Crystalline Nanoparticles and Their Impact on Electromagnetic Radiation Absorption in Advanced Clay Building Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Materials Characterization
3.2. Measurements on Composite Disks
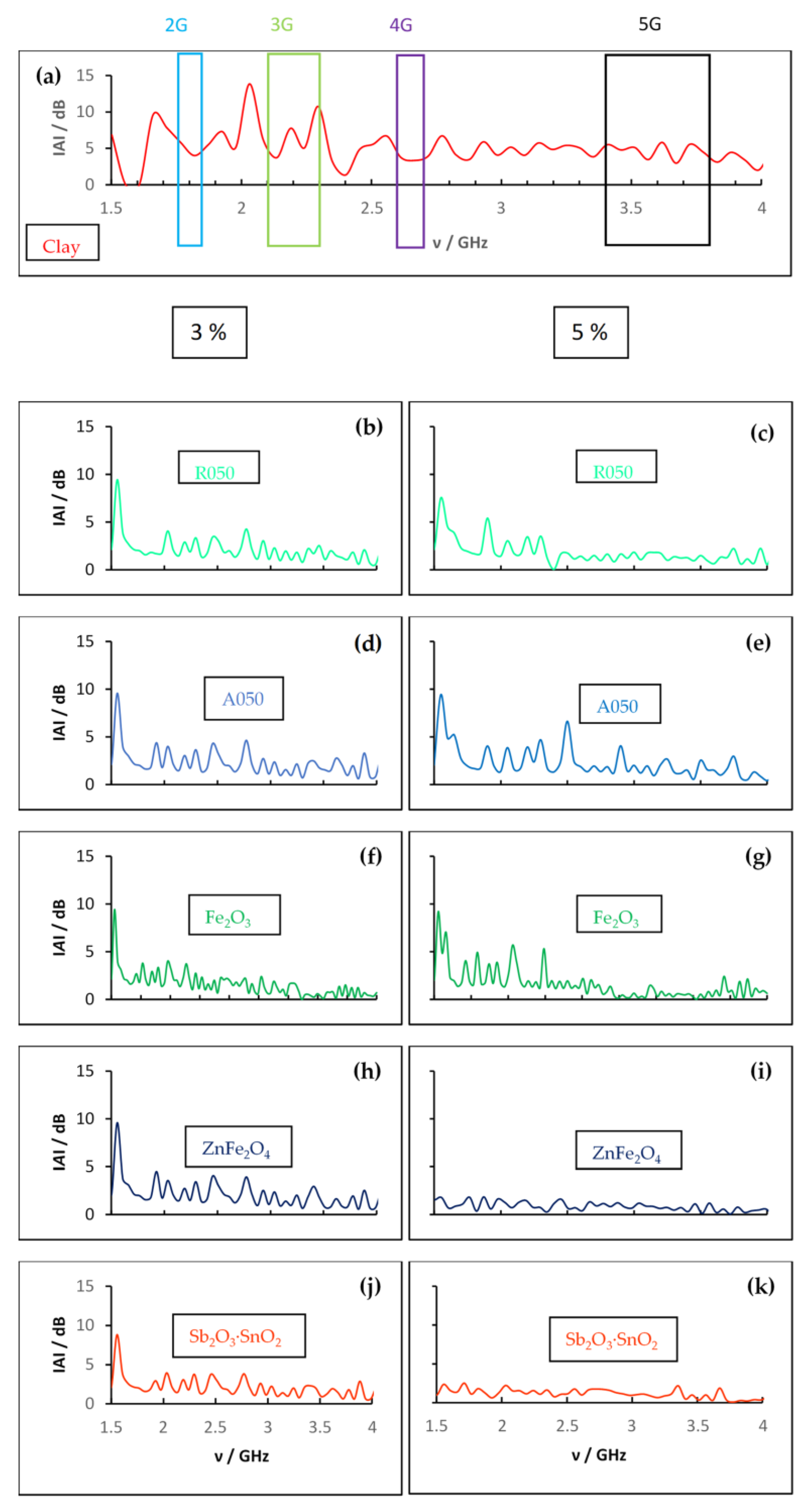
3.3. Electromagnetic Radiation Characterization of Clay and Clay-Based Composites
3.4. Discussion
| Base Material | Additive/Size/Content | EMR Measurements/Frequency Range | Conclusion | Ref. |
|---|---|---|---|---|
|
|
|
| [48] |
|
|
|
| [49] |
|
|
|
| Present work |
|
|
|
| [52] |
|
|
|
| [53] |
|
|
|
| [54,55] |
|
|
|
| Present work |
|
|
|
| [69] |
|
|
|
| [16] |
|
|
|
| Present work |
|
|
|
| [9] |
|
|
|
| Present work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATO | Antimony(III)–Tin(IV) Oxide (Sb2O3∙SnO2) |
| TGA | Thermogravimetric Analysis |
| B.E.T. | Brunauer–Emmett–Teller method |
| PXRD | Powder X-ray Diffraction |
| SEM | Scanning Electron Microscope |
| EDX | Energy-Dispersive X-ray Analyzer |
| ED–XRF | X-ray fluorescence spectrometry |
| EM | Electromagnetic |
| LTE | Long Term Evolution |
| NR | New Radio |
| TiO2 R050 | Rutile |
| TiO2 A050 | Anatase |
| EMR | Electromagnetic Radiation |
| EMI | Electromagnetic Interference |
References
- Lai, H.; Levitt, B.B. The roles of intensity, exposure duration, and modulation on the biological effects of radiofrequency radiation and exposure guidelines. Electromagn. Biol. Med. 2022, 41, 230–255. [Google Scholar] [CrossRef]
- Duhaini, I. The effects of electromagnetic fields on human health. Phys. Medica 2016, 32, 213. [Google Scholar] [CrossRef]
- Wdowiak, A.; Mazurek, P.A.; Wdowiak, A.; Bojar, I. Effect of electromagnetic waves on human reproduction. Ann. Agric. Environ. Med. 2017, 24, 13–18. [Google Scholar] [CrossRef]
- Liu, L.; Huang, B.; Lu, Y.; Zhao, Y.; Tang, X.; Shi, Y. Interactions between electromagnetic radiation and biological systems. iScience 2024, 27, 109201. [Google Scholar] [CrossRef]
- Fritzsche, H.; Phillips, M. Electromagnetic Radiation. Encyclopedia Britannica. 2025. Available online: https://www.britannica.com/science/electromagnetic-radiation (accessed on 18 June 2025).
