Abstract
Recently, a variety of nephrite containing localized pink mineral aggregates has emerged on the market, which is sometimes referred to as “peach blossom jade” by some merchants. Currently, there is limited research on this type of nephrite containing pink minerals, and its detailed mineral composition characteristics and coloration mechanisms remain unclear. In this study, four samples of nephrite containing pink minerals were systematically investigated using conventional gemological tests, as well as modern analytical techniques such as X-ray powder diffraction (XRD), infrared spectroscopy (IR), laser Raman spectroscopy, ultraviolet–visible (UV-Vis) absorption spectroscopy, electron probe microanalysis (EPMA), and X-ray fluorescence spectroscopy (XRF). These techniques were employed to elucidate the mineral composition, chemical composition, spectroscopic features, and coloration origins of the samples. The results indicate that the primary mineral constituent of the samples is tremolite, with accessory minerals including zoisite, muscovite, orthoclase, andesine, diopside, and prehnite. The major chemical components of the samples are SiO2, CaO, and MgO, along with minor amounts of Al2O3, K2O, and FeOT. The overall green hue of the samples is positively correlated with Fe content. The pink mineral present in the samples is predominantly Mn-bearing zoisite, and the pink coloration of zoisite is primarily attributed to the energy level transitions of Mn2+ at approximately 540 nm and 440 nm.
1. Introduction
China is renowned for its ancient and culturally diverse jade culture, earning the title of the “Land of Jade.” Over the course of nearly ten millennia of jade culture, nephrite has emerged as the most significant and dominant variety. Nephrite, a naturally occurring aggregate of tremolite minerals, is esteemed for its beauty, durability, rarity, and artistic craftsmanship, making it an ideal material for jewelry and ornaments. Deposits of nephrite are found worldwide, with notable occurrences not only in China but also in Russia, South Korea, Canada, New Zealand, Australia, Poland, the United States, Pakistan, and others [1,2,3,4,5,6,7,8]. The genesis of nephrite is commonly classified into two types: marble type (D-type) and serpentine type (S-type). Marble-type nephrite typically forms in the contact zones between granite/granodiorite and dolomitic marble, where the intrusive rocks supply the ore-forming hydrothermal fluids necessary for nephrite formation. In contrast, serpentinite-type nephrite originates in the contact zones between serpentinite (or peridotite) and granite (such as plagiogranite or metasedimentary rocks) [9,10,11]. Recently, several nephrite deposits have been reported to be associated with limestone metasomatism, including the Mida nephrite deposit in Xinjiang, the Xinyu nephrite deposit in Jiangxi, and others. These nephrites are closely related to limestone rather than dolomite, and some scholars refer to them as calcite-type (C-type) nephrites [12,13,14].
The predominant mineral component of nephrite is tremolite, and in addition to tremolite, a wide range of accessory minerals are also present, including actinolite, diopside, phlogopite, calcite, epidote, cerium epidote, apatite, chlorite, zoisite, scapolite, graphite, zircon, titanite, rutile, quartz, mica, pyrite, pyrrhotite, garnet, hornblende, edenite, pargasite, prehnite, fluocerite-(Ce), hexasilicate magnesium, limonite, goethite, and manganese secondary oxides [2,3,15,16,17,18]. The mineral paragenesis in nephrite holds significant importance for studying the genesis of nephrite deposits, and some accessory minerals are indicative of the deposit’s origin. For instance, fluocerite-(Ce) and hexasilicate magnesium are found in Qiemo nephrite from Xinjiang [18]; fluorophlogopite and fluorapatite is present in Kavokta nephrite from Russia [2]; and arsenopyrite in the orthorhombic form is identified in Zloty Stok nephrite from Poland [6]. Investigating the sequence of accessory mineral formation in nephrite can aid in elucidating and reconstructing the formation process of nephrite. Minerals formed at different stages provide abundant information for investigating the genesis of nephrite, playing a crucial role in understanding its formation process, fluid sources, metamorphism, mineral assemblages, formation conditions, and guiding the direction for mineral exploration [2,3,11].
Recently, a variety of nephrite with an overall yellowish-green hue and localized patches of pink has emerged on the market, garnering considerable favor among consumers. This type of nephrite is commercially termed “Peach Blossom Jade”. Its appearance exhibits distinct differences from the previously reported anthophyllite jade and rhodonite jade [19,20], which are predominantly light pink. The pink mineral assemblages in this nephrite are distributed in the form of clumps, spots, and veins within the nephrite matrix, with colors ranging from light to deep pink. Currently, there is limited research on nephrite containing such localized pink mineral assemblages, and the detailed mineralogical composition and color genesis remain unclear. This study intends to analyze the chemical composition, mineralogical composition, and spectroscopic characteristics of nephrite containing pink mineral assemblages, with the aim of enriching and refining the knowledge system of nephrite minerals and providing a reference for future related research.
