Mechanism and Air Cathode Materials of Photo-Assisted Zinc–Air Batteries for Photoelectrochemical Energy Storage
Abstract
1. Introduction
2. Features of Photoelectrochemical Energy Storage (PES) Devices
2.1. General Concept and Principles of PES Devices
2.2. PES Cathode Materials
3. PES/Photo-Assisted Zinc–Air Batteries
3.1. General Concept and Principles of PAZABs
- Charging process:
- Discharge process:
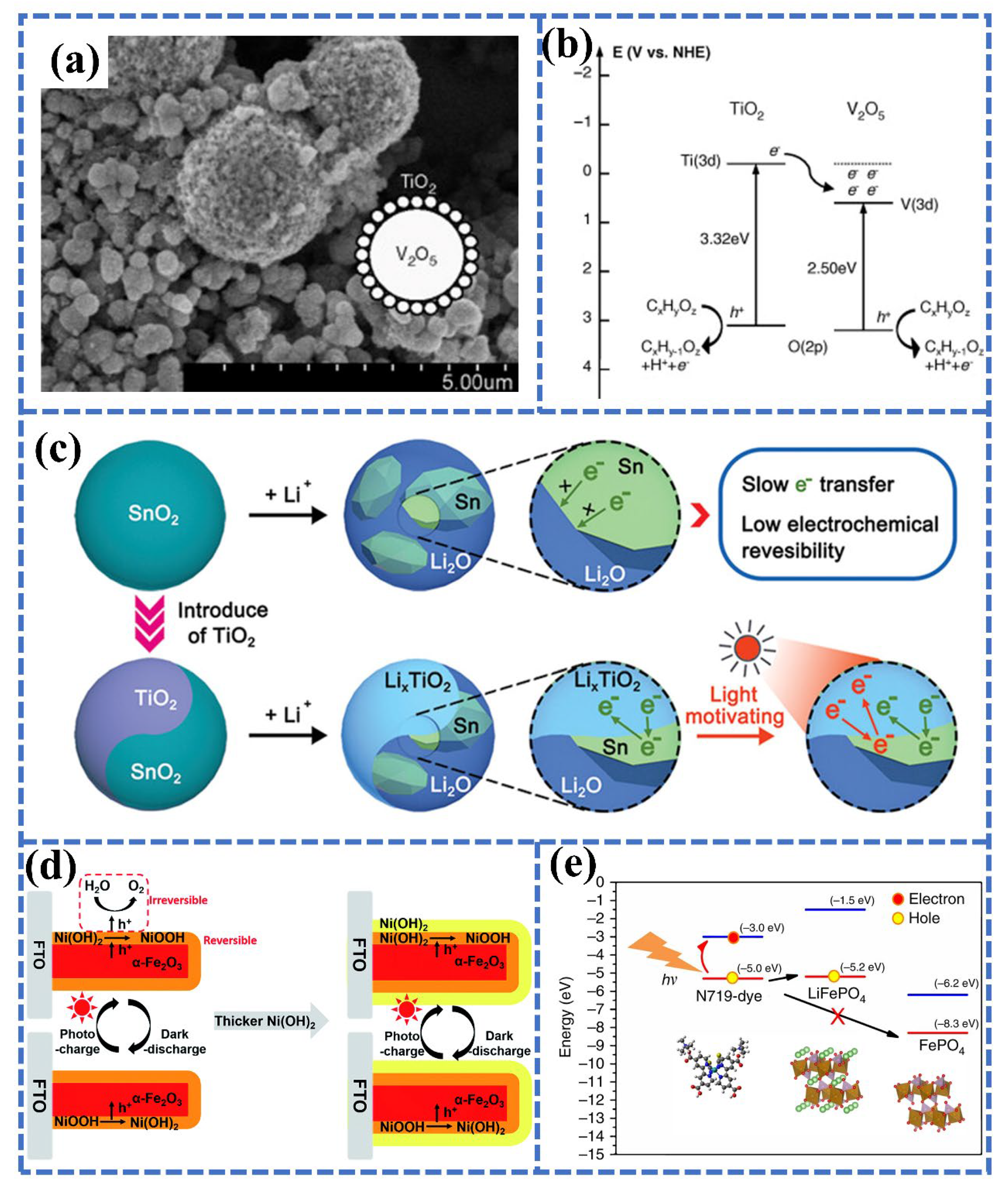
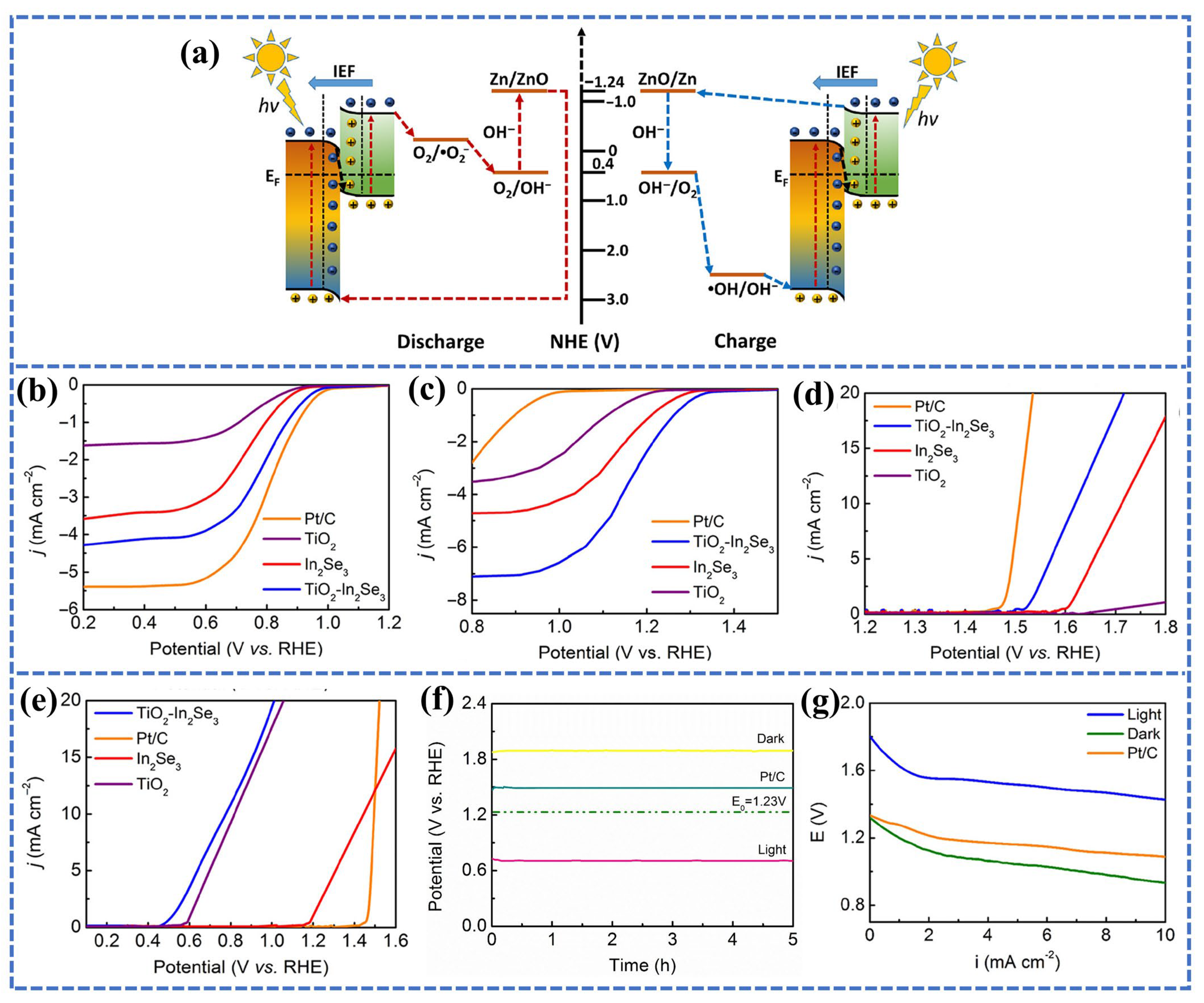
3.2. Photocathode Materials for PAZABs
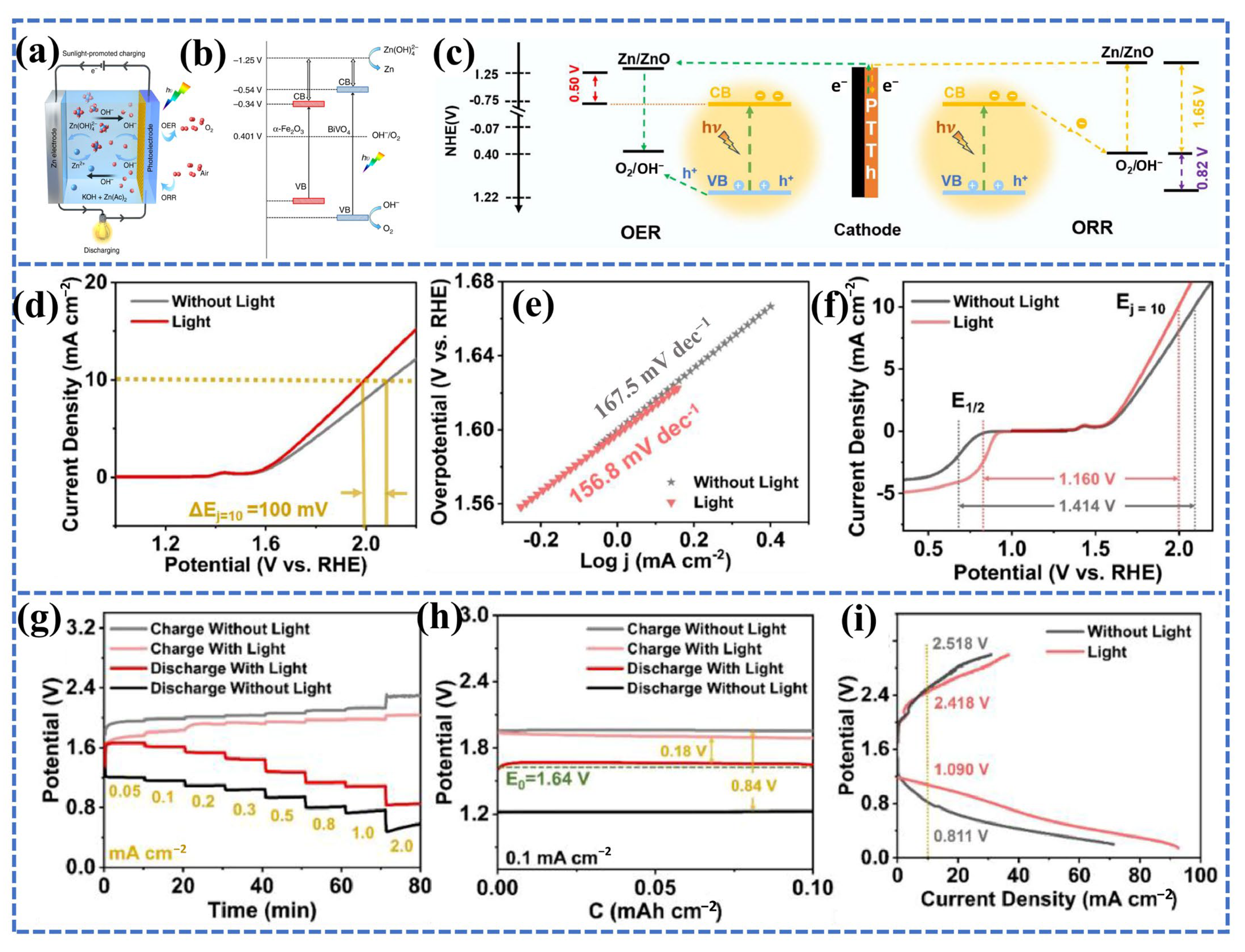
3.3. Decoupled Cathode PAZABs
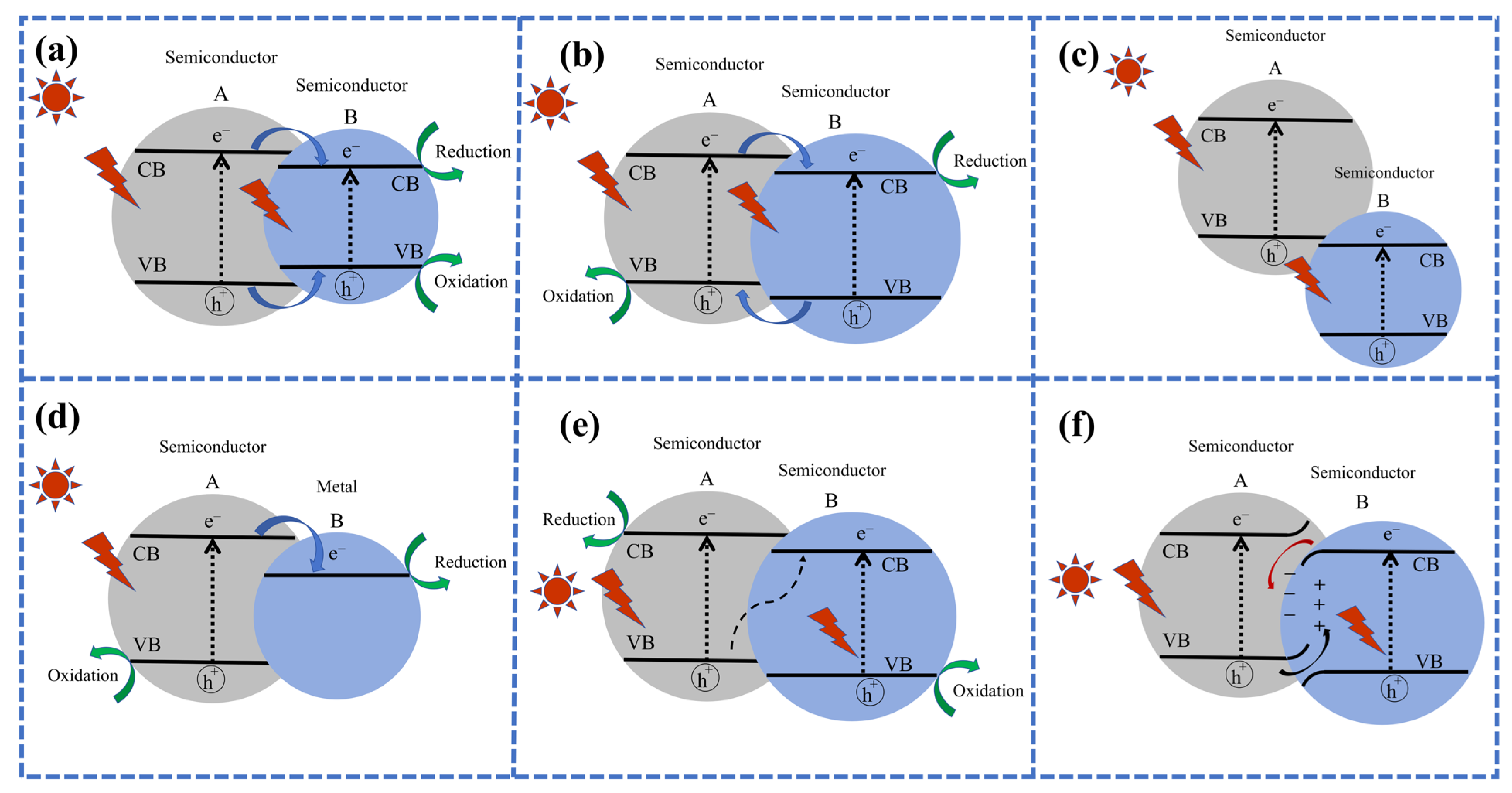
4. Interface Engineering of Photocathode Materials
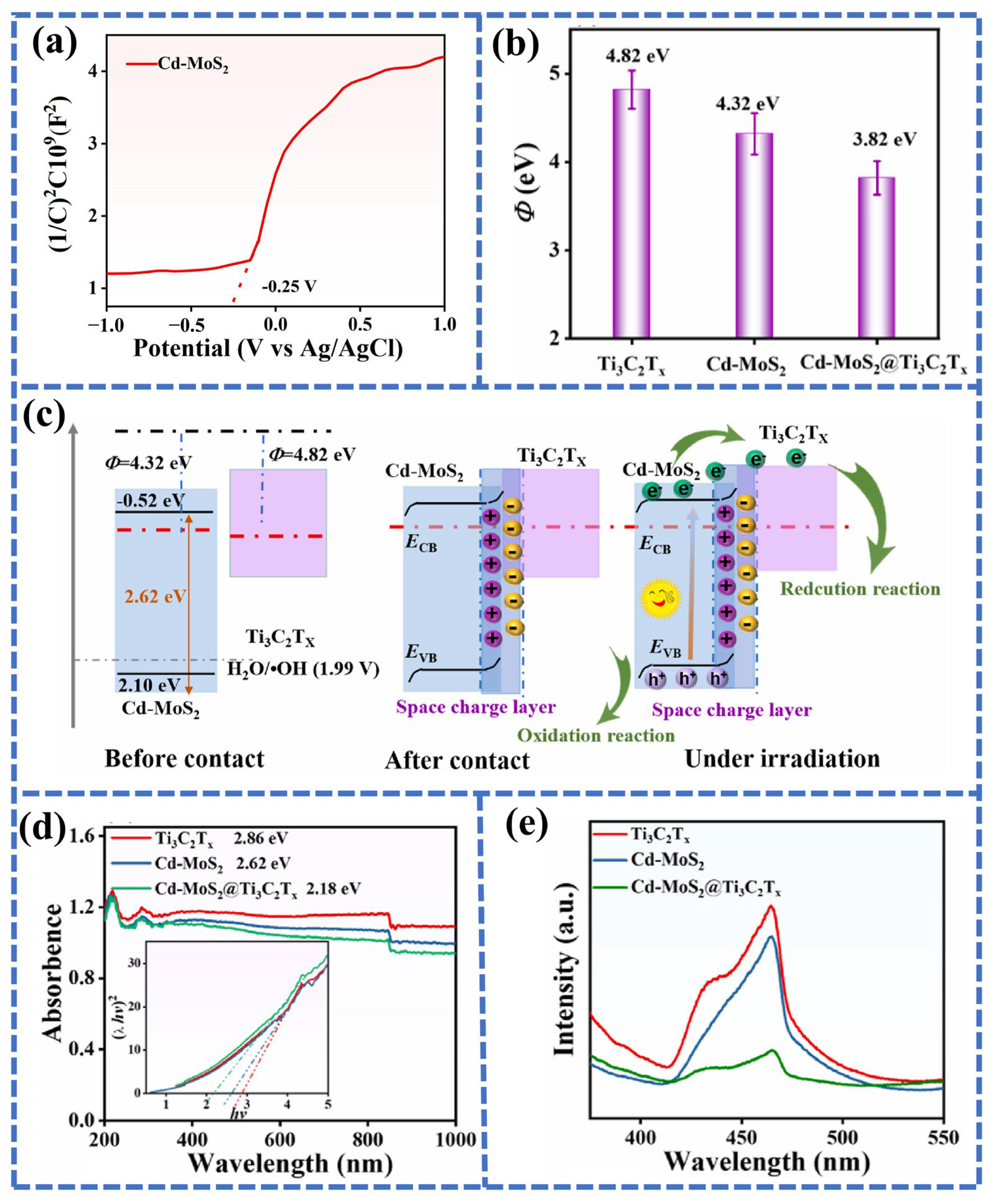
4.1. Interface Engineering Towards Heterojunction Photocathode
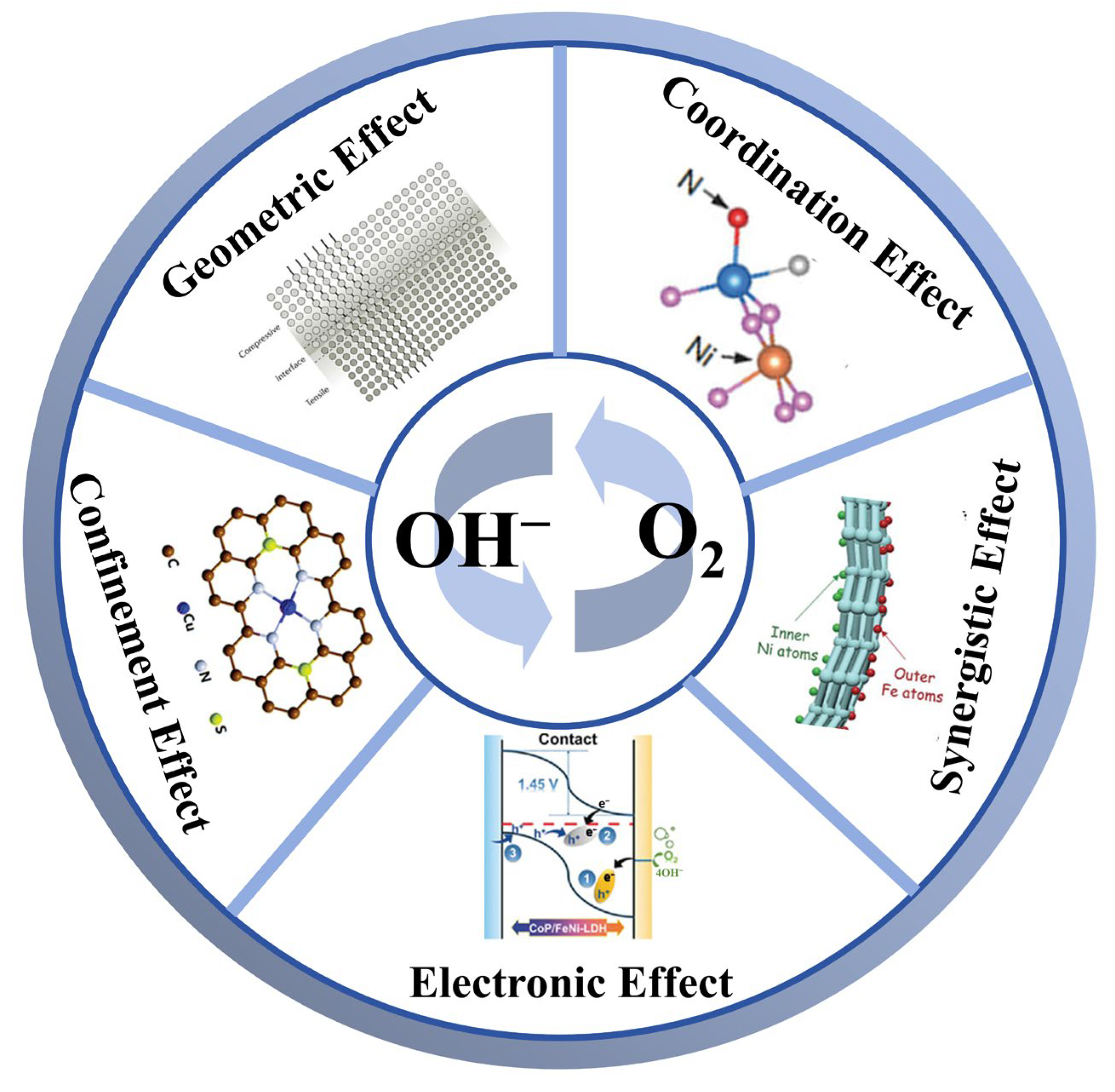
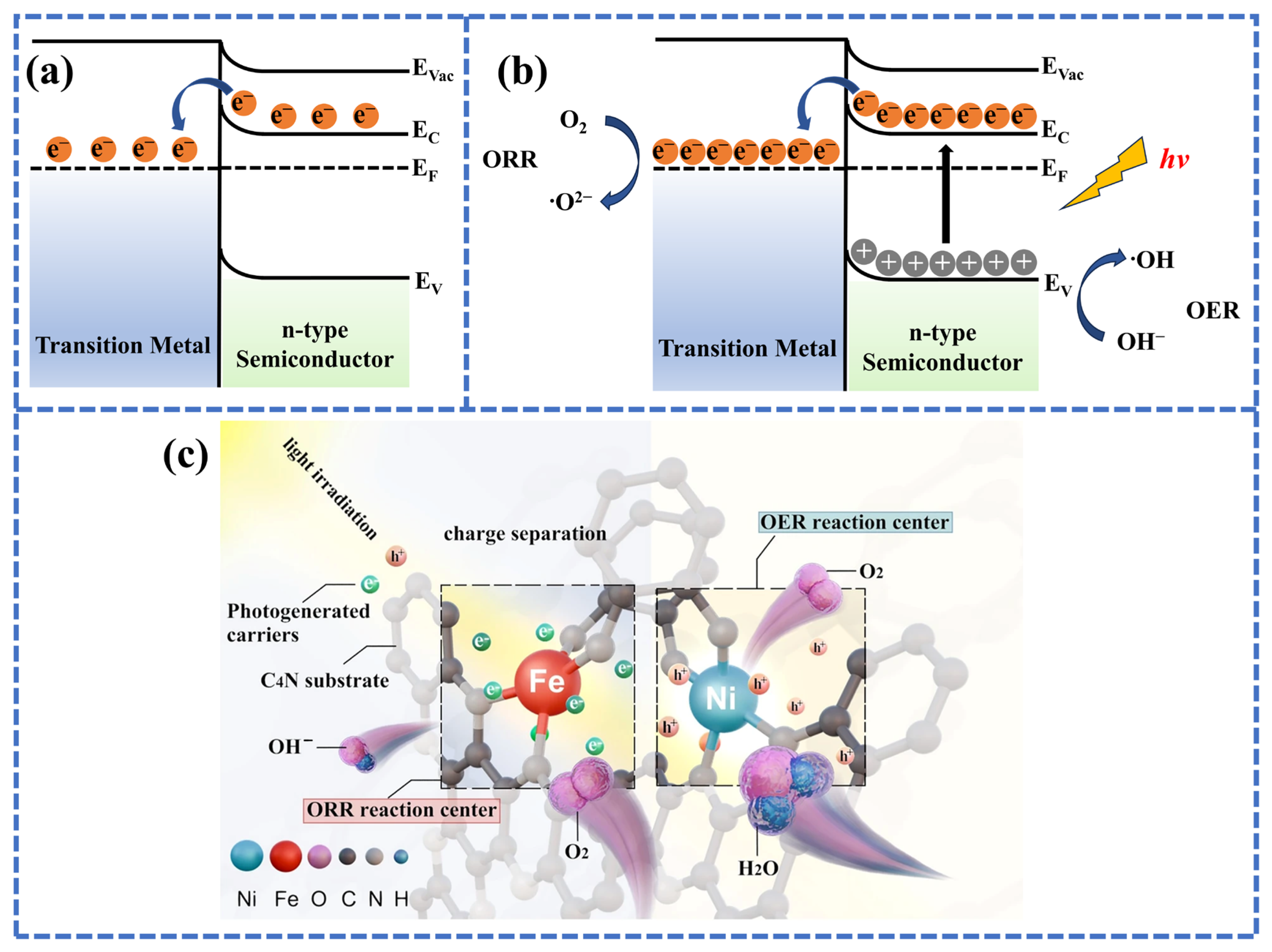
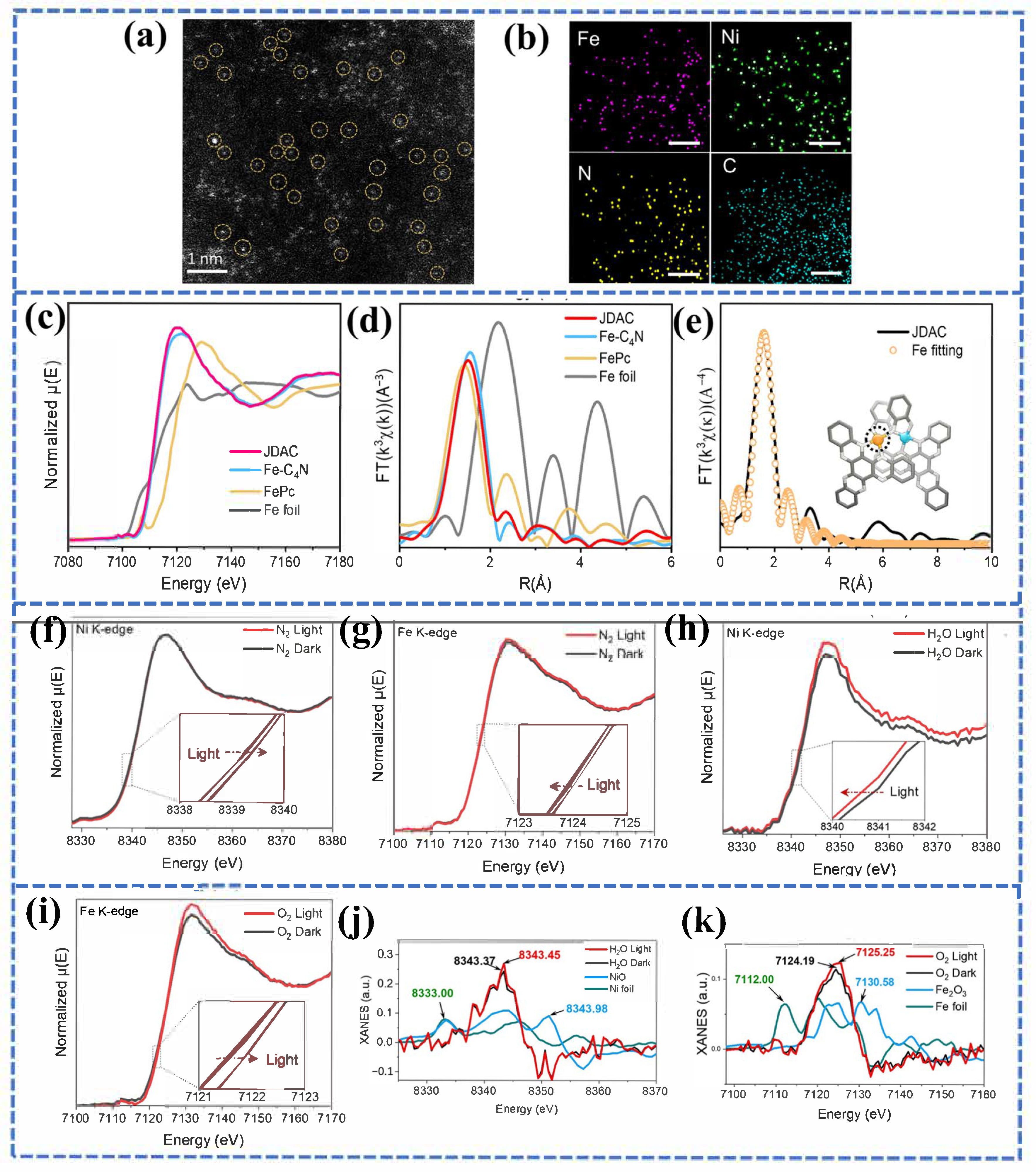
4.2. Interface Engineering Towards Single-Atom Photocathode
5. Summary and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.M.; Mou, X.N.; Zhou, X.; Wang, J.; Li, H.; Wang, C.R. Metal Compound-Based Heterostructures in Anodes Promote High Capacity and Fast Reaction Kinetic for Lithium/Sodium-Ion Storage: A Review. ChemElectroChem 2024, 11, e202300573. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Q.L.; Zhang, L.; Xu, Q.L.; Li, X.W.; Hu, G.Z. Nickel-Induced charge transfer in semicoherent Co-Ni/Co6Mo6C Heterostructures for reversible oxygen electrocatalysis. J. Colloid Interface Sci. 2024, 674, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.H.; Sun, W.P.; Xu, B.B.; Pan, H.G.; Jiang, Y.Z. Interface Engineering of Air Electrocatalysts for Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2021, 11, 2002762. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Cao, L.; Fan, L.L.; Gu, F.; Wang, S.F.; Xiong, S.X.; Gu, Y.; Yu, A.B. Tailored interface engineering of Co3Fe7/Fe3C heterojunctions for enhancing oxygen reduction reaction in zinc-air batteries. J. Colloid Interface Sci. 2024, 672, 279–286. [Google Scholar] [CrossRef]
- Liu, W.X.; Feng, J.X.; Wei, T.R.; Liu, Q.; Zhang, S.S.; Luo, Y.; Luo, J.; Liu, X.J. Active-site and interface engineering of cathode materials for aqueous Zn-gas batteries. Nano Res. 2023, 16, 2325–2346. [Google Scholar] [CrossRef]
