Influence of Fly Ash on the Macro Properties and Mineral Crystal Characteristics of Alkali-Activated Slag Grouting Materials
Abstract
1. Introduction
2. Experimental Materials and Mix Proportions
2.1. Experimental Materials
2.2. Mix Proportion Design
3. Experimental Program
3.1. Specimen Preparation and Curing
3.2. Fluidity, Setting Behavior and Compressive Strength Testing
3.3. Microscopic Analysis
4. Experimental Results and Analysis
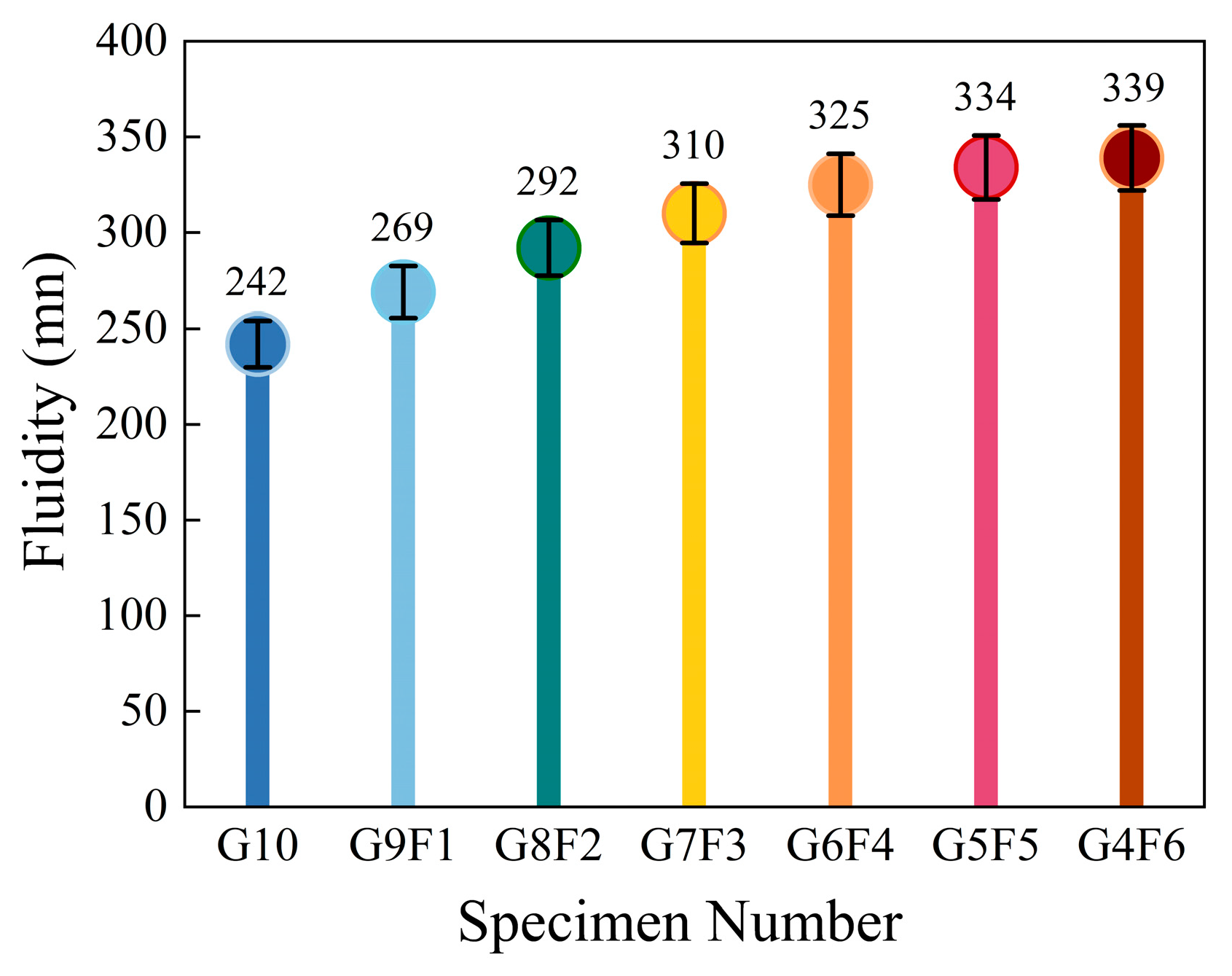
4.1. Liquidity Analysis
4.1.1. G10 Liquidity Analysis
4.1.2. Analysis of the Impact of FA on Liquidity
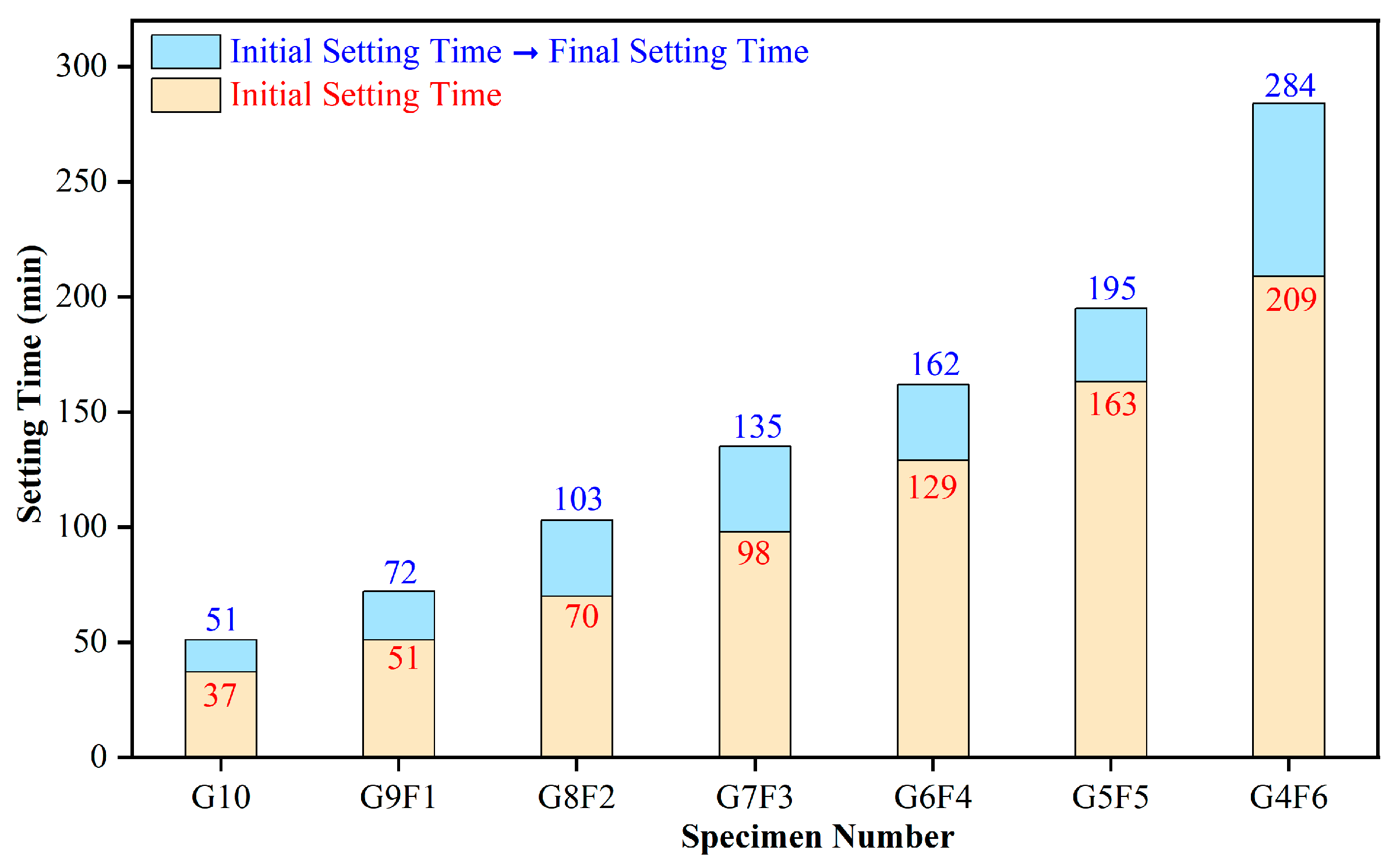
4.2. Analysis of Setting Law
4.2.1. Analysis of G10 Setting Law
4.2.2. The Influence of FA on the Setting Law
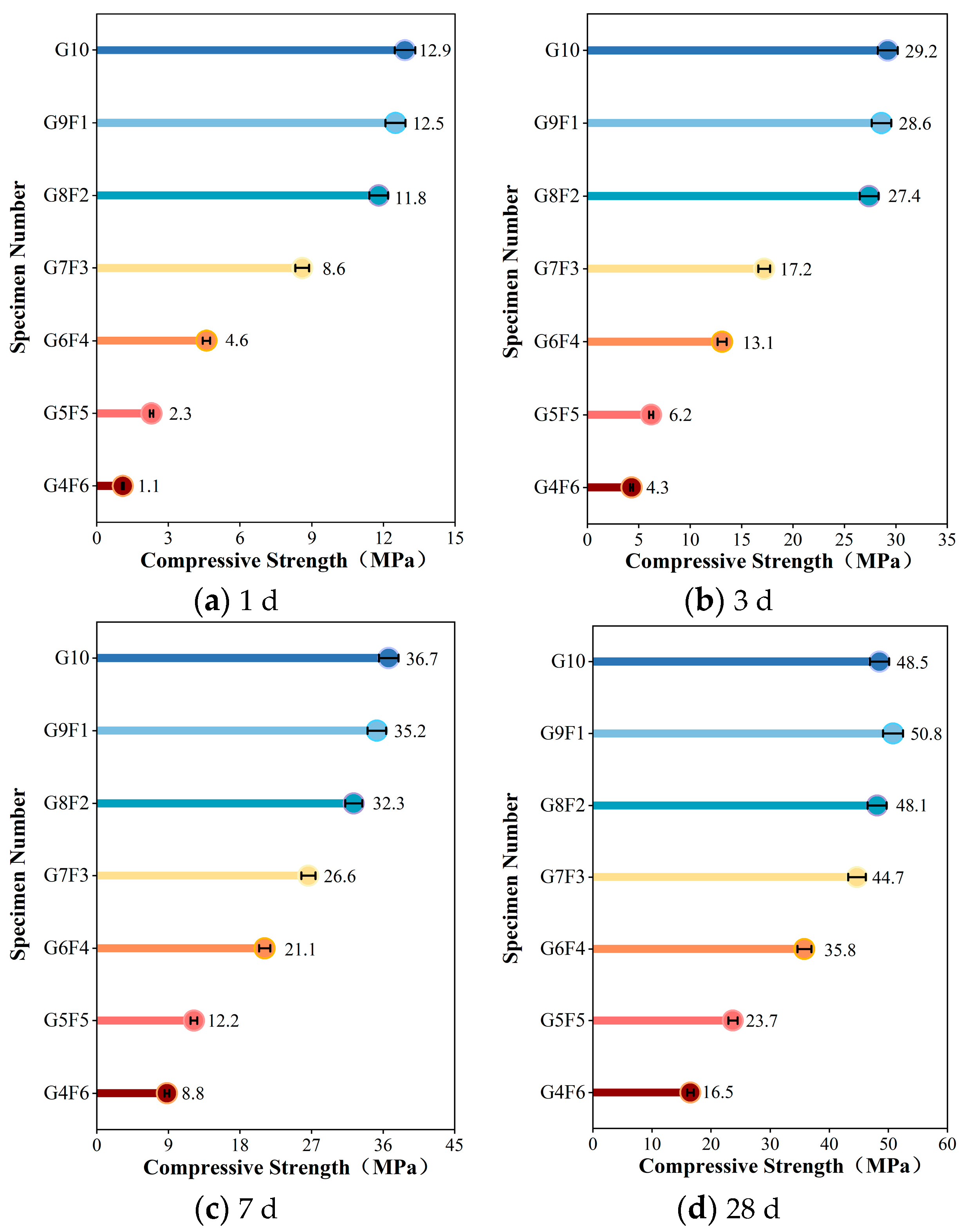
4.3. Analysis of Compressive Strength
4.3.1. G10 Compressive Strength Analysis
