Innovative Hygroscopic Material for Humidity Regulation: Diatomaceous Earth Composite Porous Ceramic
Abstract
1. Introduction
2. Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Procedure I: Preparation of DE/Boehmite Porous Ceramics
2.2.2. Procedure II: Physical-Chemical Modification of Samples
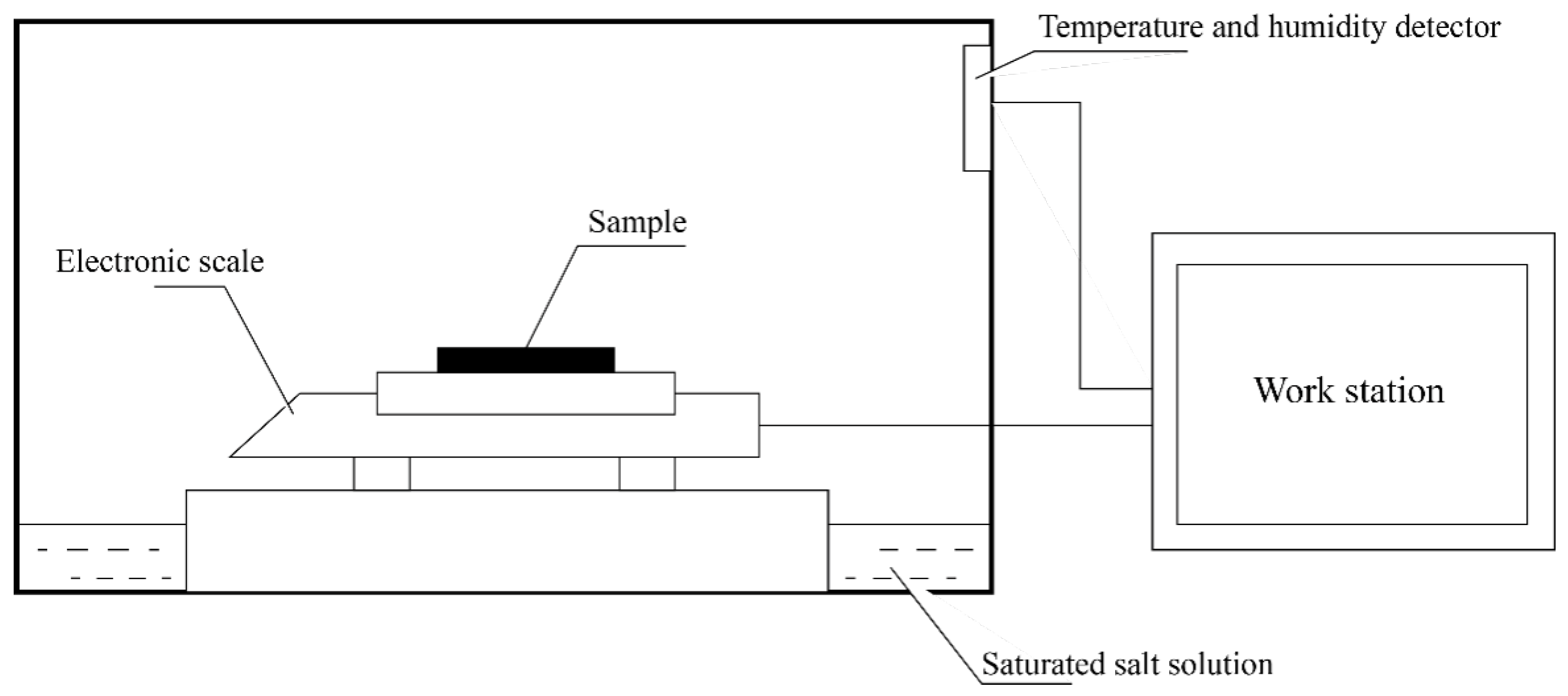
2.3. Characterization
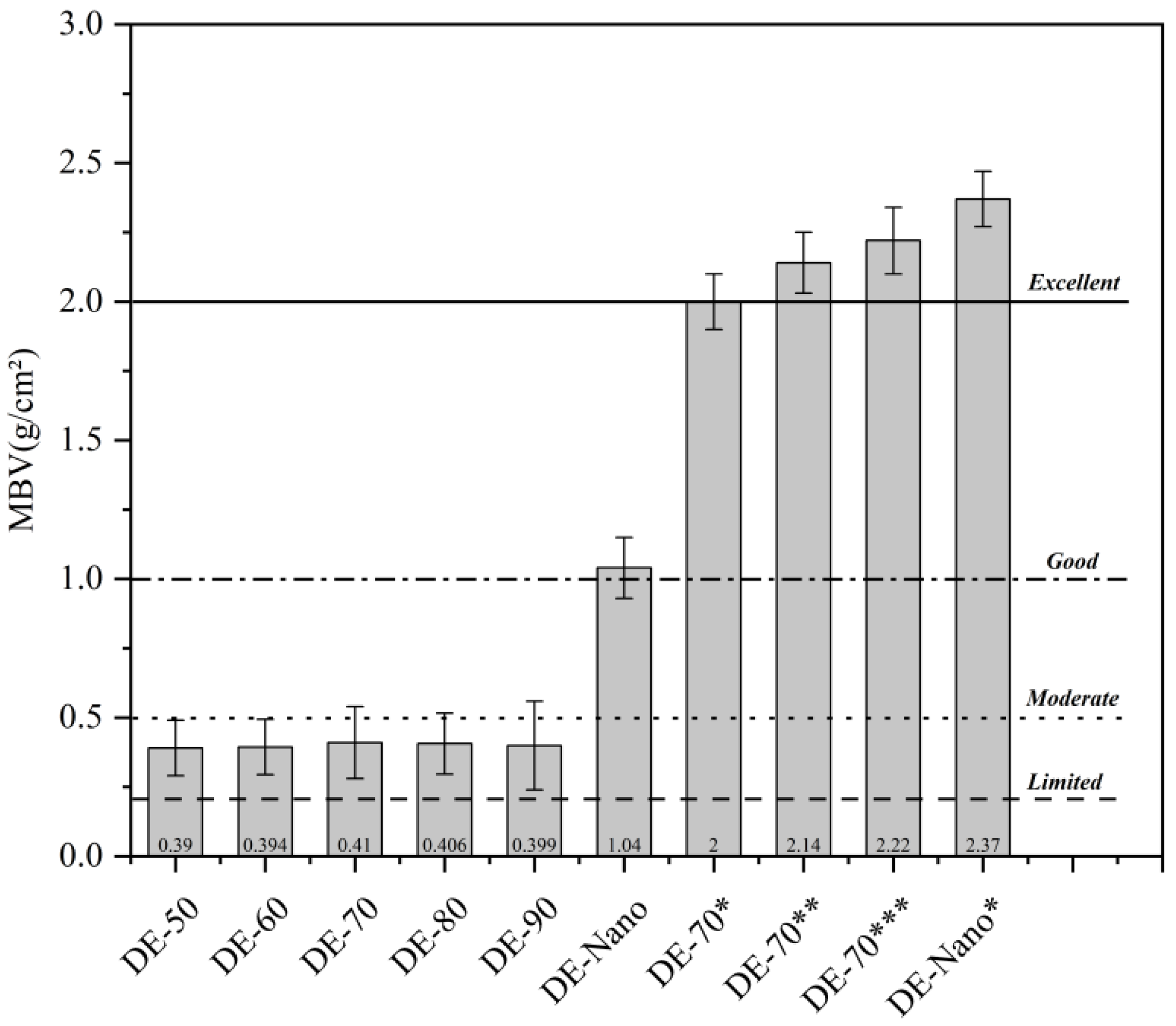
2.4. Moisture Buffer Value
3. Result and Discussion
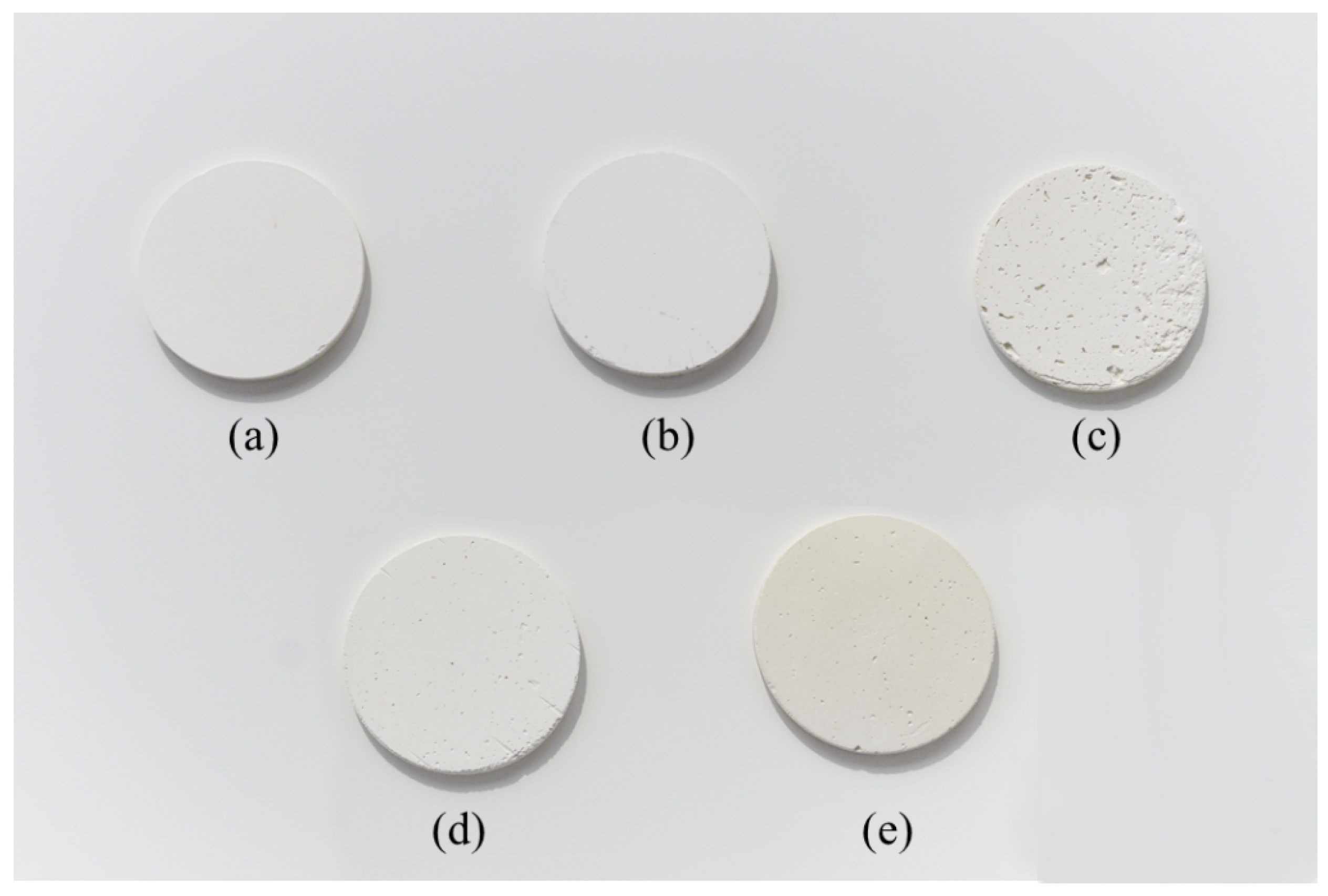
3.1. DE Composite Samples
3.2. Morphology
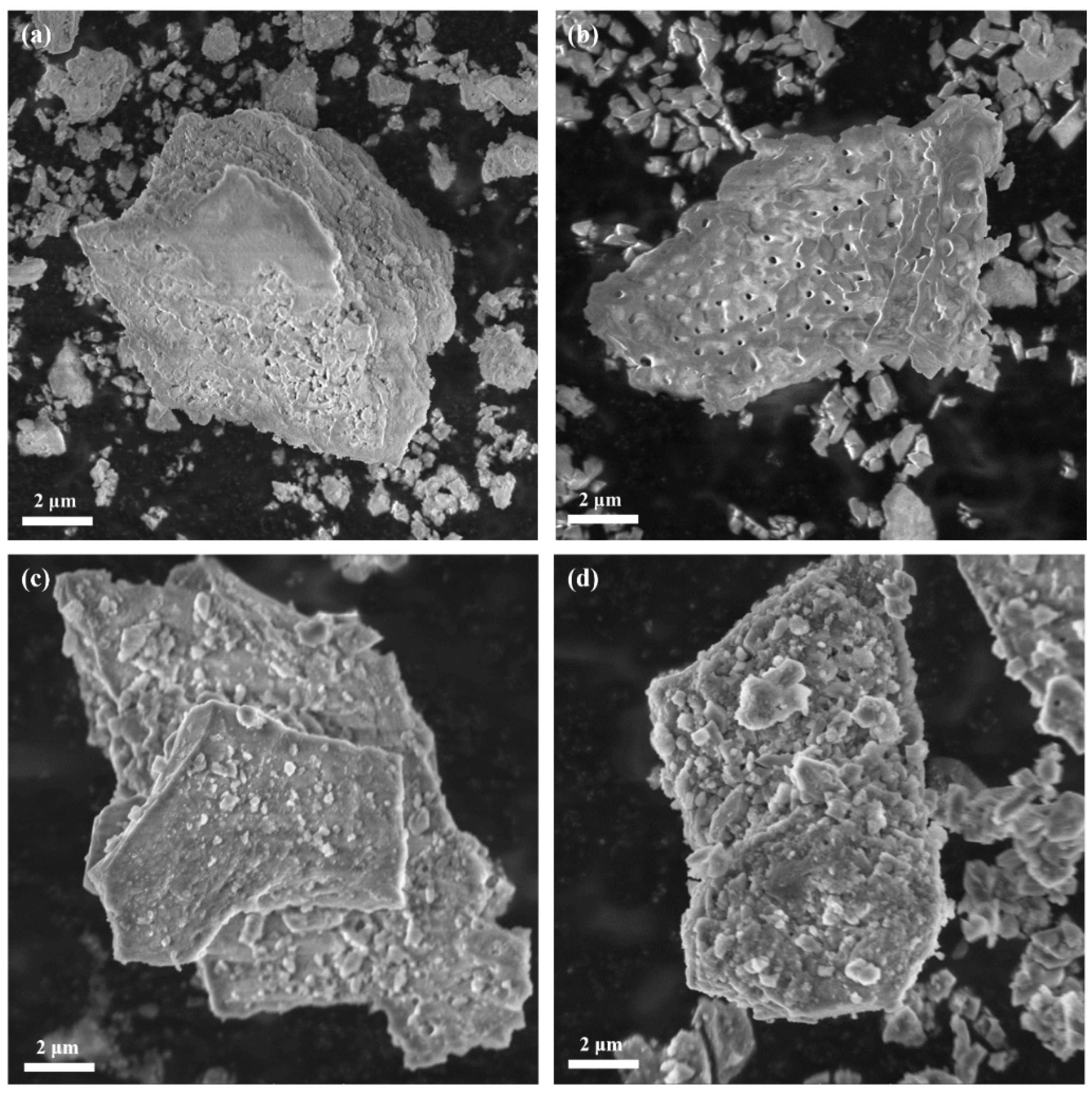
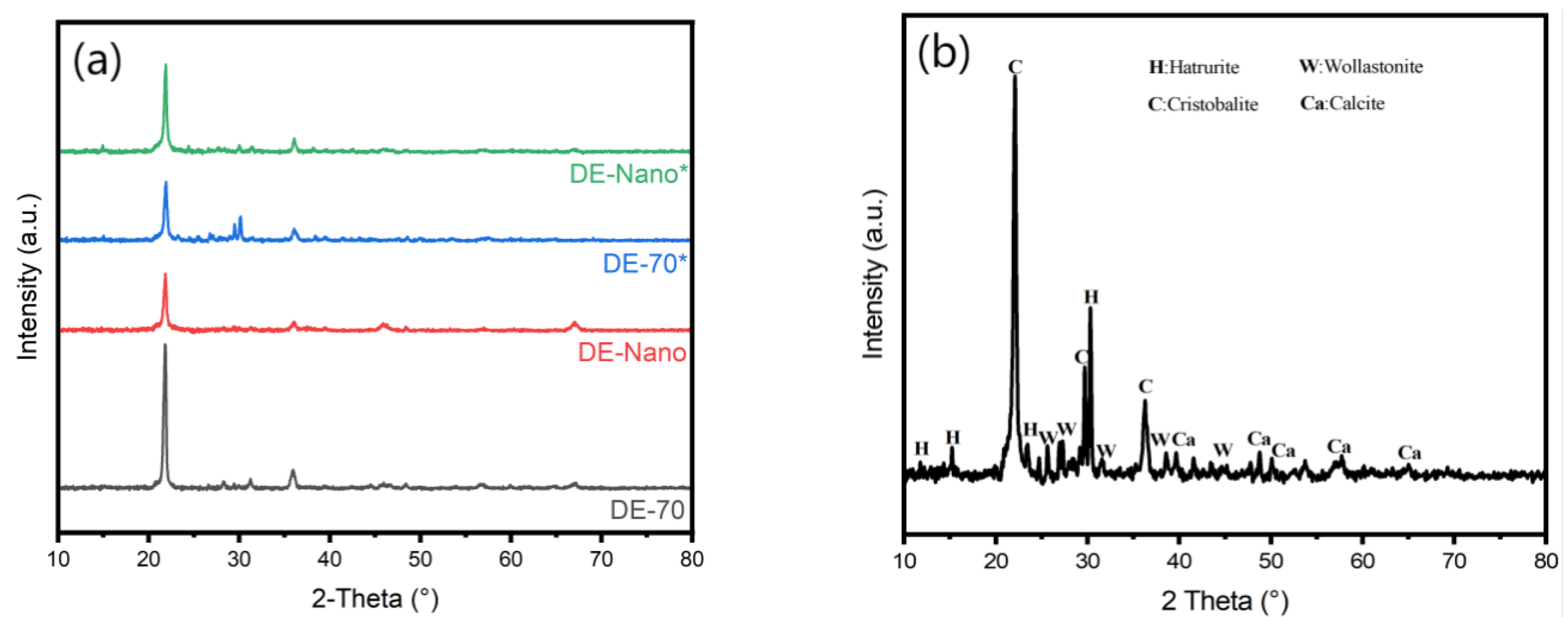
3.3. Crystal Structure
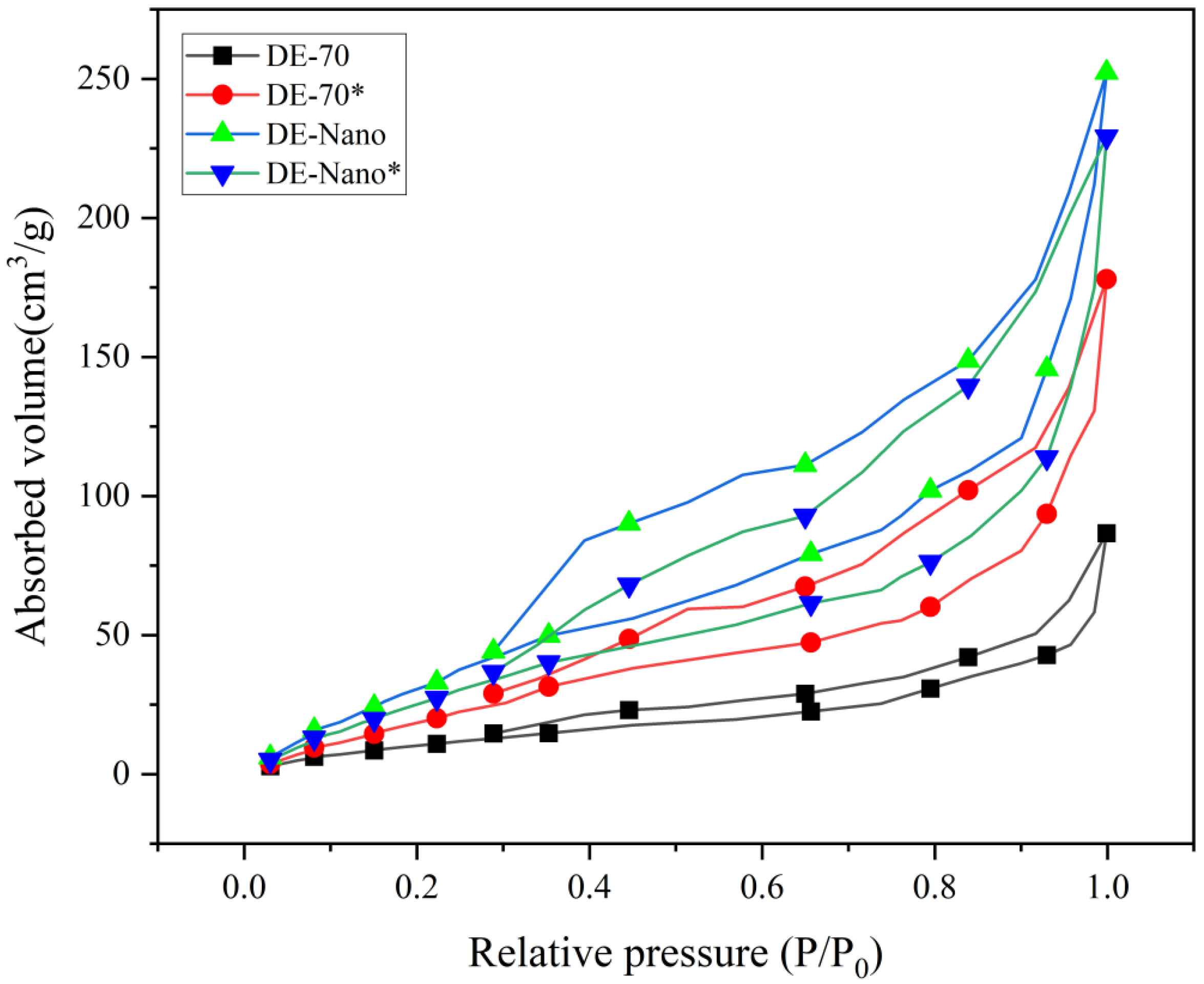
3.4. BET Specific Surface Area
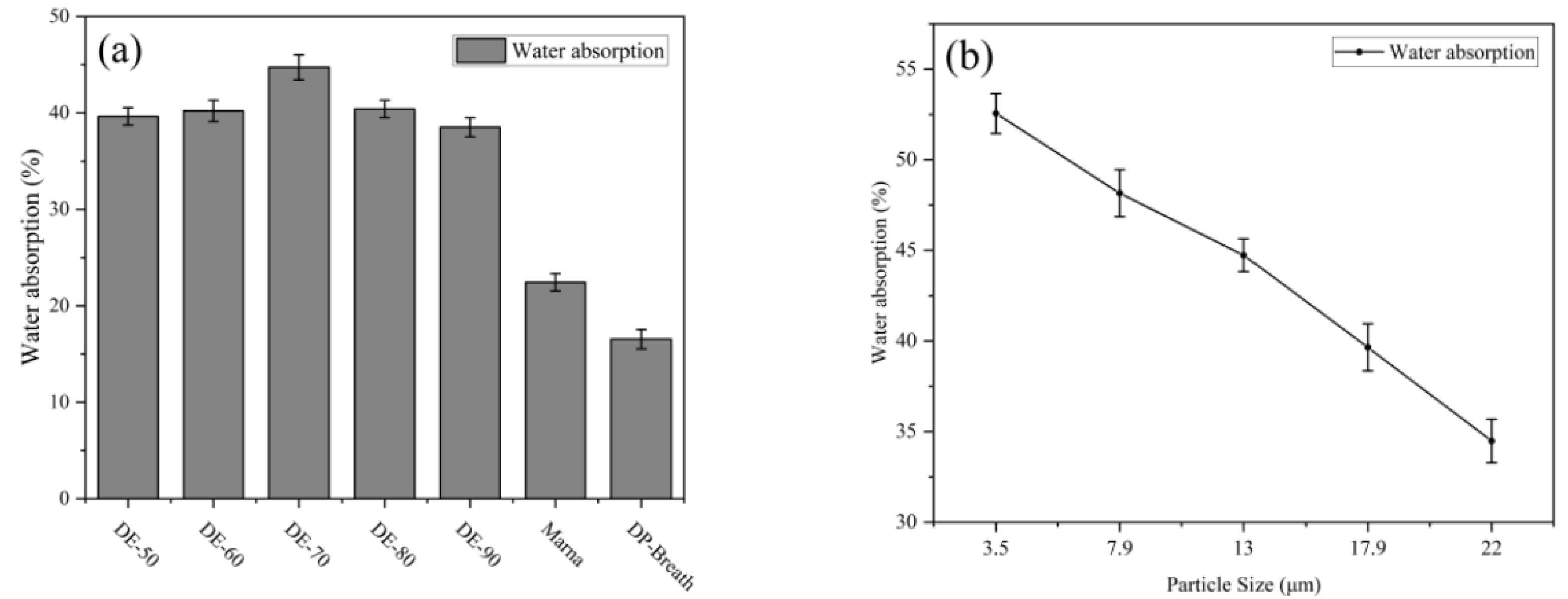
3.5. Humidity-Regulating Property
3.6. Performance Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.; Zhang, G.; Huang, Q. Field study on occupants’ thermal comfort and residential thermal environment in a hot-humid climate of China. Build. Environ. 2007, 42, 4043–4050. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhao, J. An introduction to the Chinese Evaluation Standard for the indoor thermal environment. Energy Build. 2014, 82, 27–36. [Google Scholar] [CrossRef]