- He, B.B.; Deng, Y.Z.; Wang, H.W.; Wang, R.; Jin, J.; Gong, Y.S.; Zhao, L. Metal organic framework derived perovskite/spinel heterojunction as efficient bifunctional oxygen electrocatalyst for rechargeable and flexible Zn-air batteries. J. Colloid Interface Sci. 2022, 625, 502–511. [Google Scholar] [CrossRef]
- Diao, L.C.; Zhou, W.; Zhang, B.; Shi, C.S.; Miao, Z.C.; Zhou, J.; He, C.N. NaCl sealing Strategy-Assisted synthesis CoO-Co heterojunctions as efficient oxygen electrocatalysts for Zn air batteries. J. Colloid Interface Sci. 2023, 645, 329–337. [Google Scholar] [CrossRef]
- Xu, N.N.; Zhang, Y.X.; Zhang, T.; Liu, Y.Y.; Qiao, J.L. Efficient quantum dots anchored nanocomposite for highly active ORR/OER electrocatalyst of advanced metal-air batteries. Nano Energy 2019, 57, 176–185. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhu, Y.X.; Li, Z.Y.; Wang, Y.; Wågberg, T.; Hu, G.Z. Increasing Electrocatalytic Oxygen Evolution Efficiency through Cobalt-Induced Intrastructural Enhancement and Electronic Structure Modulation. ChemSusChem 2021, 14, 467–478. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zhang, L.; Zhu, G.G.; Zhang, X.; Lu, S.Y. Open-mouth N-doped carbon nanoboxes embedded with mixed metal phosphide nanoparticles as high-efficiency catalysts for electrolytic water splitting. Nanoscale 2020, 12, 5848–5856. [Google Scholar] [CrossRef]
- Kong, C.H.; Lv, X.W.; Weng, C.C.; Ren, J.T.; Tian, W.W.; Yuan, Z.Y. Curving Engineering of Hollow Concave-Shaped Rhombic Dodecahedrons of N-Doped Carbon Encapsulated with Fe-Doped Co/Co3O4 Nanoparticles for an Efficient Oxygen Reduction Reaction and Zn-Air Batteries. ACS Sustain. Chem. Eng. 2022, 10, 11441–11450. [Google Scholar] [CrossRef]
- An, L.; Zhang, Z.Y.; Feng, J.R.; Lv, F.; Li, Y.X.; Wang, R.; Lu, M.; Gupta, R.B.; Xi, P.X.; Zhang, S. Heterostructure-Promoted Oxygen Electrocatalysis Enables Rechargeable Zinc-Air Battery with Neutral Aqueous Electrolyte. J. Am. Chem. Soc. 2018, 140, 17624–17631. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mo, Z.H.; Tang, S.T.; Li, M.Y.; Yang, H.; Long, B.; Wang, Y.; Song, S.Q.; Tong, Y.X. Photo-enhanced Zn-air batteries with simultaneous highly efficient in situ H2O2 generation for wastewater treatment. J. Mater. Chem. A. 2019, 7, 14129–14135. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, L.; Zhu, Q.L.; Ke, Z.F.; Hu, G.Z. Spatial separation strategy to construct N/S co-doped carbon nanobox embedded with asymmetrically coupled Fe-Co pair-site for boosted reversible oxygen electrocatalysis. J. Colloid Interface Sci. 2024, 653, 1577–1587. [Google Scholar] [CrossRef]