4.3.2. The Influence of FA on Compressive Strength
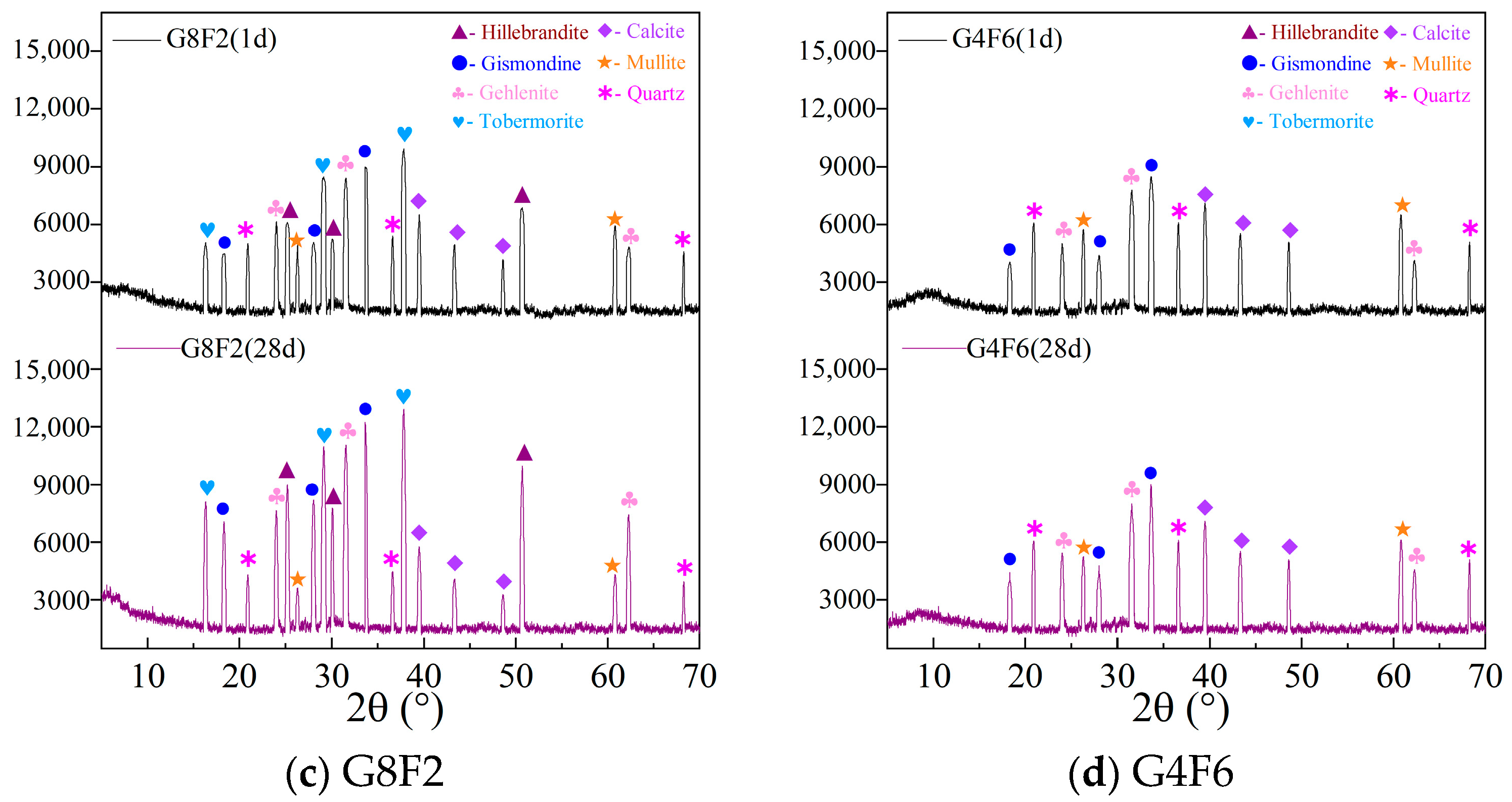
4.4. XRD Analysis
4.4.1. XRD Analysis of G10
4.4.2. FA Regulated XRD Analysis
4.5. FT-IR Analysis
4.5.1. FT-IR Analysis of G10
4.5.2. FA Regulated FT-IR Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shu, X.; Zhao, Y.; Liu, Z.; Zhao, C. A study on the mix proportion of fiber-polymer composite reinforced cement-based grouting material. Constr. Build. Mater. 2022, 328, 127025. [Google Scholar] [CrossRef]
- Malisetty, R.S.; Indraratna, B.; Qi, Y.; Rujikiatkamjorn, C. Shakedown response of recycled rubber–granular waste mixtures under cyclic loading. Geotechnique 2023, 73, 843–848. [Google Scholar] [CrossRef]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J. Experiment and Application Study on High-Performance Grouting Material Used to Solve the Floor Heave Problem of Broken Soft Rocks. Front. Phys. 2022, 10, 829681. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, X.; Liu, M.; Fang, B.; Wang, C.; Mi, H. Compatibility of sodium hydroxide, sodium silicate and calcium-enriched additives in alkali-activated materials: From the perspectives of flowability, strength and microstructure. Constr. Build. Mater. 2023, 403, 133102. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Wang, W.; Noguchi, T.; Tomoyose, A.; Zhang, Y.; Maruyama, I. Influence of volcanic glass powder on alkali-silica reaction expansion in alkali-activated slag mortars. Cem. Concr. Compos. 2024, 152, 105665. [Google Scholar] [CrossRef]
- Yu, S.; He, J.; Sang, G.; Yang, S.; Liu, G. Study on hydration process of alkali-activated slag cement activated by weakly alkaline components. Constr. Build. Mater. 2024, 413, 134716. [Google Scholar] [CrossRef]
- Ghorbani, S.; Stefanini, L.; Sun, Y.; Walkley, B.; Provis, J.L.; De Schutter, G.; Matthys, S. Characterisation of alkali-activated stainless steel slag and blast-furnace slag cements. Cem. Concr. Compos. 2023, 143, 105230. [Google Scholar] [CrossRef]
- Yang, S.; Poon, C.S.; Cui, H. Alkali-silica reactivity of lightweight aggregates in alkali-activated slag cement and ordinary Portland cement systems. J. Clean. Prod. 2023, 390, 136187. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Yan, W.; Wu, J.; Yan, F. Grouting Material Development and Dynamic Grouting Test of Broken Rock Mass. J. Mater. Civ. Eng. 2022, 34, 04022072. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, Z.; Han, D.; Song, J. Investigation and application of a high performance grouting material in water-rich silty fine sand stratum. Constr. Build. Mater. 2022, 329, 127100. [Google Scholar] [CrossRef]
- Yun-Ding, Z.; Kun, C. Research on Chemical Grouting Advance Support Technology of Silty Sand Tunnel with Low Moisture Content. Constr. Des. Proj. 2020, 145, 120–121. [Google Scholar]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Synthesis and characterization of eco-friendly alkali-activated industrial solid waste-based two-component backfilling grouts for shield tunnelling. J. Clean. Prod. 2020, 266, 21974. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; He, Y.; Li, Y.; Wei, A.; Cui, X.; Shi, C. Expansion behavior and microstructure change of alkali-activated slag grouting material in carbonate environment. Constr. Build. Mater. 2020, 262, 120593. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Granulated Blast Furnace Slag Powder Used for Cement, Mortar and Concrete. AQSIQ: Beijing, China, 2017.