- Frontczak, M.; Wargocki, P. Literature survey on how different factors influence human comfort in indoor environments. Build. Environ. 2011, 46, 922–937. [Google Scholar] [CrossRef]
- Migliore, E.; Yao, Z.; Deng, X. Porous Ceramics for the Design of Domestic Ecologies. In Proceedings of the International Conference on Human-Computer Interaction; Springer: New York, NY, USA, 2023. [Google Scholar]
- Viitanen, H.; Ritschkoff, A.C.; Peuhkuri, R. Moisture and bio-deterioration risk of building materials and structures. J. Build. Phys. 2010, 33, 201–224. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Zhang, Z. Human responses to high humidity in elevated temperatures for people in hot-humid climates. Build. Environ. 2017, 114, 257–266. [Google Scholar] [CrossRef]
- Kolokotsa, D.; Santamouris, M. Review of the indoor environmental quality and energy consumption studies for low income households in Europe. Sci. Total Environ. 2015, 536, 316–330. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Dong, S. Moisture buffering phenomenon and its impact on building energy consumption. Appl. Therm. Eng. 2017, 124, 337–345. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Zhang, L. Influence of climates and materials on the moisture buffering in office buildings: A comprehensive numerical study in China. Environ. Sci. Pollut. Res. 2022, 29, 14158–14175. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Liu, Z. Metal-organic framework MIL-100 (Fe) as a novel moisture buffer material for energy-efficient indoor humidity control. Build. Environ. 2018, 145, 234–242. [Google Scholar] [CrossRef]
- Erdoğan, S.; Akmil Başar, C.; Önal, Y. Particle size effect of raw material on the pore structure of carbon support and its adsorption capability. Part. Sci. Technol. 2017, 35, 330–337. [Google Scholar] [CrossRef]
- Bakr, H. Diatomite: Its characterization, modifications and applications. Asian J. Mater. Sci. 2010, 2, 121–136. [Google Scholar]
- Zahajská, P.; Komárek, M.; Sochorová, V. What is diatomite? Quat. Res. 2020, 96, 48–52. [Google Scholar] [CrossRef]