- Nardelli, M.B.; Yakobson, B.I.; Bernholc, J. Mechanism of strain release in carbon nanotubes. Phys. Rev. B 1998, 57, R4277–R4280. [Google Scholar] [CrossRef]
- Wang, X.R.; Liu, J.Y.; Liu, Z.W.; Wang, W.C.; Luo, J.; Han, X.P.; Du, X.W.; Qiao, S.Z.; Yang, J. Identifying the Key Role of Pyridinic-N-Co Bonding in Synergistic Electrocatalysis for Reversible ORR/OER. Adv. Mater. 2018, 30, 1800005. [Google Scholar] [CrossRef]
- Cui, M.J.; Xu, B.; Shi, X.W.; Zhai, Q.X.; Dou, Y.H.; Li, G.S.; Bai, Z.C.; Ding, Y.; Sun, W.P.; Liu, H.K.; et al. Metal-organic framework-derived single-atom catalysts for electrocatalytic energy conversion applications. J. Mater. Chem. A 2024, 12, 18921–18947. [Google Scholar] [CrossRef]
- Lv, C.H.; Li, B.B.; Ren, Y.Y.; Zhang, G.C.; Lu, Z.M.; Li, L.L.; Zhang, X.H.; Yang, X.J.; Yu, X.F. A “MOF-plus-MOF” strategy to synthesize Co-N3C1 single-atom catalyst for rechargeable Zn-air battery. Chem. Eng. J. 2024, 495, 153670. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.Y.; Zhu, Z.J.; Li, X.; Zhu, Z.; Yuan, T.; Yang, J.H.; Pang, Y.P.; Zheng, S.Y. Vacancy-defective nano-carbon matrix enables highly efficient Fe single atom catalyst for aqueous and flexible Zn-Air batteries. Chem. Eng. J. 2024, 496, 153669. [Google Scholar] [CrossRef]
- Jin, J.; Yin, J.; Liu, H.B.; Lu, M.; Li, J.Y.; Tian, M.; Xi, P.X. Transition Metal (Fe, Co and Ni)-Carbide-Nitride (M-C-N) Nanocatalysts: Structure and Electrocatalytic Applications. ChemCatChem 2019, 11, 2780–2792. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.J.; Li, A.S.; Jiang, X.C.; Zhang, Y.Z.; Cheng, C.W. Superhydrophobic Co/Fe sulfide nanoparticles and single-atoms doped carbon nanosheets composite air cathode for high-performance zinc-air batteries. J. Energy Chem. 2024, 96, 568–577. [Google Scholar] [CrossRef]
- Xu, Q.L.; Hu, J.S.; Yao, H.Y.; Lei, J.; Zhou, C.H.; Zhang, L.; Pang, H. Pyridinic-N Regulated Electron Injection to Modulate *OH Adsorption at Fe-N-C Sites for an Efficient Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2024, 11, 42352–42362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, C.; Li, L.; Yan, X.; Zhang, N.; Bao, J. Fe based MOF encapsulating triethylenediamine cobalt complex to prepare a FeN3-CoN3 dual-atom catalyst for efficient ORR in Zn-air batteries. J. Colloid Interface Sci. 2024, 676, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, J.L.; Zhang, S.L.; Sun, Y.Y.; Kong, W.L.; Geng, L.N.; Li, Y.; Dai, L.M.; Li, Z.T.; Wu, M.B. Synergistic Interactions Between Co Nanoparticles and Unsaturated Co-N2 Sites for Efficient Electrocatalysis. Adv. Funct. Mater. 