- Barbhuiya, S.; Das, B.B.; Norman, P.; Qureshi, T. A comprehensive review of radiation shielding concrete: Properties, design, evaluation, and applications. Struct. Concr. 2024, 26, 1809–1855. [Google Scholar] [CrossRef]
- Chung, D.D.L. Materials for Electromagnetic Interference Shielding. Mater. Chem. Phys. 2020, 255, 123587. [Google Scholar] [CrossRef]
- Vrdoljak, I.; Rupčić, S.; Miličević, I.; Mandrić, V. Electromagnetic field propagation through a clay composite with antimony tin oxide (ATO) additive. In Proceedings of the 2022 International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME 2022), Male, Maldives, 16–18 November 2022; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2022; Volume 5, pp. 1–5. [Google Scholar] [CrossRef]
- Brdarić, J.; Vrdoljak, I.; Rupčić, S.; Marković, B.; Miličević, I.; Mandrić, V.; Varevac, D.; Tatar, D.; Filipović, N.; Szenti, I.; et al. The Effect of Different Nanomaterials Additions in Clay-Based Composites on Electromagnetic Transmission. Materials 2022, 15, 5115. [Google Scholar] [CrossRef]
- Chung, D.D.L. Electromagnetic interference shielding effectiveness of carbon materials. Carbon 2001, 39, 279–285. [Google Scholar] [CrossRef]
- Shukla, V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019, 1, 1640–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, X.; Guo, J.; Zhao, H. Magnetite (Fe3O4)-based nanocomposites for electromagnetic wave absorption. Materials 2020, 13, 4282. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, P.; Zong, Y.; Zhao, M.; Liu, Z. Polymer-based composites with Fe3O4 nanoparticles for electromagnetic interference shielding. Compos. Part B Eng. 2021, 223, 109123. [Google Scholar] [CrossRef]
- Wanasinghe, D.; Aslani, F.; Ma, G.; Habibi, D. Review of Polymer Composites with Diverse Nanofillers for Electromagnetic Interference Shielding. Nanomaterial 2020, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chung, D.D. Combined Use of Magnetic and Electrically Conductive Fillers in a Polymer Matrix for Electromagnetic Interference Shielding. J. Electron. Mater. 2008, 37, 1088–1094. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, W. Microwave absorbing properties of double-layer cementitious composites containing Mn-Zn ferrite. Cem. Concr. Compos. 2010, 32, 726–730. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, B.; Yin, J.; Zhou, S. Optimum mix parameters of high-strength self-compacting concrete with ultrapulverized fly ash. Cem. Concr. Res. 2002, 32, 477–480. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Simon, G.P.; Suzuki, K.; Selomulya, C.; Cashion, J.D. Phase reduction of coated maghemite (γ-Fe2O3) nanoparticles under microwave-induced plasma heating for rapid heat treatment. J. Mater. Chem. 2012, 22, 617–625. [Google Scholar] [CrossRef]
- Yun, J.; Kim, H.-I. Electromagnetic interference shielding effects of polyaniline-coated multi-wall carbon nanotubes/maghemite nanocomposites. Polym. Bull. 2012, 68, 561–573. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.; Zhang, Y.; Yuan, X.; Li, Q.; Ye, F.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of reduced graphene oxide modified by maghemite colloidal nanoparticle clusters. J. Phys. Chem. C 2013, 117, 19701–19711. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Liu, B.; Yin, Z. Preparation and properties of antimony doped tin oxide nanopowders and their conductivity. Mater. Res. Bull. 2016, 83, 354–359. [Google Scholar] [CrossRef]
- Mettler Toledo GmbH. STARe Software, Version 11.04; Mettler Toledo GmbH: Schwerzenbach, Switzerland, 2009.
- ImageJ Software. Available online: https://imagej.net/ij/ij/index.html (accessed on 20 August 2024).
- Shekhawat, M.S. Thermo Gravimetric and Differential Thermal Analysis of Clay of Western Rajasthan (India). Int. J. Mod. Phys. Conf. Ser. 2013, 22, 458–465. [Google Scholar] [CrossRef]
- McCormick, J.R.; Zhao, B.; Rykov, S.; Wang, H.; Chen, J.G. Thermal Stability of Flame-Synthesized Anatase TiO2 Nanoparticles. J. Phys. Chem. B 2004, 108, 17398–17402. [Google Scholar] [CrossRef]
- Mazeina, L.; Navrotsky, A. Enthalpy of Water Adsorption and Surface Enthalpy of Goethite (α-FeOOH) and Hematite (α-Fe2O3). Chem. Mater. 2007, 19, 825–833. [Google Scholar] [CrossRef]
- Abduh, N.A.Y.; Al-Kathani, A.; Algarni, T.S. Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst. Catalysts 2023, 13, 692. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, S.; Das, B. Synthesis and structural/microstructural characteristics of antimony doped tin oxide. arXiv 2011, arXiv:1107.1807v1. [Google Scholar] [CrossRef]
- Takeda, H.; Kudoh, Y. Single-crystal X-ray diffraction study on the bond compressibility of fayalite (Fe2SiO2) and rutile (TiO2) under high pressure. Phys. B+C 1986, 139–140, 333–336. [Google Scholar] [CrossRef]
- Djerdj, I.; Tonejc, A.M. Structural Investigations of Nanocrystalline TiO2 Samples. J. Alloys Compd. 2006, 413, 159–174. [Google Scholar] [CrossRef]
- Hill, A.H.; Jiao, F.; Bruce, P.G.; Harrison, A.; Kockelmann, W.; Ritter, C. Neutron Diffraction Study of Mesoporous and Bulk Hematite, α-Fe2O3. Chem. Mater. 2008, 20, 4891–4899. [Google Scholar] [CrossRef]
- Rykov, A.I.; Tkacova, K.; Šepelák, V. Structural disordering of spinel ferrites induced by mechanical activation. Cryst. Res. Technol. 1993, 28, 53–56. [Google Scholar] [CrossRef]
- Monrós, G.; Forés, A.; Badenes, J.A.; Llusar, M.; Sorlí, S.; Tena, M.A. Synthesis and characterization of chromium–tin oxide pigments obtained from the Cr2O3–SnO2 system. Z. Für Anorg. Und Allg. Chem. 2005, 631, 2188–2191. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. IX. Die Kristallstruktur von β-Cristobalit SiO2 (bei hohen Temperaturen stabile Form). Z. Für Krist. Cryst. Mater. 1925, 62, 189–200. [Google Scholar] [CrossRef]
- Pecharroman, C.; Gonzalez-Carreno, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, gamma-Fe2O3, from reflectance measurement on pressed powders Sample: A idealized. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Julius, O.E.; Ola, O.V.; Onesmus, O.O. Characterization and Utilization of Clays from Origo and Awo, Southwestern Nigeria. Malays. J. Geosci. 2019, 3, 52–58. [Google Scholar] [CrossRef]
- García Ten, J.; Orts, M.J.; Saburit, A.; Silva, G. Thermal conductivity of traditional ceramics. Part I: Influence of bulk density and firing temperature. Ceram. Int. 2010, 36, 1951–1959. [Google Scholar] [CrossRef]
- Jaković, M.; Slaviček, I. Analiza Toplinskih Svojstava Građevnih Materijala Primjenom Metode Vrućeg Diska; University of Zagreb: Zagreb, Croatia, 2010; p. 94. [Google Scholar]
- Tehnički Propis o Racionalnoj Uporabi Energije i Toplinskoj Zaštiti u Zgradama. Available online: https://narodnenovine.nn.hr/clanci/sluzbeni/dodatni/438515.pdf (accessed on 20 July 2023).
- HRN EN 1745:2012 Zidovi i Proizvodi za Zidanje–Metode Određivanja Toplinskih Svojstava. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2008_09_110_3240.html (accessed on 20 July 2023).
- Dondi, M.; Mazzanti, F.; Principi, P.; Raimondo, M.; Zanarini, G. Thermal Conductivity of Clay Bricks. J. Mater. Civ. Eng. 2004, 16, 8–14. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Gualtieri, A.; Gagliardi, S.; Ruffini, P.; Ferrari, R.; Hanuskova, M. Thermal conductivity of fired clays: Effects of mineralogical and physical properties of the raw materials. Appl. Clay Sci. 2010, 49, 269–275. [Google Scholar] [CrossRef]
- HRN ISO 8302:1998 Toplinska Izolacija–Mjerenje Toplinskog Otpora i Srodnih Veličina u Ustaljenom Stanju–Pločasti Uređaj sa Zaštićenom Vrućom Pločom. Available online: https://repozitorij.hzn.hr/norm/HRN+ISO+8302%3A1998 (accessed on 28 July 2023).
