Mechanocatalytic Hydrogen Evolution on Centrosymmetric SnS Nanobelts: A Non-Piezoelectric Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
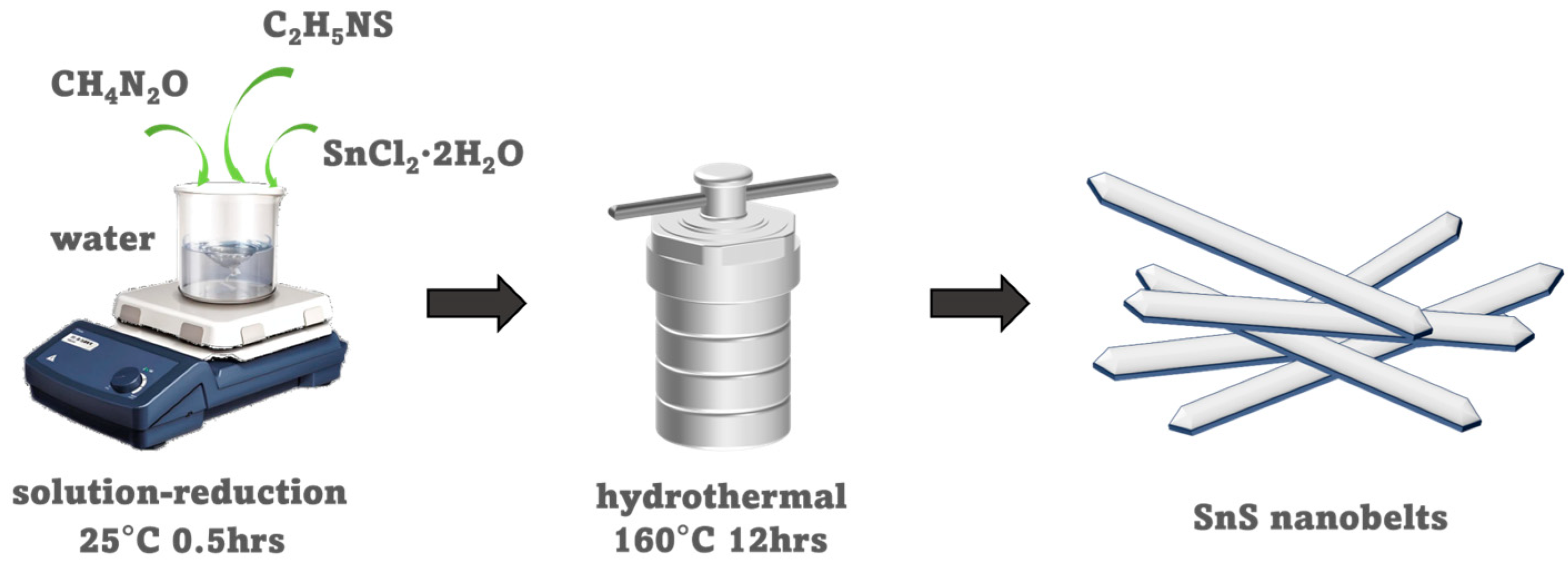
2.2. Synthesis of SnS Nanobelts
2.3. Electrochemical Measurements
3. Results
3.1. Structural, Morphological, and Compositional Characterization
3.2. Mechanocatalytic H2 Evolution Performance
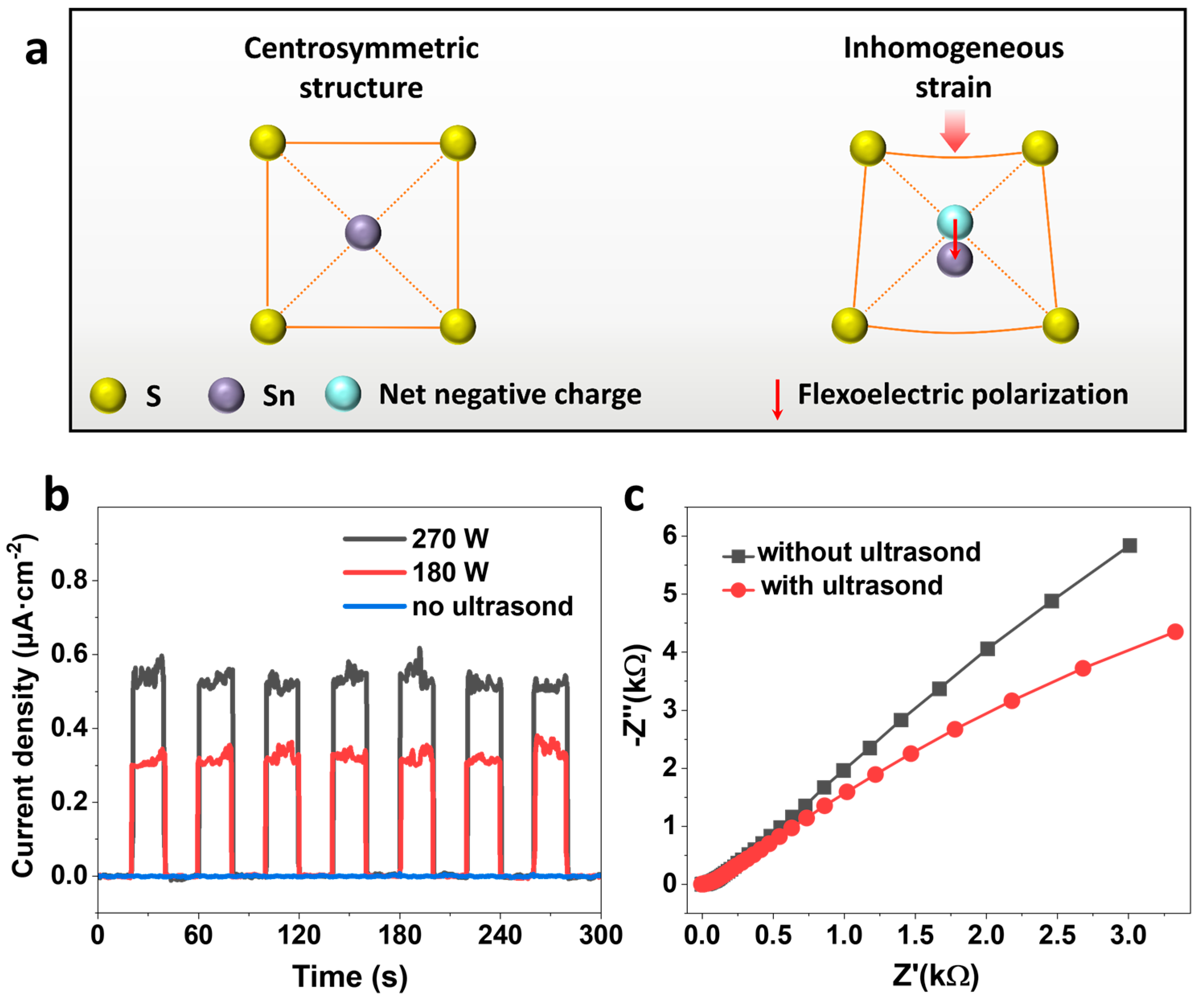
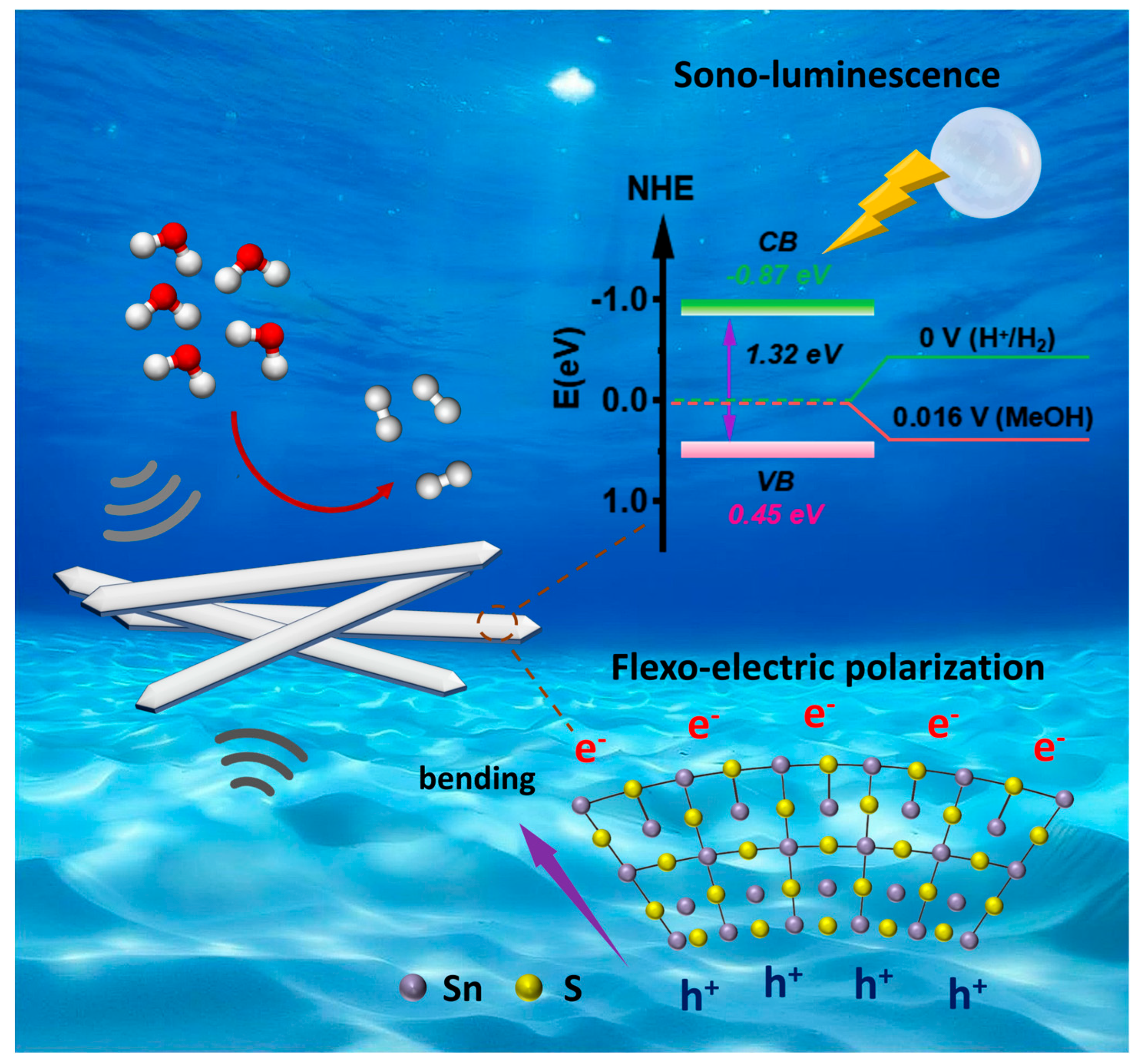
3.3. Mechanism Study of Mechanocatalytic H2 Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawson, J. Prospects for Hydrogen as an Energy Resource. Nature 1974, 249, 724–726. [Google Scholar] [CrossRef]
- De Kleijne, K.; Huijbregts, M.A.; Knobloch, F.; van Zelm, R.; Hilbers, J.P.; de Coninck, H.; Hanssen, S.V. Worldwide Greenhouse gas Emissions of Green Hydrogen Production and Transport. Nat. Energy 2024, 9, 1139–1152. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels Toward Renewable Sources. A mini review. Energy Fuel 2021, 35, 16403–16415. [Google Scholar]
- Howarth, R.W.; Jacobson, M.Z. How Green is Blue Hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Chen, S.; Pei, C.; Gong, J. Insights into Interface Engineering in Steam Reforming Reactions for Hydrogen Production. Energy Environ. Sci. 2019, 12, 3473–3495. [Google Scholar] [CrossRef]
- Sun, X.; Li, R.; Zhang, B.; Wang, H.; Cheng, Y.; Guan, J.; Liu, Q.; Chen, X. Function–Structure–Synthesis: Machine Learning Enabled Closed-Loop Design of Biomass-Derived Porous Carbon Materials. ACS Sustain. Chem. Eng. 2025, 13, 7698–7709. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Nishioka, S.; Osterloh, F.E.; Wang, X.; Mallouk, T.E.; Maeda, K. Photocatalytic Water Splitting. Nat. Rev. Methods Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Rajaambal, S.; Sivaranjani, K.; Gopinath, C.S. Recent Developments in Solar H2 Generation from Water Splitting. J. Chem. Sci. 2015, 127, 33–47. [Google Scholar] [CrossRef]