2. Materials and Methods
2.1. Samples
The four nephrite “peach blossom jade” samples (TFN-1, TFN-2, TFN-3, TFN-4) used in this study were all collected from the Shifosi Jade Market in Zhenping County, Nanyang City, with specific origins unknown. The samples were all processed into square plaques with varying shades of color. Among them, TFN-1 is white jade, TFN-2 is cela light greenish white jade, and TFN-3 and TFN-4 are bluish green jade. Each sample contains varying amounts of pink accessory minerals (Figure 1).
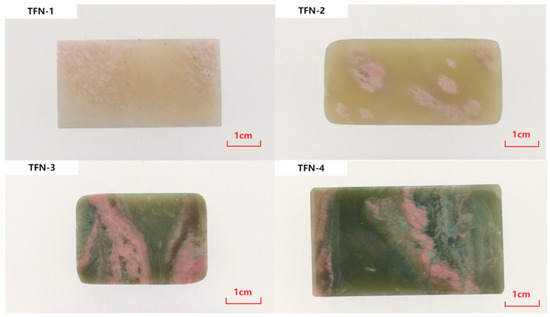
Figure 1.
Nephrite samples with pink minerals.
2.2. Methods
In the Gemstone Identification Laboratory of the Gemology Experimental Teaching Center at China University of Geosciences (Beijing, China), the refractive indices of the samples were measured using a handheld refractometer (spot measurement method), and the fluorescence characteristics of the samples were observed under a UV fluorescent lamp. The relative densities of the samples were measured using a Yitenuo ET-320S high-precision electronic balance (Beijing, China), with each sample tested three times and the average value taken.
Microscopic observations and photography of the samples were conducted at the Structure and Morphology Observation Laboratory of the Gemology Experimental Teaching Center using a Baoguang GI-MP22 gem photographic microscope (Nanjing, China). Compositional and structural observations of the samples were performed using an Olympus BX51 polarizing microscope from Tokyo, Japan.
Fourier Transform Infrared (FTIR) spectroscopy was conducted using a Bruker Tensor 27 spectrometer from Ettlingen, Germany. Testing conditions included reflection mode, 32 scans, a resolution of 4 cm−1, and a measurement range of 2000–400 cm−1. Micro-FTIR spectroscopy was performed using a Bruker LUMOS micro-FTIR spectrometer from Karlsruhe, Germany, with testing conditions including ATR mode, 32 scans, a resolution of 4 cm−1, and a measurement range of 4000–600 cm−1. These experiments were conducted at the Molecular Spectroscopy Laboratory of the Gemology Experimental Teaching Center.
Laser micro-Raman spectroscopy was performed using a Horiba LabRAM HR Evolution spectrometer from Villeneuve d’Ascq, France. The experiments were conducted at the Molecular Spectroscopy Laboratory of the Gemology Experimental Teaching Center. Testing conditions included a 532 nm laser, 50 mW power, 600 gr/mm grating, 100 confocal aperture, 5 s scan time, 2 accumulations, and a collection range of 100–1500 cm−1.
UV–Visible absorption spectroscopy was conducted using a Biaoqi GEM-3000 jewelry testing instrument (Guangzhou, China). The experiments were performed at the Molecular Spectroscopy Laboratory of the Gemology Experimental Teaching Center. Testing conditions included reflection mode, 120 ms integration time, 10 averages, 2 smoothing width, and a collection range of 225–1000 nm.
A semi-quantitative chemical analysis of the samples was performed at the Composition Analysis Laboratory of the Gemology Experimental Teaching Center using a Shimadzu EDX-7000 energy-dispersive X-ray fluorescence spectrometer from Kyoto, Japan. Testing conditions included a Rh target, a measurement element range of 11Na-92U, test voltages of 15 KV (Na-Sc) and 50 KV (Al-U), a test current of 1000 μA, a 3 mm collimator, the fundamental parameter method (FP method), and a vacuum testing environment.
An X-ray powder diffraction analysis was conducted using a Rigaku SmartLab 9 kW X-ray diffractometer from Tokyo, Japan, located at the Experimental Center of the Scientific Research Institute, China University of Geosciences (Beijing, China). Testing conditions included grinding the samples to 300 mesh, a Cu Ka target, a voltage of 40 kV, a current of 200 mA, a graphite monochromator, continuous scanning at a speed of 10°/min, and ambient conditions of 27 °C and 15% humidity.
An electron probe microanalysis (EPMA) was performed using a Shimadzu EPMA-1720 instrument at the Experimental Center of the Scientific Research Institute, China University of Geosciences (Beijing, China). Testing conditions included an accelerating voltage of 15 kV, an accelerating current of 10 nA, a beam spot diameter of 5 μm, a peak time of 20 s for major element analysis, and a background time of 10 s before and after analysis.
3. Results and Discussion
3.1. Conventional Gemological Characteristics
Upon magnified examination, the sample exhibits a fine-grained structure with pink and white minerals predominantly distributed in clumpy or spotty patterns, intertwined with the host minerals (Figure 2a,b,d). Black dot-like impurity minerals are observable within some of the pink clumps (Figure 2b), and cracks are present in a few samples (Figure 2c).
