Abstract
Olivine is a dominant constituent of the Earth’s upper mantle, and its forsterite content (Fo = 100 × Mg/(Mg + Fetotal) in molar basis) holds significant implications for indicating petrogenesis. The characteristic Raman doublet near ~820 and ~855 cm−1 shifts systematically to higher wavenumbers with increasing Fo content. Although previous studies have established general relationships between Fo content and Raman shifts in olivine, research focusing specifically on high-Fo (90–100) compositions remains limited, primarily due to a scarcity of suitable samples. This study addresses this gap by systematically investigating 45 high-Fo (90–100 olivine samples, to establish regression relationships between the Fo content and both the primary doublet (P1: 822–826 cm−1; P2: 855–858 cm−1) and three secondary peaks (P3: 881–884 cm−1, P4: 917–921 cm−1, and P5: 961–967 cm−1). Our results show that, whereas the secondary peaks (P3–P5) show weak correlations with Fo values, the doublet exhibits a strong compositional dependence, providing a reliable basis for developing calibration models. To enable the rapid screening of unknown olivines, we established a generalized linear equation (Fo = −(3547 ± 65) + (4.25 ± 0.08) P2), with P2 > 855.0 cm−1 indicating Fo > 90. For the precise quantification of these identified high-Fo samples, calibration models derived from the doublet show an excellent correlation with Fo (R2 > 0.93), with residual fluctuation within ±2.5%, a leave-one-out cross-validation root-mean-square error (LOOCV-RMSE) of ~0.7. Notably, the quadratic regression model based on the P2 peak, Fo = (346,357 ± 10,890) − (812.4 ± 287.8) P2 + (0.477 ± 0.028) P22, demonstrates exceptional predictive stability and generalization capability, with prediction errors constrained within 4 Fo units. This model provides a reliable tool for the compositional discrimination for high-Fo olivine, enriches the Raman spectral database for olivine studies, and offers a robust method for the rapid and accurate compositional analysis of both terrestrial and extraterrestrial olivine samples.
1. Introduction
Laser Raman spectroscopy is a powerful analytical technique for characterizing the structural and compositional characteristics of minerals, leveraging its distinct advantages in fingerprint identification, non-destructiveness, and high spatial resolution. The positions, quantities, and relative intensities of peaks in Raman spectra are influenced by crystal structure, atomic masses, interatomic forces, as well as ambient temperature and pressure conditions. Beyond its widespread application in terrestrial minerals, this technique holds significant promise for the characterization of extraterrestrial minerals. For instance, the Perseverance rover of the 2020 Mars mission was equipped with two Raman spectrometers to analyze the chemical composition and mineralogy of rocks and soils on the Martian surface [1,2,3,4]. Furthermore, several upcoming space exploration missions—such as the ESA’s ExoMar and China’s Tianwen-3 Mars sample return—are also scheduled to carry Raman spectrometers for in situ mineralogical characterization and the search for potential biosignatures. Laser Raman spectroscopy is also highly effective for analyzing meteorite minerals, enabling the precise identification of primary and secondary mineral phases as well as weathering product [5,6]. Given this broad applicability, research on the Raman spectral features of major rock-forming minerals (e.g., pyroxene, feldspar, olivine, garnet, and spinel [7,8,9]) is not only of great significance to geology but also provides critical technical support for extraterrestrial planetary research.
Olivine, one of the simplest silicate minerals, is a crucial rock-forming component of both terrestrial mafic-ultramafic rocks and extraterrestrial materials. As a typical orthorhombic orthosilicate (space group Pbnm) (Figure 1), Olivine [(Mg,Fe)2SiO4] forms a complete solid solution series between its end-members-forsterite (a = 4.75 Å, b = 10.20 Å, c = 5.98 Å) and fayalite (a = 4.82 Å, b = 10.48 Å, c = 6.09 Å) [10]—and typically incorporates minor substitute cations including Mn and Ni. The crystal structure of olivine consists of isolated SiO4 tetrahedra bridged by Mg2+ and Fe2+ cations. Within this framework, each silicon ion (Si4+) forms stable tetrahedral coordination with four oxygen ions (O2−), with the overall O2− framework adopting a distorted hexagonal close-packed arrangement. Si4+ ions occupy 1/8 of the tetrahedral voids within this structure. The Mg2+ and Fe2+ cations fully occupy two distinct octahedral sites, M1 and M2. The M1 site, located on an inversion center, forms a relatively regular octahedron. In contrast, the M2 site, situated on a mirror plane, exhibits a greater degree of distortion, reflected in the larger variance of its M–O bond lengths [11,12,13,14].
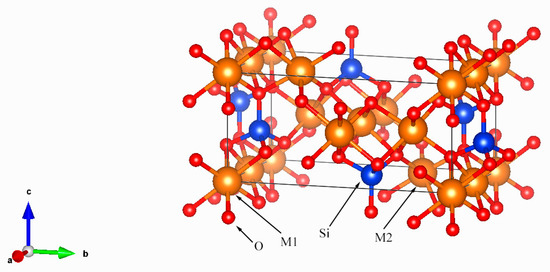
Figure 1.
Olivine (Pbnm) crystal structure with SiO4 tetrahedra with each of the tetrahedral oxygen shared by three octahedral cations.
Olivine has a total of 84 vibrational modes, of which only 36 are Raman-active: 11Ag + 11B1g + 7B2g + 7B3g [11,14]. Its Raman spectrum is conventionally divided into three regions: (1) <400 cm−1, attributed to lattice modes (rotational and translation modes of SiO4 along with M2-site cationic vibrations); (2) 400–700 cm−1, assigned to internal bending vibrational modes of the SiO4 ionic groups; and (3) 700–1050 cm−1, originating from internal stretching vibrations of the SiO4, where the doublet of olivine appears [15,16]. The most prominent feature in the Raman spectrum of olivine is a doublet, observed at approximately815–825 cm−1 and 838–857 cm−1, which are assigned to the coupled symmetric (ν1) and antisymmetric (ν3) stretching vibration modes of the SiO4 tetrahedra [11]. It is confirmed that the doublet shifts systematically to higher wavenumbers with increasing Fo content (Fo = 100 × Mg/(Mg + Fetotal) in molar basis) [17].
Significant progress has been made by previous studies in calibrating the relationship between olivine composition and its Raman spectral peaks. Following the initial exploration by Guyot et al. [18], a series of quantitative models have been developed to correlate forsterite content with Raman peak positions [19,20,21,22]. Through successive refinements, the prediction accuracy of these models has been enhanced to a level suitable for practical geological applications [22]. The model further optimized by Torre-Fdez et al. [23] through multi-omics data integration has been successfully applied to the analysis of olivine in Martian meteorites (e.g., RBT 04262 and LAR 12095) [10,24], which expands the applicability of this technology. Beyond relying solely on peak positions, Breitenfeld et al. [25] employed machine learning to fully exploit spectral information—including band shape and intensity across the 300–1500 cm−1 range—and developed a five-region partial least squares model for olivine composition prediction.
In addition to chemical composition, the Raman spectrum of olivine is also sensitive to variations in temperature and pressure [26,27,28,29,30]. As the dominant inclusion in diamonds, the residual stress in olivine quantified by Raman spectroscopy provides key insights into the origin of their host diamonds [31].
High-Mg olivine is exceptionally rare in natural environments, resulting in a persistent challenge for quantitative Raman calibration: the scarcity of samples, particularly within the Fo 95–100 range. For instance, Breitenfeld et al. reported only two natural specimens within the Fo 95–100 range [25], while other studies have relied exclusively on synthetic forsterites (Fo = 100). Consequently, existing calibration models lack precision in this compositional range and exhibit substantial prediction bias for Fo > 90 Nevertheless, high-Mg olivine represents an important component in both terrestrial and extraterrestrial mineral analyses. To address these gaps, this study systematically investigates 45 high Fo content (90–100) olivines using Raman spectrometers under varied experimental parameters. Based on the acquired dataset, we develop and optimize calibration models, with the aim of establishing a precise quantitative formulation specifically tailored to high-forsterite olivines.
2. Materials and Methods
2.1. Materials
The olivine samples analyzed in this study were derived from 15 rocks, including peridotites, marbles and dunites [32] collected from four deposits. Detailed sample provenance data are summarized in Table 1. It is noteworthy that although the rocks labeled as peridotites have been traditionally classified as such [33], the very low nickel content (0–0.06 wt%) found in their olivines indicates that these particular samples are more likely derived from a magnesium skarn. Standard petrographic thin sections were prepared from these rocks. Petrographic examination was performed using a Leica DM2700P polarizing microscope (Leica Microsystems, Wetzlar, Germany) at the National Infrastructure of Mineral, Rock and Fossil for Science and Technology. From these thin sections, 45 representative olivine grains, mostly 0.3 to 1 mm in size, were selected for subsequent analysis.

Table 1.
Lithology and Locality of Olivine-Host Rocks.