- Qian, Q.; Zhu, Y.; Ahmad, N.; Feng, Y.; Zhang, H.; Cheng, M.; Liu, H.; Xiao, C.; Zhang, G.; Xie, Y. Recent Advancements in Electrochemical Hydrogen Production via Hybrid Water Splitting. Adv. Mater. 2024, 36, 2306108. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F. Piezoelectricity of Single-Atomic-Layer MoS2 for Energy Conversion and Piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-S.; Xu, H.; Konishi, H.; Li, X. Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, Y.; Li, R.; Zhang, B.; Guan, J.; Xu, H.; Liu, Q.; Chen, X. Discovery of Piezoelectricity in Inorganic-Organic Hybrid ZnS (en)0.5 Nanosheets for Ultrahigh, Selective CO2-to-CO Conversion. Appl. Catal. B Environ. Energy 2025, 383, 126053. [Google Scholar] [CrossRef]
- Domen, K.; Ikeda, S.; Takata, T.; Tanaka, A.; Hara, M.; Kondo, J.N. Mechano-Catalytic Overall Water-Splitting into Hydrogen and Oxygen on Some Metal Oxides. Appl. Energy 2000, 67, 159–179. [Google Scholar]
- Ohta, T. Mechano-Catalytic Water-Splitting. Appl. Energy 2000, 67, 181–193. [Google Scholar] [CrossRef]
- Delogu, F. Hydrogen Generation by Mechanochemical Reaction of Quartz Powders in Water. Int. J. Hydrogen Energy 2011, 36, 15145–15152. [Google Scholar] [CrossRef]
- Yoshinari, S.; Miki, N.; Yuki, Y.; Ryota, G.; Takahiro, K.; Takahisa, M.; Tohru, T.; Miki, I.; Yuuichi, K.; Yasushi, S. Stainless-Steel-Mediated Quantitative Hydrogen Generation from Water under Ball Milling Conditions. ACS Sustain. Chem. Eng. 2015, 3, 683–689. [Google Scholar]
- Hitoki, G.; Takata, T.; Ikeda, S.; Hara, M.; Kondo, J.N.; Kakihana, M.; Domen, K. Mechano-Catalytic Overall Water Splitting on Some Mixed Oxides. Catal. Today 2000, 63, 175–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Jiang, L.; Qiang, J.; Zhang, Z.; Liu, Z.; Liu, Y.; Tian, T.; Wang, Z.; Fei, L. Boosting Hydrogen Evolution via Flexoelectric Catalysis in Gradient F-Doped Hydroxyapatite Nanowires. Chem. Sci. 2025, 16, 9905–9912. [Google Scholar] [CrossRef]
- Du, Y.; Sun, W.; Li, X.; Hao, C.; Wang, J.; Fan, Y.; Joseph, J.; Yang, C.; Gu, Q.; Liu, Y. Mechanocatalytic Hydrogen Generation in Centrosymmetric Barium Dititanate. Adv. Sci. 2024, 11, 2404483. [Google Scholar] [CrossRef]
- Mondal, S.; Das, R.C.; Du, Y.; Hou, Z.; Konstantinov, K.; Cheng, Z. Flexocatalytic Hydrogen Generation and Organics Degradation by Nano SrTiO3. Adv. Sci. 2025, 12, 2500034. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wu, J.M. Effect of Controlled Oxygen Vacancy on H2-Production Through the Piezocatalysis and Piezophototronics of Ferroelectric R3C ZnSnO3 Nanowires. Adv. Funct. Mater. 2020, 30, 1907619. [Google Scholar] [CrossRef]