2024, 34, 2410373. [Google Scholar] [CrossRef]
- Hong, J.; Chen, M.S.; Zhang, L.; Qin, L.; Hu, J.S.; Huang, X.H.; Zhou, C.H.; Zhou, Y.T.; Wågberg, T.; Hu, G.Z. Asymmetrically coupled Co Single-atom and Co nanoparticle in Double-shelled Carbon-based nanoreactor for enhanced reversible oxygen catalysis. Chem. Eng. J. 2023, 455, 140401. [Google Scholar] [CrossRef]
- Rong, J.; Chen, W.Y.; Gao, E.H.; Wu, J.; Ao, H.S.; Zheng, X.D.; Zhang, Y.Z.; Li, Z.Y.; Kim, M.; Yamauchi, Y.; et al. Design of Atomically Dispersed CoN4 Sites and Co Clusters for Synergistically Enhanced Oxygen Reduction Electrocatalysis. Small 2024, 11, 2402323. [Google Scholar] [CrossRef]
- Chen, C.L.; Chai, J.; Sun, M.R.; Guo, T.Q.; Lin, J.; Zhou, Y.R.; Sun, Z.Y.; Zhang, F.; Zhang, L.; Chen, W.X.; et al. An asymmetrically coordinated ZnCoFe hetero-trimetallic atom catalyst enhances the electrocatalytic oxygen reaction. Energy Environ. Sci. 2024, 17, 2298–2308. [Google Scholar] [CrossRef]
- Lv, J.Q.; Abbas, S.C.; Huang, Y.Y.; Liu, Q.; Wu, M.X.; Wang, Y.B.; Dai, L.M. A photo-responsive bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nano Energy 2018, 43, 130–137. [Google Scholar] [CrossRef]
- Zhang, R.; Jian, W.; Yang, Z.D.; Bai, F.Q. Insights into the photocatalytic mechanism of the C4N/MoS2 heterostructure: A first-principle study. Chin. Chem. Lett. 2020, 31, 2319–2324. [Google Scholar] [CrossRef]
- Lv, J.Q.; Xie, J.F.; Mohamed, A.G.A.; Zhang, X.; Wang, Y.B. Photoelectrochemical energy storage materials: Design principles and functional devices towards direct solar to electrochemical energy storage. Chem. Soc. Rev. 2022, 51, 1511–1528. [Google Scholar] [CrossRef]
- Tan, Y.X.; Zhang, X.; Lin, J.; Wang, Y.B. A perspective on photoelectrochemical storage materials for coupled solar batteries. Energy Environ. Sci. 2023, 16, 2432–2447. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.Y.; Zhang, X.; Chen, J.S.; Wang, Y.B. An organic-halide perovskite-based photo-assisted Li-ion battery for photoelectrochemical storage. Nanoscale 2022, 14, 10903–10909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.B.; Zhang, X.; Zhu, L.; Yuan, D.Q.; Wang, Y.B. A Solar Responsive Battery Based on Charge Separation and Redox Coupled Covalent Organic Framework. Adv. Funct. Mater. 2023, 33, 2213667. [Google Scholar] [CrossRef]