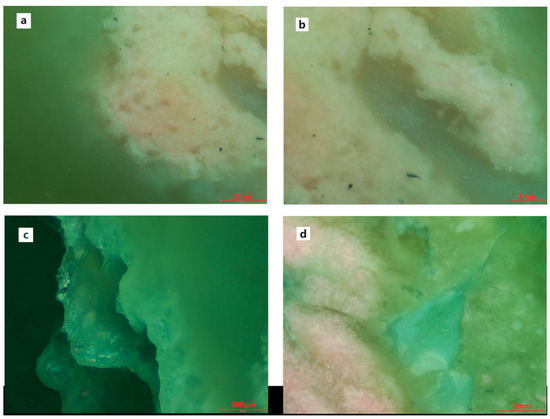
Figure 2.
Magnification features of samples. (a,b,d) The pink and white minerals are distributed in clumpy or spotty patterns; (b) Black dot-like impurity minerals are observable within some of the pink clumps; (c) Cracks in the sample.
Other conventional gemological tests indicate that the refractive index of the sample ranges from approximately 1.61 to 1.62 (spot measurement), with a relative density between 2.94 and 2.96. The samples exhibit inert behavior under ultraviolet fluorescence lighting. The conventional gemological characteristics of the sample are summarized in Table 1.

Table 1.
Gemological characteristics of samples.
3.2. Polarized Light Microscopy
Under polarized light microscopy, it can be observed that tremolite exhibits a typical felted, fibrous interwoven structure. Under crossed polars, the interference colors range from second-order blue to blue-green, with a positive relief and predominantly radial or bundle-like aggregates composed of columnar fibers (as shown in Figure 3a). Zoisite appears as first-order gray, with a high positive relief, and is distributed in the form of granular-columnar aggregates within the tremolite crystals (Figure 3b).
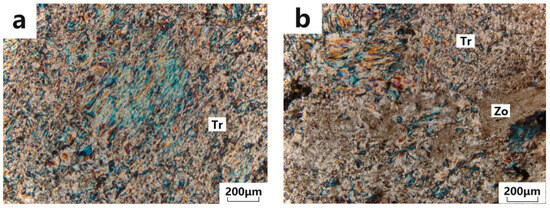
Figure 3.
Microphotographs under polarized light microscope. (a) Tremolite exhibits a typical felted, fibrous interwoven structure; (b) Zoisite occurs as granular-columnar aggregates distributed within the tremolite crystals. Tr: tremolite; Zo: zoisite.
3.3. XRD Testing
The X-ray diffraction patterns obtained from the experiments are presented in Figure 4. The results indicate that the major intense diffraction peaks in the patterns of TFN-1, TFN-2, and TFN-4 closely match the standard diffraction peaks of tremolite at 8.380 Å (110), 3.121 Å (310), and 2.705 Å (151) (ICDD PDF#71-1058), confirming that the primary mineral component of the samples is tremolite. Additionally, the samples contain small amounts of other minerals. Specifically, TFN-1 exhibits characteristic lines of zoisite at 2.86 Å and 2.69 Å (ICDD PDF#85-1631), as well as plagioclase at 4.04 Å and 3.20 Å (ICDD PDF#83-1607). TFN-2 shows characteristic lines of muscovite at 9.97 Å, 3.63 Å, 3.06 Å, and 2.56 Å (ICDD PDF#71-1885), along with weak characteristic lines of prehnite at 3.48 Å, 3.03 Å, and 2.56 Å (ICDD PDF#72-1374). In TFN-4, apart from the accessory mineral muscovite, trace amounts of plagioclase lines are also observed.
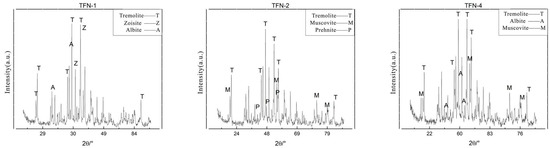
Figure 4.
XRD patterns of nephrite samples.
3.4. Chemical Composition Analysis
X-ray fluorescence spectrometry (XRF) tests were conducted on both the matrix and pink mineral portions of four nephrite samples, with the results presented in Table 2 and Table 3. The primary chemical components of the matrix portions are SiO2, CaO, and MgO, whose contents closely match the standard chemical composition of tremolite. As the samples progress from white nephrite to light greenish white nephrite and finally to bluish green nephrite, the green hue deepens gradually, accompanied by an increase in FeOT content, indicating that the green tone of the sample matrix is predominantly influenced by the content of the Fe element.

Table 2.
XRF test results of the sample matrix portions (wt%).

Table 3.
XRF test results of pink minerals (wt%).
Due to the uneven distribution of pink mineral aggregates within each sample, three points were selected for testing in the pink regions of each sample, and the average values were calculated. The test results reveal that, in addition to the main chemical components of tremolite, the pink regions also contain Al2O3 and K2O, presumably associated with the presence of accessory minerals such as zoisite, muscovite, and feldspar in the samples.