- Gashti, M.P.; Eslami, S. Optical and electromagnetic characteristics of clay–iron oxide nanocomposites. Res. Chem. Intermed. 2011, 37, 771–784. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Titanium Dioxide Polymorphs: Rutile vs. Anatase. Available online: https://www.samaterials.com/titanium-dioxide-polymorphs-rutile-vs-anatase.html (accessed on 26 August 2025).
- Bunea, G.; Alexa-Stratulat, S.-M.; Mihai, P.; Toma, I.-O. Use of Clay and Titanium Dioxide Nanoparticles in Mortar and Concrete—A State-of-the-Art Analysis. Coatings 2023, 13, 506. [Google Scholar] [CrossRef]
- Lu, L.; He, Y.; Ping, B.; Wang, F.; Hu, S. TiO2 containing electromagnetic wave absorbing aggregate and its application in concrete. Constr. Build. Mater. 2017, 134, 602–609. [Google Scholar] [CrossRef]
- Li, Z.; Dong, S.; Wang, X.; Yu, X.; Han, B. Electromagnetic Wave-Absorbing Property and Mechanism of Cementitious Composites with Different Types of Nano Titanium Dioxide. J. Mater. Civ. Eng. 2020, 32, 04020073. [Google Scholar] [CrossRef]
- Chicot, D.; Mendoza, J.; Zaoui, A.; Louis, G.; Lepingle, V.; Roudet, F.; Lesage, J. Mechanical Properties of Magnetite (Fe3O4), Hematite (α-Fe2O3) and Goethite (α-FeOOH) by Instrumented Indentation and Molecular Dynamics Analysis. Mater. Chem. Phys. 2011, 129, 862–870. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Almousa, N.; Elsafi, M. Preparation of Mortar with Fe2O3 Nanoparticles for Radiation Shielding Application. Coatings 2022, 12, 1329. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yang, C.S.; Cheng, H.; Huang, X.H.; Zhu, Y.H. The Electromagnetic Wave Absorption Performance and Mechanical Properties of Cement-Based Composite Material Mixed with Functional Aggregates with High Fe2O3 and SiC. Rev. Compos. Matér. Adv.-J. Compos. Adv. Mater. 2021, 31, 249–255. [Google Scholar] [CrossRef]