- He, J.; Yi, Z.; Chen, Q.; Li, Z.; Hu, J.; Zhu, M. Harvesting Mechanical Energy Induces Piezoelectric Polarization of MIL-100 (Fe) for Cocatalyst-Free Hydrogen Production. Chem. Commun. 2022, 58, 10723–10726. [Google Scholar] [CrossRef]
- Wang, M.; Zuo, Y.; Wang, J.; Wang, Y.; Shen, X.; Qiu, B.; Cai, L.; Zhou, F.; Lau, S.P.; Chai, Y. Remarkably Enhanced Hydrogen Generation of Organolead Halide Perovskites via Piezocatalysis and Photocatalysis. Adv. Energy Mater. 2019, 9, 1901801. [Google Scholar] [CrossRef]
- Yu, C.; He, J.; Tan, M.; Hou, Y.; Zeng, H.; Liu, C.; Meng, H.; Su, Y.; Qiao, L.; Lookman, T. Selective Enhancement of Photo-piezocatalytic Performance in BaTiO3 via Heterovalent ion Doping. Adv. Funct. Mater. 2022, 32, 2209365. [Google Scholar] [CrossRef]
- Tu, C.-Y.; Wu, J.M. Localized Surface Plasmon Resonance Coupling with Piezophototronic Effect for Enhancing Hydrogen Evolution Reaction with Au@MoS2 Nanoflowers. Nano Energy 2021, 87, 106131. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Zhang, S.; Chen, L.-Q. Flexoelectricity in Solids: Progress, Challenges, and Perspectives. Prog. Mater. Sci. 2019, 106, 100570. [Google Scholar] [CrossRef]
- Wu, P.Y.; Le, K.T.; Lin, H.-Y.; Chen, Y.-C.; Wu, P.-H.; Wu, J.M. Flexoelectric Catalysts Based on Hierarchical Wrinkling Surface of Centrosymmetric High-Entropy Oxide. Acs Nano 2023, 17, 17417–17426. [Google Scholar] [PubMed]
- Shao, P.-W.; Lin, M.-C.; Zhuang, Q.; Huang, J.; Liu, S.; Chen, H.-W.; Liu, H.-L.; Lu, Y.-J.; Hsu, Y.-J.; Wu, J.-M. Flexo-Phototronic Effect in Centro-Symmetric BiVO4 Epitaxial Films. Appl. Catal. B Environ. 2022, 312, 121367. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, P.H.; Liao, Y.S.; Chou, J.P.; Wu, J.M. Defect Engineering Centrosymmetric 2D Material Flexocatalysts. Small 2024, 20, 2401116. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, H.; Kong, F.; Wang, M. Piezocatalytic Oxidation of 5-hydroxymethylfurfural to 5-formyl-2-furancarboxylic acid over Pt decorated Hydroxyapatite. Appl. Catal. B Environ. 2022, 309, 121281. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2 Ultrathin Nanosheets Integrated on CdS Nanorods to Promote Charge Separation and Migration and Improve Solar-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 1563–1570. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical Layered WS2/graphene-Modified CdS Nanorods for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Tekalgne, M.; Nguyen, T.P.; Van Le, Q.; Ahn, S.H.; Kim, S.Y. Electrocatalysts Based on MoS2 and WS2 for Hydrogen Evolution Reaction: An overview. Battery Energy 2023, 2, 20220057. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C.; Wang, X.; Xi, Y.; Li, X. Synthesis and Photosensitivity of SnS Nanobelts. J. Alloys Compd. 2012, 513, 1–5. [Google Scholar] [CrossRef]
- Vaughn, D.D.; Hentz, O.D.; Chen, S.; Wang, D.; Schaak, R.E. Formation of SnS Nanoflowers for Lithium Ion Batteries. Chem. Commun. 2012, 48, 5608–5610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.; Shen, S.; Xu, H.; Wang, Q. Ultralarge Single Crystal SnS Rectangular Nanosheets. Chem. Commun. 2011, 47, 5226–5228. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, L.; Jamil, S.; Xie, J.; Yan, H.; Yuan, Y.; Zhang, Y.; Nie, S.; Pan, J.; Wang, X. Free-Standing SnS/C nanofiber Anodes for Ultralong Cycle-Life Lithium-Ion Batteries and Sodium-Ion Batteries. Energy Storage Mater. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Sun, L.; Lee, S.W.; Park, H.H.; Kim, S.B.; Yang, C.; Gordon, R.G. Overcoming Efficiency Limitations of SnS-Based Solar Cells. Adv. Energy Mater. 2014, 4, 1400496. [Google Scholar]
- Dutta, P.K.; Sen, U.K.; Mitra, S. Excellent Electrochemical Performance of Tin Monosulphide (SnS) as a Sodium-Ion Battery Anode. RSC Adv. 2014, 4, 43155–43159. [Google Scholar] [CrossRef]
- Manh Hung, N.; Nguyen, C.V.; Arepalli, V.K.; Kim, J.; Duc Chinh, N.; Nguyen, T.D.; Seo, D.-B.; Kim, E.-T.; Kim, C.; Kim, D. Defect-induced Gas-Sensing Properties of a Flexible SnS Sensor Under UV Illumination at Room Temperature. Sensors 2020, 20, 5701. [Google Scholar] [CrossRef]
- Chia, X.; Lazar, P.; Sofer, Z.; Luxa, J.; Pumera, M. Layered SnS Versus SnS2: Valence and Structural Implications on Electrochemistry and Clean Energy Electrocatalysis. J. Phys. Chem. C 2016, 120, 24098–24111. [Google Scholar]
- Mishra, R.K.; Choi, G.J.; Verma, R.; Jin, S.H.; Bhardwaj, R.; Arya, S.; Singh, J.; Gwag, J.S. Revolutionizing Energy Evolution: SnS-Sn2S3 Layered Structures as Exceptional Electrocatalytic Materials for H2 and O2 Generation. Mater. Sci. Eng. B 2024, 303, 117292. [Google Scholar]