2.2. Methods
The chemical composition of olivine grains was analyzed using a SHIMADZU EPMA-1720 wavelength-dispersive electron microprobe (Shimadzu Corporation, Tokyo, Japan) at the Electron Probe Laboratory, China University of Geosciences (Beijing). Operating conditions were as follows: beam current of 10 nA, accelerating voltage of 15 kV, excitation time of 10 s, excitation beam spot diameter of 5 µm. The instrument was calibrated using 52 standard minerals from the SPI Supplies (West Chester, PA, USA), and the correction method employed was ZAF 3. All analyzed spots were positioned at the core of the olivine grains to ensure compositional representativeness. The obtained chemical compositions are presented in Table A1 (Appendix A). The corresponding cation numbers, calculated on the basis of 4 oxygen atoms, and the resulting forsterite (Fo = 100 × Mg/(Mg + Fetotal) in molar basis) contents are compiled in Table A2 (Appendix A). The forsterite content of the 45 analyzed olivines ranges from 90.00 to 99.86 (Figure 2). The total amount of trace components detected such as MnO, NiO and CaO in each sample was less than 1 wt%.
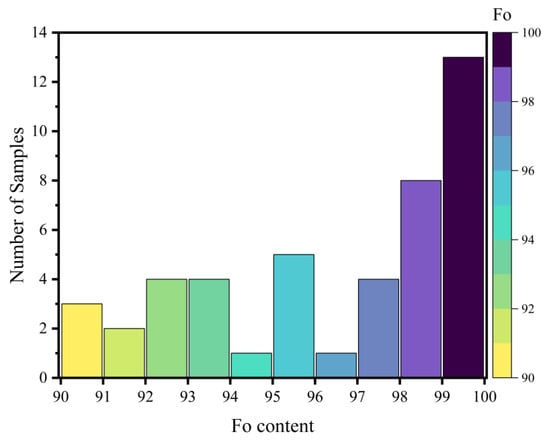
Figure 2.
Histogram of 45 Olivine Samples (Fo: 90–100).
Raman measurements were performed using a Horiba HR-Evolution Raman Spectrometer (Horiba Scientific, Villeneuve d’Ascq, France) at the School of Gemmology, China University of Geosciences (Beijing). The samples were excited using a 532 nm laser with a power of 100 mw and a spot size of 1 μm. Every Raman spectrum was acquired with a scanning time of 20 s, and an accumulation of 3 times. Before the measurements, the Raman shift was initially corrected to a Si wafer shift of 520.7 cm−1. Furthermore, to mitigate wavenumber drift caused by room temperature and power supply fluctuations, a single-crystal silicon wafer was used repeatedly for calibration throughout the testing process. To assess the impact of spectral resolution, measurements were conducted using two different gratings: an 1800 lines/mm grating for samples from the Houxianyu and Ji’an deposits (yielding a resolution of ~0.65 cm−1), and a 600 lines/mm grating for the other two localities (resulting in a resolution of ~1.5 cm−1). The measurement data were recorded and processed using LabSpec 6 software version 6.5.6.1 [34]. The positions of the five Raman bands between 700 and 1050 cm−1 for all 45 olivine grains are listed in Table A3 (Appendix A).
In this study, we used two key parameters for model evaluation: the coefficient of determination (R2) to assess the fitting degree of regression models, and the root mean square error (RMSE) to measure prediction accuracy. The R2 range is between 0 and 1, with values closer to 1 indicating a more perfect model fit (calculation formula shown in Equation (1)). Unlike R2, RMSE quantifies the differences between predicted and true values. The smaller the RMSE, the higher the fitting accuracy of the model to the training data. To evaluate model performance, we implemented leave-one-out cross-validation (LOOCV), an approach particularly suitable for small sample sizes (n ≤ 50). In LOOCV, each iteration uses one sample as the test set and all remaining samples as the training set; this process repeats n times (where n equals the total sample size), with the final performance metrics derived from averaging all n test results (calculation formula provided in Equation (2)).
3. Results
3.1. Raman Measurements
The specific assignment of Raman modes in olivine has been summarized in previous studies [11,16,25]. Figure 3 shows the characteristic doublet peaks from the 45 olivine samples in this study, clearly illustrating a systematic shift to higher wavenumbers with increasing Fo values. Specifically, the peak near 820 cm−1 shifts from 822.8 to 825.6 cm−1, while the peak near 850 cm−1 varies between 855.2 and 857.8 cm−1. The observed increase in Raman wavenumber with Mg content is attributed to the shortening of the average Si-O bond lengths and hence, stronger Si-O force constant. As Fe2+ is substituted by Mg2+, the forsterite number (Fo) increases, leading to a decrease in both the atomic mass at octahedral sites and the polyhedral volumes. The consequent shortening of M1-O and M2-O bond lengths induces positional shifts of oxygen atoms bridging SiO4 tetrahedra with M1/M2 sites [35]. This leads to shorter Si-O bonds and higher vibrational frequencies, thereby causing the observed high-wavenumber shifts in the Raman peaks. The degree of coupling between the symmetric and antisymmetric stretching modes of the SiO4 groups may also influence the Raman shifts [11,16,20,25]. In addition to the peak positions, the relative intensity of the doublet is also affected by the crystallographic orientation of the measured areas [36].
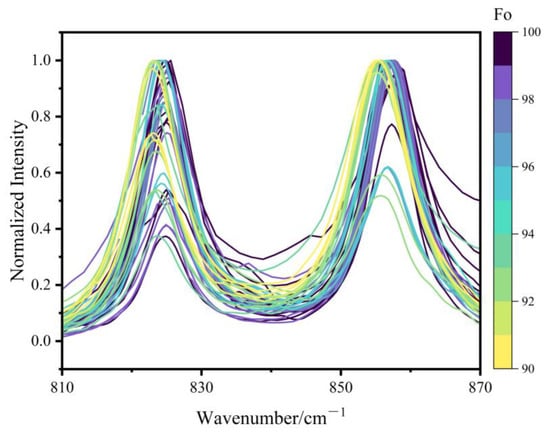
Figure 3.
Raman spectra of olivine doublet of 45 samples. All spectra were baseline-corrected and normalized to the maximum intensity of 1.
3.2. Peak Fitting
While octahedral substitution occurs, the resulting spectral shifts are not uniformly evident across all Raman-active regions. The spectral regions < 400 cm−1 and 400–700 cm−1 are characterized by weak signal intensity and poor resolvability in mixed-phase spectra, which limits their practical application. In contrast, the peaks in the 700–1050 cm−1 region show high intensity, enabling the clear identification of olivine even in complex mineral mixtures. Therefore, this study focused primarily on this high-frequency region. Beyond the characteristic doublet, we observed three secondary peaks at approximately 881–883 cm−1, 918–921 cm−1, and 962–967 cm−1 (Figure 4), all of which also exhibit systematic shifts. These three secondary peaks are attributed to the Si-O antisymmetric stretching vibration ν3 [11,22,35].
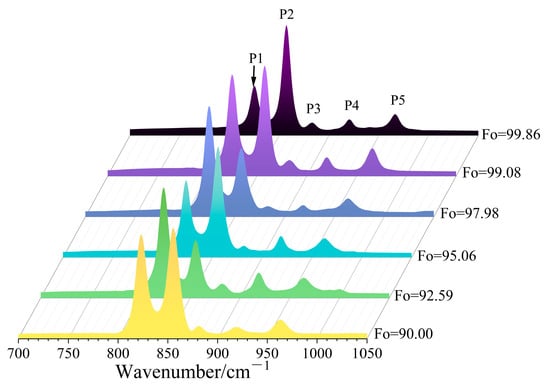
Figure 4.
Raman spectra (700–1050 cm−1) of six olivines with different Fo values.
We denote these five peaks as P1 (815–825 cm−1), P2 (838–857 cm−1), P3 (881–883 cm−1), P4 (918–921 cm−1), and P5 (962–967 cm−1). Both linear and quadratic fittings were applied to the relationship between the position of each peak (Pi) and the Fo content. The general expressions for the regression models, established using the least-squares method, are as follows:
where k1, k2, k3, b1, and b2 represent the regression parameters. The detailed equations and corresponding R2 values for all models are listed in Table A4 (Appendix A). The goodness of fit for the doublet (P1 and P2) was significantly better than that for the secondary peaks (P3, P4 and P5). The R2 values for both P1 and P2 exceeded 0.93, whereas those for P3, P4, and P5 were substantially lower, generally below 0.80 (P3: 0.561–0.657; P4: 0.755–0.783; P5: 0.776–0.781), indicating relatively weak correlation. Consequently, we recommend that the doublet be prioritized for subsequent quantitative analyses, while the secondary peaks may serve as supplementary indicators. Both linear and quadratic regressions revealed strong correlations between Fo content and the doublet positions (Figure 5a,b). Notably, the goodness of fit for P2 (R2 = 0.938–0.942) were marginally better than those for P1 (R2 = 0.935–0.940). Furthermore, the quadratic model exhibited better performance than the linear model for both peaks.
