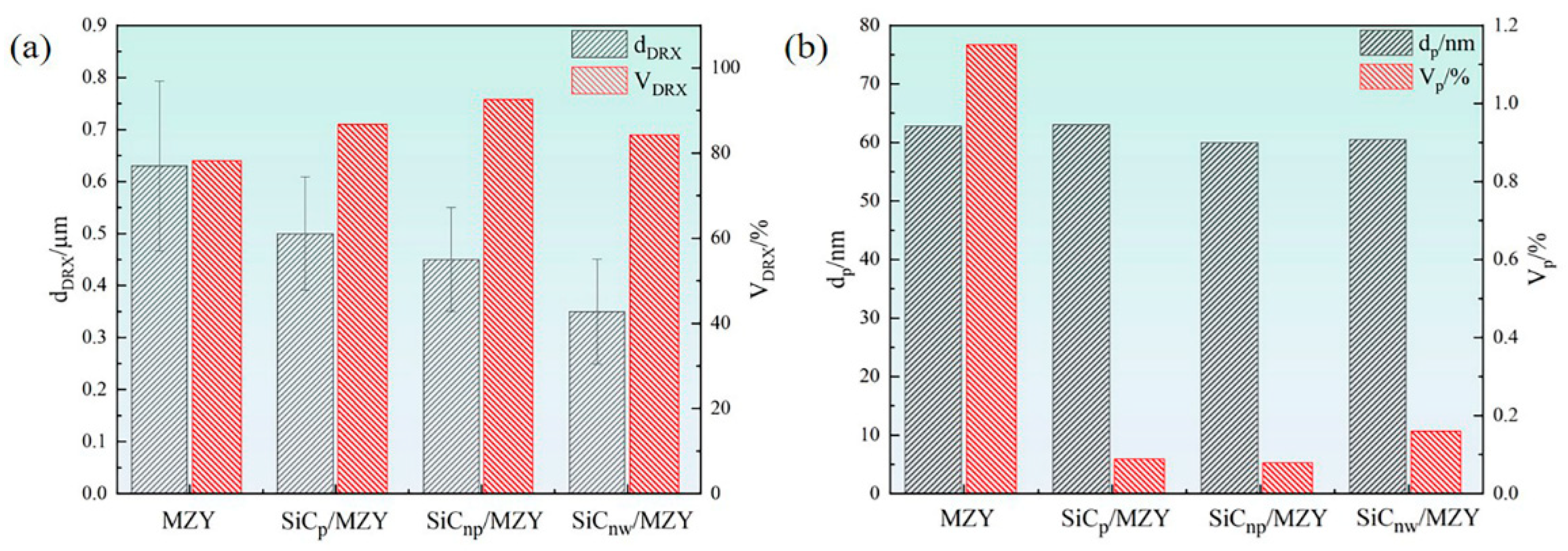
3.1. Microstructure
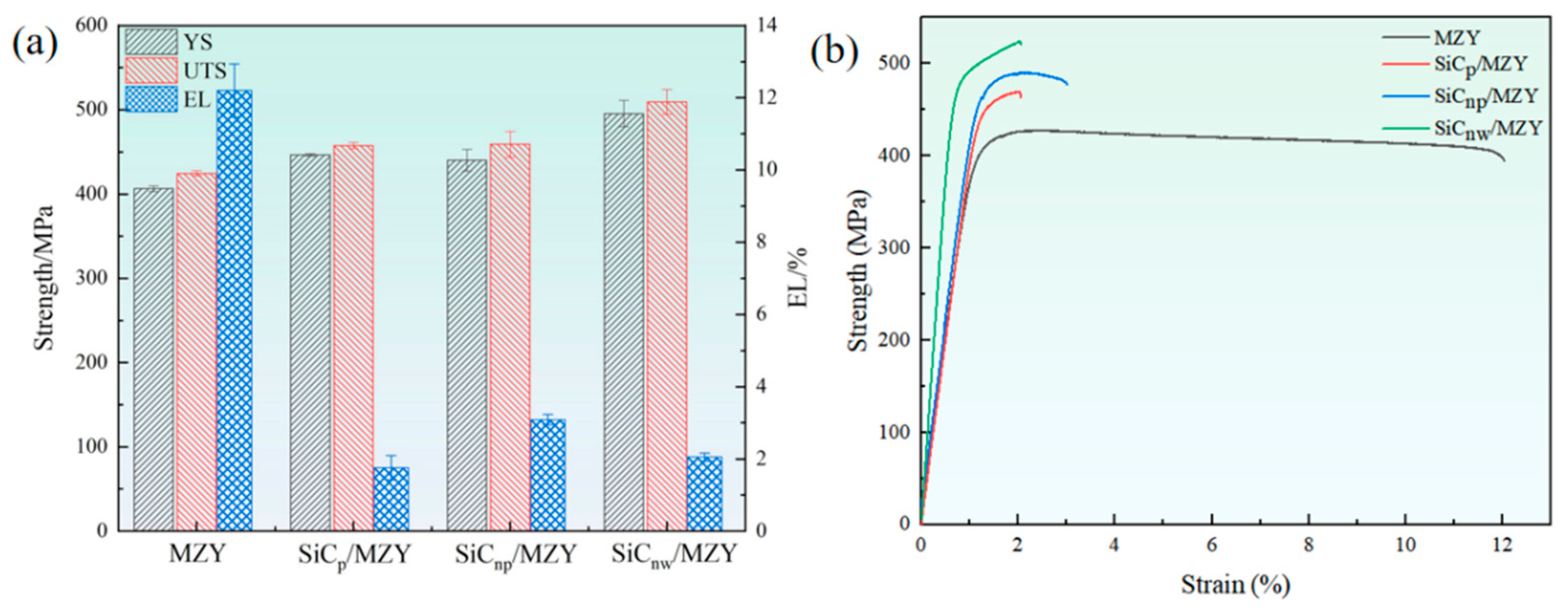
3.2. Mechanical Properties
3.3. Corrosion Behaviors
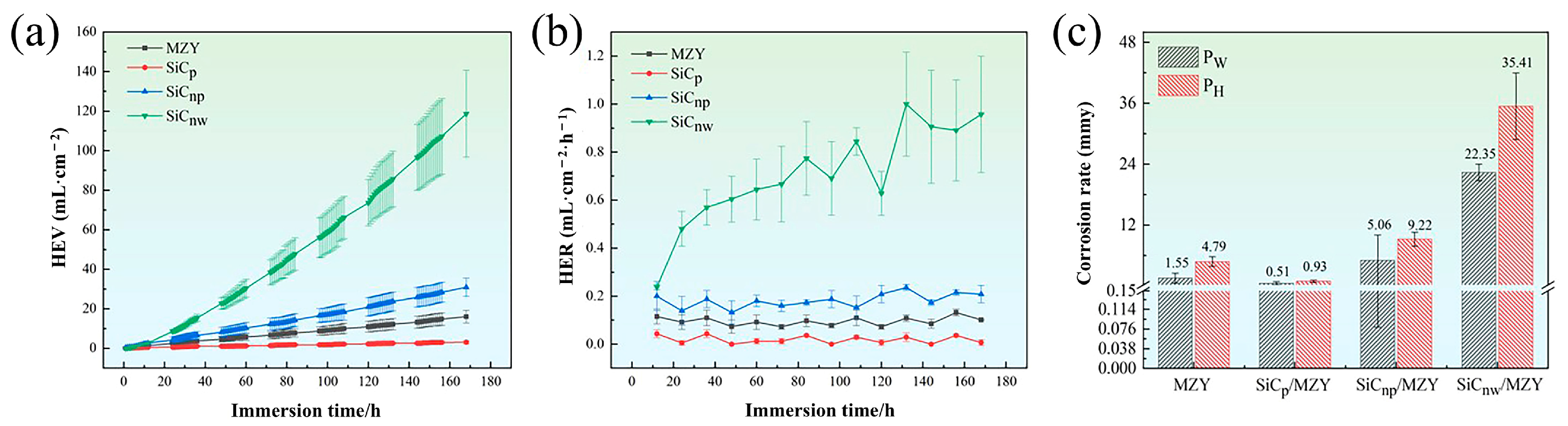
3.3.1. Hydrogen Evolution and Weight Loss
3.3.2. Electrochemical Impedance Spectra (EIS) Tests
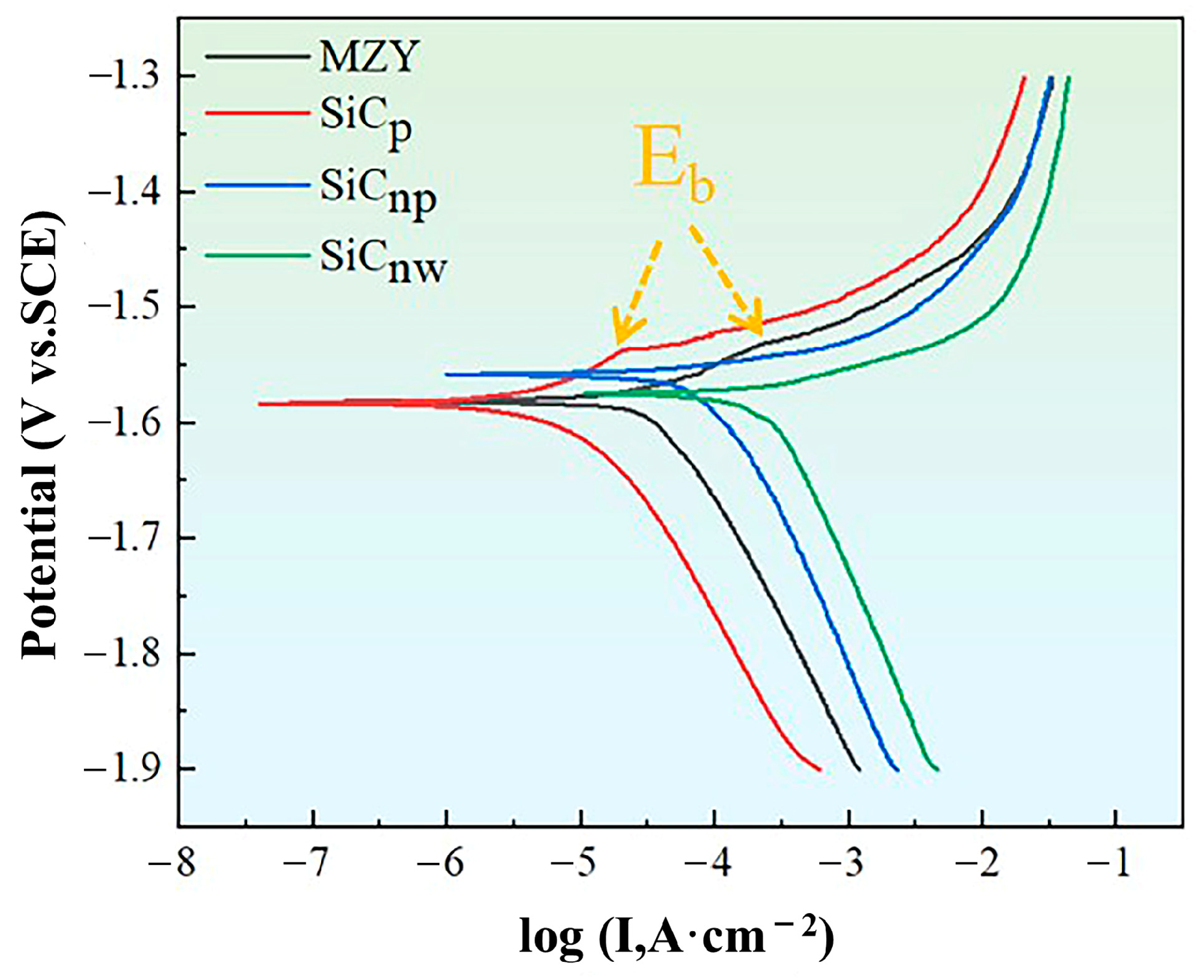
3.3.3. Potentiodynamic Polarization Measurements
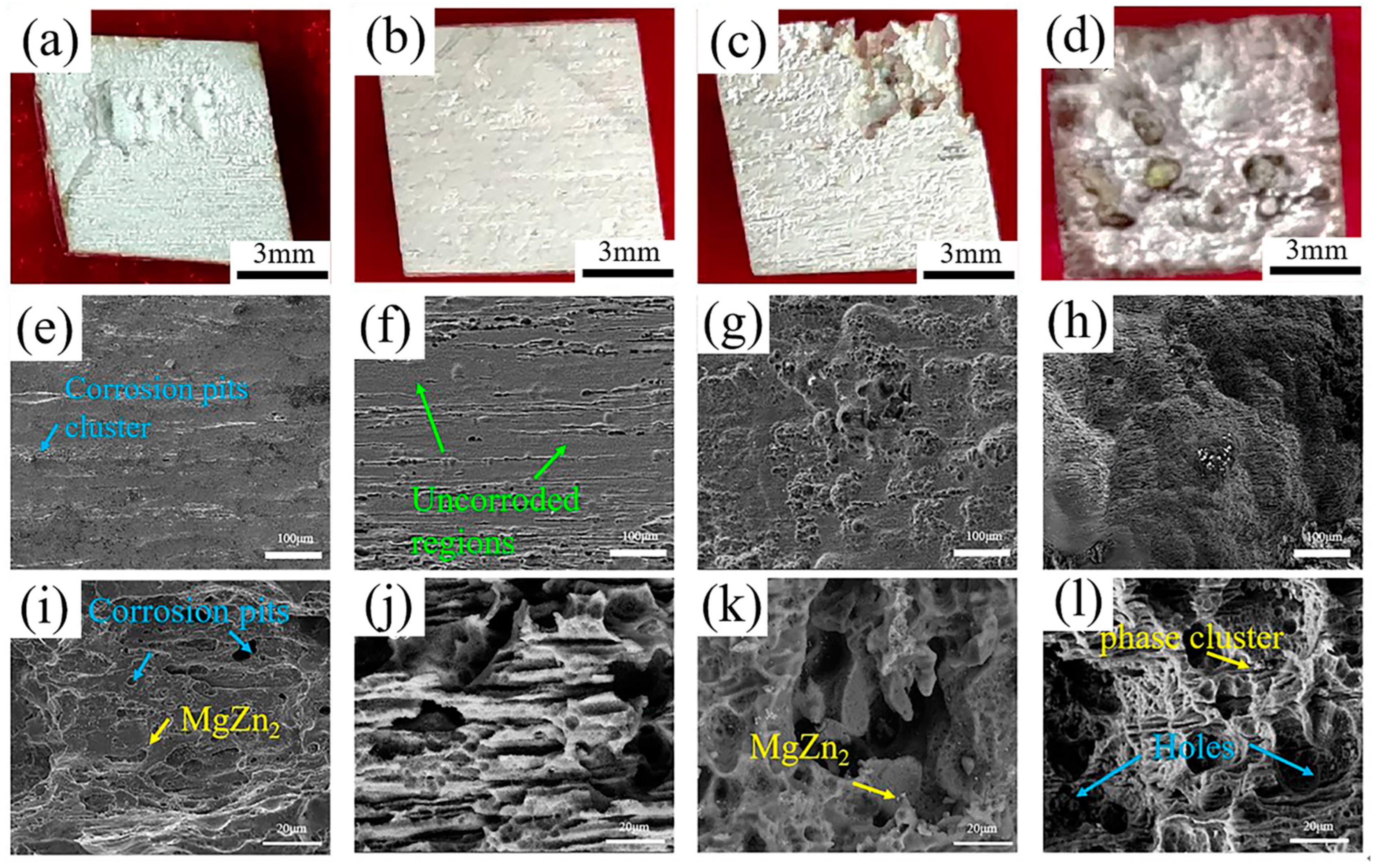
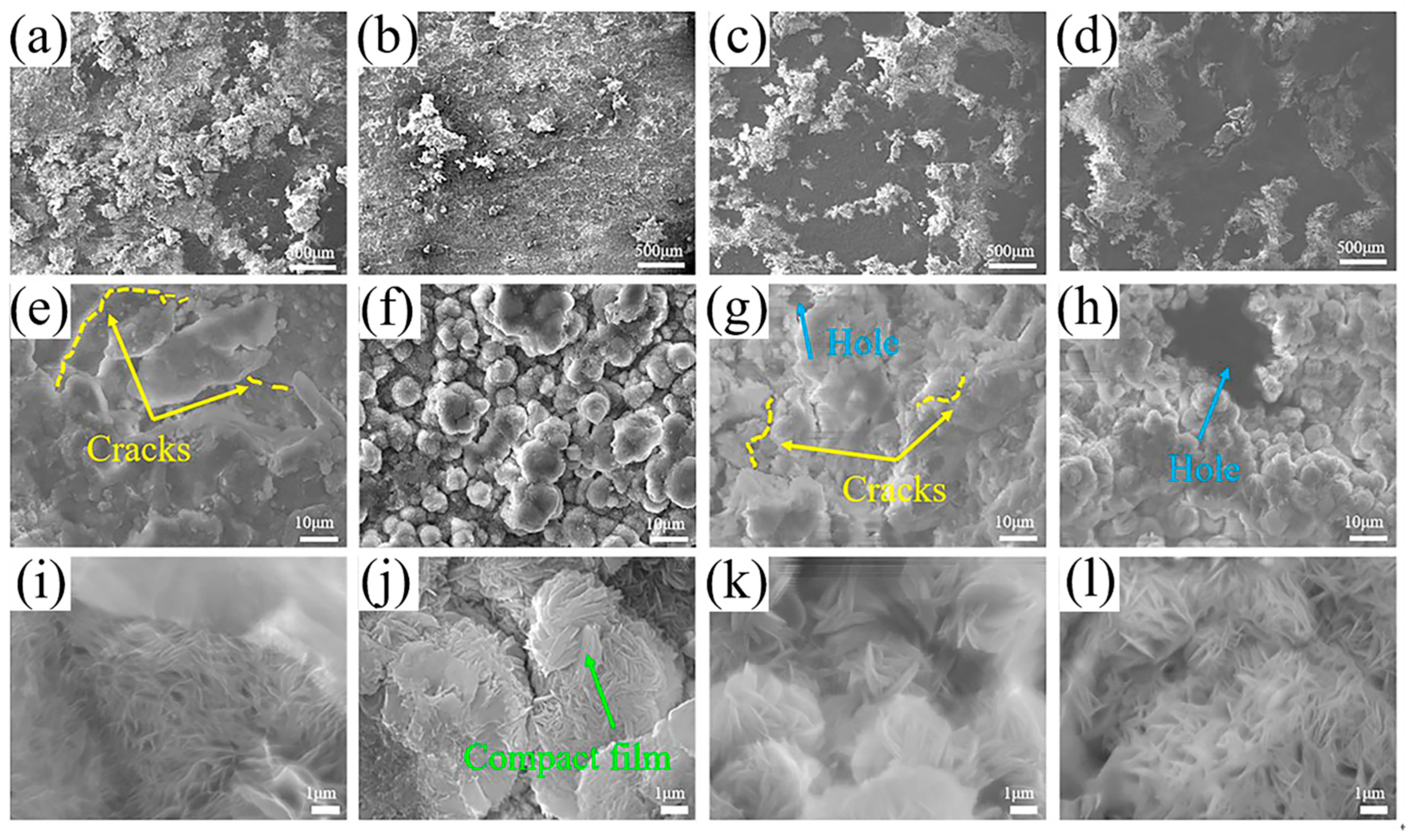
3.3.4. Corrosion Morphologies
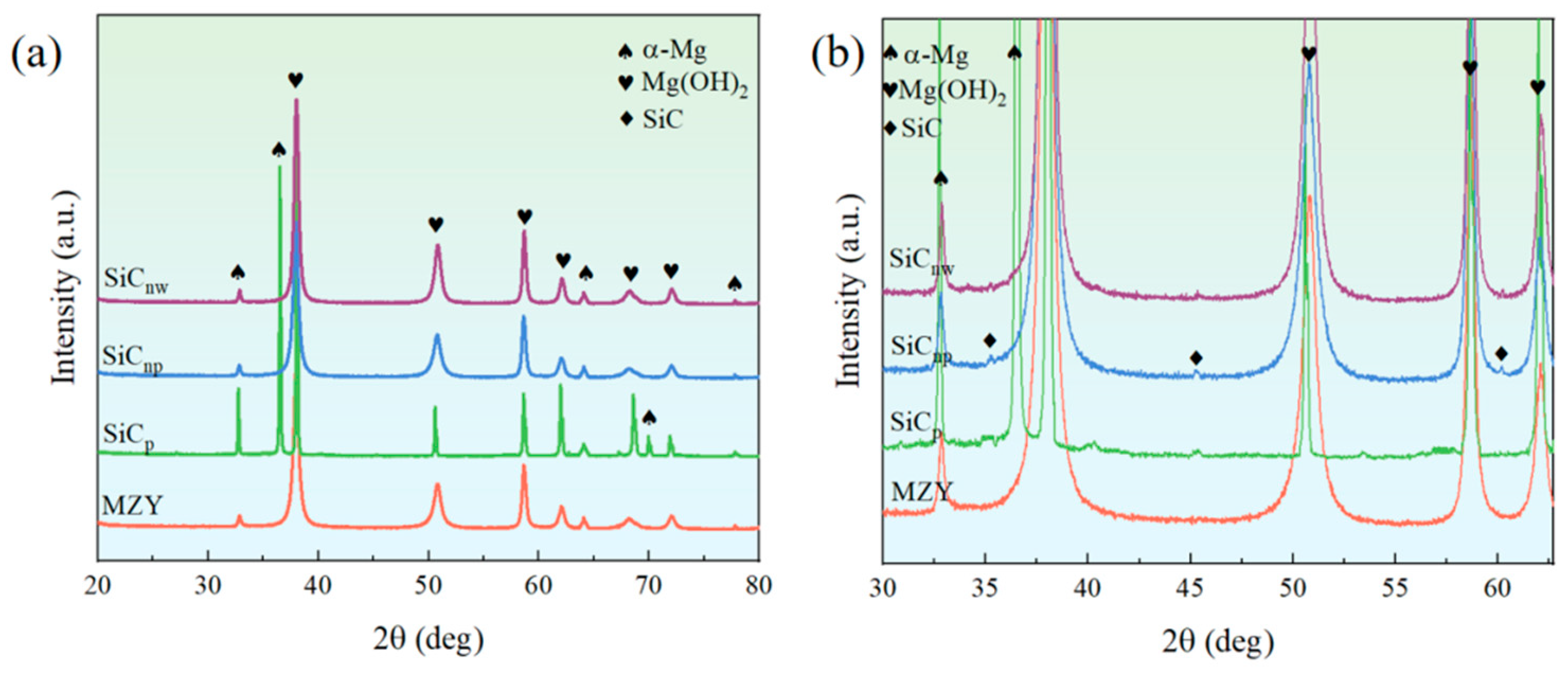
3.3.5. Corrosion Product
3.3.6. KPFM Measurements
4. Discussion
4.1. Effect of SiC Morphologies on Mechanical Properties of MZY Alloy
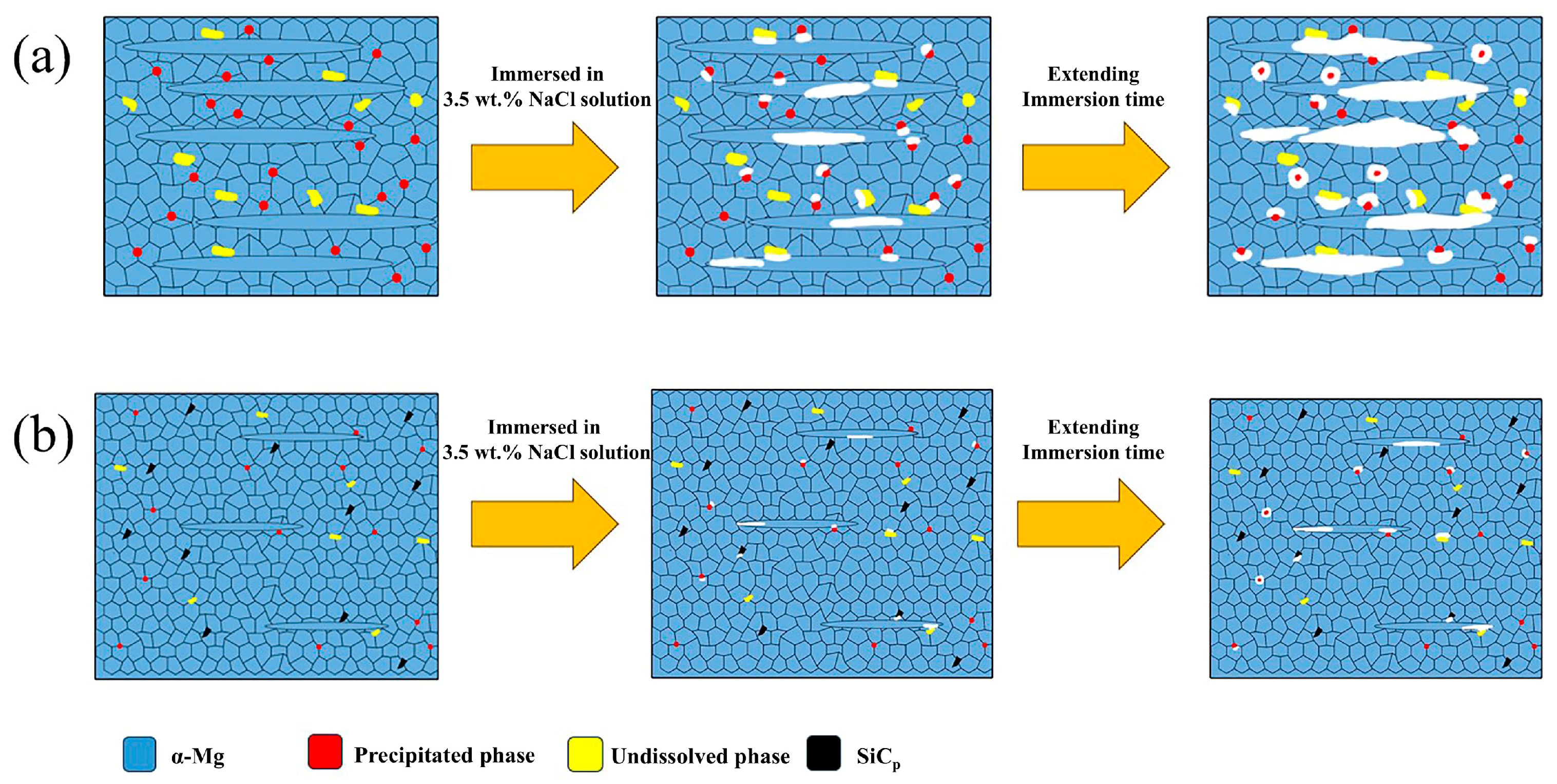
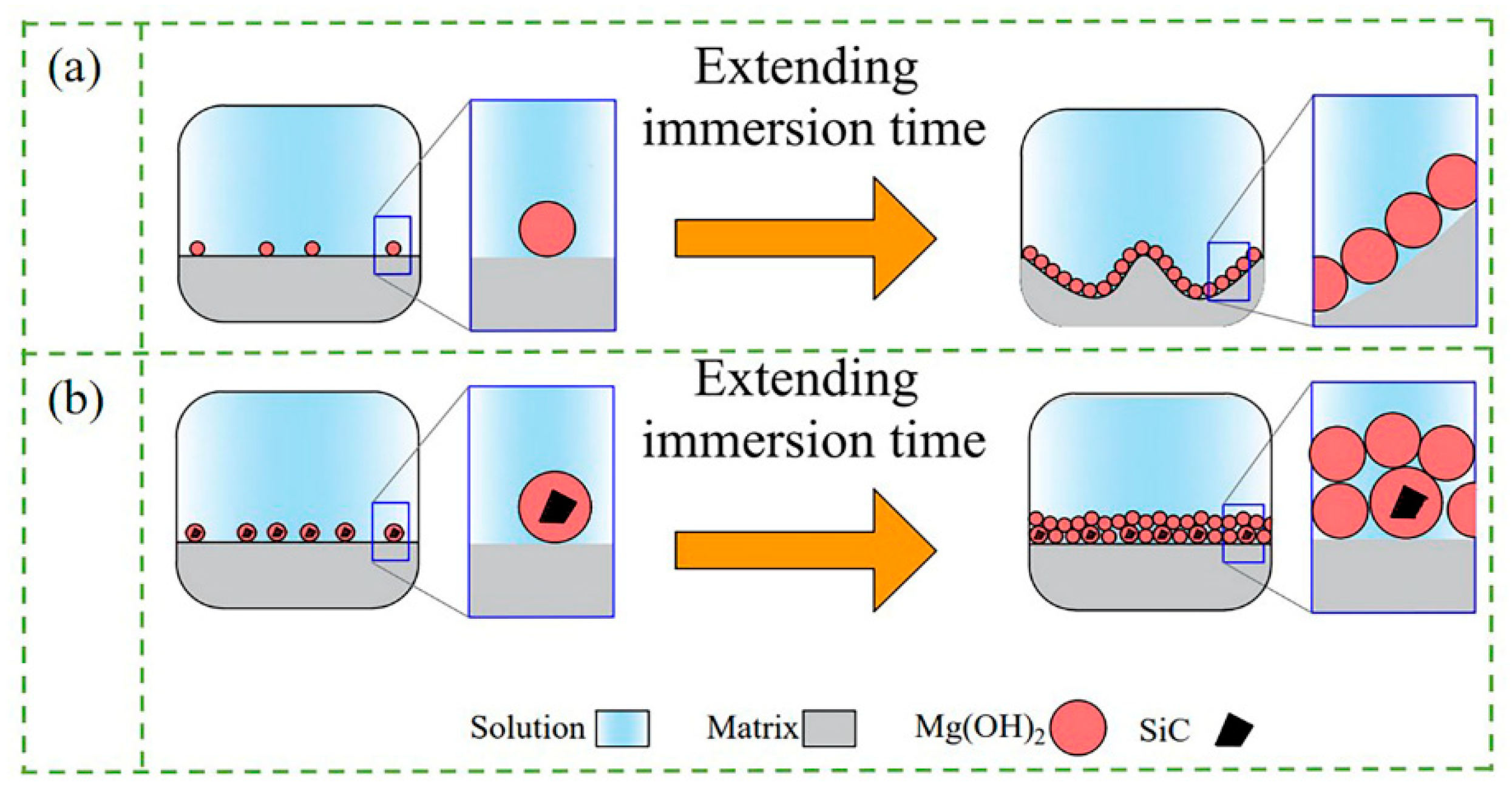
4.2. Effect of SiC Morphologies on Corrosion Properties of MZY Alloy
5. Conclusions
- (1)
- After extrusion, visible dynamic crystallization occurred in the alloy and composites, and the recrystallization degree of SiCnp/MZY was the highest, reaching 92.6%. The addition of SiC reduced the grain size and decreased the number of second phases significantly. The SiCnw/MZY composite had the finest grains and the SiCnp/MZY composite had the lowest number of second phases;
- (2)
- Compared with MZY, the strength of SiC/MZY was improved, with a YS of more than 440 MPa and an ultimate tensile strength of more than 450 MPa. SiCnw/MZY had the highest strengths, with YS and UTS values of ~495.5 MPa and ~509.4 MPa, respectively. For different composites, the SiC reinforcements promoted fine grain strengthening. However, SiCnw could improve the strength through load transfer due to its large aspect ratio. A large number of small SiCnp had a pinning effect on the dislocations, and improved the strength of the composites through Orowan strengthening. On the other hand, submicron SiCp with a larger size improved the strength through dislocations;
- (3)
- Compared with alloy, SiCp/MZY was benefited by the presence of few galvanic coupling pairs and reduced potential difference, resulting in a hydrogen evolution corrosion rate (PH) that was 81% lower than that of MZY alloy (PH 0.93 mm/y, PW 0.51 mm/y). On the other hand, SiCnp/MZY increased the corrosion rate due to the large number of reinforcements-to-matrix interfaces and high energy grain boundaries (PH 9.22 mm/y, PW 5.06 mm/y). The increase in second phase and the decrease in the recrystallization rate in SiCnw/MZY resulted in a faster corrosion rate (PH 35.41 mm/y, PW 22.35 mm/y) compared to SiCnp/MZY;
