Variation in Carbon Content During the Melting of γ-TiAl in a Graphite Crucible
Abstract
1. Introduction
2. Experimental Procedure
2.1. Production of the Investment Mold
2.2. Induction Melting and Centrifugal Casting
2.3. Characterization of Castings and Graphite Crucible
3. Results and Discussion
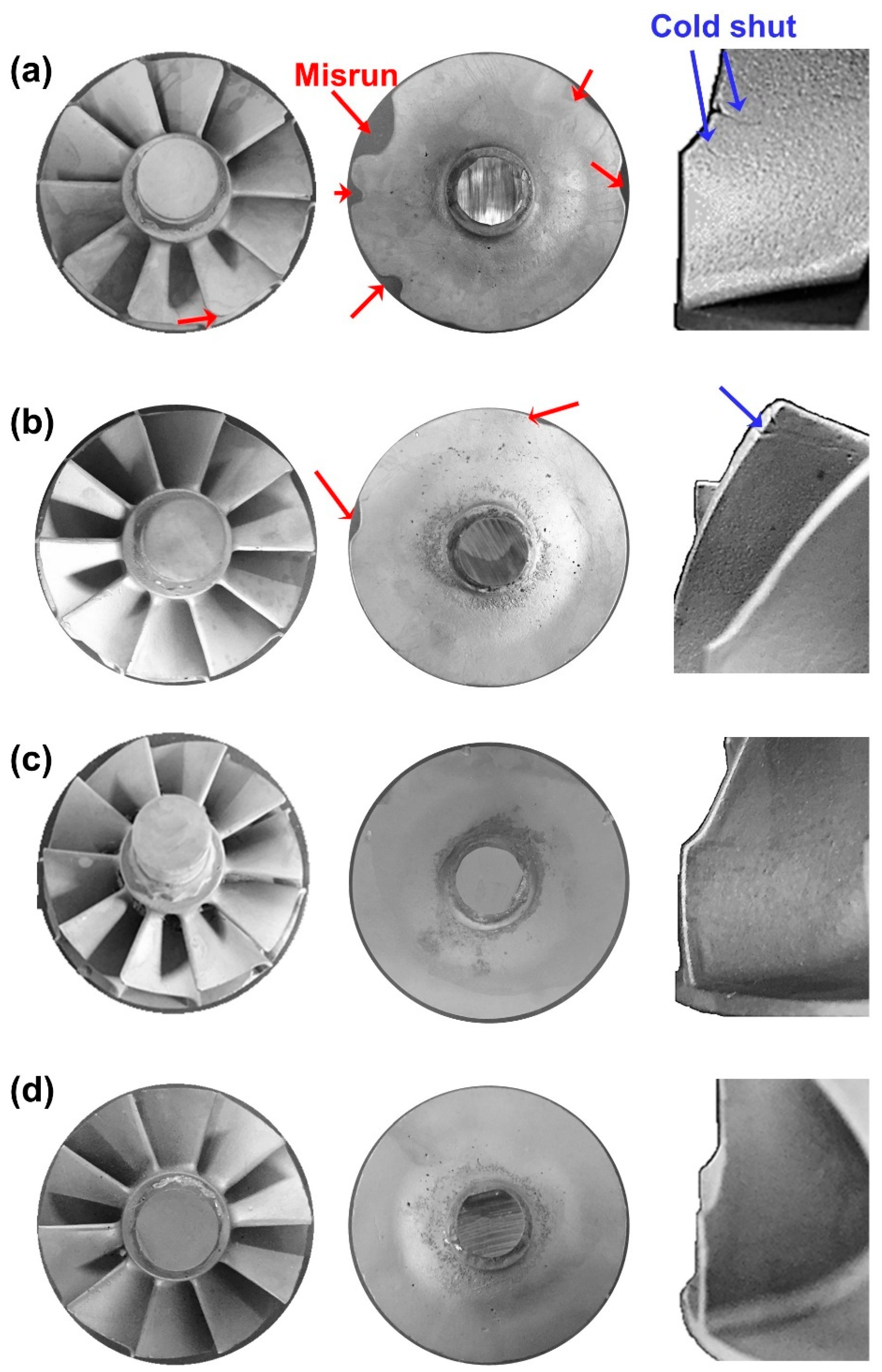
3.1. Optimum Degree of Superheating (ΔT) for Mold Filling of Turbocharger Castings
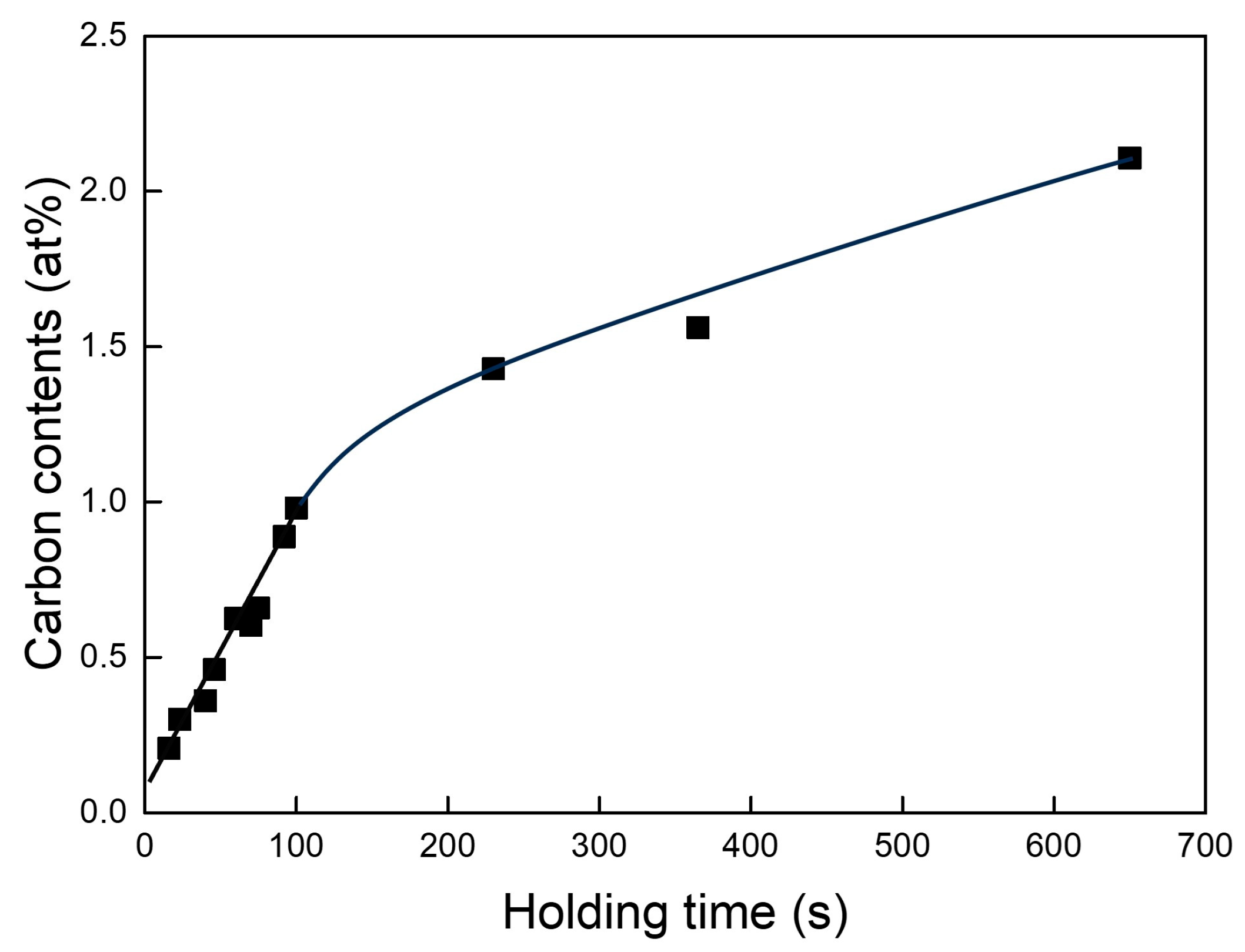
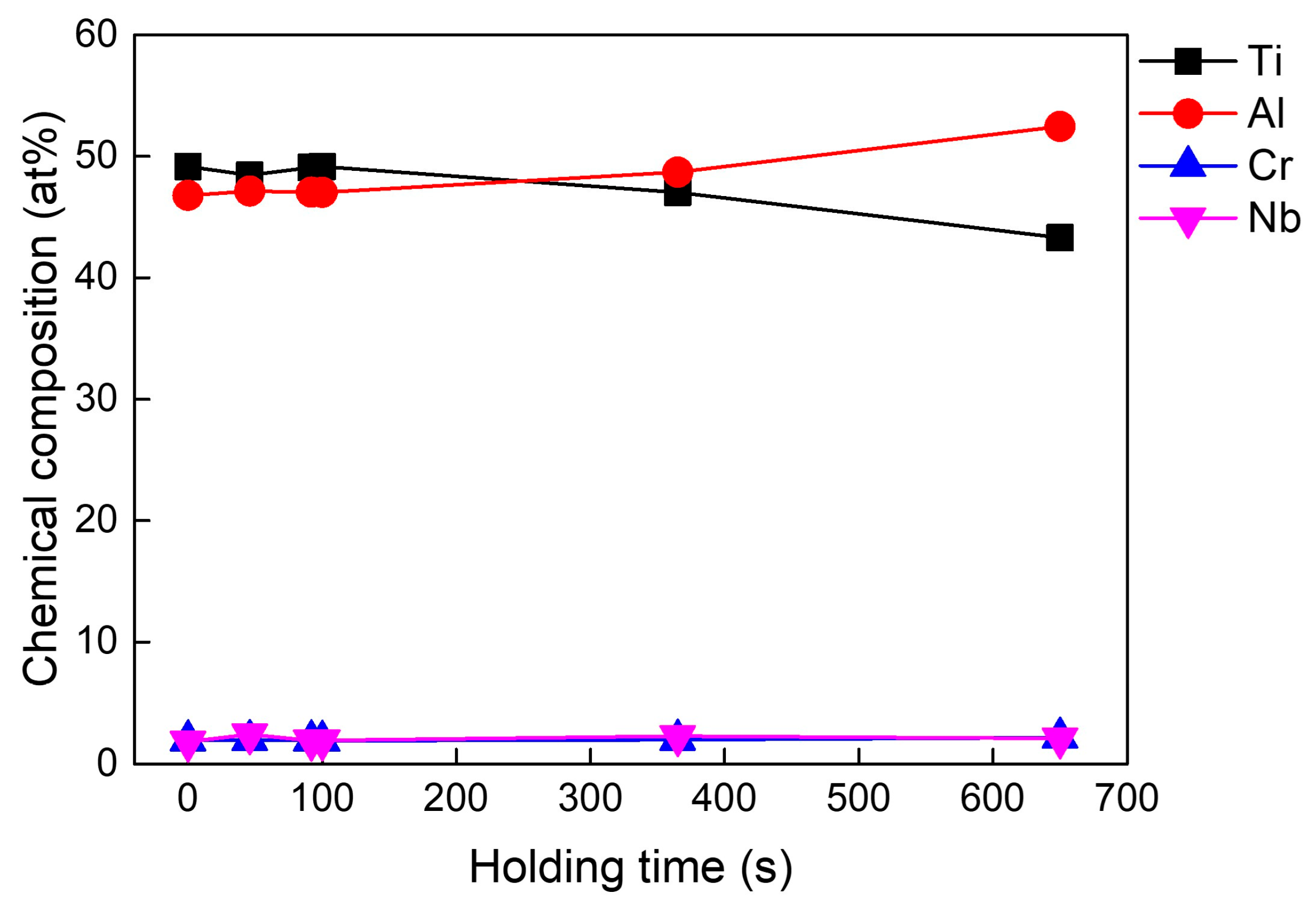