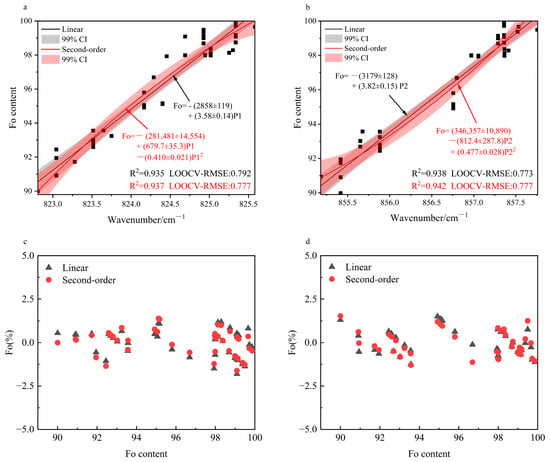
Figure 5.
(a) Linear and Quadratic Fit of P1 Peak vs. Fo (R2 & LOOCV-RMSE); (b) Linear and Quadratic Fit of P2 Peak vs. Fo (R2 & LOOCV-RMSE); (c) Residuals of the Linear and Quadratic Fits for P1; (d) Residuals of the Linear and Quadratic Fits for P2.
The regression residual plots (Figure 5c,d) indicate that the residuals of all fitted models fall within a narrow band of ±2.5%, confirming the high accuracy of the models. A notable systematic pattern is observed, however: predictions based on P1 tend to overestimate the Fo content, whereas those based on P2 consistently yield underestimations.
Given the small sample size (n = 45), leave-one-out cross-validation (LOOCV) was employed to evaluate model performance, consistent with the methodology of Breitenfeld et al. for univariate predictive models [25]. The LOOCV results (Figure 5a,b) demonstrate the robust predictive capability of the doublet-based models, with those for P2 again slightly outperforming those for P1. For P1, the quadratic model (LOOCV-RMSE = 0.777) shows a slight improvement over the linear model (LOOCV-RMSE = 0.792), suggesting that a moderate increase in model complexity can enhance prediction accuracy. In contrast, the opposite trend was observed for P2. A direct comparison of the LOOCV-RMSE values reported by Breitenfeld et al. demonstrates the superior predictive performance of our calibration models [25].
4. Discussion
4.1. Comparative Analysis of Calibration Models
Previous studies have reported distinct distributions of Fo content in olivine samples. Specifically, the study by Kuebler et al. [20] covered the complete solid solution from fayalite to forsterite, whereas Ishibashi et al. [22] focused on medium-to-high Mg olivines (Fo: 62.8–100). The work of Yasuzuka et al. [31] is further confined to the high-Mg interval (Fo: 70–100). A systematic review of published data, visualized in the Fo value versus doublet scatter plots (Figure 6), reveals a pronounced scarcity of samples in the high-Mg range (Fo: 90–100). This reveals a significant data gap in this end-member region. This study specifically addresses this gap through systematically data supplementation in this critical interval, thereby providing essential support for establishing a comprehensive composition-spectral evolution model.
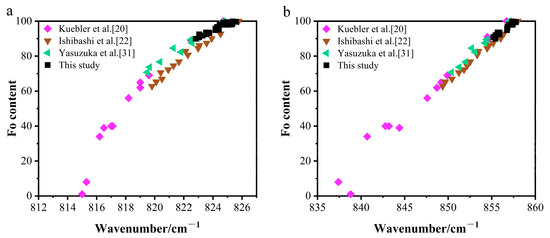
Figure 6.
Raman doublet peak positions versus Fo content. (a) The 815–826 cm−1 peak versus Fo content. (b) The 837–858 cm−1 peak versus Fo content. Data sources: This study, Kuebler et al., Yasuzuka et al., Ishibashi et al. [20,22,31].
To enable straightforward and efficient estimation of Fo values in olivine samples of unknown composition, this study integrated data from both published sources [20,22,31] and our own measurements. We developed linear calibration models for olivines with Fo > 60 based on the P1 and P2 peaks in their Raman spectra (Figure 7). While the relationship between the P1 peak position and Fo content exhibits mild nonlinearity, a linear approximation still offers a practically useful estimate suitable for initial screening purposes.
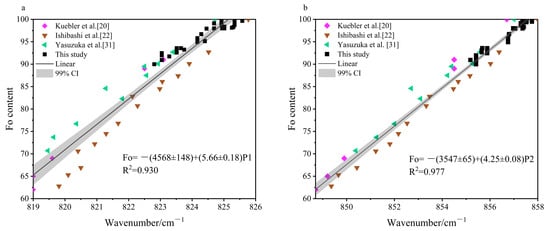
Figure 7.
Multi-source linear calibration of Raman peaks for Fo > 60 olivines. (a) Fo vs. P1 peak position. (b) Fo vs. P2 peak position [20,22,31].
The P2-based calibration displays a stronger linear correlation, achieving a better fit (R2 = 0.977) compared to the P1-based model (R2 = 0.930). Furthermore, within the Fo > 60 composition range, the P2 peak spans a broader Raman shift interval (848.7–858.0 cm−1) than the P1 peak (819.0–825.8 cm−1), reflecting its greater sensitivity to compositional variation and consequently enhanced robustness and generalizability across diverse analytical conditions. Based on these results, we recommend the P2-derived linear equation, Fo = −(3547 ± 65) + (4.25 ± 0.08) P2, as the method of choice for rapid Fo estimation in unknown olivine samples. This model directly leads to a simple and robust discriminant: a P2 peak position exceeding 855.0 cm−1 reliably predicts Fo > 90. The combination of high goodness-of-fit (R2 = 0.977) and this practical criterion, which incorporates regression uncertainties, ensures the practical reliability of this equation for screening purposes.
4.2. Performance Evaluation: Comparative Prediction of High-Forsterite (Fo > 90)
Four primary types of calibration models have been established in previous studies to quantify the relationship between Fo content and Raman peak shifts: (1) Single-peak linear models [31,36]; (2) Single-peak quadratic models [19,20,21,23]; (3) Doublet quadratic models [25]; and (4) Peak-separation models [21,36]. We compiled data from the single-peak linear and the quadratic models (Figure 8), and observed significant differences in their predictions of Fo content. A key factor contributing to these discrepancies is the varying calibration ranges of the models. Notably, the linear model from Yasuzuka et al. [31] was calibrated exclusively for Fo values between 70 and 100, while all other models were developed using the full compositional range (Fo0-100).
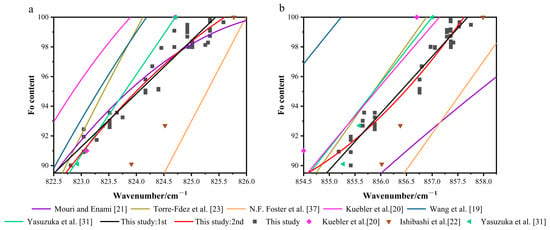
Figure 8.
Calibration models of the doublet positions vs. Fo: this study model vs. existing models. (a) P1 peak position vs. Fo content. (b) P2 peak position vs. Fo content. Note: N.F. Foster et al. [37] and Yasuzuka et al. [31] used linear fitting; Wang et al. [19], Kuebler et al. [20], and Mouri and Enami [21] adopted quadratic fitting; Torre-Fdez et al. [23] employed a quadratic fitting curve with Fo as the abscissa and peak shift as the ordinate, which has been inversely processed here. The specific equations and their statistical parameters are provided in the respective original publications.
In the 815–825 cm−1 spectral region, the models proposed by Mouri and Enami [21] and Yasuzuka et al. [31] agree well with the experimental data. In contrast, the model by Kuebler et al. [20] displays a consistent positive bias, characterized by a fitted curve that lies systematically above the data cluster, indicating a systematic overestimation. Conversely, the model developed by N.F. Foster et al. [37] shows a clear negative bias, as its predictions are generally lower than the observed values, reflecting a systematic underestimation.
In the 838–857 cm−1 spectral region, the model by Wang et al. [19] shows significant deviations from others, with a maximum discrepancy reaching 7 Fo units. In contrast, the calibrated models developed by Kuebler et al. [20] and Torre-Fdez et al. [23] perform notably well in this range, consistently maintaining prediction errors within 3 Fo units. Those of Mouri and Enami [21] and N.F. Foster et al. [37], however, exhibit systematic negative bias, consistently underestimating Fo content.
To more accurately evaluate the generalization capabilities of existing and proposed models, we applied them to predict Fo content in five high-forsterite (Fo = 90–100) datasets compiled from Kuebler et al., Ishibashi et al., and Yasuzuka et al. [20,22,31]. The prediction results (see Table A5 and Table A6 in the Appendix A) align with the degree of fit shown in Figure 7, revealing that each model possesses distinct generalization abilities and systematic deviations to varying degrees. It should be noted that samples with similar Fo content from Ishibashi et al. [22] and Yasuzuka et al. [31] exhibit significant differences in their Raman doublet positions. Whereas most calibration models could only fit the experimental dataset from a single researcher well, but perform poorly on other datasets. Furthermore, only the models proposed in this study simultaneously match multi-source data and deliver significantly better performance than other calibration models, with both P2-based models constraining prediction errors within 4 Fo units.
The systematic over- or underestimation observed across different calibration models stems from a combination of factors, including Raman measurement conditions, analytical methodologies, and sample coverage. These variables lead to substantial performance variation when existing models are applied to independent datasets. In practice, the selection of an appropriate model should be guided by the target Fo range. For the range of 60–100, the model by Ishibashi et al. [22] is recommended, while the Yasuzuka et al. [31] model is better suited for the 70–100 range. For the high-value interval (90–100), the model proposed in this study, owing to its excellent consistency across multiple datasets, can serve as a more reliable choice.