- (4)
- The presence of SiC particles on the composite surface prevented corrosive ions from entering the matrix, and provided nucleation sites for Mg(OH)2 to form a thicker protective film more rapidly. However, the protective films on the surfaces of SiCnp/MZY and SiCnw/MZY were more prone to cracking and further expansion with the extension of the corrosion time. The larger size of submicron SiCp could not only improve the formation of protective film, but also reduce the internal galvanic corrosion, which further ensured the integrity and protection of the surface film;
- (5)
- The high-strength and corrosion-resistant materials prepared by the semi-solid stirring method in this study exhibited excellent performance and relatively low cost. The combination of high strength, corrosion resistance, and cost-effectiveness makes these materials highly valuable for a wide range of marine and offshore engineering applications. This study provides valuable insights and design ideas for future material development and applications in these fields.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, S.-H.; Lee, S.W.; Moon, B.G.; Kim, H.S.; Park, S.H. Variation in dynamic deformation behavior and resultant yield asymmetry of AZ80 alloy with extrusion temperature. J. Mater. Sci. Technol. 2020, 46, 225–236. [Google Scholar] [CrossRef]
- Guo, R.; Le, Q.; Zhou, W.; Wang, T.; Ren, L.; Jiang, Y.; Zou, Q.; Liao, Q.; Yu, F.; Aranas, C., Jr. Effect of SiCp on the microstructure and tensile properties of the Mg–3Y magnesium alloy. J. Mater. Res. Technol. 2023, 24, 4232–4241. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, S.-H.; Lee, D.H.; Kim, H.J.; Kim, Y.M.; Park, S.H. Variations in microstructure and bending formability of extruded Mg–Al–Zn–Ca–Y–MM alloy with precompression and subsequent annealing treatment conditions. J. Magnes. Alloys 2021, 10, 2475–2490. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.-H.; Kim, Y.M.; You, B.S. Improving mechanical properties of extruded Mg–Al alloy with a bimodal grain structure through alloying addition. J. Alloys Compd. 2015, 646, 932–936. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, J.U.; Kim, Y.J.; Jung, J.-G.; Park, S.H. Controlling the microstructure and improving the tensile properties of extruded Mg-Sn-Zn alloy through Al addition. J. Alloys Compd. 2018, 751, 1–11. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, S.H. Underlying mechanisms of drastic reduction in yield asymmetry of extruded Mg-Sn-Zn alloy by Al addition. Mater. Sci. Eng. A 2018, 733, 285–290. [Google Scholar] [CrossRef]
- Yang, J.; Heogh, W.; Ju, H.; Kang, S.; Jang, T.-S.; Jung, H.-D.; Jahazi, M.; Han, S.C.; Park, S.J.; Kim, H.S.; et al. Functionally graded structure of a nitride-strengthened Mg2Si-based hybrid composite. J. Magnes. Alloys 2024, 12, 1239–1256. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Zhang, K.; Shi, Y.; Ren, J. Effect of extrusion on corrosion behavior and corrosion mechanism of Mg-Y alloy. J. Rare Earths 2016, 34, 315–327. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, Y.; Guan, L.; Ye, L.; Guo, X.; Luo, A. Effect of grain size and crystal orientation on the corrosion behavior of as-extruded Mg-6Gd-2Y-0.2Zr alloy. Corros. Sci. 2020, 164, 108338. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, C.; Wang, D.-W.; Li, M.-X.; Ma, Y.-L.; Hua, Z.-M.; Zhang, L.-Y.; Li, J.; Wang, H.-Y. Effects of solute redistribution during heat treatment on micro-galvanic corrosion behavior of dilute Mg-Al-Ca-Mn alloy. Corros. Sci. 2023, 213, 110971–110987. [Google Scholar] [CrossRef]
- Atrens, A.; Chen, X.; Shi, Z. Mg Corrosion-Recent Progress. Corros. Mater. Degrad. 2022, 3, 566–597. [Google Scholar] [CrossRef]
- AbdelGawad, M.; Usman, C.A.; Shunmugasamy, V.C.; Karaman, I.; Mansoor, B. Corrosion behavior of Mg-Zn-Zr-RE alloys under physiological environment-Impact on mechanical integrity and biocompatibility. J. Magnes. Alloy. 2022, 10, 1542–1572. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloy. 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.-L.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnes. Alloys 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research-A review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, L.; Huang, Z.; Wang, F. Improving the electrochemical corrosion resistance of a high-Ca heat-resistant magnesium alloy by enhancing the barrier effect of the cathodic phase skeleton. J. Alloys Compd. 2025, 1010, 177728. [Google Scholar] [CrossRef]