- Ng, J.P.S.; Sum, Y.L.; Soong, B.H.; Maier, M.; Monteiro, P.J.M. Electromagnetic Wave Propagation through Composite Building Materials in Urban Environments at Mid-Band 5G Frequencies. IET Microw. Antennas Propag. 2022, 16, 627–638. [Google Scholar] [CrossRef]
- Ng, J.P.S.; Sum, Y.L.; Soong, B.H.; Monteiro, P.J.M. Effects of Iron (III) Oxide-Enhanced Building Materials on RF Communications. In Proceedings of the 2022 IEEE Region 10 Conference (TENCON), Hong Kong, China, 1–4 November 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Ostermann, R.; Zieba, R.; Rudolph, M.; Schlettwein, D.; Smarsly, B.M. Electrospun Antimony-Doped Tin Oxide (ATO) Nanofibers as a Versatile Conducting Matrix. Chem. Commun. 2011, 47, 12119–12121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Englund, S.; Kubart, T.; Keller, J.; Scragg, J.J.S.; Platzer-Björkman, C. Antimony-Doped Tin Oxide as Transparent Back Contact in Cu2ZnSnS4 Thin-Film Solar Cells. Phys. Status Solidi (A) 2019, 216, 1900542. [Google Scholar] [CrossRef]
- Rani, J.; Sood, S.; Sharma, A. Tin Oxide for Optoelectronic, Photovoltaic and Energy Storage Devices: A Review. J. Mater. Chem. A 2021, 9, 15754–15799. [Google Scholar] [CrossRef]
- Koebel, M.M.; Nadargi, D.Y.; Jimenez-Cadena, G.; Romanyuk, Y.E. Transparent, Conducting ATO Thin Films by Epoxide-Initiated Sol–Gel Chemistry: A Highly Versatile Route to Mixed-Metal Oxide Films. ACS Appl. Mater. Interfaces 2012, 4, 2464–2473. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Gerhardt, R.A. Thin Films Made from Colloidal Antimony Tin Oxide Nanoparticles for Transparent Conductive Applications. MRS Proc. 2013, 1494, 61–66. [Google Scholar] [CrossRef]