4.3. The Optimized Model for Precise Determination of High-Fo Olivines
The development of the calibration model was guided by two key priorities: high predictive accuracy and robust generalizability across diverse datasets.
Previous studies have clearly confirmed that variations in instrument configurations and experimental parameters (e.g., laser wavelength, spectral resolution) during Raman spectroscopic measurements can lead to observable shifts in Raman peak positions [25]. To mitigate systematic errors arising from a single instrument or fixed analytical conditions, and to thereby develop robust calibration models adaptable to different experimental setups, this study conducted systematic measurements using two distinct grating densities: 600 lines/mm and 1800 lines/mm.
The accurate identification of the weak peaks P3, P4, and P5 was constrained by two principal challenges. First, it was observed that when the grating line number was 1800, the signal noise became significantly more pronounced, which complicated the accurate identification of weak peaks such as P3, P4, and P5. A further inherent limitation arose from the samples themselves: the natural variation in olivine crystal orientation led to changes in the relative intensities of these peaks, causing them to appear indistinct or entirely absent in a subset of the data. Together, these factors rendered subsequent fitting of P3–P5 highly unreliable. Therefore, this study focuses on developing calibration models based on the P1 and P2 peaks.
We also systematically integrated our dataset with published Fo 90–100 olivine Raman spectra featuring the characteristic doublet from Kuebler et al. [20], Ishibashi et al. [22], and Yasuzuka et al. [31]. Both linear and quadratic regression analyses were performed on the combined dataset (Figure 9). However, the performance of the integrated model did not meet expectations. The coefficients of determination for the integrated fits were in the range of R2 = 0.88–0.92, significantly lower than those of the model from our dataset alone (R2 > 0.94). This outcome demonstrates that merely expanding the sample size does not guarantee improved accuracy and may instead introduce non-negligible systematic biases. Thus, our subsequent evaluation centers on the P1 and P2-based calibration models derived exclusively from our dataset.
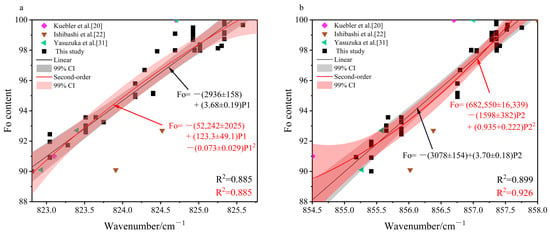
Figure 9.
Fitting results for the integrated dataset (Fo 90–100): (a) P1 peak position vs. Fo; (b) P2 peak position vs. Fo [20,22,31].
Regarding the fitting performance, the calibration models established based on P1 and P2 showed no significant differences in the coefficient of determination (R2), residual distribution, or leave-one-out cross-validated root mean square error (LOOCV-RMSE).
Regarding the prediction performance in this study, both P1-based calibration models performed excellently on datasets from Kuebler et al. [20] and Yasuzuka et al. [31], with prediction errors consistently below 1%, indicating high precision. However, its prediction error exceeded 4% in the dataset from Ishibashi et al. [22]. This suggests that the P1 is highly sensitive to variations in experimental conditions. In contrast, both P2-based calibration models maintained stable prediction errors within 2–4 Fo units across the Ishibashi et al. data [22], displaying superior predictive capability compared to both P1-based models. Although the P2-based model showed slightly lower predictive accuracy than the P1-based model on the datasets from Kuebler et al. [20] and Yasuzuka et al. [31], its stability and consistency across diverse datasets make its overall predictive performance more robust and reliable. Furthermore, the quadratic model of P2 provided marginally better prediction results than its linear counterpart.
Therefore, based on comprehensive evaluation metrics including the R2, regression residual, LOOCV-RMSE, and prediction performance, the quadratic model of P2, formulated as: Fo = (346,357 ± 10,890) − (812.4 ± 287.8) P2+ (0.477 ± 0.028) P22 was identified as the optimal predictive method for determining Fo values in the range of 90–100.
Comprehensive systematic studies of olivine composition are expected to overcome the limitations of conventional analytical approaches, enabling real-time mineral identification and precise compositional analysis in extremely complex environments (e.g., deep-sea, deep-earth, or high-altitude settings) by leveraging the rapid response and accuracy of portable Raman spectrometers.
This Raman calibration model established in this study provides a rapid method of estimating the Fo content of olivine. However, its applicability is strictly confined to the forsterite-fayalite [(Mg,Fe)2SiO4] solid-solution series. Before applying this model, we recommend preliminary screening using rapid, in situ techniques that provide semi-quantitative compositional data, such as Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM-EDS) or micro-X-ray Fluorescence (μ-EDXRF). This step confirms the characteristically low calcium content of the [(Mg,Fe)2SiO4] series and ensures analytical reliability.
5. Conclusions
This study focuses on the relationships between Raman peak shifts and composition for five Raman peaks in the 700–1050 cm−1 Raman spectral range of high-Fo olivines (Fo: 90–100). The findings reveal that the three secondary peaks (881–883 cm−1, 918–921 cm−1, and 962–967 cm−1) exhibit weak correlations with Fo content (R2 < 0.80). In contrast, the calibration models based on the doublet peaks (815–825 cm−1 and 838–857 cm−1) shows exceptional potential for quantitative analysis, with R2 > 0.93, regression residual constrained within ±2.5%, and LOOCV-RMSE values at approximately 0.7—all indicative of high reliability and accuracy. To facilitate practical application, we established a generalized linear equation (Fo = −(3547 ± 65) + (4.25 ± 0.08) P2) for rapid screening of Fo > 60 olivines, where P2 > 855.0 cm−1 serves as a reliable indicator of high-forsterite content (Fo > 90). Among all tested models, both P2-based calibration models exhibited the most prominent performance in prediction, with errors maintained below 4 Fo units. The quadratic regression model, Fo = (346,357 ± 10,890) − (812.4 ± 287.8) P2 + (0.477 ± 0.028) P22, thus provides a reliable spectroscopic approach for determining the composition of high-Mg olivines.
Author Contributions
Conceptualization, M.H. and M.Y.; data curation, T.W. and J.W.; formal analysis, T.W. and N.W.; funding acquisition, M.H.; investigation, T.W. and N.W.; methodology, T.W. and B.P.; resources, M.H. and J.W.; writing—original draft preparation, T.W. and B.P.; writing—review and editing, T.W. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Deep Earth Probe and Mineral Resources Exploration-National Science and Technology Major Project under Grant 2025ZD1008600; National Mineral Rock and Fossil Specimens Resource Center (Grant No. NCSTI-RMF202501).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed towards the corresponding author.
Acknowledgments
This present work was supported by the National Mineral Rock and Fossil Specimens Resource Center. Special thanks to the School of Gemmology and the Electron Probe Laboratory, China University of Geosciences (Beijing) for their experimental assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| R2 | Coefficient of determination |
| LOOCV | Leave-one-out cross-validation |
| RMSE | Root Mean Square Error |
Appendix A

Table A1.
Major element composition of the olivine grains used for study.
Table A1.
Major element composition of the olivine grains used for study.