- Zhang, X.; Su, K.Z.; Mohamed, A.G.A.; Liu, C.P.; Sun, Q.F.; Yuan, D.Q.; Wang, Y.M.; Xue, W.H.; Wang, Y.B. Photo-assisted charge/discharge Li-organic battery with a charge-separated and redox-active C60@porous organic cage cathode. Energy Environ. Sci. 2022, 15, 780–785. [Google Scholar] [CrossRef]
- Peng, Z.; Abbas, S.C.; Lv, J.Q.; Yang, R.; Wu, M.X.; Wang, Y. Mixed-metal organic framework-coated ZnO nanowires array for efficient photoelectrochemical water oxidation. Int. J. Hydrogen Energy 2019, 44, 2446–2453. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, S.J.; Song, H.W. Photon management to reduce energy loss in perovskite solar cells. Chem. Soc. Rev. 2021, 50, 7250–7329. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Zhang, X.F.; Song, W.L.; Tu, J.G.; Wang, J.X.; Wang, M.Y.; Jiao, S.Q. A Review of Integrated Systems Based on Perovskite Solar Cells and Energy Storage Units: Fundamental, Progresses, Challenges, and Perspectives. Adv. Sci. 2021, 8, 2100552. [Google Scholar] [CrossRef]
- Tan, W.C.; Saw, L.H.; Thiam, H.S.; Xuan, J.; Cai, Z.S.; Yew, M.C. Overview of porous media/metal foam application in fuel cells and solar power systems. Renew. Sust. Energ. Rev. 2018, 96, 181–197. [Google Scholar] [CrossRef]
- Gurung, A.; Qiao, Q.Q. Solar Charging Batteries: Advances, Challenges, and Opportunities. Joule 2018, 2, 1217–1230. [Google Scholar] [CrossRef]
- Gurung, A.; Chen, K.; Khan, R.; Abdulkarim, S.S.; Varnekar, G.; Pathak, R.; Naderi, R.; Qiao, Q.Q. Highly Efficient Perovskite Solar Cell Photocharging of Lithium Ion Battery Using DC-DC Booster. Adv. Energy Mater. 2017, 7, b1602105. [Google Scholar] [CrossRef]
- Nagai, H.; Suzuki, T.; Takahashi, Y.; Sato, M. Photovoltaic lithium-ion battery fabricated by molecular precursor method. Funct. Mater. Lett. 2016, 9, 1650046. [Google Scholar] [CrossRef]
- Gong, H.; Xue, H.R.; Gao, B.; Li, Y.; Yu, X.Y.; Fan, X.L.; Zhang, S.T.; Wang, T.; He, J.P. Solar-enhanced hybrid lithium-oxygen batteries with a low voltage and superior long-life stability. Chem. Commun. 2020, 56, 13642–13645. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.D.; Wen, B.; De Volder, M. Light Rechargeable Lithium-Ion Batteries Using V2O5 Cathodes. Nano Lett. 2021, 21, 3527–3532. [Google Scholar] [CrossRef]