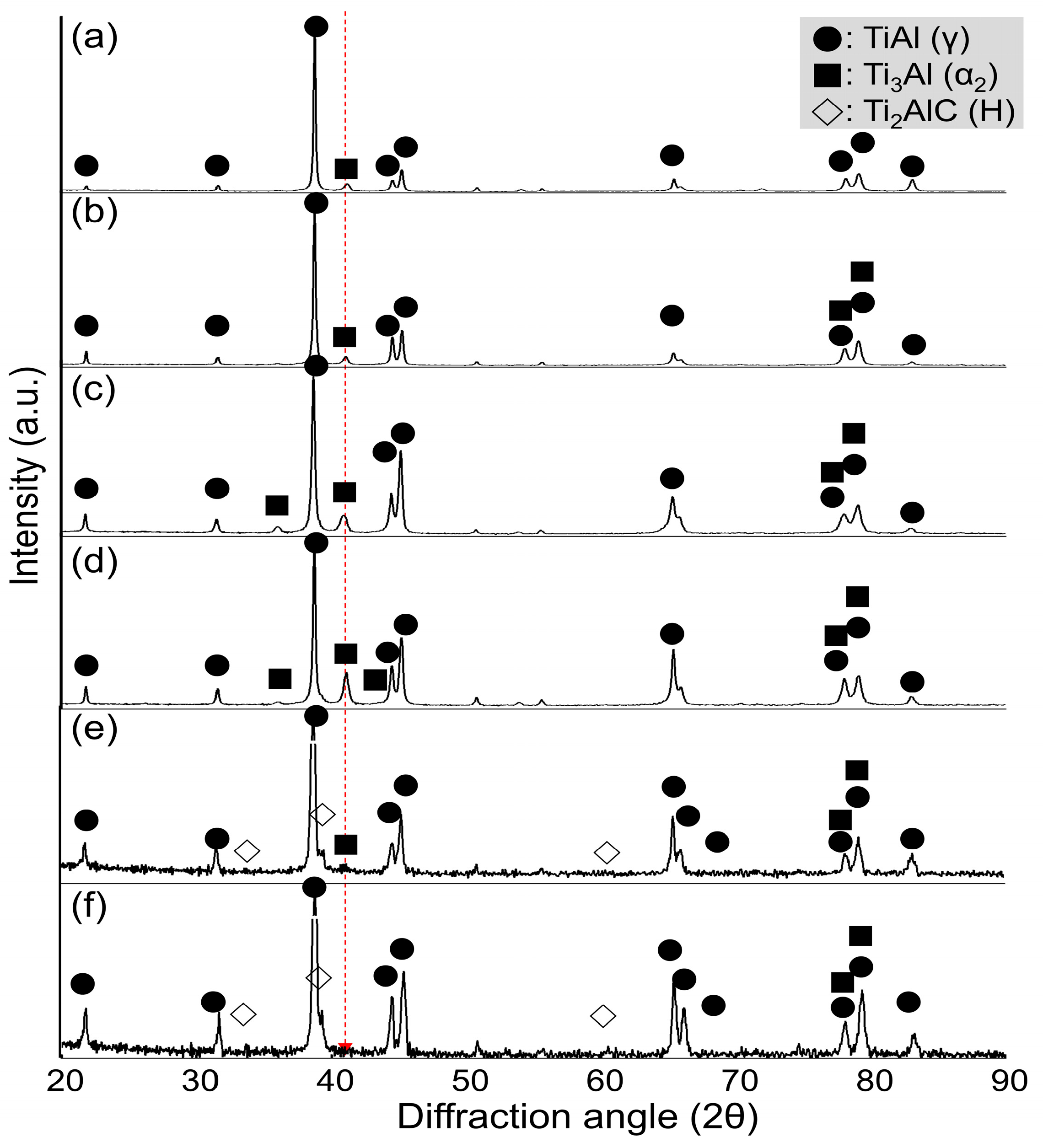
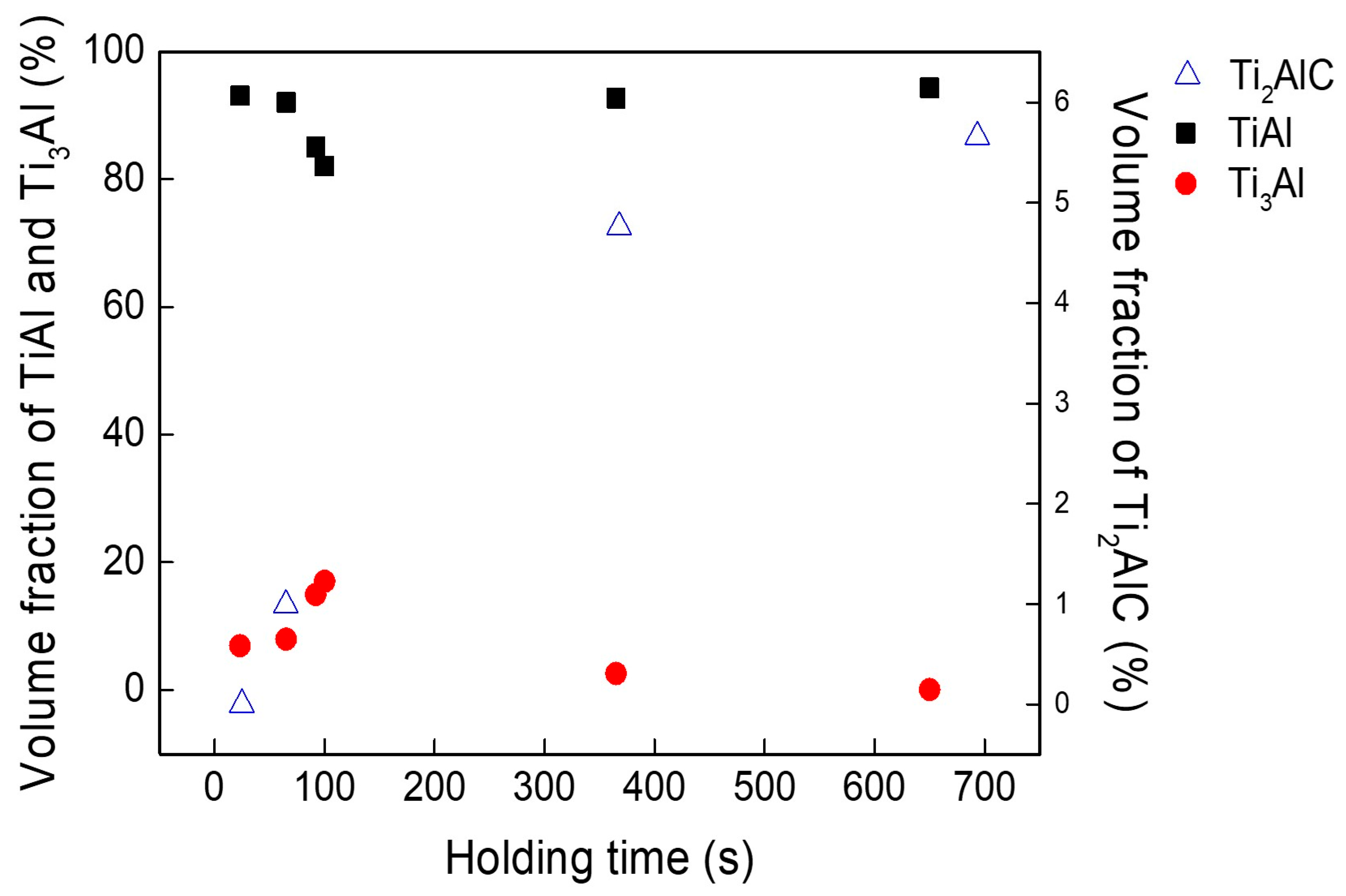
3.2. Variation in Carbon Content and Chemical Composition with Respect to Holding Time (Δt)
3.3. Compositional Change in Castings and Variation in Carbon Contamination Rate
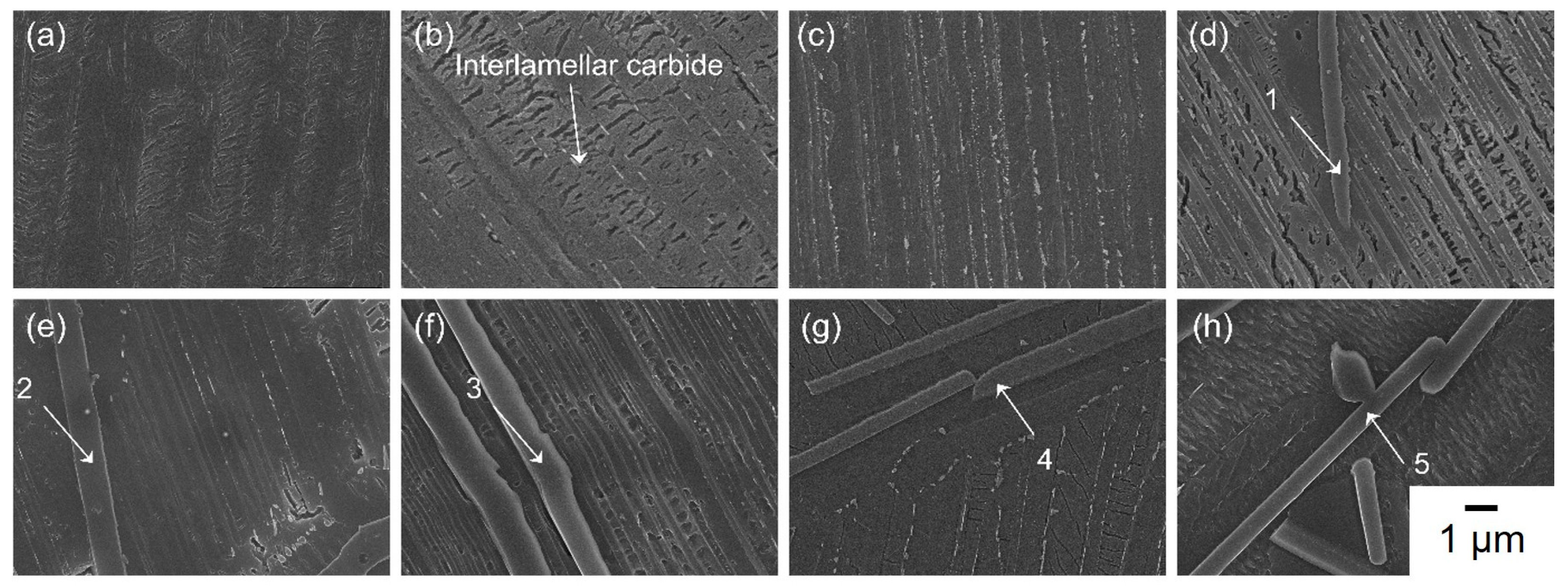
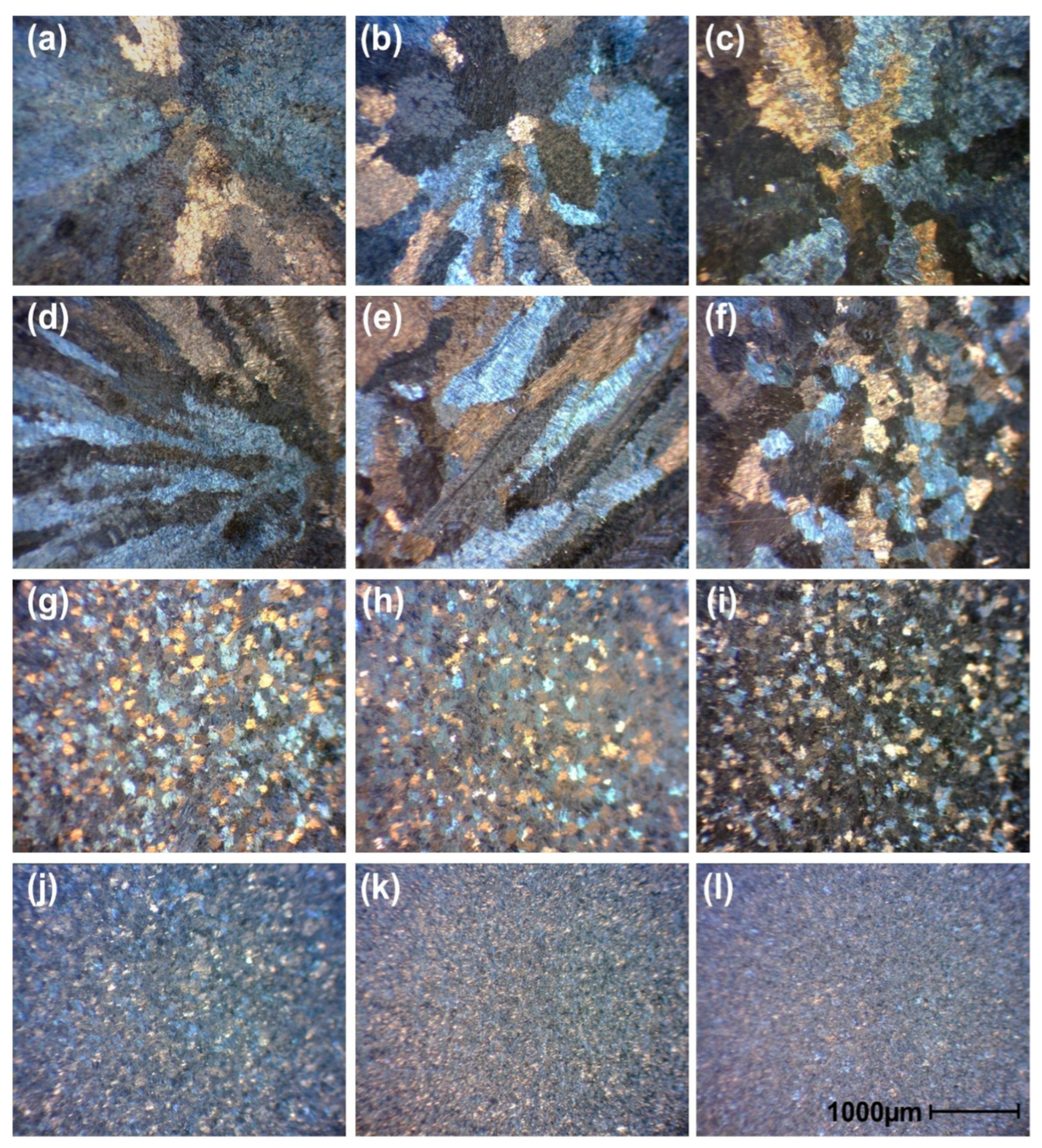
3.4. Microstructural Evolution of TiAl Castings as a Function of Holding Time, Δt
4. Conclusions