| Point | TiO2 | SiO2 | Cr2O3 | MgO | FeO | MnO | NiO | K2O | CaO | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| HXY18-20101 | 0.00 | 40.33 | 0.01 | 49.74 | 9.86 | 0.27 | 0.00 | 0.01 | 0.02 | 100.26 |
| HXY 18-20102 | 0.00 | 40.57 | 0.00 | 50.47 | 9.00 | 0.19 | 0.02 | 0.02 | 0.01 | 100.26 |
| HXY 18-20201 | 0.00 | 40.95 | 0.01 | 50.66 | 9.00 | 0.24 | 0.00 | 0.03 | 0.00 | 100.89 |
| HXY 18-20103 | 0.00 | 40.55 | 0.00 | 50.27 | 8.08 | 0.23 | 0.00 | 0.01 | 0.00 | 99.14 |
| HXY 18-20202 | 0.00 | 40.64 | 0.01 | 50.63 | 7.91 | 0.32 | 0.04 | 0.01 | 0.00 | 99.55 |
| S27-0403 | 0.00 | 41.18 | 0.00 | 51.27 | 7.47 | 0.17 | 0.03 | 0.00 | 0.02 | 100.14 |
| S27-0101 | 0.03 | 41.56 | 0.02 | 51.74 | 7.38 | 0.17 | 0.02 | 0.01 | 0.00 | 100.97 |
| S27-0102 | 0.00 | 41.32 | 0.00 | 51.95 | 7.31 | 0.15 | 0.02 | 0.01 | 0.01 | 100.77 |
| S27-0303 | 0.00 | 40.85 | 0.00 | 50.82 | 7.02 | 0.12 | 0.01 | 0.01 | 0.00 | 98.83 |
| S27-0201 | 0.02 | 41.05 | 0.00 | 51.08 | 6.83 | 0.19 | 0.00 | 0.00 | 0.00 | 99.16 |
| S27-0203 | 0.06 | 40.90 | 0.02 | 51.47 | 6.64 | 0.13 | 0.00 | 0.02 | 0.00 | 99.23 |
| S27-0301 | 0.00 | 40.83 | 0.01 | 51.58 | 6.33 | 0.14 | 0.00 | 0.01 | 0.01 | 98.91 |
| RR32-1 | 0.03 | 42.31 | 0.02 | 50.38 | 6.17 | 0.25 | 0.04 | 0.01 | 0.02 | 99.28 |
| wy33-0106 | 0.00 | 41.07 | 0.00 | 53.00 | 5.06 | 0.16 | 0.00 | 0.00 | 0.00 | 99.29 |
| WY330102 | 0.00 | 41.14 | 0.00 | 52.67 | 4.88 | 0.12 | 0.04 | 0.01 | 0.01 | 98.87 |
| wy33-0107 | 0.00 | 41.20 | 0.00 | 53.95 | 4.00 | 0.18 | 0.06 | 0.02 | 0.00 | 100.45 |
| wy33-0308 | 0.00 | 41.48 | 0.00 | 53.93 | 4.94 | 0.09 | 0.01 | 0.00 | 0.01 | 100.45 |
| wy33-0307 | 0.00 | 41.32 | 0.05 | 54.67 | 4.95 | 0.05 | 0.01 | 0.00 | 0.01 | 101.04 |
| wy33-0105 | 0.00 | 41.35 | 0.00 | 55.60 | 4.35 | 0.13 | 0.00 | 0.01 | 0.00 | 101.44 |
| D10 | 0.14 | 37.73 | 0.00 | 58.80 | 3.58 | 0.16 | 0.02 | 0.05 | 0.03 | 100.60 |
| s18-0605 | 0.00 | 41.29 | 0.00 | 56.21 | 2.12 | 0.04 | 0.00 | 0.12 | 0.05 | 99.82 |
| s18-0305 | 0.00 | 41.88 | 0.02 | 56.20 | 2.07 | 0.04 | 0.01 | 0.02 | 0.01 | 100.25 |
| s18-0603 | 0.02 | 41.50 | 0.01 | 56.13 | 2.05 | 0.09 | 0.00 | 0.03 | 0.01 | 99.83 |
| RR07-4.1 | 0.00 | 41.82 | 0.00 | 55.05 | 2.01 | 0.17 | 0.04 | 0.00 | 0.00 | 99.14 |
| RR07-5.3 | 0.00 | 41.01 | 0.01 | 55.72 | 2.02 | 0.11 | 0.00 | 0.00 | 0.01 | 99.90 |
| s18-0304 | 0.05 | 41.91 | 0.00 | 56.33 | 1.91 | 0.00 | 0.01 | 0.03 | 0.01 | 100.31 |
| s18-0701 | 0.00 | 41.54 | 0.03 | 56.91 | 1.93 | 0.00 | 0.00 | 0.00 | 0.02 | 100.44 |
| s18-0106 | 0.07 | 42.33 | 0.00 | 56.44 | 1.75 | 0.00 | 0.03 | 0.00 | 0.02 | 100.65 |
| s18-0604 | 0.00 | 41.93 | 0.00 | 56.33 | 1.67 | 0.00 | 0.01 | 0.02 | 0.01 | 99.97 |
| s18-0606 | 0.00 | 41.92 | 0.00 | 56.25 | 1.33 | 0.00 | 0.00 | 0.08 | 0.04 | 99.62 |
| RR06-1 | 0.00 | 43.26 | 0.00 | 55.91 | 1.27 | 0.06 | 0.00 | 0.02 | 0.02 | 100.57 |
| s21-0203 | 0.00 | 41.78 | 0.00 | 56.46 | 1.03 | 0.03 | 0.06 | 0.03 | 0.01 | 99.40 |
| s19-0502 | 0.05 | 42.78 | 0.01 | 56.62 | 1.01 | 0.00 | 0.00 | 0.01 | 0.01 | 100.49 |
| s21-0204 | 0.00 | 42.07 | 0.00 | 56.24 | 0.93 | 0.02 | 0.01 | 0.00 | 0.03 | 99.28 |
| HYG13-1-2 | 0.02 | 43.62 | 0.02 | 55.55 | 0.92 | 0.19 | 0.05 | 0.01 | 0.01 | 100.40 |
| s16-0201 | 0.00 | 41.28 | 0.02 | 56.86 | 0.93 | 0.09 | 0.03 | 0.00 | 0.00 | 99.20 |
| HY07-1 | 0.02 | 42.93 | 0.01 | 55.62 | 0.89 | 0.09 | 0.00 | 0.00 | 0.03 | 99.62 |
| HY07-4 | 0.04 | 42.99 | 0.02 | 56.41 | 0.89 | 0.10 | 0.00 | 0.01 | 0.02 | 100.52 |
| s19-0102 | 0.00 | 41.22 | 0.00 | 57.12 | 0.88 | 0.01 | 0.05 | 0.01 | 0.01 | 99.30 |
| HY07-2 | 0.00 | 43.25 | 0.00 | 56.69 | 0.84 | 0.09 | 0.02 | 0.02 | 0.01 | 100.97 |
| s21-0304 | 0.00 | 41.90 | 0.00 | 56.44 | 0.64 | 0.00 | 0.04 | 0.00 | 0.00 | 99.02 |
| s21-0501 | 0.08 | 41.06 | 0.01 | 58.61 | 0.54 | 0.00 | 0.00 | 0.00 | 0.01 | 100.34 |
| JLJA01-1 OL1 | 0.03 | 41.05 | 0.00 | 58.20 | 0.35 | 0.01 | 0.02 | 0.02 | 0.02 | 99.82 |
| RR09-1.1 | 0.00 | 41.79 | 0.00 | 58.02 | 0.30 | 0.06 | 0.00 | 0.00 | 0.01 | 100.19 |
| RR20-1 | 0.09 | 43.25 | 0.04 | 56.33 | 0.14 | 0.00 | 0.03 | 0.01 | 0.01 | 99.94 |
The experiments were performed at the Electron Probe Laboratory, China University of Geosciences (Beijing).

Table A2.
Cation numbers (on the basis of 4 oxygen atoms) of the studied olivine grains.
Table A2.
Cation numbers (on the basis of 4 oxygen atoms) of the studied olivine grains.
| Point | Ti | Si | Cr3+ | Mg | Fe2+ a | Mn2+ | Ni | K | Ca | Total | Fo b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HXY18-20101 | 0.000 | 0.988 | 0.000 | 1.816 | 0.202 | 0.006 | 0.000 | 0.000 | 0.000 | 3.012 | 90.00 |
| HXY 18-20102 | 0.000 | 0.989 | 0.000 | 1.834 | 0.183 | 0.004 | 0.000 | 0.000 | 0.000 | 3.011 | 90.91 |
| HXY 18-20201 | 0.000 | 0.992 | 0.000 | 1.829 | 0.182 | 0.005 | 0.000 | 0.001 | 0.000 | 3.009 | 90.94 |
| HXY 18-20103 | 0.000 | 0.995 | 0.000 | 1.839 | 0.166 | 0.005 | 0.000 | 0.000 | 0.000 | 3.005 | 91.73 |
| HXY 18-20202 | 0.000 | 0.993 | 0.000 | 1.845 | 0.162 | 0.007 | 0.001 | 0.000 | 0.000 | 3.007 | 91.95 |
| S27-0403 | 0.000 | 0.997 | 0.000 | 1.851 | 0.151 | 0.003 | 0.000 | 0.000 | 0.000 | 3.003 | 92.45 |
| S27-0101 | 0.001 | 0.997 | 0.000 | 1.851 | 0.148 | 0.004 | 0.000 | 0.000 | 0.000 | 3.002 | 92.59 |
| S27-0102 | 0.000 | 0.994 | 0.000 | 1.862 | 0.147 | 0.003 | 0.000 | 0.000 | 0.000 | 3.007 | 92.68 |
| S27-0303 | 0.000 | 1.000 | 0.000 | 1.854 | 0.144 | 0.003 | 0.000 | 0.000 | 0.000 | 3.000 | 92.81 |
| S27-0201 | 0.000 | 1.000 | 0.000 | 1.856 | 0.139 | 0.004 | 0.000 | 0.000 | 0.000 | 2.999 | 93.02 |
| S27-0203 | 0.001 | 0.996 | 0.000 | 1.868 | 0.135 | 0.003 | 0.000 | 0.000 | 0.000 | 3.003 | 93.25 |
| S27-0301 | 0.000 | 1.00 | 0.000 | 1.876 | 0.129 | 0.003 | 0.000 | 0.000 | 0.000 | 3.004 | 93.56 |
| RR32-1 | 0.001 | 1.024 | 0.000 | 1.818 | 0.125 | 0.005 | 0.000 | 0.000 | 0.001 | 2.974 | 93.57 |
| wy33-0106 | 0.000 | 0.992 | 0.000 | 1.910 | 0.102 | 0.003 | 0.000 | 0.000 | 0.000 | 3.008 | 94.92 |
| WY330102 | 0.000 | 0.997 | 0.000 | 1.903 | 0.099 | 0.003 | 0.001 | 0.000 | 0.000 | 3.003 | 95.06 |
| wy33-0107 | 0.000 | 0.985 | 0.000 | 1.924 | 0.100 | 0.004 | 0.002 | 0.000 | 0.000 | 3.015 | 95.06 |