- Reka, A.; Zuberi, A.; Fatima, S. Diatomaceous earth: A literature review. J. Nat. Sci. Math. UT 2022, 7, 256–268. [Google Scholar]
- Akhtar, F.; Rehman, Y.; Bergström, L. A study of the sintering of diatomaceous earth to produce porous ceramic monoliths with bimodal porosity and high strength. Powder Technol. 2010, 201, 253–257. [Google Scholar] [CrossRef]
- Aggrey, P.; Andoh, S.; Amoako, P. The structure and phase composition of nano-silicon as a function of calcination conditions of diatomaceous earth. Mater. Today Proc. 2020, 33, 1884–1892. [Google Scholar] [CrossRef]
- Kang, Y.; Chang, S.J.; Kim, S. Hygrothermal behavior evaluation of walls improving heat and moisture performance on gypsum boards by adding porous materials. Energy Build. 2018, 165, 431–439. [Google Scholar] [CrossRef]
- Grommersch, B.M.; Pant, J.; Hopkins, S.P.; Goudie, M.J.; Handa, H. Biotemplated Synthesis and Characterization of Mesoporous Nitric Oxide-Releasing Diatomaceous Earth Silica Particles. ACS Appl. Mater. Interfaces 2018, 10, 2291–2301. [Google Scholar] [CrossRef]