- At superheating of 130 K and 170 K, casting defects were observed at the disk and blade tip. However, the misrun and cold shut were resolved at temperatures above ΔT of 200 K. Considering contamination by impurities, it was found that superheating of 200 K is appropriate for casting a sound γ-TiAl turbocharger component.
- As the TiC and Ti2AlC phases, which are richer in titanium, adhered to the crucible walls during casting, less titanium remained in the melt. Consequently, the proportion of aluminum in the melts increased, leading to higher aluminum concentration in the castings.
- The outer layers of solid TiC and Ti2AlC, which enclosed the molten volume during the melting process, limited the increase in carbon contamination by creating a stable barrier that prevented carbon diffusion from the crucible into the melt. This reduced the rate of carbon contamination.
- As holding time increased, fine secondary carbides formed first due to differences in carbon solubility between the γ and α2 phases—specifically, the γ phase could dissolve more carbon than the α2 phase. As a result, carbon began to precipitate as fine secondary carbides, followed by the crystallization of primary carbides as the process continued. Both the fine secondary and coarse primary carbides were identified as Ti2AlC phases, ensuring precise phase identification. This sequence led to a transformation of the columnar structure into an equiaxed one through TiC crystallization. Additionally, increased carbon contamination promoted more heterogeneous carbide nuclei in the molten state, resulting in refined grain size.
- The volume fraction of the α2 phase increased as carbon acted as an α-phase stabilizer, reaching a maximum at a holding time of 100 s. As the holding time increased beyond 100 s, the volume fraction of the α2 phase decreased sharply. Simultaneously, the γ phase region expanded due to the removal of Ti2AlC and TiC from the melt, as these carbides adhered to the crucible wall.