- Yu, F.; Peng, Y.; Liu, F.; Peng, P.; Zhang, C.; Long, S.; Zhang, J.; Yang, Q.; Dai, Q.; She, J.; et al. Synergistic optimization of mechanical properties and corrosion resistance in Sm-doped Mg-Gd-Zn-Zr biodegradable magnesium alloys. J. Rare Earths 2024. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.; Le, Q.; Wang, T.; Liao, Q.; Liu, L. Achieving the improvement of tensile properties and corrosion resistance of Mg-Gd-Sn-Y-Zn-Mn alloy by increasing the concentration of solute elements in nearly equal-amplitude. J. Alloys Compd. 2025, 1010, 178323. [Google Scholar] [CrossRef]
- Wan, D.Q.; Sun, Y.M.; Xue, Y.D.; Dong, S.Y.; Han, G.L.; Wang, Y.; Yang, F.; Tang, H.; Wang, Y.Y. Improvement in corrosion resistance of Mg97Zn1Y2 alloy by Zr addition. China Foundry 2024, 21, 634–640. [Google Scholar] [CrossRef]
- Patil, A.; Nartu, M.S.K.K.Y.; Ozdemir, F.; Banerjee, R.; Gupta, R.K.; Borkar, T. Enhancement of the mechanical properties of graphene nanoplatelet (GNP) reinforced nickel matrix nanocomposites. Mater. Sci. Eng. A 2021, 817, 141324. [Google Scholar] [CrossRef]
- Guo, Y.; Nie, K.; Deng, K.; Liu, Z.; Shi, Q. Strength-plasticity-matched (GNPs+GFs)/Mg–3Zn-0.1Ymagnesium matrix composites with high modulus through liquid-phase dispersion and multistep deformation. Compos. Commun. 2024, 45, 101774. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, T.-T.; Chu, Y.; Song, B.; Zhao, J.; Chen, X.-H.; Zheng, K.-H.; Pan, F.-S. Effect of Ti Particles on the Microstructure and Mechanical Properties of AZ91 Magnesium Matrix Composites. Acta Metall. Sin. (Engl. Lett.) 2024, 37, 513–524. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Z.; Guo, S.; Zhou, Z.; Miao, Z.; Xie, S.; Huang, R.; Li, L. Hyperbranched graphene oxide structure-based epoxy nanocomposite with simultaneous enhanced mechanical properties, thermal conductivity, and superior electrical insulation. Compos. Sci. Technol. 2022, 217, 109082. [Google Scholar] [CrossRef]
- He, H.; Fan, G.; Saba, F.; Tan, Z.; Su, Z.; Xiong, D.; Li, Z. Enhanced distribution and mechanical properties of high content nanoparticles reinforced metal matrix composite prepared by flake dispersion. Compos. Part B 2023, 252, 110514. [Google Scholar] [CrossRef]