- Hong, T.D.; Jenkins, N.E.; Ellis, R.H. Saturated salt solutions for humidity control and the survival of dry powder and oil formulations of Beauveria bassiana conidia. J. Invertebr. Pathol. 2005, 89, 136–143. [Google Scholar] [CrossRef]
- Lu, T.; Chen, C. Uncertainty evaluation of humidity sensors calibrated by saturated salt solutions. Measurement 2007, 40, 591–599. [Google Scholar] [CrossRef]
- Simonova, I.A.; Pavlov, A.R.; Kislyakov, I.M. Water sorption on composite “silica modified by calcium nitrate”. Microporous Mesoporous Mater. 2009, 122, 223–228. [Google Scholar] [CrossRef]
- Cortés, F.; Trujillo, A.; Gil, A. Water adsorption on zeolite 13X: Comparison of the two methods based on mass spectrometry and thermogravimetry. Adsorption 2010, 16, 141–146. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.Z.; Oliveira, R.G. The performance of two adsorption ice making test units using activated carbon and a carbon composite as adsorbents. Carbon 2006, 44, 2671–2680. [Google Scholar] [CrossRef]
- Gong, L.; Wang, R.Z.; Oliveira, R.G. Adsorption equilibrium of water on a composite adsorbent employing lithium chloride in silica gel. J. Chem. Eng. Data 2010, 55, 2920–2923. [Google Scholar] [CrossRef]