- Lv, J.Q.; Tan, Y.X.; Xie, J.F.; Yang, R.; Yu, M.X.; Sun, S.S.; Li, M.D.; Yuan, D.Q.; Wang, Y.B. Direct Solar-to-Electrochemical Energy Storage in a Functionalized Covalent Organic Framework. Angew. Chem.-Int. Edit. 2018, 57, 12716–12720. [Google Scholar] [CrossRef]
- Boruah, B.D.; Mathieson, A.; Wen, B.; Feldmann, S.; Dose, W.M.; De Volder, M. Photo-rechargeable zinc-ion batteries. Energy Environ. Sci. 2020, 13, 2414–2421. [Google Scholar] [CrossRef]
- Boruah, B.D.; Mathieson, A.; Park, S.K.; Zhang, X.; Wen, B.; Tan, L.F.; Boies, A.; De Volder, M. Vanadium Dioxide Cathodes for High-Rate Photo-Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100115. [Google Scholar] [CrossRef]
- Boruah, B.D.; Wen, B.; De Volder, M. Molybdenum Disulfide-Zinc Oxide Photocathodes for Photo-Rechargeable Zinc-Ion Batteries. ACS Nano 2021, 15, 16616–16624. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Liao, K.M.; Li, Q.; Ishida, M.; Zhou, H.S. Lowering the charge voltage of Li-O2 batteries via an unmediated photoelectrochemical oxidation approach. J. Mater. Chem. A 2016, 4, 12411–12415. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, J.; Qiao, Y.; Wang, D.; He, P.; Li, Q.; Wu, S.C.; Zhou, H.S. Solar-driven efficient Li2O2 oxidation in solid-state Li-ion O2 batteries. Energy Storage Mater. 2018, 11, 170–175. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, X.X.; Li, F.; Zheng, L.J.; Xu, J.J.; Yu, J.H. A Bifunctional Photo-Assisted Li-O2 Battery Based on a Hierarchical Heterostructured Cathode. Adv. Mater. 2020, 32, 1907098. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.Y.; Zhang, F.; She, L.N.; Li, Q.; He, X.X.; Sun, J.; Lei, Z.B.; Liu, Z.H. Ultra-Large Sized Siloxene Nanosheets as Bifunctional Photocatalyst for a Li-O2 Battery with Superior Round-Trip Efficiency and Extra-Long Durability. Angew. Chem.-Int. Edit. 2021, 60, 11257–11261. [Google Scholar] [CrossRef]
- Lv, Q.L.; Zhu, Z.; Zhao, S.; Wang, L.B.; Zhao, Q.; Li, F.J.; Archer, L.A.; Chen, J. Semiconducting Metal-Organic Polymer Nanosheets for a Photoinvolved Li-O2 Battery under Visible Light. J. Am. Chem. Soc. 2021, 143, 1941–1947. [Google Scholar] [CrossRef]
- Liu, X.R.; Yuan, Y.F.; Liu, J.; Liu, B.; Chen, X.; Ding, J.; Han, X.P.; Deng, Y.D.; Zhong, C.; Hu, W.B. Utilizing solar energy to improve the oxygen evolution reaction kinetics in zinc-air battery. Nat. Commun. 2019, 10, 4767. [Google Scholar] [CrossRef]