- GB/T 1596-2017; Fly Ash Used for Cement and Concrete. AQSIQ: Beijing, China, 2017.
- GB/T 17671-2021; Method of Testing Cements—Determination of Strength. AQSIQ: Beijing, China, 2021.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. AQSIQ: Beijing, China, 2005.
- GB/T 1346-2024; Test Methods for Water Requirement of Standard Consistency, Setting Time Andsoundness of the Portland Cement. AQSIQ: Beijing, China, 2024.
- Zhang, S.; He, Y.; Zhang, H.; Chen, J.; Liu, L. Effect of fine sand powder on the rheological properties of one-part alkali-activated slag semi-flexible pavement grouting materials. Constr. Build. Mater. 2022, 333, 127328. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Z.; Zhang, K.; Tang, X.; Luo, Y. Preparation and characterization of the greener alkali-activated grouting materials based on multi-index optimization. Constr. Build. Mater. 2021, 269, 121328. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, Z.; Xu, Y.; Wang, S.; Chen, H.; Huang, J.; Xue, B.; Wang, J.; Chen, J.; Yang, C. High-Fluidization, Early Strength Cement Grouting Material Enhanced by Nano-SiO2: Formula and Mechanisms. Materials 2021, 14, 6144. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Davoodabadi, M.; Liebscher, M.; Hampel, S. Multi-walled carbon nanotube dispersion methodologies in alkaline media and their influence on mechanical reinforcement of alkali-activated nanocomposites. Compos. Part B Eng. 2021, 209, 108559. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, J.; Pang, G.; Li, R.; Zhang, P.; Xu, S. Influence of the Activator Concentration and Solid/Liquid Ratio on the Strength and Shrinkage Characteristics of Alkali-Activated Slag Geopolymer Pastes. Adv. Civ. Eng. 2021, 2021, 6631316. [Google Scholar] [CrossRef]
- Ran, W.; Qiu, H.; Ai, X.; Zhang, S.; Wang, Y. Experimental study on the dynamic modulus of an asphalt roadbed grouting mixture under the influence of complex and multiple factors. Buildings 2023, 13, 1969. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, H. Analysis of the grouting fullness and road performance of hotrecycled semi-flexible pavement materials. Case Stud. Constr. Mater. 2024, 20, e2755. [Google Scholar]
- Jin-Jin, Z.; Bo, L.I.; Chuang, Y.U.; Mao-Yu, Z. Mechanical properties of slag-fly ash based geopolymer stabilized sandy soil. Rock Soil Mech. 2022, 43, 2421–2430. [Google Scholar]
- Duan, D.; Wu, H.; Wei, F.; Song, H.; Ma, Z.; Chen, Z.; Cheng, F. Preparation, characterization, and rheological analysis of eco-friendly geopolymer grouting cementitious materials based on industrial solid wastes. J. Build. Eng. 2023, 78, 107451. [Google Scholar] [CrossRef]
- Li, M.; Du, M.; Wang, F.; Xue, B.; Fang, H. Study on the mechanical properties of polyurethane (PU) grouting material of different geometric sizes under uniaxial compression. Constr. Build. Mater. 2020, 259, 119797. [Google Scholar] [CrossRef]