- Sager, A.; Esen, I.; Ahlatçi, H.; Turen, Y. Characterization and corrosion behavior of composites reinforced with ZK60, AlN, and SiC particles. Eng. Sci. Technol. 2023, 41, 101389. [Google Scholar] [CrossRef]
- Yang, L.; Shi, X.-H.; Feng, G.-H.; Tian, X.-F.; Wang, J.-C.; Qi, L.-H. Effect of SiC doping on corrosion behavior of Cf/Mg composites. Ceram. Int. 2020, 46, 22564–22574. [Google Scholar] [CrossRef]
- Singla, S.; Kang, A.S.; Sidhu, T. Characterization and electrochemical corrosion behaviour of FSPed WE43/nano-SiC surface composite. Mater. Today Proc. 2020, 26, 3138–3144. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Esmaily, M.; Mortazavi, N.; Svensson, J.; Halvarsson, M.; Jarfors, A.; Wessén, M.; Arrabal, R.; Johansson, L. On the microstructure and corrosion behavior of AZ91/SiC composites produced by rheocasting. Mater. Chem. Phys. 2016, 180, 29–37. [Google Scholar] [CrossRef]
- Velavan, K.; Palanikumar, K.; Dhanush, V.; Rajapandiyan, S.; Kumar, U.T.; Aishwarya, M. Corrosion and microstructure studies on magnesium alloy composite reinforced with mSiCp fabricated via powder metallurgy. Mater. Today Proc. 2023, 72, 2132–2138. [Google Scholar] [CrossRef]
- Zhou, Q.; Dai, X.; Zhang, X.; Liu, X.; Rao, J.; Zhang, Y.; Hou, Y. Core-shell Co-doped NiFe LDH@SiCnw nanocomposite towards efficiency microwave absorption with corrosion resistance. Appl. Surf. Sci. 2024, 655, 159633. [Google Scholar] [CrossRef]
- Lv, X.; Deng, K.; Wang, C.; Nie, K.; Shi, Q.; Niu, H. The corrosion resistance and discharge performance of as-extruded AZ91 alloy synergistically improved by the addition of submicron SiCp. Electrochim. Acta 2023, 441, 141843. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Peng, C.; Cai, Z. Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x = 5, 8, 11 wt.%). Corros. Sci. 2020, 167, 108487. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gao, X.Y.; Nie, K.B.; Li, Y.N.; Deng, K.K. Different effects of SiC dimensions on the microstructure and mechanical properties of magnesium matrix composites. Mater. Sci. Eng. A 2022, 847, 108487. [Google Scholar] [CrossRef]