- Reddy, N.P.; Muniramaiah, R.; Santhosh, R.; Fernandes, J.M.; Padmanaban, D.B.; Maharana, G.; Kovendhan, M.; Joseph, D.P.; Murali, B. Cost-Effective Sb-Doped SnO2 Films as Stable and Efficient Alternative Transparent Con-ducting Electrodes for Dye-Sensitized Solar Cells. J. Mater. Chem. C 2022, 10, 7997–8008. [Google Scholar] [CrossRef]
- Ramanathan, R.; Nagarajan, S.; Sanhiyamoorthy, S.; Manavaimaran, B.; Barshilia, H.C.; Mallik, R.C. A highly sensitive and room temperature ethanol gas sensor based on spray deposited Sb doped SnO2 thin films. Mater. Adv. 2024, 5, 293–305. [Google Scholar] [CrossRef]
- Kostishin, V.G.; Isaev, I.M.; Salogub, D.V. Radio-Absorbing Magnetic Polymer Composites Based on Spinel Ferrites: A Review. Polymers 2024, 16, 1003. [Google Scholar] [CrossRef]
- Ochmann, M.; Vrba, V.; Kopp, J.; Ingr, T.; Malina, O.; Machala, L. Microwave-Enhanced Crystalline Properties of Zinc Ferrite Nanoparticles. Nanomaterials 2022, 12, 2987. [Google Scholar] [CrossRef]
- Lorenz, M.; Brandt, M.; Mexner, K.; Brachwitz, K.; Ziese, M.; Esquinazi, P.; Hochmuth, H.; Grundmann, M. Ferri-magnetic ZnFe2O4 Thin Films on SrTiO3 Single Crystals with Highly Tunable Electrical Conductivity. Phys. Status Solidi (RRL) 2011, 5, 438–440. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Ye, Z.-G.; Xie, Z.; Zheng, L. Partially Inverse Spinel ZnFe2O4 with High Saturation Magnetization Synthesized via a Molten Salt Route. Appl. Phys. Lett. 2011, 99, 202505. [Google Scholar] [CrossRef]