An electron probe microanalysis (EPMA) was performed on four pink mineral-bearing areas of thin sections from two samples, TFN-1 and TFN-4, with the calculation results and backscattered electron (BSE) images presented in Table 4 and Figure 5. The analytical data reveal the presence of tremolite and zoisite in both samples. Both tremolite and actinolite are calcic amphiboles within the amphibole group; the primary difference between them lies in the relative contents of Mg and Fe2+, and they can be clearly distinguished by calculating their Mg/(Mg + Fe2+) ratios [21]. Additionally, TFN-4 contains muscovite, while TFN-1 contains chlorite and andesine. As shown in Table 4, the calculation results indicate that the chlorite present in the sample is clinochlorite [22]. The ω(SiO2) content of tremolite in the samples ranges from 52.56% to 55.52%, with an average of 54.04%; the ω(CaO) content ranges from 14.35% to 14.56%, with an average of 14.46%, slightly higher than the theoretical value; and the ω(MgO) content ranges from 24.99% to 25.95%, with an average of 25.47%. For the zoisite in the samples, the ω(SiO2) content is 36.22% to 38.22%, with an average of 37.22%; the ω(CaO) content is 25.44% to 26.38%, with an average of 25.91%; and the ω(Al2O3) content is 32.89% to 34.82%, with an average of 33.86%, all of which are close to the theoretical values.

Table 4.
Results of electron microprobe testing of representative minerals in the sample (wt%).
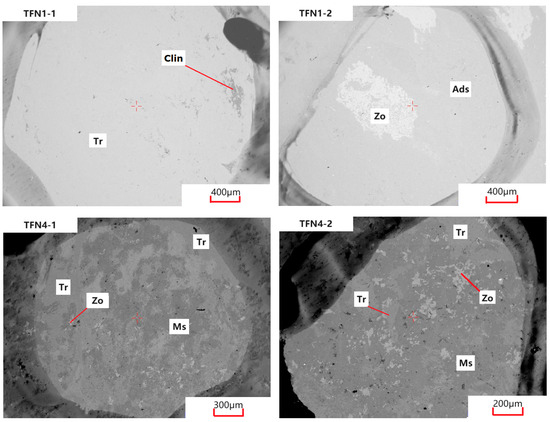
Figure 5.
BSE images of samples. Tr: tremolite; Clin: clinochlore; Zo: zoisite; Ads: andesine; Ms: muscovite.
3.5. Infrared Spectroscopy
Infrared diffuse reflectance measurements were conducted on the substrate portions of four samples, with the spectra presented in Figure 6 (after Kubelka–Munk transformation). The infrared spectra of the substrate portions of the four samples exhibit a general consistency with the standard spectrum of tremolite, characterized by absorption bands at 1152, 1092, 1040, 993, and 929 cm−1, which are attributed to the antisymmetric stretching vibrations of O-Si-O and Si-O-Si, as well as the symmetric stretching vibration of O-Si-O. Absorption bands at 767, 688, 665, and 598 cm−1 are ascribed to the symmetric stretching vibration of O-Si-O, while those near 538, 513, and 464 cm−1 arise from the bending vibrations of Si-O [23,24].
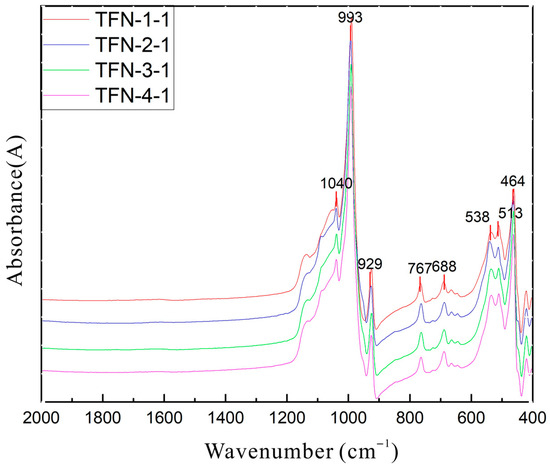
Figure 6.
Infrared spectra of samples by diffuse reflection (after Kramers–Kronig transformation).
Due to the samples being cryptocrystalline aggregates with fine mineral grains, the spectra of the pink portions often mix with the tremolite peaks of the substrate during infrared diffuse reflectance measurements. To further ascertain the mineral composition and structure of the pink portions of the samples, micro-infrared spectroscopy was performed, with the spectra shown in Figure 7. A comprehensive analysis of the obtained spectra reveals that, in addition to tremolite, characteristic structural peaks of zoisite, muscovite, and prehnite are also present in the pink clump regions. Specifically, the peak near 3677 cm−1 is attributed to the antisymmetric stretching vibration of OH in tremolite, the band at 3618 cm−1 in the muscovite spectrum corresponds to OH stretching vibration, and the bands at 1019 cm−1 and 982 cm−1 represent Si-O stretching vibrations. In the zoisite infrared spectrum, the band at 3323 cm−1 is due to OH stretching vibration, while the bands at 1137 cm−1, 1110 cm−1, 978 cm−1, and 894 cm−1 are associated with the asymmetric stretching vibrations of Si-O-Si and Si-O(Al). The band at 3483 cm−1 in the prehnite infrared spectrum is caused by OH stretching vibration. The positions of the infrared absorption bands arising from OH vibrations in these minerals are distinctly different, facilitating their differentiation [25,26].