- Vignesh, P.; Ramanathan, S.; Ashokkumar, M.; Sonar, T.; Ananthi, V. Microstructure, Mechanical, and Electrochemical Corrosion Performance of Ti/HA (Hydroxyapatite) Particles Reinforced Mg-3Zn Squeeze Casted Composites. Int. J. Met. 2023, 18, 1348–1360. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J.; Yu, X.; Xie, Y.; Zhang, J.; Yu, S.; Fan, D.; Liu, J. Surface microstructure and corrosion characterization of AZ31 magnesium alloys fabricated by laser surface-modification. J. Alloys Compd. 2024, 994, 174708. [Google Scholar] [CrossRef]
- Yin, S.; Duan, W.; Liu, W.; Wu, L.; Yu, J.; Zhao, Z.; Liu, M.; Wang, P.; Cui, J.; Zhang, Z. Influence of specific second phases on corrosion behaviors of Mg-Zn-Gd-Zr alloys. Corros. Sci. 2020, 166, 108419. [Google Scholar] [CrossRef]
- Gu, J.; Hu, Z.; Zhu, H.; Lu, D. Effect of laser cavitation peening on surface integrity and electrochemical corrosion behavior of magnesium alloy. Mater. Today Commun. 2024, 38, 107806. [Google Scholar] [CrossRef]
- Ren, Y.; Wan, T.; Xu, Y.; Zhang, K.; Zhang, M.; Li, J. Effects of stress aging treatment on the microstructure, mechanical properties and electrochemical corrosion behavior of Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2024, 997, 174686. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Song, G.-L.; Wu, P.-P.; Zheng, D.-J.; Wang, Z.-M. A burnished and Al-alloyed magnesium surface with improved mechanical and corrosion properties. Corros. Sci. 2021, 184, 109395. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; Liu, H.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Microstructure and corrosion behavior of Mg-Sc binary alloys in 3.5 wt.% NaCl solution. Corros. Sci. 2020, 174, 108831. [Google Scholar] [CrossRef]
- Feng, Y.-J.; Wei, L.; Chen, X.-B.; Li, M.-C.; Cheng, Y.-F.; Li, Q. Unexpected cathodic role of Mg41Sm5 phase in mitigating localized corrosion of extruded Mg-Sm-Zn-Zr alloy in NaCl solution. Corros. Sci. 2019, 159, 108133. [Google Scholar] [CrossRef]
- Wu, L.P. Potentiostatic preparation and in vitro characterization of functional hazenite conversion coatings on AZ31 magnesium alloy. Surf. Coat. Technol. 2024, 476, 130215. [Google Scholar] [CrossRef]
- Bahmani, A.; Shin, K.S. Controlling the microstructure and texture using multidirectional forging to develop a low corrosion rate Mg alloy. Corrosion 2020, 76, 750–765. [Google Scholar] [CrossRef]
- Li, W.; Nie, K.; Liu, Z.; Deng, K.; Li, Y.; Tong, X. Effect of hot extrusion on the microstructure and mechanical properties of SiCNWs/Mg-2Zn-0.1Y composite. Mater. Charact. 2022, 189, 111970. [Google Scholar] [CrossRef]