| wy33-0308 | 0.000 | 0.990 | 0.000 | 1.919 | 0.099 | 0.002 | 0.000 | 0.000 | 0.000 | 3.010 | 95.12 |
| wy33-0307 | 0.000 | 0.981 | 0.001 | 1.936 | 0.098 | 0.001 | 0.000 | 0.000 | 0.000 | 3.018 | 95.17 |
| wy33-0105 | 0.000 | 0.977 | 0.000 | 1.958 | 0.086 | 0.003 | 0.000 | 0.000 | 0.000 | 3.023 | 95.80 |
| D10 | 0.003 | 0.906 | 0.000 | 2.105 | 0.072 | 0.003 | 0.000 | 0.002 | 0.001 | 3.092 | 96.69 |
| s18-0605 | 0.000 | 0.981 | 0.000 | 1.991 | 0.042 | 0.001 | 0.000 | 0.004 | 0.001 | 3.021 | 97.93 |
| s18-0305 | 0.000 | 0.989 | 0.000 | 1.979 | 0.041 | 0.001 | 0.000 | 0.001 | 0.000 | 3.011 | 97.98 |
| s18-0603 | 0.000 | 0.985 | 0.000 | 1.986 | 0.041 | 0.002 | 0.000 | 0.001 | 0.000 | 3.015 | 97.99 |
| RR07-4.1 | 0.000 | 0.998 | 0.000 | 1.959 | 0.040 | 0.004 | 0.001 | 0.000 | 0.000 | 3.002 | 97.99 |
| RR07-5.3 | 0.000 | 0.995 | 0.000 | 1.967 | 0.040 | 0.002 | 0.000 | 0.000 | 0.000 | 3.005 | 98.01 |
| s18-0304 | 0.001 | 0.989 | 0.000 | 1.982 | 0.038 | 0.000 | 0.000 | 0.001 | 0.000 | 3.011 | 98.13 |
| s18-0701 | 0.000 | 0.980 | 0.001 | 2.001 | 0.038 | 0.000 | 0.000 | 0.000 | 0.001 | 3.020 | 98.13 |
| s18-0106 | 0.001 | 0.994 | 0.000 | 1.975 | 0.034 | 0.000 | 0.000 | 0.000 | 0.000 | 3.005 | 98.29 |
| s18-0604 | 0.000 | 0.991 | 0.000 | 1.985 | 0.033 | 0.000 | 0.000 | 0.001 | 0.000 | 3.009 | 98.37 |
| s18-0606 | 0.000 | 0.993 | 0.000 | 1.986 | 0.026 | 0.000 | 0.000 | 0.002 | 0.001 | 3.008 | 98.69 |
| RR06-1 | 0.000 | 1.012 | 0.000 | 1.950 | 0.025 | 0.001 | 0.001 | 0.000 | 0.000 | 2.989 | 98.74 |
| s21-0203 | 0.000 | 0.991 | 0.000 | 1.996 | 0.020 | 0.001 | 0.001 | 0.001 | 0.000 | 3.010 | 98.99 |
| s19-0502 | 0.001 | 1.001 | 0.000 | 1.975 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 2.998 | 99.01 |
| s21-0204 | 0.000 | 0.997 | 0.000 | 1.987 | 0.018 | 0.000 | 0.000 | 0.000 | 0.001 | 3.003 | 99.08 |
| HYG13-1-2 | 0.000 | 1.020 | 0.000 | 1.936 | 0.018 | 0.004 | 0.004 | 0.001 | 0.000 | 2.980 | 99.08 |
| s16-0201 | 0.000 | 0.982 | 0.000 | 2.016 | 0.018 | 0.002 | 0.002 | 0.000 | 0.000 | 3.018 | 99.09 |
| HY07-1 | 0.000 | 1.012 | 0.000 | 1.955 | 0.017 | 0.002 | 0.002 | 0.000 | 0.001 | 2.987 | 99.11 |
| HY07-4 | 0.001 | 1.005 | 0.000 | 1.967 | 0.017 | 0.002 | 0.002 | 0.000 | 0.000 | 2.994 | 99.12 |
| s19-0102 | 0.000 | 0.979 | 0.000 | 2.023 | 0.017 | 0.000 | 0.000 | 0.001 | 0.000 | 3.021 | 99.14 |
| HY07-2 | 0.000 | 1.007 | 0.000 | 1.967 | 0.016 | 0.002 | 0.002 | 0.000 | 0.000 | 2.993 | 99.18 |
| s21-0304 | 0.000 | 0.995 | 0.000 | 1.997 | 0.013 | 0.000 | 0.000 | 0.001 | 0.000 | 3.005 | 99.37 |
| s21-0501 | 0.001 | 0.966 | 0.000 | 2.055 | 0.011 | 0.000 | 0.000 | 0.000 | 0.000 | 3.033 | 99.49 |
| JLJA01-1 OL1 | 0.001 | 0.970 | 0.000 | 2.050 | 0.007 | 0.000 | 0.000 | 0.000 | 0.001 | 3.030 | 99.66 |
| RR09-1.1 | 0.000 | 0.981 | 0.000 | 2.031 | 0.006 | 0.001 | 0.001 | 0.000 | 0.000 | 3.019 | 99.71 |
| RR20-1 | 0.002 | 1.013 | 0.001 | 1.967 | 0.003 | 0.000 | 0.000 | 0.001 | 0.000 | 2.985 | 99.86 |
a All Fe assumed to be Fe2+. b Fo = 100 × Mg/(Mg + Fe) in molar basis.

Table A3.
Peak positions of the Raman spectra for 45 olivine grains in the 700–1050 cm−1 region.
Table A3.
Peak positions of the Raman spectra for 45 olivine grains in the 700–1050 cm−1 region.
| Point | 815–825 cm−1 | 838–857 cm−1 | 881–883 cm−1 | 918–921 cm−1 | 962–967 cm−1 | Fo |
|---|---|---|---|---|---|---|
| HXY18-20101 | 822.8 | 855.4 | 881.6 | 917.9 | 962.5 | 90.00 |
| HXY 18-20102 | 823.0 | 855.4 | 881.1 | 917.9 | 962.3 | 90.91 |
| HXY 18-20201 | 823.0 | 855.2 | 881.1 | 918.8 | 963.2 | 90.94 |
| HXY 18-20103 | 823.3 | 855.4 | 881.1 | 918.4 | 962.7 | 91.73 |
| HXY 18-20202 | 823.0 | 855.4 | 881.6 | 918.4 | 965.1 | 91.95 |
| S27-0403 | 823.0 | 855.9 | - | 919.3 | 963.2 | 92.45 |
| S27-0101 | 823.5 | 855.9 | 882.5 | 918.8 | 963.7 | 92.59 |
| S27-0102 | 823.5 | 855.6 | 881.6 | 918.8 | 961.3 | 92.68 |
| S27-0303 | 823.5 | 855.9 | 881.6 | 918.8 | 962.7 | 92.81 |
| S27-0201 | 823.5 | 855.6 | 881.6 | 918.8 | 963.7 | 93.02 |
| S27-0203 | 823.7 | 855.9 | 882.0 | 918.4 | 962.7 | 93.25 |
| S27-0301 | 823.5 | 855.9 | 881.6 | 919.3 | 962.3 | 93.56 |
| RR32-1 | 823.6 | 855.7 | 883.8 | 917.8 | 967.7 | 93.57 |
| wy33-0106 | 824.2 | 856.8 | 882.1 | 920.0 | 964.6 | 94.92 |
| WY330102 | 824.2 | 856.8 | 882.9 | 920.0 | 963.9 | 95.06 |
| wy33-0107 | 824.2 | 856.8 | 882.1 | 919.6 | 962.3 | 95.06 |
| wy33-0308 | 824.4 | 856.8 | 882.1 | 919.6 | 964.2 | 95.12 |
| wy33-0307 | 824.4 | 856.8 | 881.7 | 920.0 | 963.7 | 95.17 |
| wy33-0105 | 824.2 | 856.8 | 883.5 | 919.6 | 965.6 | 95.80 |
| D10 | 824.3 | 856.8 | 882.1 | 919.9 | 965.8 | 96.69 |
| s18-0605 | 824.5 | 857.1 | 882.0 | 918.8 | 966.4 | 97.93 |
| s18-0305 | 825.0 | 857.4 | 883.4 | 919.9 | 965.9 | 97.98 |
| s18-0603 | 824.7 | 857.1 | 882.5 | 920.2 | 966.0 | 97.99 |
| RR07-4.1 | 824.9 | 857.0 | 882.2 | 920.7 | 965.6 | 97.99 |
| RR07-5.3 | 824.9 | 857.0 | - | 919.0 | 969.0 | 98.01 |
| s18-0304 | 825.3 | 857.4 | 883.2 | 921.1 | 967.5 | 98.13 |
| s18-0701 | 825.0 | 857.4 | 883.0 | 920.4 | 965.9 | 98.13 |
| s18-0106 | 825.3 | 857.4 | 882.8 | 920.4 | 966.7 | 98.29 |
| s18-0604 | 825.0 | 857.4 | 883.0 | 920.9 | 965.9 | 98.37 |
| s18-0606 | 824.9 | 857.3 | 883.0 | 920.7 | 966.9 | 98.69 |
| RR06-1 | 825.3 | 857.4 | 882.6 | 919.4 | 966.0 | 98.74 |
| s21-0203 | 824.9 | 857.3 | 882.0 | 920.2 | 966.4 | 98.99 |
| s19-0502 | 824.9 | 857.3 | 882.5 | 919.3 | 967.4 | 99.01 |
| s21-0204 | 824.9 | 857.3 | 882.4 | 920.2 | 966.0 | 99.08 |
| HYG13-1-2 | 825.3 | 857.4 | 882.6 | 919.4 | 966.0 | 99.08 |
| s16-0201 | 824.7 | 857.3 | 882.5 | 920.7 | 967.0 | 99.09 |
| HY07-1 | 825.3 | 857.4 | 884.2 | 921.0 | 967.7 | 99.11 |
| HY07-4 | 825.3 | 857.4 | 882.6 | 921.0 | 971.0 | 99.12 |
| s19-0102 | 824.9 | 857.3 | 882.0 | 920.7 | 966.4 | 99.14 |
| HY07-2 | 825.3 | 857.4 | 884.2 | 920.2 | 967.7 | 99.18 |
| s21-0304 | 824.9 | 857.5 | 883.0 | 920.7 | 968.3 | 99.37 |
| s21-0501 | 824.9 | 857.8 | 883.4 | 920.5 | 966.7 | 99.49 |
| JLJA01-1 OL1 | 825.6 | 857.5 | 882.7 | 921.1 | 967.6 | 99.66 |
| RR09-1.1 | 825.3 | 857.4 | 882.2 | 920.7 | 965.6 | 99.71 |
| RR20-1 | 825.3 | 857.4 | 882.6 | 919.4 | 967.7 | 99.86 |

Table A4.