- Wang, Z.; Du, M.; Fang, H.; Zhang, C.; Li, M.; Shi, M. Influence of different corrosion environments on mechanical properties of a roadbed rehabilitation polyurethane grouting material under uniaxial compression. Constr. Build. Mater. 2021, 301, 124092. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, W.; Guo, C.; Du, X.; Pan, Y.; Gao, J.; Gao, X. Surface free energy theory for evaluating moisture damage in expandable polyurethane grouting materials. Adv. Eng. Mater. 2022, 24, 2200409. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, W.; Fang, H.Y.; Wang, J.; Wang, C.X.; Du, M.R.; Zhao, P.; Wang, L.; Wang, F.M. Research progress of the cell structure characteristics and compressive properties of polyurethane foam grouting rehabilitation materials. Mater. Rep. 2024, 38, 229–242. [Google Scholar]
- Shuang, G.; Juan, W.; Yu, H. Experimental study on the influence of temperature and humidity on the fracture properties of polyurethane grouting materials. J. Mater. Res. Technol. 2024, 32, 1299–1309. [Google Scholar] [CrossRef]
- Liang, J.; Du, X.; Fang, H.; Du, M.; Shi, M.; Gao, X.; Han, Y. Numerical and experimental study of diffusion law of foamed polymer grout in fracture considering viscosity variation of slurry. Tunn. Undergr. Space Technol. 2022, 128, 10467. [Google Scholar] [CrossRef]
- Weng, L.; Wu, Z.; Zhang, S.; Liu, Q.; Chu, Z. Real-time characterization of the grouting diffusion process in fractured sandstone based on the low-field nuclear magnetic resonance technique. Int. J. Rock Mech. Min. Sci. 2022, 152, 105060. [Google Scholar] [CrossRef]
- Chen, C.; Lai, H.; Liu, Y. Study on the working performance and microscopic mechanism of high-performance grouting material (HPGM) reinforced dense fine sand layer. Constr. Build. Mater. 2024, 449, 138518. [Google Scholar] [CrossRef]



| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | Others | Loss | |
|---|---|---|---|---|---|---|---|---|---|---|
| GBFS | 32.48 | 20.82 | 0.82 | 34.55 | 6.16 | 1.15 | 0.65 | 0.13 | 2.06 | 1.18 |
| FA | 55.32 | 30.34 | 5.89 | 1.87 | 1.36 | 0.82 | 0.36 | 0.05 | 2.64 | 1.35 |
| GBFS | FA | NaOH | Liquid Sodium Silicate | Water | Liquid-Solid Ratio | |
|---|---|---|---|---|---|---|
| G10 | 1000 | 0 | 45 | 200 | 470 | 0.6 |
| G9F1 | 900 | 100 | 45 | 200 | 470 | 0.6 |
| G8F2 | 800 | 200 | 45 | 200 | 470 | 0.6 |
| G7F3 | 700 | 300 | 45 | 200 | 470 | 0.6 |
| G6F4 | 600 | 400 | 45 | 200 | 470 | 0.6 |
| G5F5 | 500 | 500 | 45 | 200 | 470 | 0.6 |
| G4F6 | 400 | 600 | 45 | 200 | 470 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Xu, J.; Qi, J.; Zhang, F.; Dou, B. Influence of Fly Ash on the Macro Properties and Mineral Crystal Characteristics of Alkali-Activated Slag Grouting Materials. Crystals 2025, 15, 999. https://doi.org/10.3390/cryst15110999
Huang G, Xu J, Qi J, Zhang F, Dou B. Influence of Fly Ash on the Macro Properties and Mineral Crystal Characteristics of Alkali-Activated Slag Grouting Materials. Crystals. 2025; 15(11):999. https://doi.org/10.3390/cryst15110999
Chicago/Turabian StyleHuang, Guodong, Jiahao Xu, Jun Qi, Fengan Zhang, and Baoxuan Dou. 2025. "Influence of Fly Ash on the Macro Properties and Mineral Crystal Characteristics of Alkali-Activated Slag Grouting Materials" Crystals 15, no. 11: 999. https://doi.org/10.3390/cryst15110999
APA StyleHuang, G., Xu, J., Qi, J., Zhang, F., & Dou, B. (2025). Influence of Fly Ash on the Macro Properties and Mineral Crystal Characteristics of Alkali-Activated Slag Grouting Materials. Crystals, 15(11), 999. https://doi.org/10.3390/cryst15110999