- Le, T.; Mao, P.; Hu, W.; Le, Q. The electrochemical corrosion behaviors and Mg-air battery anode performance of Mg–1Sn–1Ca-0.3Mn alloy with different grain size and Mg2Ca morphology. J. Power Sources 2024, 608, 234297. [Google Scholar] [CrossRef]
- Rahmati, M.; Zahrani, E.M.; Atapour, M.; Nezhad, A.N.; Hakimizad, A.; Alfantazi, A. In situ synthesis and electrochemical corrosion behavior of plasma electrolytic oxidation coating containing an osteoporosis drug on AZ31 magnesium alloy. Mater. Chem. Phys. 2024, 315, 128983. [Google Scholar] [CrossRef]
- Nunez-Lopez, C.; Habazaki, H.; Skeldon, P.; Thompson, G.; Karimzadeh, H.; Lyon, P.; Wilks, T. An investigation of microgalvanic corrosion using a model magnesium–silicon carbide metal matrix composite. Corros. Sci. 1996, 38, 1721–1729. [Google Scholar] [CrossRef]
- Tiwari, S.; Balasubramaniam, R.; Gupta, M. Corrosion behavior of SiC reinforced magnesium composites. Corros. Sci. 2007, 49, 711–725. [Google Scholar] [CrossRef]
- Greer, A.; Bunn, A.; Tronche, A.; Evans, P.; Bristow, D. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Li, W.-J.; Deng, K.-K.; Zhang, X.; Wang, C.-J.; Kang, J.-W.; Nie, K.-B.; Liang, W. Microstructures, tensile properties and work hardening behavior of SiCp/Mg-Zn-Ca composites. J. Alloys Compd. 2017, 695, 2215–2223. [Google Scholar] [CrossRef]
- Savaedi, Z.; Mirzadeh, H.; Aghdam, R.M.; Mahmudi, R. Effect of grain size on the mechanical properties and bio-corrosion resistance of pure magnesium. J. Mater. Res. Technol. 2022, 19, 3100–3109. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constantphase-element behavior caused by resistivity distributions in films II, Applications. J. Electrochem. Soc. 2010, 157, 458. [Google Scholar] [CrossRef]
- Li, W.; Liu, K.; Wu, J.; Lian, Q.; Qiang, Y.; Pan, J.; Jin, Y. Numerical simulation of carbon steel atmospheric corrosion under varying electrolyte-film thickness and corrosion product porosity. NPJ Mater. Degrad. 2023, 7, 3. [Google Scholar] [CrossRef]




| Zn | Y | Mg | Reinforcements | |
|---|---|---|---|---|
| MZY | 2 wt. % | 0.1 wt. % | Balance | |
| SiCp/MZY | 2 wt. % | 0.1 wt. % | Balance | 0.3 wt. % |
| SiCnp/MZY | 2 wt. % | 0.1 wt. % | Balance | 0.3 wt. % |
| SiCnw/MZY | 2 wt. % | 0.1 wt. % | Balance | 0.3 wt. % |