The order, equation and determination coefficient (R2) of the model based on five peaks.
Table A4.
The order, equation and determination coefficient (R2) of the model based on five peaks.
| Peak | Order | Equation | R2 |
|---|---|---|---|
| P1 | Linear | −(2858 ± 119) + (3.58 ± 0.14) P1 | 0.935 |
| Quadratic | −(281,481 ± 14,554) + (679.7 ± 35.3) P1 − (0.410 ± 0.021) P12 | 0.940 | |
| P2 | Linear | −(3179 ± 128) + (3.82 ± 0.15) P2 | 0.938 |
| Quadratic | (346,357 ± 10,890) − (812.4 ± 287.8) P2+ (0.477 ± 0.028) P22 | 0.942 | |
| P3 | Linear | −(2668± 386) + (3.13 ± 0.44) P3 | 0.561 |
| Quadratic | −(1,243,000 ± 37,590) + (2815 ± 825) P3 − (1.59 ± 0.48) P32 | 0.657 | |
| P4 | Linear | −(2508 ± 229) + (2.83± 0.25) P4 | 0.755 |
| Quadratic | −(403,589 ± 12,583) +(875.2 ± 391.2) P4 − (0.474 ± 0.267) P42 | 0.783 | |
| P5 | Linear | −(1193 ± 107) + (1.34 ± 0.11) P5 | 0.776 |
| Quadratic | −(56,645 ± 4814) +(116.3 ± 100.5) P5 − (0.060 ± 0.042) P52 | 0.781 |

Table A5.
Prediction results and errors of P1 Raman peak for five datasets: current vs. previous models.
Table A5.
Prediction results and errors of P1 Raman peak for five datasets: current vs. previous models.
| Fo | P1 | This Study | N.F. Foster et al. [37] | Wang et al. [19] | Kuebler et al. [20] | Mouri and Enami [21] | Torre-Fdez et al. [23] | |
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | Linear | Quadratic | Quadratic | Quadratic | Quadratic | ||
| 91 [20] | 823.1 cm−1 | 91.59 (0.59) * | 91.35 (0.35) | 79.55 (−11.45) | 93.72 (2.72) | 96.32 (5.32) | 92.13 (1.13) | 92.78 (1.78) |
| 92.7 [20] | 824.52 cm−1 | 96.68 (3.98) | 96.93 (4.23) | 89.60 (−3.1) | 101.73 (9.03) | 102.27 (9.57) | 97.02 (4.32) | 102.72 (10.02) |
| 90.1 [22] | 823.91 cm−1 | 94.5 (4.4) | 94.74 (4.64) | 85.28 (−4.82) | 98.50 (8.4) | 100.07 (9.97) | 95.19 (5.09) | 98.55 (8.45) |
| 92.7 [22] | 823.4 cm−1 | 92.67 (−0.03) | 92.67 (−0.03) | 81.67 (−11.03) | 95.56 (2.86) | 97.82 (5.12) | 93.35 (0.65) | 94.95 (2.25) |
| 90.1 [31] | 822.93 cm−1 | 90.98 (0.88) | 90.58 (0.48) | 78.34 (−11.76) | 92.65 (2.55) | 95.42 (5.32) | 91.40 (1.3) | 91.54 (1.44) |
* The prediction residual (predicted − reference Fo) in wt%.

Table A6.
Prediction results and errors of P2 Raman peak for five datasets: current vs. previous models.
Table A6.
Prediction results and errors of P2 Raman peak for five datasets: current vs. previous models.
| Fo | P2 | This Study | N.F. Foster et al. [37] | Wang et al. [19] | Kuebler et al. [20] | Mouri and Enami [21] | Torre-Fdez et al. [23] | |
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | Linear | Quadratic | Quadratic | Quadratic | Quadratic | ||
| 91 [20] | 854.5 cm−1 | 87.81 (−3.19) | 89.34 (−1.66) | 79.96 (−11.04) | 96.86 (5.86) | 89.18 (−1.82) | 84.68 (−6.32) | 88.31 (−2.69) |
| 92.7 [20] | 856.38 cm−1 | 94.99 (2.29) | 94.69 (1.99) | 89.13 (−3.57) | 104.68 (11.98) | 96.95 (4.25) | 90.63 (−2.07) | 97.53 (4.83) |
| 90.1 [22] | 856.02 cm−1 | 93.62 (3.52) | 93.40 (3.3) | 87.38 (−2.72) | 103.24 (13.14) | 95.48 (5.38) | 89.53 (−0.57) | 95.77 (5.67) |
| 92.7 [22] | 855.58 cm−1 | 91.94 (−0.76) | 92.00 (−0.7) | 85.23 (−7.47) | 101.45 (8.75) | 93.68 (0.98) | 88.16 (4.54) | 93.62 (0.92) |
| 90.1 [31] | 855.27 cm−1 | 90.75 (0.65) | 91.12 (1.02) | 83.72 (−6.38) | 100.15 (10.05) | 92.40 (2.3) | 87.18 (−2.92) | 92.10 (2.00) |
References
- Bernard, S.; Beyssac, O.; Manrique, J.A.; Lopez-Reyes, G.; Ollila, A.M.; Le Mouélic, S.; Beck, P.; Pilleri, P.; Forni, O.; Julve-Gonzalez, S.; et al. Ageing of Organic Materials at the Surface of Mars: A Raman Study Aboard Perseverance. Geochem. Perspect. Lett. 2025, 34, 25–30. [Google Scholar] [CrossRef]
- Wiens, R.C.; Udry, A.; Beyssac, O.; Quantin-Nataf, C.; Mangold, N.; Cousin, A.; Mandon, L.; Bosak, T.; Forni, O.; McLennan, S.M.; et al. Compositionally and Density Stratified Igneous Terrain in Jezero Crater, Mars. Sci. Adv. 2022, 8, eabo3399. [Google Scholar] [CrossRef] [PubMed]
- Clavé, E.; Benzerara, K.; Meslin, P.-Y.; Forni, O.; Royer, C.; Mandon, L.; Beck, P.; Quantin-Nataf, C.; Beyssac, O.; Cousin, A.; et al. Carbonate Detection with SuperCam in Igneous Rocks on the Floor of Jezero Crater, Mars. J. Geophys. Res. Planets 2023, 128, e2022JE007463. [Google Scholar] [CrossRef]
- Clavé, E.; Beyssac, O.; Bernard, S.; Royer, C.; Lopez-Reyes, G.; Schröder, S.; Rammelkamp, K.; Forni, O.; Fau, A.; Cousin, A.; et al. Radiation-Induced Alteration of Apatite on the Surface of Mars: First in Situ Observations with SuperCam Raman Onboard Perseverance. Sci. Rep. 2024, 14, 11284. [Google Scholar] [CrossRef]
- Huidobro, J.; Aramendia, J.; García-Florentino, C.; Ruíz-Galende, P.; Torre-Fdez, I.; Castro, K.; Arana, G.; Madariaga, J.M. Mineralogy of the RBT 04262 Martian Meteorite as Determined by Micro-Raman and Micro-X-ray Fluorescence Spectroscopies. J. Raman Spectrosc. 2022, 53, 450–462. [Google Scholar] [CrossRef]
- Cloutis, E.; Turenne, N.; Sidhu, S.; Connell, S.; Applin, D. A Raman Spectroscopy-Compositional-Structural Investigation of Lunar Surface Materials and Analogues. J. Chemom. 2023, 37, e2429. [Google Scholar] [CrossRef]
- Zhu, Q.; Cook, N.J.; Xie, G.; Ciobanu, C.L.; Ji, Y. Determination of Skarn Garnet Compositions Using Raman Spectroscopy. J. Raman Spectrosc. 2023, 54, 217–224. [Google Scholar] [CrossRef]
- Huang, S.; Xue, B.; Zhao, Y.; Yang, J. Characterization of Primary Silicate Minerals in Earth-like Bodies via Raman Spectroscopy. J. Raman Spectrosc. 2024, 55, 625–636. [Google Scholar] [CrossRef]
- Torre-Fdez, I.; García-Florentino, C.; Huidobro, J.; Coloma, L.; Ruiz-Galende, P.; Aramendia, J.; Castro, K.; Arana, G.; Madariaga, J.M. Characterization of Olivines and Their Metallic Composition: Raman Spectroscopy Could Provide an Accurate Solution for the Active and Future Mars Missions. J. Raman Spectrosc. 2023, 54, 340–350. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman: New York, NY, USA, 1992; ISBN 0-582-30094-0. [Google Scholar]