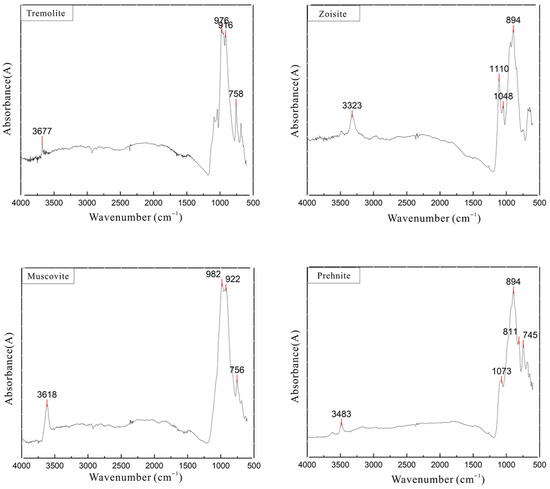
Figure 7.
Micro-infrared spectra of pink mineral aggregates.
3.6. Raman Spectroscopy
A Raman spectroscopic analysis was conducted on both the substrate and pink portions of four samples, with the resulting laser Raman spectra presented in Figure 8. The Raman spectra of the substrate portions exhibit spectral peaks that are largely consistent with the characteristic peaks of tremolite. Specifically, the Raman peaks below 400 cm−1, such as those near 178 cm−1 and 224 cm−1, are attributed to lattice vibrations. The peak near 530 cm−1 corresponds to Si-O bending vibrations, while the strongest peak at 673 cm−1 is assigned to the symmetric stretching vibration of Si-O-Si. The peaks near 930 cm−1, 1030 cm−1, and 1060 cm−1 are attributed to Si-O stretching vibrations [27].
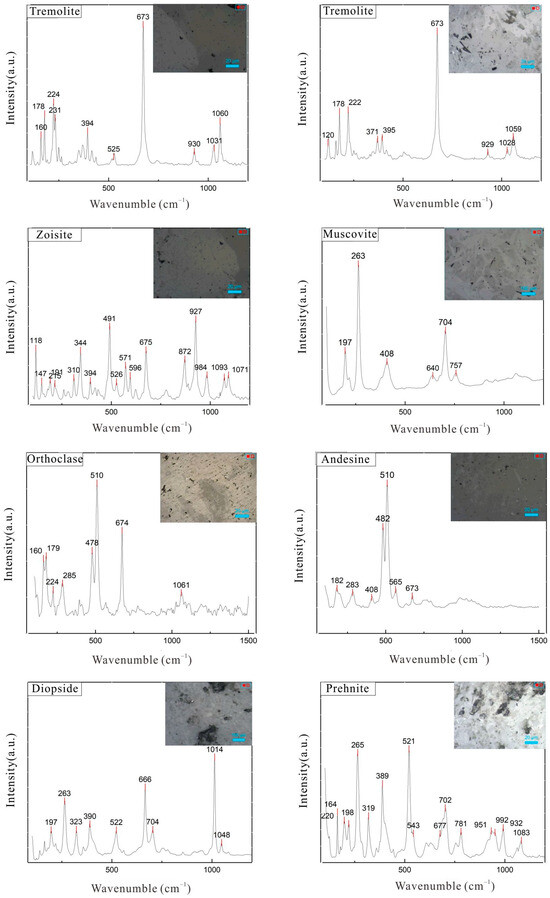
Figure 8.
Laser Raman spectra of samples.
The Raman spectra of the pink portions reveal characteristic peaks at 257 cm−1, 282 cm−1, 310 cm−1, 344 cm−1, 432 cm−1, 491 cm−1, 571 cm−1, 675 cm−1, 872 cm−1, 927 cm−1, 984 cm−1, and 1093 cm−1, which are largely consistent with the standard spectrum of zoisite, indicating that the constituent mineral is zoisite. Among these, the Raman peaks at 257 cm−1, 282 cm−1, and 310 cm−1 can be attributed to lattice vibrations; the peak at 344 cm−1 is assigned to SiO4 rotational or M-O translational vibrations; the peaks at 491 cm−1, 571 cm−1, and 675 cm−1 are attributed to Si-O bending vibrations; and the peaks at 872 cm−1, 927 cm−1, 984 cm−1, and 1093 cm−1 are attributed to Si-O stretching vibrations. Additionally, characteristic structural peaks of other minerals such as muscovite, orthoclase, andesine, diopside, and prehnite were also detected in the samples [28,29].