- Tian, W.; Li, N.; Chen, D.; Xu, Q.; Li, H.; Yan, C.; Lu, J. Vibration-Driven Reduction of CO2 to Acetate with 100% Selectivity by SnS Nanobelt Piezocatalysts. Angew. Chem. Int. Ed. 2023, 135, e202306964. [Google Scholar]
- Khan, H.; Mahmood, N.; Zavabeti, A.; Elbourne, A.; Rahman, M.A.; Zhang, B.Y.; Krishnamurthi, V.; Atkin, P.; Ghasemian, M.B.; Yang, J. Liquid Metal-Based Synthesis of High Performance Monolayer SnS Piezoelectric Nanogenerators. Nat. Commun. 2020, 11, 3449. [Google Scholar] [PubMed]
- Tian, Z.; Guo, C.; Zhao, M.; Li, R.; Xue, J. Two-Dimensional SnS: A Phosphorene Analogue with Strong in-Plane Electronic Anisotropy. ACS Nano 2017, 11, 2219–2226. [Google Scholar]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen Evolution Catalyzed by MoS3 and MoS2 Particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar]
- Sivakumar, V.; Mohan, R. Measurement and Mapping of Cavitation Energy in Leather and Material Processing Vessels Using an Ultrasonic Horn. Appl. Phys. A 2022, 128, 27. [Google Scholar]
- Ren, Z.; Chen, F.; Zhao, Q.; Zhao, G.; Li, H.; Sun, W.; Huang, H.; Ma, T. Efficient CO2 Reduction to Reveal the Piezocatalytic Mechanism: From Displacement Current to Active Sites. Appl. Catal. B Environ. 2023, 320, 122007. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Yao, K.; Zhang, C.; Wu, Q.; Hu, T.; Shan, S. Dye-Sensitized NH2-UiO-66 Anchored with Copper Ions for Tandem Visible-Light-Driven Hydrogen Evolution. J. Environ. Chem. Eng. 2023, 11, 111349. [Google Scholar]
- Wang, C.; Cui, D.; Yang, X.; Zhang, T.; Sun, Z.; Li, Q.; Li, F. H3PMo12O40@ ZIF-67-Derived CoMoC/ZnIn2S4 Schottky Heterojunction for Enhanced Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2024, 77, 666–676. [Google Scholar]
- Zheng, L.-L.; Zhang, L.-S.; Chen, Y.; Tian, L.; Jiang, X.-H.; Chen, L.-S.; Xing, Q.-J.; Liu, X.-Z.; Wu, D.-S.; Zou, J.-P. A New Strategy for the Fabrication of Covalent Organic Framework-Metal-Organic Framework Hybrids via In-Situ Functionalization of Ligands for Improved Hydrogen Evolution Reaction Activity. Chin. J. Catal. 2022, 43, 811–819. [Google Scholar] [CrossRef]