- Princivalle, F.; Secco, L. Crystal Structure Refinement of 13 Olivines in the Forsterite-Fayalite Series from Volcanic Rocks and Ultramafic Nodules. Mineral. Petrol. 1985, 34, 105–115. [Google Scholar] [CrossRef]
- Chopelas, A. Single Crystal Raman Spectra of Forsterite, Fayalite, and Monticellite. Am. Mineral. 1991, 76, 1101–1109. [Google Scholar]
- Kądziołka-Gaweł, M.; Dulski, M.; Kalinowski, L.; Wojtyniak, M. The Effect of Gamma Irradiation on the Structural Properties of Olivine. J. Radioanal. Nucl. Chem. 2018, 317, 261–268. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Zubkova, N.V.; Koshlyakova, N.N.; Sidorov, E.G. Natural Forsterite Strongly Enriched by Arsenic and Phosphorus: Chemistry, Crystal Structure, Crystal Morphology and Zonation. Phys. Chem. Miner. 2019, 46, 889–898. [Google Scholar] [CrossRef]
- Mohanan, K.; Sharma, S.K. A Raman Spectral Study of Forsterite-Monticellite Solid Solutions. Am. Mineral. 1993, 78, 42–48. [Google Scholar]
- Kolesov, B.A.; Geiger, C.A. A Raman Spectroscopic Study of Fe-Mg Olivines. Phys. Chem. Miner. 2004, 31, 142–154. [Google Scholar] [CrossRef]
- Do Nascimento-Dias, B.L. Overview about Raman Spectroscopy of Types of Olivine Group Minerals: A Brief Review. J. Raman Spectrosc. 2022, 53, 1942–1946. [Google Scholar] [CrossRef]
- Guyot, F.; Boyer, H.; Madon, M. Comparison of the Raman Microprobe Spectra of (Mg, Fe)2SiO4 and Mg2GeO4 with Olivine and Spinel Structures. Phys. Chem. Miner. 1986, 13, 91–95. [Google Scholar] [CrossRef]
- Wang, A.; Kuebler, K.; Jolliff, B.; Haskin, L.A. Mineralogy of a Martian Meteorite as Determined by Raman Spectroscopy. J. Raman Spectrosc. 2004, 35, 504–514. [Google Scholar] [CrossRef]
- Kuebler, K.E.; Jolliff, B.L.; Wang, A.; Haskin, L.A. Extracting Olivine (Fo-Fa) Compositions from Raman Spectral Peak Positions. Geochim. Cosmochim. Acta 2006, 70, 6201–6222. [Google Scholar] [CrossRef]
- Mouri, T.; Enami, M. Raman Spectroscopic Study of Olivine-Group Minerals. J. Mineral. Petrol. Sci. 2009, 103, 100–104. [Google Scholar] [CrossRef]
- Ishibashi, H.; Arakawa, M.; Yamamoto, J.; Kagi, H. Precise Determination of Mg/Fe Ratios Applicable to Terrestrial Olivine Samples Using Raman Spectroscopy. J. Raman Spectrosc. 2012, 43, 331–337. [Google Scholar] [CrossRef]
- Torre-Fdez, I.; Ruiz-Galende, P.; Aramendia, J.; Gomez-Nubla, L.; Castro, K.; Arana, G.; Madariaga, J.M. New quantitative model to determine fayalite-forsterite content in olivine minerals by Raman spectroscopy. In Proceedings of the 50th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2019; p. 2486. [Google Scholar]
- Huidobro, J.; Aramendia, J.; García-Florentino, C.; Población, I.; Castro, K.; Arana, G.; Madariaga, J.M. Mineralogy of the LAR 12095 Martian Shergottite as Determined by Micro-Raman and Micro-X-ray Fluorescence Spectroscopies. J. Raman Spectrosc. 2023, 54, 1229–1241. [Google Scholar] [CrossRef]
- Breitenfeld, L.B.; Dyar, M.D.; Carey, C.J.; Tague, T.J., Jr.; Wang, P.; Mullen, T.; Parente, M. Predicting Olivine Composition Using Raman Spectroscopy Through Band Shift and Multivariate Analyses. Am. Mineral. 2018, 103, 1827–1836. [Google Scholar] [CrossRef]
- Reynard, B.; Remy, C.; Takir, F. High-Pressure Raman Spectroscopic Study of Mn2GeO4, Ca2GeO4, Ca2SiO4, and CaMgGeO4 Olivines. Phys. Chem. Miner. 1997, 24, 77–84. [Google Scholar] [CrossRef]
- Durben, D.J.; McMillan, P.F. Raman Study of the High-Pressure Behavior of Forsterite (Mg2SiO4) Crystal and Glass. Am. Mineral. 1993, 78, 1143–1148. [Google Scholar]
- Weber, I.; Böttger, U.; Pavlov, S.G.; Jessberger, E.K.; Hübers, H.-W. Mineralogical and Raman Spectroscopy Studies of Natural Olivines Exposed to Different Planetary Environments. Planet. Space Sci. 2014, 104, 163–172. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.-Q.; Yan, S.-Y.; Cui, Q.-L.; Zhao, Y.-N.; Zhang, D.-G.; Chen, Y. The Laser Raman Spectroscopic Study of Olivine at High Pressure. Chin. J. Light Scatt. 2006, 1, 10–15. [Google Scholar]
- Yan, S.-Y.; Zhou, Y.-Q.; Chen, Y. The Laser Raman Spectroscopic Study of Pyroxene and Olivine at the High-Temperature. Chin. J. Light Scatt. 2004, 4, 325–331. [Google Scholar]
- Yasuzuka, T.; Ishibashi, H.; Arakawa, M.; Yamamoto, J.; Kagi, H. Simultaneous Determination of Mg# and Residual Pressure in Olivine Using Micro-Raman Spectroscopy. J. Mineral. Petrol. Sci. 2009, 104, 395–400. [Google Scholar]
- Peng, B.; He, M.; Yang, M.; Liu, X.; Sui, X.; Sun, K.; Wu, S. Petrogenesis of Jian Forsterite Jade Solely Composed of End-Member Forsterite (Fo 99.8): Constrained by Trace Element and Oxygen Isotope. Ore Geol. Rev. 2022, 150, 105167. [Google Scholar] [CrossRef]
- Dong, A.; Zhu, X.; Li, Z.; Kendall, B.; Li, S.; Wang, Y.; Tang, C. A Multi-Isotope Approach towards Constraining the Origin of Large-Scale Paleoproterozoic B-(Fe) Mineralization in NE China. Precam. Res. 2017, 292, 115–129. [Google Scholar] [CrossRef]
- Horiba Scientific. LabSpec 6 Spectroscopy Suite; Version 6; Horiba Scientific: Kyoto, Japan, 2020; Available online: https://www.horiba.com/usa/scientific/products/detail/action/show/Product/labspec-6-spectroscopy-suite-software-1843/ (accessed on 20 October 2025).
- Lam, P.K.; Yu, R.; Lee, M.W.; Sharma, S.K. Structural Distortions and Vibrational Modes in Mg2SiO4. Am. Mineral. 1990, 75, 109–119. [Google Scholar]
- Ishibashi, H.; Arakawa, M.; Ohi, S.; Yamamoto, J.; Miyake, A.; Kagi, H. Relationship between Raman Spectral Pattern and Crystallographic Orientation of a Rock-Forming Mineral: A Case Study of Fo89Fa11 Olivine. J. Raman Spectrosc. 2008, 39, 1653–1659. [Google Scholar] [CrossRef]
- Foster, N.J.; Wozniakiewicz, P.J.; Price, M.C.; Kearsley, A.T.; Burchell, M.J. Identification by Raman Spectroscopy of Mg-Fe Content of Olivine Samples after Impact at 6 km s−1 onto Aluminium Foil and Aerogel: In the Laboratory and in Wild-2 Cometary Samples. Geochim. Cosmochim. Acta 2013, 121, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).