- Pino, L.; Ghibaudi, E.; Torchio, M.F. Composite materials based on zeolite 4A for adsorption heat pumps. Adsorption 1997, 3, 33–40. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Deng, X. Preparation of a novel diatomite-based PCM gypsum board for temperature-humidity control of buildings. Build. Environ. 2022, 226, 109732. [Google Scholar] [CrossRef]
- Latif, E.; Tucker, S.; Ciupala, M.A.; Wijeyesekera, D.C. Moisture buffer potential of experimental wall assemblies incorporating formulated hemp-lime. Build. Environ. 2015, 93, 199–209. [Google Scholar] [CrossRef]
- Rode, C.; Peuhkuri, R.; Hansen, K.K. Moisture Buffering of Building Materials; BYG Report No. R-127; Technical University of Denmark, Department of Civil Engineering: Kongens Lyngby, Denmark, 2005. [Google Scholar]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 3–9. [Google Scholar] [CrossRef]

| Sample | Ingredient (DE/Boehmite) | Post-Processing | Procedure |
|---|---|---|---|
| DE-50 | 5/5 | - | |
| DE-60 | 6/4 | - | |
| DE-70 | 7/3 | - | I |
| DE-80 | 8/2 | - | |
| DE-90 | 9/1 | - | |
| DE-Nano | 4/3/3 (DE/Boehmite/Nano-() ) | Add nano-() | |
| DE-Salt | 7/3 | Pickling, Hygroscopic salt loading (32%) | |
| DE-Nano * | 4/3/3 (DE/Boehmite/Nano-() ) | Add nano-(), salt loading (32%) | II |
| DE-70 * | 7/3 | Load salt concentration: 32% | |
| DE-70 ** | 7/3 | Load salt concentration: 64% | |
| DE-70 *** | 7/3 | Load salt concentration: 96% |
| Standard | Value |
|---|---|
| Negligible | 0.0–0.2 |
| Limited | 0.2–0.5 |
| Moderate | 0.5–1.0 |
| Good | 1.0–2.0 |
| Excellent | 2.0–upwards |
| Sample | Dpc (nm) | ||
|---|---|---|---|
| DE-70 | 17.6 ± 0.2 | 0.154 ± 0.01 | 18.8 ± 0.1 |
| DE-70 * | 12.4 ± 0.2 | 0.11 ± 0.01 | 17.8 ± 0.1 |
| DE-Nano | 19.8 ± 0.2 | 0.127 ± 0.01 | 12.4 ± 0.1 |
| DE-Nano * | 14.7 ± 0.2 | 0.072 ± 0.01 | 11.6 ± 0.1 |
| Sample | Concentration (LiCl) | Over-Absorbing Time (h) | Water Absorption (%) |
|---|---|---|---|
| DE-70 * | 96% | 12 ± 0.1 | 31.5 ± 0.3 |
| DE-70 ** | 64% | 19 ± 0.2 | 30.3 ± 0.2 |
| DE-70 *** | 32% | - | 28.1 ± 0.2 |
| DE-Nano | 32% | 9 ± 0.2 | 32.6 ± 0.1 |
| Sample | (g) | (g/cm) | Standard |
|---|---|---|---|
| DE-70 | 0.495 ± 0.1 | 0.41 ± 0.03 | Limited |