- Musa, E.N.; Yadav, A.K.; Srichareonkul, M.; Thampetraruk, D.; Frechette, E.; Thiele, H.C.; Stylianou, K.C. What Up with MOFs in Photocatalysis: Exploring the Influence of Experimental Conditions on the Reproducibility of Hydrogen Evolution Rates. ACS Appl. Mater. Interfaces 2024, 16, 70675–70684. [Google Scholar] [CrossRef]
- Wang, S.; Ai, Z.; Niu, X.; Yang, W.; Kang, R.; Lin, Z.; Waseem, A.; Jiao, L.; Jiang, H.L. Linker Engineering of Sandwich-Structured Metal–Organic Framework Composites for Optimized Photocatalytic H2 Production. Adv. Mater. 2023, 35, 2302512. [Google Scholar] [CrossRef]
- Ding, M.; Li, M.; Wang, J.; Jin, Z. Visible-Light-Induced Photocatalytic Hydrogen Evolution Performance of Graphdiyne-Alkyne Phosphating Mo–Metal-Organic Frameworks Heterojunction. Solar RRL 2024, 8, 2400041. [Google Scholar] [CrossRef]
- Ranjan, A.; Hsiao, K.-Y.; Lin, C.-Y.; Tseng, Y.-H.; Lu, M.-Y. Enhanced Piezocatalytic Activity in Bi1/2Na1/2TiO3 for Water Splitting by Oxygen Vacancy Engineering. ACS Appl. Mater. Interfaces 2022, 14, 35635–35644. [Google Scholar] [PubMed]
- Long, Y.; Xu, H.; He, J.; Li, C.; Zhu, M. Piezoelectric Polarization of BiOCl via Capturing Mechanical Energy for Catalytic H2 Evolution. Surf. Interfaces 2022, 31, 102056. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, J.; Lu, Z.; Hu, J.; Hao, A.; Cao, Y. Insight into the Effect of OH Modification on the Piezo-Photocatalytic Hydrogen Production Activity of SrTiO3. J. Colloid Interface Sci. 2022, 612, 111–120. [Google Scholar] [CrossRef]
- Yu, C.; Tan, M.; Li, Y.; Liu, C.; Yin, R.; Meng, H.; Su, Y.; Qiao, L.; Bai, Y. Ultrahigh Piezocatalytic Capability in Eco-Friendly BaTiO3 Nanosheets Promoted by 2D Morphology Engineering. J. Colloid Interface Sci. 2021, 596, 288–296. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, L.; Wu, Z.; Jia, Y.; Ye, X.; Wang, F.; Yuan, B.; Yu, Y.; Huang, H.; Zou, G. Harvesting Vibration Energy to Piezo-catalytically Generate Hydrogen Through Bi2WO6 Layered-Perovskite. Nano Energy 2020, 78, 105351. [Google Scholar]
- Zhang, M.; Zhao, S.; Zhao, Z.; Li, S.; Wang, F. Piezocatalytic Effect Induced Hydrogen Production from Water Over Non-Noble Metal Ni Deposited Ultralong GaN Nanowires. ACS Appl. Mater. Interfaces 2021, 13, 10916–10924. [Google Scholar] [CrossRef]