- The carbon content was within 0.5 at% in the castings for a melt holding time of up to 50 s at a degree of superheating of 200 K. When the holding time was less than 20 s, carbon was present only as a solid solution in the castings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perrut, M.; Caron, P.; Thomas, M.; Couret, A. High temperature materials for aerospace applications: Ni-based superalloys and γ-TiAl alloys. C. R. Phys. 2018, 19, 657–671. [Google Scholar] [CrossRef]
- Yuan, C.; Cheng, X.; Holt, G.S.; Shevchenko, D.; Withey, P.A. Investment casting of Ti–46Al–8Nb–1B alloy using molds with CaO-stabilized zirconia face coat at various mold pre-heat temperatures. Ceram. Int. 2015, 41, 4129–4139. [Google Scholar] [CrossRef]
- Čegan, T.; Szurman, I.; Kursa, M.; Holešinský, J.; Vontorová, J. Preparation of TiAl-based alloys by induction melting in graphite crucibles. Kov. Mater. 2015, 53, 69–78. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, C.; Blackburn, S.; Withey, P.A. The influence of binder systems in Y2O3–ZrO2 face coat material on investment casting slurries and shell properties. J. Eur. Ceram. Soc. 2014, 34, 3061–3068. [Google Scholar] [CrossRef]
- Malakizadi, A.; Mallipeddi, D.; Dadbakhsh, S.; M’Saoubi, R.; Krajnik, P. Post-processing of additively manufactured metallic alloys—A review. Int. J. Mach. Tools Manuf. 2022, 179, 103908. [Google Scholar] [CrossRef]
- Larsen, D.E. Status of investment cast gamma titanium aluminides in the USA. Mater. Sci. Eng. A 1996, 213, 128–133. [Google Scholar] [CrossRef]
- Kuang, J.P.; Harding, R.A.; Campbell, J. Examination of defects in gamma titanium aluminide investment castings. Int. J. Cast Met. Res. 2000, 13, 125–134. [Google Scholar] [CrossRef]
- Dlouhý, A.; Zemčík, L.; Válek, R. Near-gamma TiAl investment casting and its optimization. In Gamma Titanium Aluminides 2003; Kim, Y.W., Clemens, H., Rosenberger, A.H., Eds.; TMS: Warrendale, PA, USA, 2003; pp. 291–296. [Google Scholar]
- Fashu, S.; Lototskyy, M.; Davids, M.W.; Pickering, L.; Linkov, V.; Tai, S.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Des. 2020, 186, 108295. [Google Scholar] [CrossRef]
- Tetsui, T.; Kobayashi, T.; Mori, T.; Kishimoto, T.; Harada, H. Evaluation of yttria applicability as a crucible for induction melting of TiAl alloy. Mater. Trans. 2010, 51, 1656–1662. [Google Scholar] [CrossRef]
- Gomes, F.; Puga, H.; Barbosa, J.; Ribeiro, C.S. Effect of melting pressure and superheating on chemical composition and contamination of yttria-coated crucible induction melted titanium alloys. J. Mater. Sci. 2011, 46, 4922–4936. [Google Scholar] [CrossRef]
- Jovanović, M.T.; Bobić, I.; Mišković, Z.; Zec, S. Precision Cast Ti-based alloys—Microstructure and mechanical properties. J. Met. 2009, 15, 53–69. [Google Scholar]
- Dlouhý, A.; Dočekalová, K.; Zemčík, L. Vacuum induction melting and investment casting technologies tailored to near-gamma TiAl alloys. Mater. Sci. Forum 2007, 539, 1463–1468. [Google Scholar] [CrossRef]
- Fan, J.; Liu, J.; Wu, S.; Tian, S.; Gao, H.; Wang, S.; Wang, X. Microstructure formation and interface characteristics of directionally solidified TiAl–Si alloys in alumina crucibles with Y2O3 skull-aided technology. Sci. Rep. 2017, 7, 45198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, X.; Zhou, C.; Zhang, H.; Zhang, S. Comparison of directional solidification of γ-TiAl alloys in conventional Al2O3 and novel Y2O3-coated Al2O3 crucibles. J. Eur. Ceram. Soc. 2013, 33, 925–934. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Nan, H.; Feng, X.; Ding, X. Effect of carbon addition on as-cast microstructures of high Nb containing TiAl alloys. Metals 2019, 9, 1201. [Google Scholar] [CrossRef]
- Lapin, J.; Štamborská, M.; Kamyshnykova, K.; Pelachová, T.; Klimová, A.; Bajana, O. Room temperature mechanical behaviour of cast In-Situ TiAl matrix composite reinforced with carbide particles. Intermetallics 2019, 105, 113–123. [Google Scholar] [CrossRef]
- Lapin, J.; Štamborská, M.; Pelachová, T.; Bajana, O. Fracture behaviour of cast In-Situ TiAl matrix composite reinforced with carbide particles. Mater. Sci. Eng. A 2018, 721, 1–7. [Google Scholar] [CrossRef]
- Rösler, J.; Evans, A.G. The effect of reinforcement size on the creep strength of intermetallic matrix composites. In High Temperature Aluminides and Intermetallics; Elsevier: Amsterdam, The Netherlands, 1992; pp. 438–443. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Xiao, L.; Xu, L.; Tian, J.; Chen, Y. Effects of carbon and boron addition on microstructure and mechanical properties of TiAl alloys. J. Alloys Compd. 2017, 728, 206–221. [Google Scholar] [CrossRef]