| DE-Nano | 1.263 ± 0.1 | 1.04 ± 0.06 | Good |
| DE-70 * | 2.43 ± 0.1 | 2.00 ± 0.07 | Good |
| DE-Nano * | 2.88 ± 0.1 | 2.37 ± 0.03 | Excellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Migliore, E.; Galluzzi, M.; Liu, J. Innovative Hygroscopic Material for Humidity Regulation: Diatomaceous Earth Composite Porous Ceramic. Crystals 2025, 15, 158. https://doi.org/10.3390/cryst15020158
Yao Z, Migliore E, Galluzzi M, Liu J. Innovative Hygroscopic Material for Humidity Regulation: Diatomaceous Earth Composite Porous Ceramic. Crystals. 2025; 15(2):158. https://doi.org/10.3390/cryst15020158
Chicago/Turabian StyleYao, Zhennan, Enza Migliore, Massimiliano Galluzzi, and Jingze Liu. 2025. "Innovative Hygroscopic Material for Humidity Regulation: Diatomaceous Earth Composite Porous Ceramic" Crystals 15, no. 2: 158. https://doi.org/10.3390/cryst15020158
APA StyleYao, Z., Migliore, E., Galluzzi, M., & Liu, J. (2025). Innovative Hygroscopic Material for Humidity Regulation: Diatomaceous Earth Composite Porous Ceramic. Crystals, 15(2), 158. https://doi.org/10.3390/cryst15020158