- Fang, Z.S.; Li, Y.; Li, J.; Shu, C.H.; Zhong, L.F.; Lu, S.L.; Mo, C.S.; Yang, M.J.; Yu, D.S. Capturing Visible Light in Low-Band-Gap C4N-Derived Responsive Bifunctional Air Electrodes for Solar Energy Conversion and Storage. Angew. Chem.-Int. Edit. 2021, 60, 17615–17621. [Google Scholar] [CrossRef]
- Du, D.F.; Zhao, S.; Zhu, Z.; Li, F.J.; Chen, J. Photo-excited Oxygen Reduction and Oxygen Evolution Reactions Enable a High-Performance Zn-Air Battery. Angew. Chem.-Int. Edit. 2020, 59, 18140–18144. [Google Scholar] [CrossRef]
- Kalasina, S.; Phattharasupakun, N.; Sawangphruk, M. A new energy conversion and storage device of cobalt oxide nanosheets. J. Mater. Chem. A 2018, 6, 36–40. [Google Scholar] [CrossRef]
- Boruah, B.D.; Mathieson, A.; Wen, B.; Jo, C.S.; Deschler, F.; De Volder, M. Photo-Rechargeable Zinc-Ion Capacitor Using 2D Graphitic Carbon Nitride. Nano Lett. 2020, 20, 5967–5974. [Google Scholar] [CrossRef]
- Dong, W.J.; Cho, W.S.; Lee, J.L. Indium Tin Oxide Branched Nanowire and Poly (3-hexylthiophene) Hybrid Structure for a Photorechargeable Supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 22676–22683. [Google Scholar] [CrossRef]
- Wang, H.; Cao, J.J.; Zhou, Y.J.; Wang, X.; Huang, H.; Liu, Y.; Shao, M.W.; Kang, Z.H. Carbon dots modified Ti3C2Tx-based fibrous supercapacitor with photo-enhanced capacitance. Nano Res. 2021, 14, 3886–3892. [Google Scholar] [CrossRef]
- Fu, Y.P.; Wu, H.W.; Ye, S.Y.; Cai, X.; Yu, X.; Hou, S.C.; Kafafy, H.; Zou, D.C. Integrated power fiber for energy conversion and storage. Energy Environ. Sci. 2013, 6, 805–812. [Google Scholar] [CrossRef]
- Chen, J.C.; Luo, J.L.; Xiang, Y.L.; Yu, Y.J. Light-assisted rechargeable zinc-air battery: Mechanism, progress, and prospects. J. Energy Chem. 2024, 91, 178–193. [Google Scholar] [CrossRef]
- Wu, Y.J.; Ding, Y.; Chen, M.Y.; Zhang, H.; Yu, J.; Jiang, T.T.; Wu, M.Z. A Photo-Assisted Zinc-Air Battery with MoS2/Oxygen Vacancies Rich TiO2 Heterojunction Photocathode. Small 2024, 20, 2408627. [Google Scholar] [CrossRef]
- Liang, S.; Zheng, L.J.; Song, L.N.; Wang, X.X.; Tu, W.B.; Xu, J.J. Accelerated Confined Mass Transfer of MoS2 1D Nanotube in Photo-Assisted Metal-Air Batteries. Adv. Mater. 2024, 36, 2307790. [Google Scholar] [CrossRef]