| Points | Elements/at% | Possible Compounds | ||||
|---|---|---|---|---|---|---|
| Mg | Zn | Y | Si | C | ||
| A | 98.1 | 1.9 | 0 | 0 | 0 | MgZn2 |
| B | 98.2 | 1.8 | 0 | 0 | 0 | MgZn2 |
| C | 97.8 | 2.2 | 0 | 0 | 0 | MgZn2 |
| D | 97.6 | 2.4 | 0 | 0 | 0 | MgZn2 |
| E | 0 | 0 | 0 | 9.45 | 90.55 | SiC |
| F | 93.1 | 0.8 | 0 | 1.5 | 4.6 | MgZn2, SiC |
| G | 77.5 | 0.7 | 0 | 8.4 | 13.4 | MgZn2, SiC |
| H | 0 | 0 | 0 | 13.35 | 86.65 | SiC |
| I | 99.31 | 0.69 | 0 | 0 | 0 | MgZn2 |
| J | 96.9 | 1.4 | 0 | 0 | 1.7 | MgZn2 |
| K | 85.6 | 0.7 | 0 | 4.6 | 9 | MgZn2, SiC |
| L | 0 | 0 | 0 | 36.93 | 63.07 | SiC |
| Materials | (Ω) | ) | ) | ) | ) | L (H) | ) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MZY | 7.57 | 0.79 | 0.960 | 26.37 | 0.26 | 0.943 | 35.32 | 311.2 | 431.9 | 61.69 |
| SiCp/MZY | 7.78 | 1.71 | 0.915 | 1516 | 0.21 | 0.779 | 79.27 | 550.2 | 13,400 | 1595.27 |
| SiCnp/MZY | 8.63 | 2.02 | 0.896 | 31.05 | 0.36 | 1 | 29.30 | 122.4 | 197.2 | 60.35 |
| SiCnw/MZY | 6.83 | 2.13 | 0.978 | 6.722 | 0.99 | 0.981 | 3.658 | 45.44 | 44.74 | 10.38 |
| Materials | (V vs. SCE) | (mV/dec) | ) | Corrosion Rate (mm/y) |
|---|---|---|---|---|
| MZY | −1.581 | −227.9 0.36 | 53.82 | 1.23 |
| SiCp/MZY | −1.583 | −197.9 1.02 | 5.076 | 0.11 |
| SiCnp/MZY | −1.558 | −269.9 0.57 | 117.4 | 2.68 |
| SiCnw/MZY | −1.574 | −275.8 0.56 | 277.8 | 6.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Nie, K.; Deng, K.; Li, Y. Regulating the Mechanical and Corrosion Properties of Mg-2Zn-0.1Y Alloy by Trace SiC with Different Morphologies. Crystals 2025, 15, 166. https://doi.org/10.3390/cryst15020166
Guo F, Nie K, Deng K, Li Y. Regulating the Mechanical and Corrosion Properties of Mg-2Zn-0.1Y Alloy by Trace SiC with Different Morphologies. Crystals. 2025; 15(2):166. https://doi.org/10.3390/cryst15020166
Chicago/Turabian StyleGuo, Furong, Kaibo Nie, Kunkun Deng, and Yanan Li. 2025. "Regulating the Mechanical and Corrosion Properties of Mg-2Zn-0.1Y Alloy by Trace SiC with Different Morphologies" Crystals 15, no. 2: 166. https://doi.org/10.3390/cryst15020166
APA StyleGuo, F., Nie, K., Deng, K., & Li, Y. (2025). Regulating the Mechanical and Corrosion Properties of Mg-2Zn-0.1Y Alloy by Trace SiC with Different Morphologies. Crystals, 15(2), 166. https://doi.org/10.3390/cryst15020166