3.7. UV–Visible Spectroscopy
The pink regions of the four samples were tested using a Biaoqi GEM3000 UV-Vis spectrophotometer (Guangzhou, China), with the results presented in Figure 9. The samples exhibit strong absorption bands near 540 nm in the green region and near 440 nm in the blue region, which are close to the characteristic spectral bands of Mn at 0.45 μm and 0.55 μm reported in the literature [30]. The formation of these two broad absorption bands is attributed to energy level transitions related to the crystal field strength Dq of Mn2+. Mn2+, with a typical d⁵ electron configuration, exhibits weak transitions that are spin-forbidden, resulting in the pale color commonly observed in Mn2+ compounds, such as pink [29,31]. Based on the chemical composition analysis data of the samples, Mn2+ is considered the primary reason for the pink coloration of the samples.
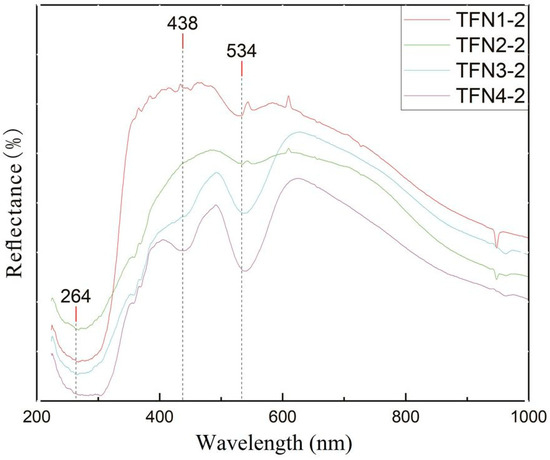
Figure 9.
UV-Vis spectra of pink mineral aggregates.
The absorption band near 265 nm in the samples is related to Fe in the substrate mineral tremolite, primarily due to charge transfer between O2− and Fe3+. As the Fe content in the samples increases, the green hue of the samples gradually deepens, and the absorption band in the range of 260 nm to 270 nm in the UV-Vis absorption spectrum shifts towards the red region.
3.8. Formation Mechanism
Based on the preceding test results, the primary mineral composition of the sample is tremolite, with accessory minerals including zoisite, diopside, feldspar, muscovite, chlorite, and prehnite, typical of magnesian marble-type nephrite [2,9,32]. Diopside is replaced by tremolite and exists as isolated single grains within the matrix (Figure 7 and Figure 8), appearing as white spots in the sample, indicating its formation preceded that of tremolite. Given its similar chemical composition to tremolite and its frequent occurrence in magnesian skarn deposits, diopside is also considered one of the main material sources for the formation of nephrite [2,3,33]. Zoisite, often found in skarn zones, is another important mineral formed through contact metasomatic processes. In the sample, zoisite is observed in irregular and embayed shapes, replaced by muscovite and tremolite, and chlorite is also seen being replaced by tremolite (Figure 5).
Based on previous research findings, the formation of diopside occurs during the early skarn stage, specifically the transition from dolomite marble to diopside skarn [34]. With the introduction of meteoric water and the subsequent decrease in temperature and pressure, hydrous minerals such as zoisite, coarse-grained tremolite, and chlorite are formed. Under continuous hydrothermal metasomatism and tectonic processes, these minerals, including zoisite, coarse-grained tremolite, and chlorite, are then replaced by fine-grained tremolite, ultimately leading to the formation of nephrite containing pink mineral assemblages. The reaction can be represented as follows:
CaMg(CO3)2 + 2SiO2 → CaMgSi2O6 + 2CO2
2CaMg(CO3)2 + Al2O3 + 3SiO2 + H2O → Ca2Al3(SiO4)3(OH) + 2MgO + 4CO2
5CaMgSi2O6 + H2O + 3CO2 + 4O2 → Ca2Mg5(Si4O11)2(OH)2 + 3CaCO3 + 6SiO2
Ca2Al3(SiO4)3(OH) + 5MgO + 4 SiO2 → Ca2Mg5(Si4O11)2(OH)2 + Al2O3
Mg5Al2Si3O10(OH)8 + 2CaO + 5SiO2 → Ca2Mg5(Si4O11)2(OH)2 + Al2O3 + 4H2O
4. Conclusions
Through conventional gemological testing and a variety of modern analytical techniques, the mineral compositional characteristics of the newly emerged pink mineral-bearing nephrite on the market have been determined, and the origins of its color have been explored. The following conclusions have been drawn:
- (1)
- The pink mineral-bearing nephrite samples generally exhibit a color range from yellowish-white to light green, with a greasy luster. Pink patches of varying shades are distributed across the base, and the green hue of the base is positively correlated with its Fe content. The refractive index of the samples is approximately 1.61–1.62 (spot measurement), with a relative density ranging between 2.94 and 2.96. All samples are inert under ultraviolet fluorescence lighting.