- Szkliniarz, W.; Szkliniarz, A. Microstructure and properties of TiAl-based alloys melted in graphite crucible. Metals 2021, 11, 669. [Google Scholar] [CrossRef]
- Barbosa, J.J.; Ribeiro, C.S. Influence of crucible material on the level of contamination in TiAl using induction melting. Int. J. Cast Met. Res. 2000, 12, 293–301. [Google Scholar] [CrossRef]
- Barbosa, J.; Ribeiro, C.A.S.; Monteiro, C. The production of TiAl by foundry processes. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2002; Volume 230–232, pp. 106–109. [Google Scholar] [CrossRef]
- Lee, S.; Shin, M.S.; Kim, Y.J. Development of non-reactive mold for investment casting of Ti-48Al-2Cr-2Nb alloys. Metall. Mater. Trans. B 2020, 51, 861–869. [Google Scholar] [CrossRef]
- Zhang, Z.; Frenzel, J.; Neuking, K.; Eggeler, G. On the reaction between NiTi melts and crucible graphite during vacuum induction melting of NiTi shape memory alloys. Acta Mater. 2005, 53, 3971–3985. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Hallstedt, B.; Bondar, A.A.; Hecht, U.; Sleptsov, S.V.; Velikanova, T.Y. Thermodynamic description of the Al–C–Ti system. J. Alloys Compd. 2015, 623, 480–496. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Rashkova, B.; Clemens, H.; Stark, A.; Mayer, S. Effect of carbon addition on solidification behavior, phase evolution and creep properties of an intermetallic β-stabilized γ-TiAl-based alloy. Intermetallics 2014, 46, 173–184. [Google Scholar] [CrossRef]
- Bolotin, K.; Brazhnik, D. Bottom Induction Stirrer for Induction Crucible Furnace with Graphite Crucible. In Proceedings of the International Russian Automation Conference (RusAutoCon 2019), Sochi, Russia, 8–14 September 2019; Radionov, A.A., Karandaev, A.S., Eds.; Lecture Notes in Electrical Engineering; Springer: Cham, Switzerland, 2020; Volume 641, pp. 1158–1166. [Google Scholar] [CrossRef]
- Wu, L.; Ek, M.; Song, M.; Du, S. The Effect of Solid Particles on Liquid Viscosity. Steel Res. Int. 2011, 82, 388–397. [Google Scholar] [CrossRef]
- Klimová, A.; Lapin, J. Effect of Al content on microstructure of Ti-Al-Nb-C-Mo composites reinforced with carbide particles. Kov. Mater. 2019, 57, 377–387. [Google Scholar] [CrossRef]
- Menand, A.; Huguet, A.; Nérac-Partaix, A. Interstitial solubility in γ and α2 phases of TiAl-based alloys. Acta Mater. 1996, 44, 4729–4737. [Google Scholar] [CrossRef]
- McCullough, C.; Valencia, J.J.; Levi, C.G.; Mehrabian, R. Phase equilibria and solidification in Ti–Al alloys. Acta Metall. 1989, 37, 1321–1336. [Google Scholar] [CrossRef]
- Perdrix, F.; Trichet, M.F.; Bonnentien, J.L.; Cornet, M.; Bigot, J. Relationships between interstitial content, microstructure and mechanical properties in fully lamellar Ti–48Al alloys, with special reference to carbon. Intermetallics 2001, 9, 807–815. [Google Scholar] [CrossRef]


| Region | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| C K | 50.7 | 18.7 | 43.4 | 24.2 | 51.9 | 17.7 |
| Al K | 0.4 | 32.7 | 0.4 | 25.8 | 2.3 | 14.9 |
| Ti K | 42.4 | 42.6 | 55.4 | 50.0 | 45.8 | 56.6 |
| Cr K | 1.6 | 1.7 | ||||
| Nb L | 4.9 | 4.3 | 0.8 | 10.8 | ||
| Totals | 100 | 100 | 100 | 100 | 100 | 100 |
| Region | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| C K | 22.6 | 30.7 | 29.9 | 26.2 | 31.7 |
| Al K | 27.8 | 25.9 | 24.2 | 24.0 | 27.1 |
| Ti K | 49.1 | 41.4 | 44.0 | 49.8 | 39.2 |
| Cr K | 0.5 | 0.1 | 1.9 | 0.3 | |
| Nb L | 0.1 | 1.7 | |||
| Totals | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Ha, T.; Lee, S.; Kim, Y. Variation in Carbon Content During the Melting of γ-TiAl in a Graphite Crucible. Crystals 2025, 15, 1006. https://doi.org/10.3390/cryst15121006
Kang B, Ha T, Lee S, Kim Y. Variation in Carbon Content During the Melting of γ-TiAl in a Graphite Crucible. Crystals. 2025; 15(12):1006. https://doi.org/10.3390/cryst15121006
Chicago/Turabian StyleKang, Byungil, Taekyu Ha, Seul Lee, and Youngjig Kim. 2025. "Variation in Carbon Content During the Melting of γ-TiAl in a Graphite Crucible" Crystals 15, no. 12: 1006. https://doi.org/10.3390/cryst15121006
APA StyleKang, B., Ha, T., Lee, S., & Kim, Y. (2025). Variation in Carbon Content During the Melting of γ-TiAl in a Graphite Crucible. Crystals, 15(12), 1006. https://doi.org/10.3390/cryst15121006