- Su, R.; Wang, Z.; Zhu, L.; Pan, Y.; Zhang, D.; Wen, H.; Luo, Z.D.; Li, L.; Li, F.T.; Wu, M. Strain-Engineered Nano-Ferroelectrics for High-Efficiency Piezocatalytic Overall Water Splitting. Angew. Chem. Int. Ed. 2021, 60, 16019–16026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, S.; Mofarah, S.S.; Niu, R.; Sun, Y.; Rawal, A.; Ma, H.; Xue, K.; Fang, X.; Toe, C.Y. Efficient and Stable Piezo-Photocatalytic Splitting of Water and Seawater by Interfacial Engineering of Na0.5Bi0.5TiO3/Na0.5Bi4.5Ti4O15 Self-Generated Heterojunctions. Nano Energy 2023, 116, 108830. [Google Scholar] [CrossRef]
- Qiu, P.; Park, B.; Choi, J.; Thokchom, B.; Pandit, A.B.; Khim, J. A Review on Heterogeneous Sonocatalyst for Treatment of Organic Pollutants in Aqueous Phase Based on Catalytic Mechanism. Ultrason. Sonochem. 2018, 45, 29–49. [Google Scholar] [CrossRef]
- Wu, T.; Liu, K.; Liu, S.; Feng, X.; Wang, X.; Wang, L.; Qin, Y.; Wang, Z.L. Highly Efficient Flexocatalysis of Two-Dimensional Semiconductors. Adv. Mater. 2023, 35, 2208121. [Google Scholar]
- Liu, Z.; Wen, X.; Wang, Y.; Jia, Y.; Wang, F.; Yuan, G.; Wang, Y. Robust Flexo-Catalysis in Centrosymmetric Nanoparticles. Adv. Mater. Technol. 2022, 7, 2101484. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, S.; Cheng, Z. Flexocatalysis of Nanoscale Titanium Dioxide. Nano Energy 2024, 127, 109731. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, X.; Hu, H.; Qin, A.; Huang, H.; Yao, Y.; Zhang, Y.; Ma, T. Harvesting Vibration Energy for Efficient Cocatalyst-Free Sonocatalytic H2 Production over Magnetically Separable Ultra-Low-Cost Fe3O4. Materials 2024, 17, 1463. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Tong, W.; Li, X.; Zhang, Y. Microstructure Designed Flexoelectric Materials and Tip Force for Multifunctional Applications. Nano Energy 2025, 133, 110442. [Google Scholar]





| Type of Reaction | Catalysts | Driving Source | Reaction System | Catalytic Activity |
|---|---|---|---|---|
| Photoatalysis | EY-6Cu-NU-66 [50] | 300 W Xe lamp | TEOA (aq) | H2: 3579.8 μmol g−1 h−1 |
| Photoatalysis | CoMoC/ZnIn2S4 [51] | 300 W Xe lamp | TEOA (aq) | H2: 2232 μmol g−1 h−1 |
| Photoatalysis | B-CTF-C-Ti-MOF [52] | 300 W Xe lamp | TEOA (aq) | H2: 1975 μmol g−1 h−1 |
| Photoatalysis | MIL-125-NH2/Ni2P [53] | 300 W Xe lamp | TEOA (aq) | H2: 4327 μmol g−1 h−1 |
| Photoatalysis | UiO-66-NH2@ Pt@UiO-66-H [54] | 300 W Xe lamp | TEOA (aq) | H2: 2708.2 μmol g−1 h−1 |