- (2)
- The primary mineral component of the samples is tremolite, with no actinolite observed. Additionally, minor amounts of zoisite, muscovite, feldspar, diopside, and prehnite are present, typical of magnesian marble-type nephrite. Based on the results of an electron probe microanalysis, infrared spectroscopy, Raman spectroscopy, and ultraviolet–visible absorption spectroscopy, the pink mineral in the samples is primarily identified as Mn-bearing zoisite.
- (3)
- The ultraviolet–visible absorption spectroscopy results reveal that the main coloration mechanism of the pink zoisite is attributed to the energy level transitions of Mn2+ at 540 nm and 440 nm.
Author Contributions
Y.Y. and M.S. designed the experiments; Y.Y. and Y.L. performed the experiments; M.S. resources; Y.L. investigation; Y.Y. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by National Natural Science Foundation of China (Grant No.42002156), Excellent youth project of Hebei GEO University (Grant No. YQ202404), and Doctoral research start-up fund project of Hebei GEO University (Grant No. BQ2024008). The APC was funded by the Doctoral research start-up fund project of Hebei GEO University, grant number [BQ2024008].
Data Availability Statement
All data are contained within the work.
Acknowledgments
We sincerely appreciate the valuable feedback and constructive suggestions provided by the reviewers, which have greatly enhanced the quality of our work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Deng, J.; Shi, G.H.; Lu, T.; Wang, Q. Chemical Zone of Nephrite in Alamas, Xinjiang, China. Resour. Geol. 2010, 60, 249–259. [Google Scholar] [CrossRef]
- Kislov, E.V. Kavokta deposit, Middle Vitim mountain country, Russia: Composition and genesis of dolomite type nephrite. Geosciences 2024, 14, 303. [Google Scholar] [CrossRef]
- Kislov, E.V.; Goncharuk, I.S.; Vanteev, V.V. Mineral composition and formation model of dolomite type nephrite, Voimakan deposit, Middle-Vitim mountain country. Lithosphere 2024, 24, 609–628. [Google Scholar] [CrossRef]
- Aitchison, C.; Ireland, R.; Blake, C.; Flood, P.G. 530 Ma zircon age for ophiolite from the New England Orogen: Oldest rocks known from eastern Australia. Geology 1992, 20, 125–128. [Google Scholar] [CrossRef]
- Cooper, F. Nephrite and Metagabbro in the Haast Schist at Muddy Creek, Northwest Otago, New Zealand. N. Z. J. Geol. Geop. 1995, 56, 402–410. [Google Scholar] [CrossRef]
- Gil, G. Petrographic and microprobe study of nephrites from Lower Silesia (SW Poland). Geol. Q. 2013, 57, 395–404. [Google Scholar] [CrossRef]
- Burtseva, M.V.; Ripp, G.S.; Posokhov, V.F.; Murzintseva, A.E. Nephrites of East Siberia: Geochemical features and problems of genesis. Russ. Geol. Geop. 2015, 56, 402–410. [Google Scholar] [CrossRef]
- Feng, Y.H.; He, X.M.; Jing, Y.T. A new model for the formation of nephrite deposits: A case study of the Chuncheon nephrite deposit, South Korea. Ore Geol. Rev. 2022, 141, 104655. [Google Scholar]
- Harlow, G.; Sorensen, S. Jade (nephrite and jadeitite) and serpentinite: Metasomatic connections. Int. Geol. Rev. 2005, 47, 113–146. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.Q.; Zhang, Z.Y.; Shi, G.H.; Zhang, Q.C.; Abuduwayiti, M.; Liu, J.H. Mineral inclusions and SHRIMP U-Pb dating of zircons from the Alamas nephrite and granodiorite: Implications for the genesis of a magnesian skarn deposit. Lithos 2015, 212, 128–144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.Q.; Abuduwayiti, M.; Wang, C.; Zhang, S.P.; Shen, C.H.; Zhang, Z.Y.; He, M.Y.; Zhang, Y.; Yang, X.D. SHRIMP U-Pb zircon ages, mineral compositions and geochemistry of placer nephrite in the Yurungkash and Karakash River deposits, West Kunlun, Xinjiang, northwest China: Implication for a magnesium skarn. Ore. Geol. Rev. 2016, 72, 699–727. [Google Scholar] [CrossRef]
- Yang, L.; Lin, J.H.; Wang, L.; Tan, J.; Wang, B. Petrochemical characteristics and genesic significance of Luodian jade from Guizhou. J. Mineral. Petrol. 2012, 32, 12–19. [Google Scholar]
- Jiang, T.L.; Shi, G.H.; Ye, D.N.; Zhang, X.C.; Zhang, L.J.; Han, H.W. A new type of white nephrite from limestone replacement along the Kunlun–Altyn Tagh mountains: A case from the Mida deposit, Qiemo county, Xinjiang, China. Crystals 2023, 13, 1677. [Google Scholar] [CrossRef]