- Rani, A.; Yadav, A.; Kumar, R.; Singh, D.; Yadav, R.S. Effect of Fuel Type and Synthesis Temperature on Magnetic Properties of ZnFe2O4 Nanomaterials Synthesized by Sol–Gel Method. J. Sol-Gel Sci. Technol. 2024, 111, 783–793. [Google Scholar] [CrossRef]
- Abu Ayana, Y.M.; El-Sawy, S.M.; Salah, S.H. Zinc–Ferrite Pigment for Corrosion Protection. Anti-Corros. Methods Mater. 1997, 44, 381–388. [Google Scholar] [CrossRef]
- Li, X.; Kang, Q.; Zhou, C. Research on Absorbing Properties of the Concrete Shielding Material at 3 mm Wave Bands. In Proceedings of the Asia-Pacific Conference on Environmental Electromagnetics (CEEM 2003), Hangzhou, China, 4–7 November 2003; pp. 536–540. [Google Scholar] [CrossRef]
- Meng, X.; Xu, W.; Ren, X.; Zhu, M. Progress and Challenges of Ferrite Matrix Microwave Absorption Materials. Materials 2024, 17, 2315. [Google Scholar] [CrossRef] [PubMed]
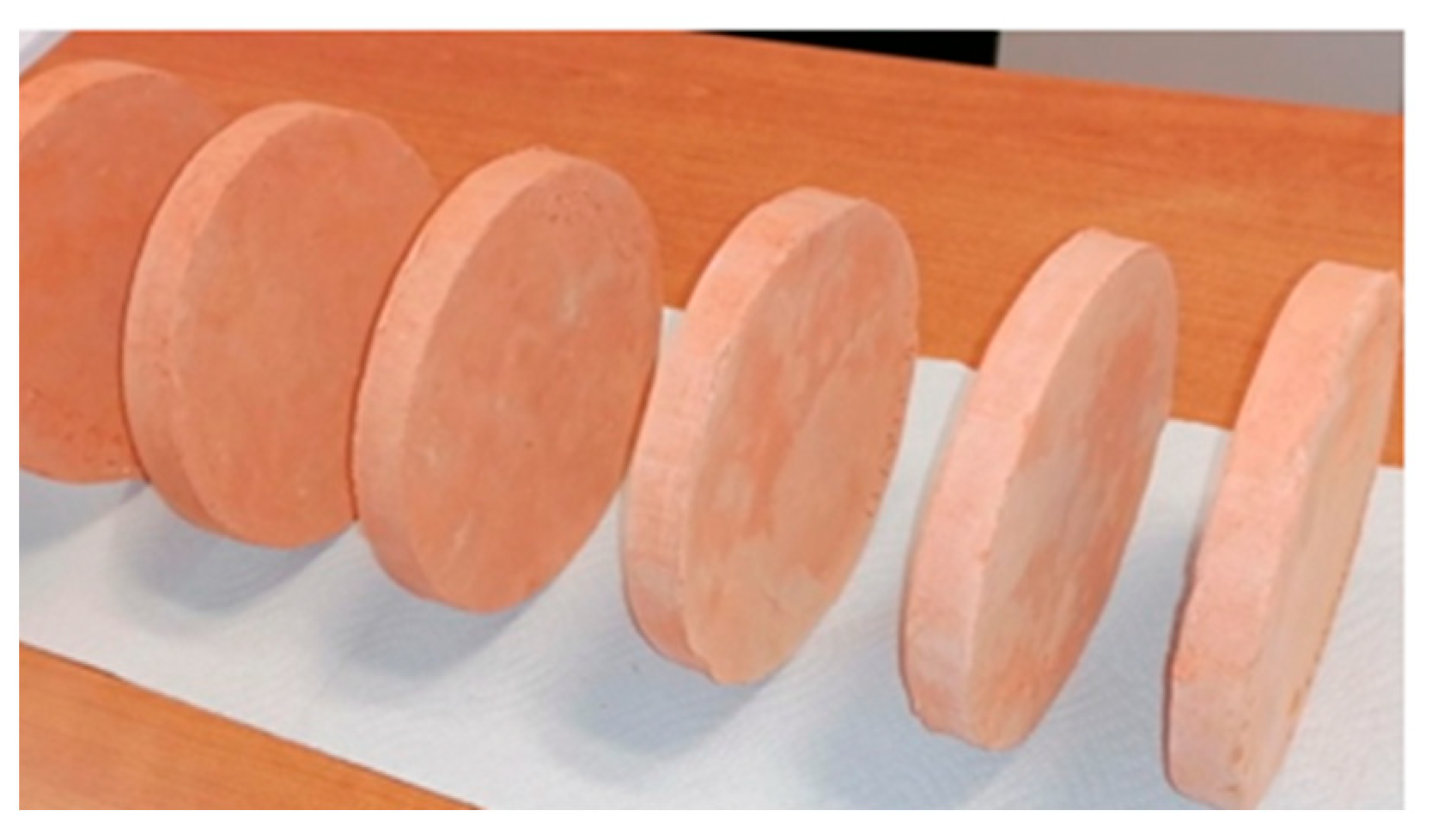
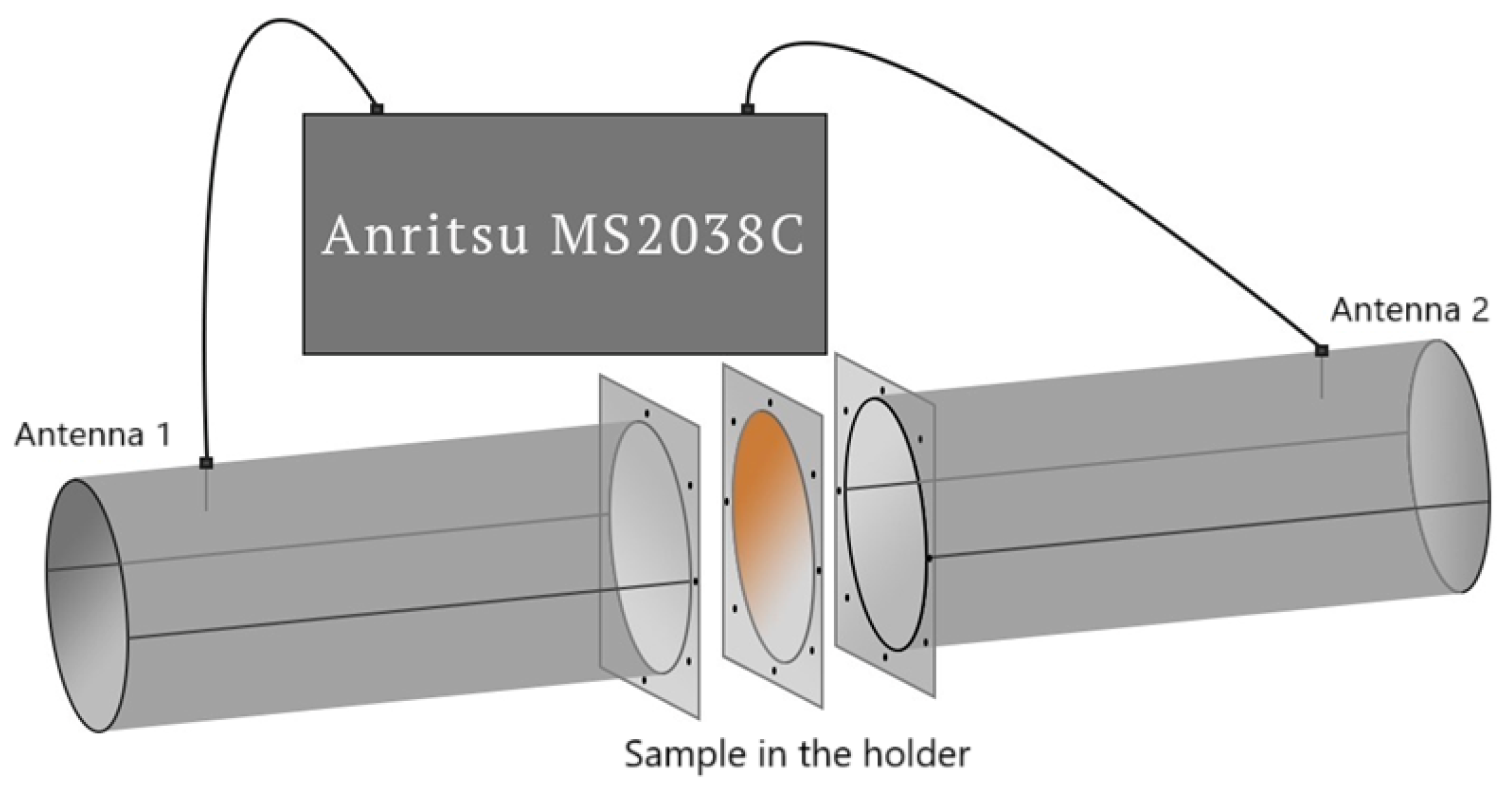
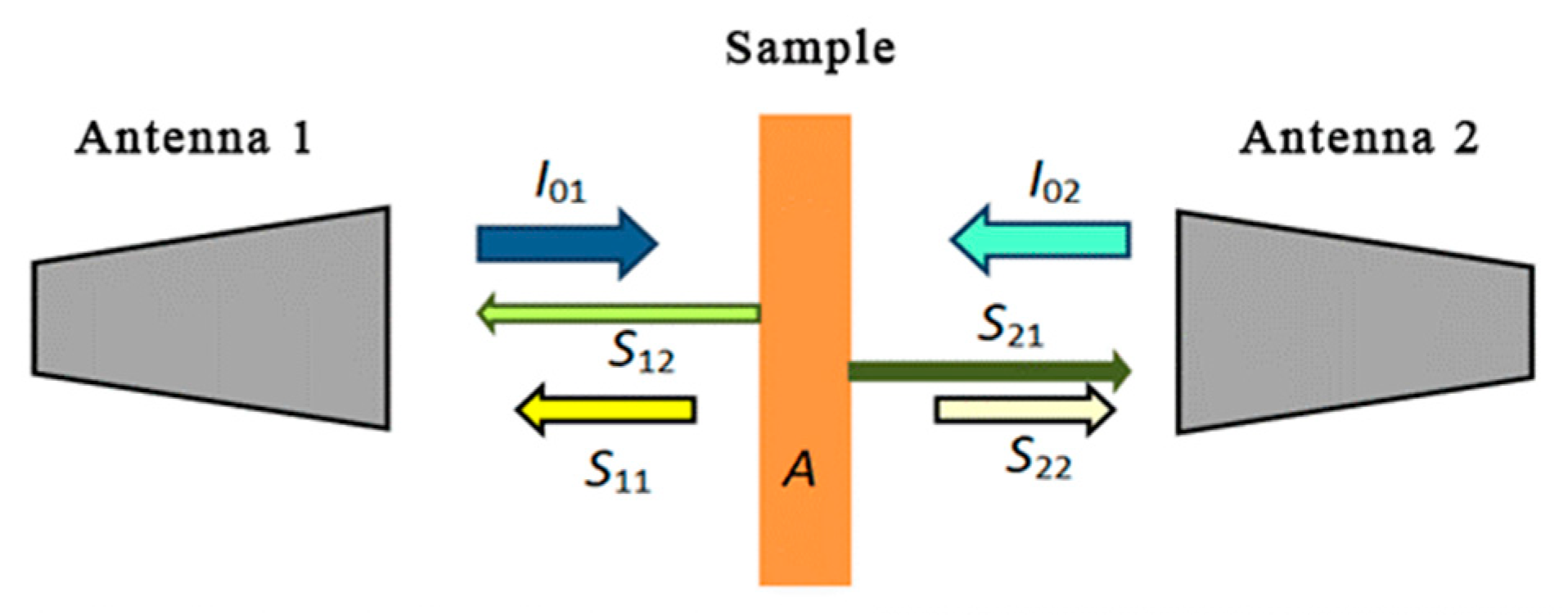
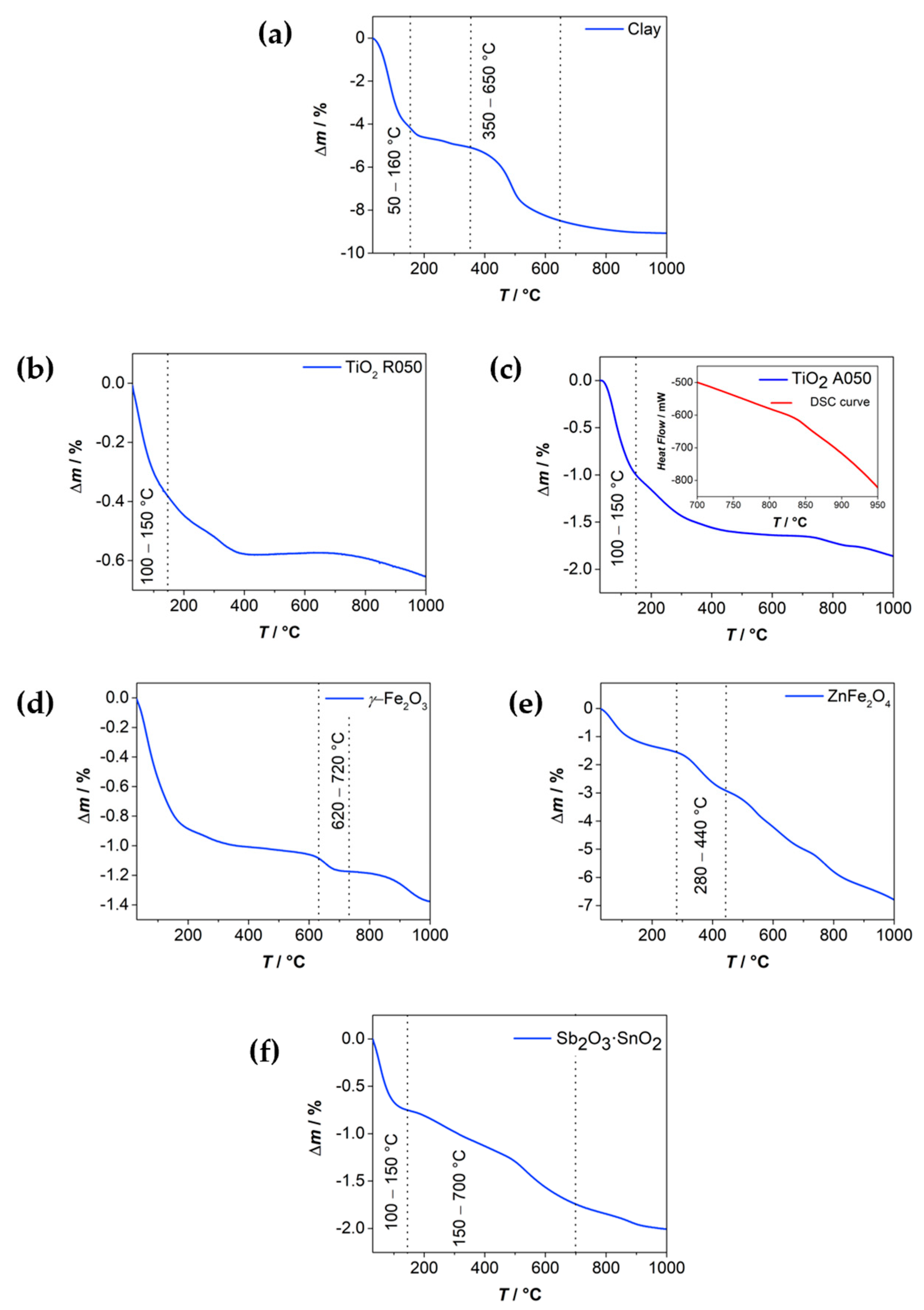

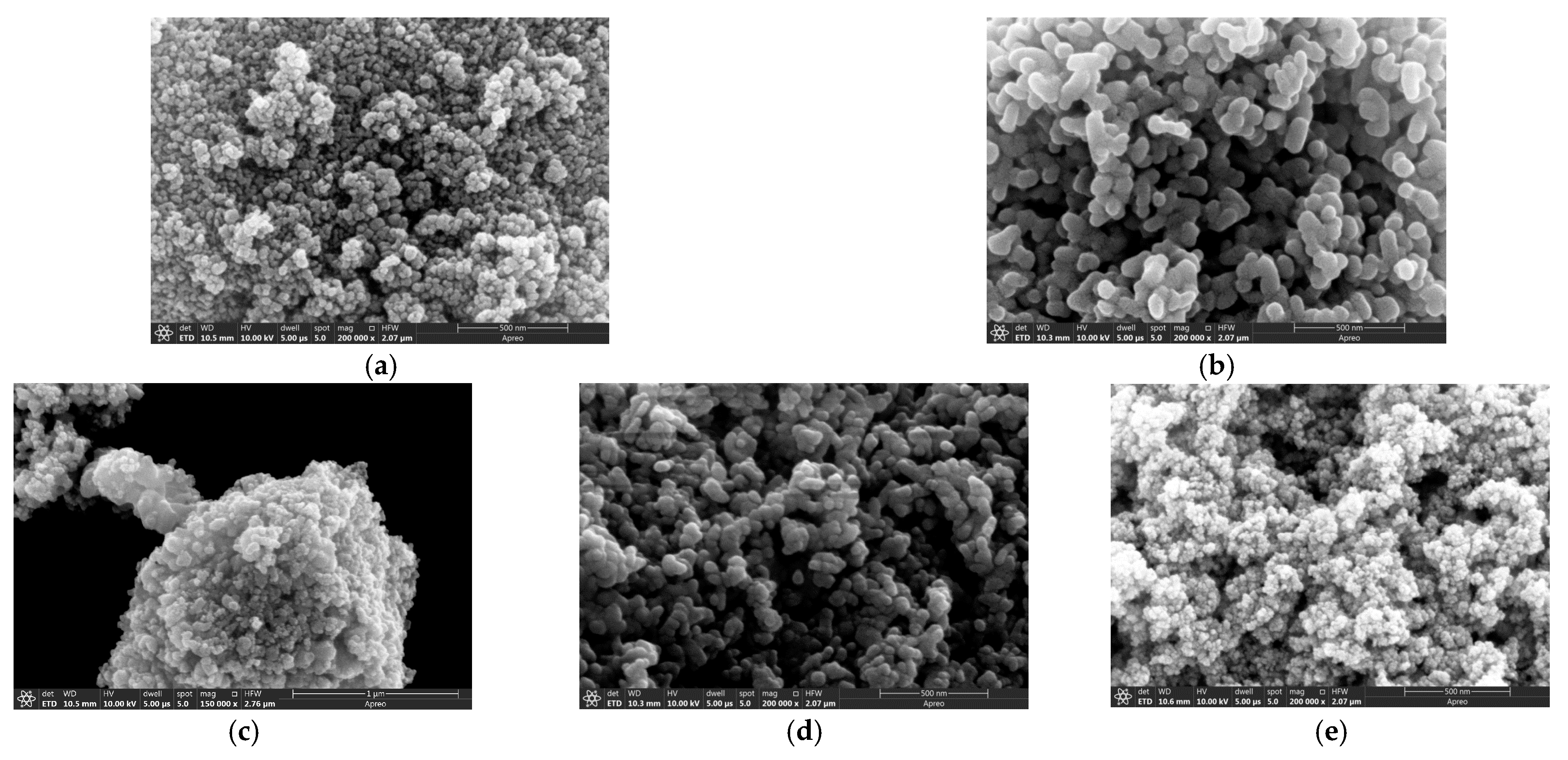
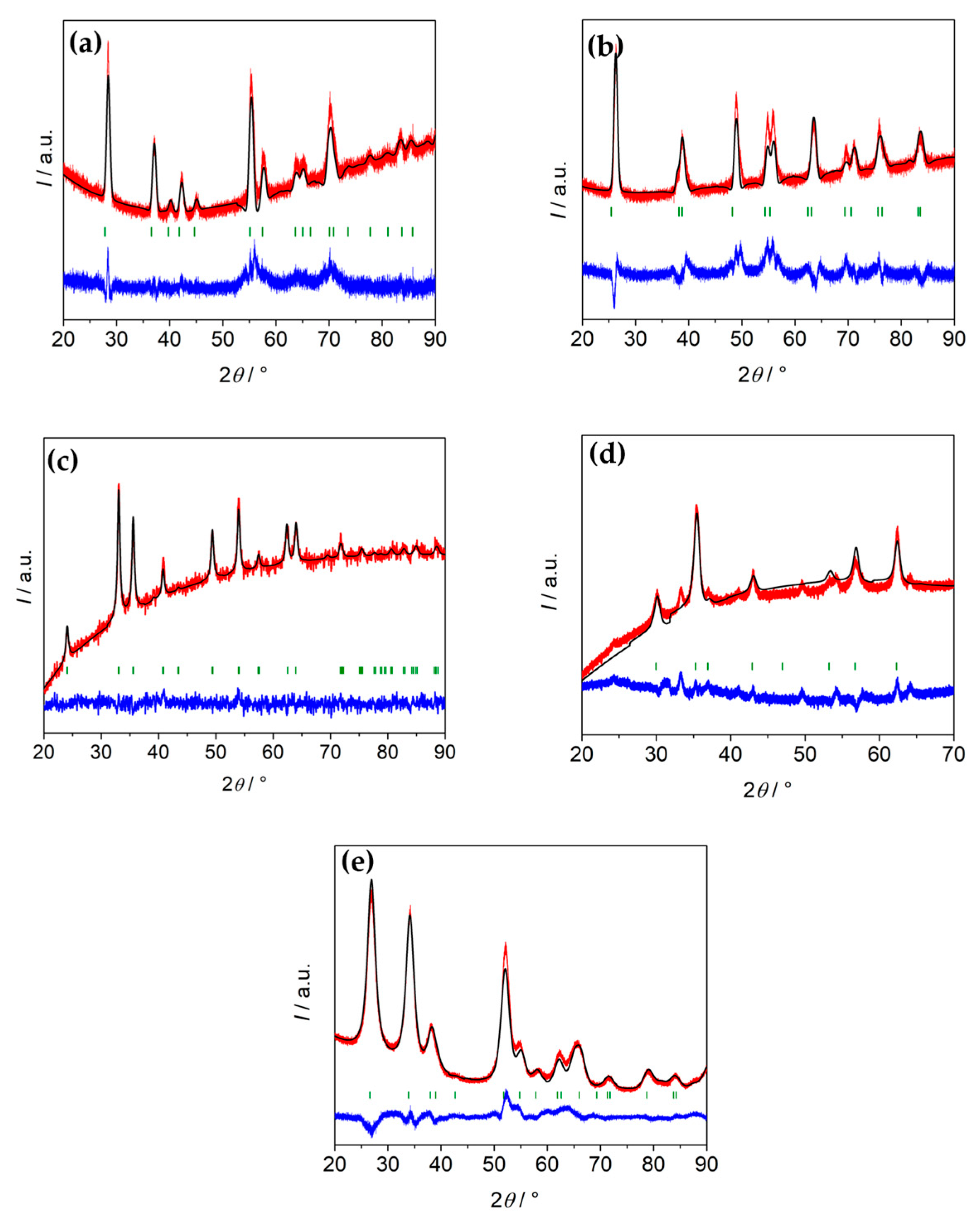
| Material | Chemical Formula | Manufacturer |
|---|---|---|
| Clay | - | Wienerberger, Đakovo, Croatia |
| Titanium dioxide R050 | TiO2 (rutile) | MK Nano, Canada |
| Titanium dioxide A050 | TiO2 (anatase) | |
| Iron(III) oxide | γ-Fe2O3 | Iolitec, Germany |
| Zinc Ferrite | ZnFe2O4 | Nanografi, Turkey |
| Antimony(III) Tin(IV) oxide (ATO) | Sb2O3·SnO2 | EPRUI, China |
| Materials | Specific Surface Area/m2g−1 | Particle Size/nm * | Particle Size/nm *** |
|---|---|---|---|
| Clay | 39 | ** | ** |
| TiO2 R050 | 25 | 50 | 74 ± 13 |