| Photoatalysis | PMF/G-25 [55] | Mutichannel reaction system | TEOA (aq) | H2: 1688.5 μmol g−1 h−1 |
| Piezocatalysis | Bi0.5Na0.5TiO3 [56] | 100 W, 40 kHz vibration | MOH (aq) | H2: 506.7 μmol g−1 h−1 |
| Piezocatalysis | BiOCl nanosheets [57] | 120 W, 40 kHz vibration | MOH (aq) | H2: 975.4 μmol g−1 h−1 |
| Piezocatalysis | Au/MoS2 Nanoflowers [26] | vibration | MOH (aq) | H2: 2981 μmol g−1 h−1 |
| Piezocatalysis | OH-SrTiO3 [58] | 300 W, 40 kHz vibration | MOH (aq) | H2: 321.5 μmol g−1 h−1 |
| Piezocatalysis | MIL-100(Fe) [23] | 40 W, 120 kHz vibration | MOH (aq) | H2: 2800 μmol g−1 h−1 |
| Piezocatalysis | BTO Nanosheets [59] | 40 W, 100 kHz vibration | TEOA (aq) | H2: 92 μmol g−1 h−1 |
| Piezocatalysis | Bi2WO6 nanosheets [60] | 40 kHz vibration | TEOA (aq) | H2: 191.3 μmol g−1 h−1 |
| Piezocatalysis | GaN nanowires [61] | 110 W, 40 kHz vibration | TEOA (aq) | H2: 88.3 μmol g−1 h−1 |
| Piezocatalysis | Li-doped BTO Nanosheets [25] | 40 kHz vibration | TEOA (aq) | H2: 3700 μmol g−1 h−1 |
| Piezocatalysis | BTO NPs [62] | 40 kHz vibration | H2O | H2: 159 μmol g−1 h−1 |
| Piezocatalysis | Na0.5Bi0.5TiO3/ Na0.5Bi4.5Ti4O15 [63] | 400 W, 40 kHz vibration | H2O | H2: 128 μmol g−1 h−1 |
| Mechano- catalysis | SnS (This work) | 630 W, 80 kHz vibration | MOH (aq) | CO: 3889 μmol g−1 h−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Chen, M.; Zhang, B.; Liu, Y.; Liu, Q.; Chen, X. Mechanocatalytic Hydrogen Evolution on Centrosymmetric SnS Nanobelts: A Non-Piezoelectric Pathway. Crystals 2025, 15, 940. https://doi.org/10.3390/cryst15110940
Sun X, Chen M, Zhang B, Liu Y, Liu Q, Chen X. Mechanocatalytic Hydrogen Evolution on Centrosymmetric SnS Nanobelts: A Non-Piezoelectric Pathway. Crystals. 2025; 15(11):940. https://doi.org/10.3390/cryst15110940
Chicago/Turabian StyleSun, Xiaotong, Mingyang Chen, Bowen Zhang, Yawei Liu, Qi Liu, and Xiaoqing Chen. 2025. "Mechanocatalytic Hydrogen Evolution on Centrosymmetric SnS Nanobelts: A Non-Piezoelectric Pathway" Crystals 15, no. 11: 940. https://doi.org/10.3390/cryst15110940
APA StyleSun, X., Chen, M., Zhang, B., Liu, Y., Liu, Q., & Chen, X. (2025). Mechanocatalytic Hydrogen Evolution on Centrosymmetric SnS Nanobelts: A Non-Piezoelectric Pathway. Crystals, 15(11), 940. https://doi.org/10.3390/cryst15110940