- Wei, X.; Shi, G.H.; Zhang, X.C.; Zhang, J.J.; Shih, M.Y. A new nephrite occurrence in Jiangxi province, China: Its characterization and gemological significance. Minerals 2024, 14, 432. [Google Scholar] [CrossRef]
- Ling, X.X.; Schmädicke, E.; Li, Q.L.; Gose, J.; Wu, R.H.; Wang, S.Q.; Liu, Y.; Tang, G.Q.; Li, X.H. Age determination of nephrite by in-situ SIMS U-Pb dating syngenetic titanite: A case study of the nephrite deposit from Luanehuan, Henan, China. Lithos 2015, 220–223, 289–299. [Google Scholar] [CrossRef]
- Shi, G.H.; Jia, R.; Santosh, M.; Liang, H.; He, H.Y. First report of a nephrite deposit from Somaliland, Africa: Characterization and geological and archaeological implications. GSA Bull. 2024, 136, 661–672. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, G.H.; Xu, L.G.; Li, X.L. Mineralogy and geochemistry of nephrite jade from Yinggelike deposit, Altyn Tagh (Xinjiang, NW China). Minerals 2020, 10, 418. [Google Scholar] [CrossRef]
- Gao, K. Study on the Metallogenic Mechanism of Tashisayi Nephrite from Xinjiang. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2018. [Google Scholar]
- Wang, C.B.; Yuan, X.Q.; Lei, T.; Zheng, Y.L. Gemmological characteristic of “anthophyllite jade” from Xinjiang, China. J. Gems Gemmol. 2018, 20, 37–45, (In Chinese with English Abstract). [Google Scholar]
- Tan, Q.M.; Lei, T.; He, L.J.; Ruan, L.; Ruan, Q.F. Comparative study on gemological characteristics of rhodonite jade in Brazil and Xinjiang of China. J. Superhard Mater. 2021, 33, 52–57. [Google Scholar]
- Hawthorne, F.C.; Oberti, R. Classification of the amphiboles. Rev. Mineral. Geochem. 2007, 67, 55–88. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; The Mineralogical Society: London, UK, 2013; pp. 208–215. [Google Scholar]
- Lu, W. Infrared Spectroscopy of Minerals; Chongqing University Press: Chongqing, China, 1989; pp. 81–89. [Google Scholar]
- Ren, J.H.; Shi, G.H.; Zhang, J.H.; Yuan, Y.; Gao, K.; Wang, M.L.; Li, X.L.; Long, C. Infrared spectra of grayish green nephrite and gray nephrite: Characteristics and significance. Spectrosc. Spect. Anal. 2019, 39, 772–777, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.H.; Wang, Y.; Zhao, J.; Pei, Y. Gemmological characteristics and color genesis of a new pink clinozoisite Jade. J. Gems Gemol. 2020, 158, 43–49, (In Chinese with English Abstract). [Google Scholar]
- Ross, L.; Detrie, A.; Liu, Z.X. High-pressure Raman and infrared spectroscopic study of prehnite. Minerals 2020, 10, 312. [Google Scholar] [CrossRef]
- Yang, X.D.; Shi, G.H.; Liu, Y. Vibrational spectra of black species of Hetian nephrite (tremolite Jade) and its color genesis. Spectrosc. Spect. Anal. 2012, 32, 681–685, (In Chinese with English Abstract). [Google Scholar]
- Zhang, Y.Y. Study on Gemmological and Mineralogical Characteristic of Pink Dushan Jade from Nanyang. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2020. [Google Scholar]
- Wang, W.N.; Chen, Q.; Zhou, Z.Y.; Shang, J.C.; Liu, Y.C. Research on gemological and spectroscopic characteristics of the nephrite containing pink minerals appeared in markets recently. China Gems Jades 2021, 169, 2–7, (In Chinese with English Abstract). [Google Scholar]
- Gan, F.P.; Wang, R.S.; Ma, A.N. Spectral identification tree (sit) for mineral extraction based on spectral characteristics of minerals. Geosci. Front. 2003, 10, 44–451. [Google Scholar]
- Schwarzinger, C. The heat treatment of pink zoisite. Minerals 2022, 12, 1472. [Google Scholar] [CrossRef]
- Tang, Y.L.; Chen, B.Z.; Jiang, R.H. Chinese Hetian Nephrite; Xinjiang People’s Publishing House: Xinjiang, China, 1994; pp. 103–206, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.; Deng, J.; Shi, G.H.; Sun, X.; Yang, L.Q. Geochemistry and petrogenesis of placer nephrite from Hetian, Xinjiang, Northwest China. Ore. Geol. Rev. 2011, 41, 122–132. [Google Scholar] [CrossRef]
- Jing, Y.T.; Liu, Y.; Zhang, Y.; Abuduwayiti, M. Metallogenic age, formation process and prospecting direction of marble-related nephrite deposit in China. Acta Petrol. Mineral. 2022, 41, 651–667, (In Chinese with English Abstract). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).