| TiO2 A050 | 64 | 50 | 42 ± 7 |
| γ-Fe2O3 | 39 | 30 | 49 ± 7 |
| ZnFe2O4 | 74 | 15 | 45 ± 8 |
| Sb2O3·SnO2 | 60 | 50 | 24 ± 5 |
| Clay | |
|---|---|
| Oxides | wt.% |
| SiO2 | 64.73 |
| Al2O3 | 22.70 |
| CaO | 2.40 |
| Fe2O3 | 4.59 |
| SO3 | 0.06 |
| MgO | 1.39 |
| K2O | 2.16 |
| Na2O | 1.02 |
| TiO2 | 0.96 |
| MnO | 0.01 |
| P2O5 | <0.01 |
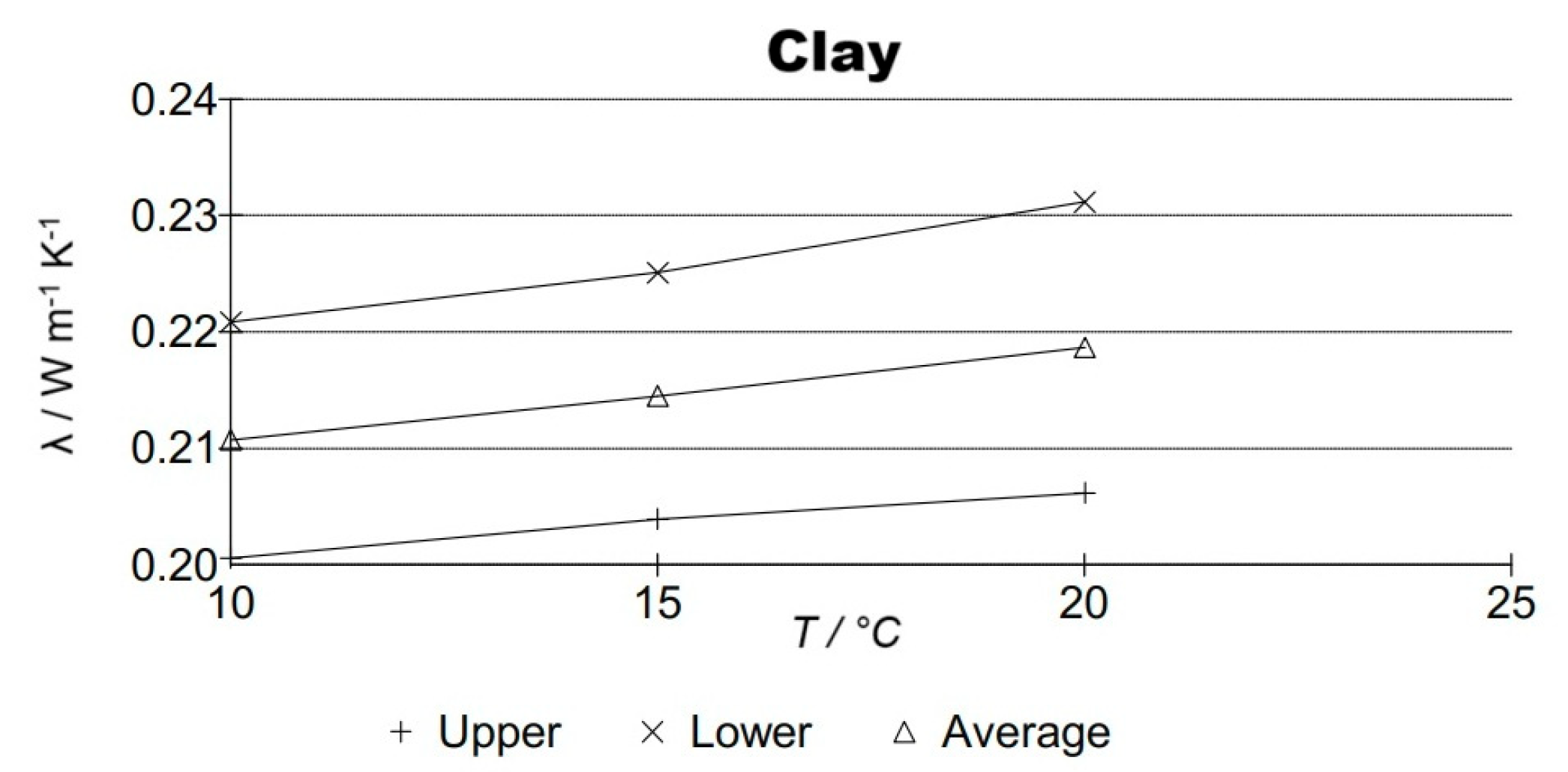
| Sample | wt.% | λ (W m−1 K−1) | R (m2 K/W) |
|---|---|---|---|
| Clay | 0.2146 ± 0.010 | 0.0890 | |
| TiO2—anatase | 3 | 0.2002 ± 0.006 | 0.0962 |
| 5 | 0.1970 ± 0.009 | 0.0987 | |
| TiO2—rutile | 3 | 0.2231 ± 0.006 | 0.0848 |
| 5 | 0.2458 ± 0.013 | 0.0773 | |
| ZnFe2O4 | 3 | 0.2187 ± 0.004 | 0.0853 |
| 5 | 0.2279 ± 0.004 | 0.083 | |
| γ-Fe2O3 | 3 | 0.2305 ± 0.009 | 0.0835 |
| 5 | 0.2208 ± 0.003 | 0.0864 | |
| Sb2O3·SnO2 | 3 | 0.2185 ± 0.011 | 0.0866 |
| 5 | 0.2656 ± 0.010 | 0.0737 |
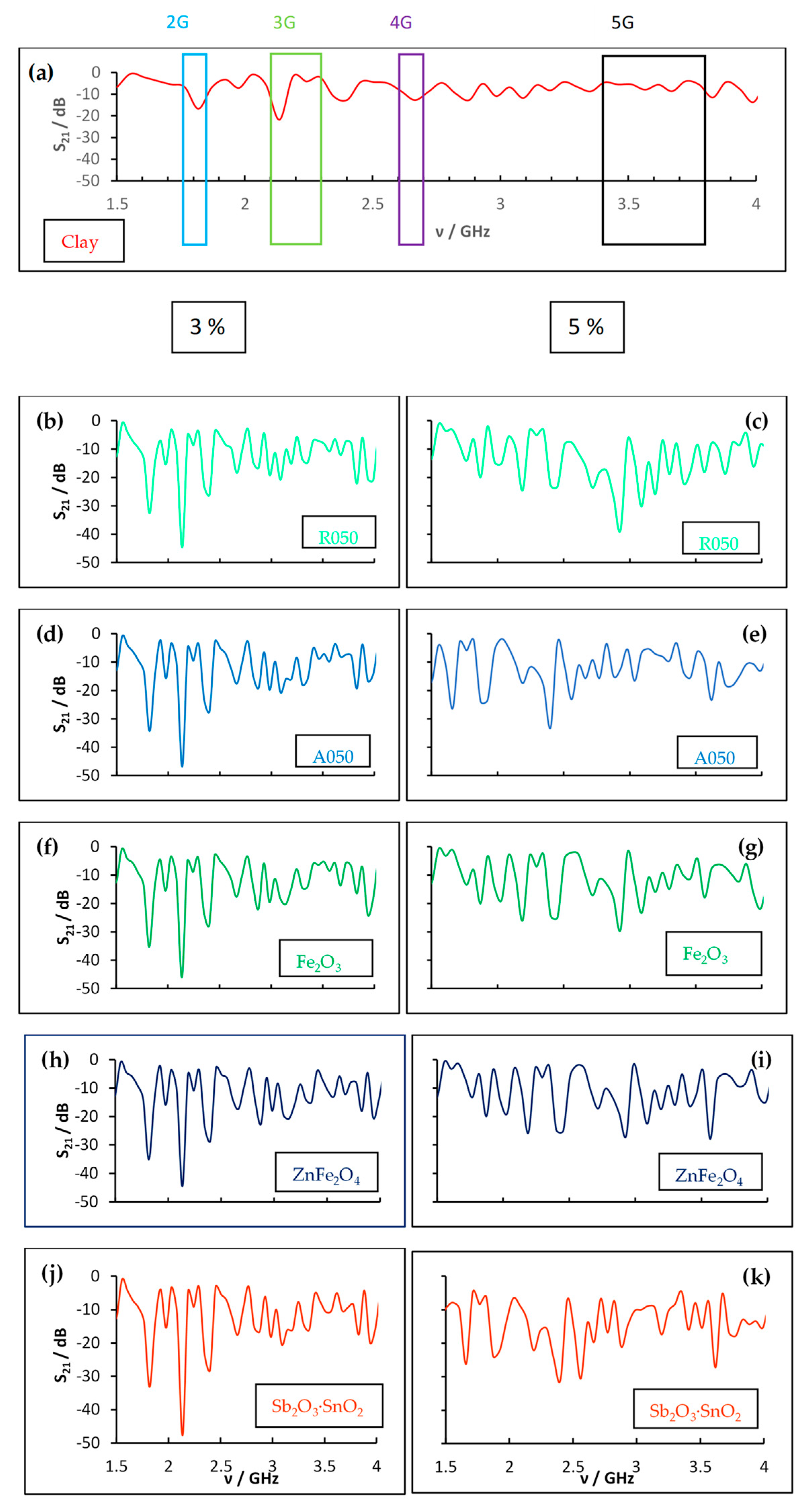
| Sample | wt.% | 2G Area (1.75–1.85 GHz) | 3G Area (2.10–2.30 GHz) | 4G Area (2.60–2.70 GHz) | 5G Area (3.40–3.80 GHz) |
|---|---|---|---|---|---|
| Clay | 1.156 | 1.548 | 1.065 | 2.428 | |
| TiO2 R050 | 3 | 2.294 | 3.243 | 1.411 | 3.465 |
| 5 | 2.084 | 2.188 | 1.501 | 5.408 | |
| TiO2 A050 | 3 | 2.367 | 3.379 | 1.471 | 3.988 |
| 5 | 1.577 | 2.569 | 1.342 | 5.725 | |
| Fe2O3 | 3 | 2.416 | 3.331 | 1.434 | 3.009 |
| 5 | 2.134 | 2.344 | 0.815 | 3.737 | |
| ZnFe2O4 | 3 | 2.408 | 3.347 | 1.513 | 3.628 |
| 5 | 1.982 | 2.293 | 0.881 | 4.323 | |
| Sb2O3·SnO2 | 3 | 2.312 | 3.377 | 1.462 | 3.478 |
| 5 | 1.861 | 3.278 | 1.616 | 5.624 |
| Sample | wt.% | 2G Area (1.75–1.85 GHz) | 3G Area (2.10–2.30 GHz) | 4G Area (2.60–2.70 GHz) | 5G Area (3.40–3.80 GHz) |
|---|---|---|---|---|---|
| Clay | 0.495 | 0.628 | 0.363 | 1.857 | |
| TiO2 R050 | 3 | 0.181 | 0.434 | 0.167 | 0.643 |
| 5 | 0.176 | 0.469 | 0.121 | 0.492 | |
| TiO2 A050 | 3 | 0.181 | 0.467 | 0.169 | 0.735 |
| 5 | 0.176 | 0.558 | 0.163 | 0.641 | |
| Fe2O3 | 3 | 0.180 | 0.455 | 0.169 | 0.733 |
| 5 | 0.167 | 0.482 | 0.272 | 0.545 | |
| ZnFe2O4 | 3 | 0.177 | 0.432 | 0.160 | 0.553 |
| 5 | 0.109 | 0.215 | 0.093 | 0.220 | |
| Sb2O3·SnO2 | 3 | 0.178 | 0.476 | 0.157 | 0.596 |
| 5 | 0.151 | 0.276 | 0.143 | 0.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brdarić Kosanović, J.; Marković, B.; Miličević, I.; Stanković, A.; Tatar, D. Crystalline Nanoparticles and Their Impact on Electromagnetic Radiation Absorption in Advanced Clay Building Materials. Crystals 2025, 15, 959. https://doi.org/10.3390/cryst15110959
Brdarić Kosanović J, Marković B, Miličević I, Stanković A, Tatar D. Crystalline Nanoparticles and Their Impact on Electromagnetic Radiation Absorption in Advanced Clay Building Materials. Crystals. 2025; 15(11):959. https://doi.org/10.3390/cryst15110959
Chicago/Turabian StyleBrdarić Kosanović, Jelena, Berislav Marković, Ivana Miličević, Anamarija Stanković, and Dalibor Tatar. 2025. "Crystalline Nanoparticles and Their Impact on Electromagnetic Radiation Absorption in Advanced Clay Building Materials" Crystals 15, no. 11: 959. https://doi.org/10.3390/cryst15110959
APA StyleBrdarić Kosanović, J., Marković, B., Miličević, I., Stanković, A., & Tatar, D. (2025). Crystalline Nanoparticles and Their Impact on Electromagnetic Radiation Absorption in Advanced Clay Building Materials. Crystals, 15(11), 959. https://doi.org/10.3390/cryst15110959