- Wang, J.B.; Liu, X.J.; Zhang, Y.N.; Wang, Q.Q.; Lv, P.F.; Wei, Q.F. Bionic Leaf: Ultrahigh energy efficiency in a defect Engineered MoS2 based photo induced charging of flexible Zn-Air battery. Chem. Eng. J. 2024, 499, 156600. [Google Scholar] [CrossRef]
- Feng, H.G.; Zhang, C.M.; Liu, Z.Y.; Sang, J.X.; Xue, S.L.; Chu, P. A light-activated TiO2@In2Se3@Ag3PO4 cathode for high-performance Zn-Air batteries. Chem. Eng. J. 2022, 434, 134650. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, Z.; Feng, K.Y.; Deng, F.J.; Yu, Y.J. Electrostatically connected Fe2O3@Ni-MOF nanosheet array heterojunction for high-performance light-assisted zinc-air batteries. Compos. Pt. B-Eng. 2025, 289, 111936. [Google Scholar] [CrossRef]
- Zhu, T.; Xia, C.F.; Wu, B.; Pan, J.; Yang, H.R.; Zhang, W.B.; Xia, B.Y. Inbuilt photoelectric field of heterostructured cobalt/iron oxides promotes oxygen electrocatalysis for high-energy-efficiency zinc-air batteries. Appl. Catal. B-Environ. Energy 2024, 357, 124315. [Google Scholar] [CrossRef]
- Wang, C.T.; Huang, H.H. Photo-chargeable titanium/vanadium oxide composites. J. Non-Cryst. Solids 2008, 354, 3336–3342. [Google Scholar] [CrossRef]
- Hu, C.; Chen, L.; Hu, Y.J.; Chen, A.P.; Chen, L.; Jiang, H.; Li, C.Z. Light-Motivated SnO2/TiO2 Heterojunctions Enabling the Breakthrough in Energy Density for Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2103558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.J.; Zhu, G.X.; Wang, J.; Zhu, J.X.; Sun, G.Z.; Zhang, Y.; Li, P.; Zhu, Y.F.; Luo, W.J.; Zou, Z.G.; et al. Direct storage of holes in ultrathin Ni(OH)2 on Fe2O3 photoelectrodes for integrated solar charging battery-type supercapacitors. J. Mater. Chem. A 2018, 6, 21360–21367. [Google Scholar] [CrossRef]
- Paolella, A.; Faure, C.; Bertoni, G.; Marras, S.; Guerfi, A.; Darwiche, A.; Hovington, P.; Commarieu, B.; Wang, Z.R.; Prato, M.; et al. Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat. Commun. 2017, 8, 14643. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Yang, X.M. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Ren, Z.F.; Fan, H.J.; Yang, X.M. Elementary photocatalytic chemistry on TiO2 surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D.Y. Core-shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef]
- Usui, H.; Koseki, K.; Tamura, T.; Domi, Y.; Sakaguchi, H. Light energy storage in TiO2/MnO2 composite electrode for photoelectrochemical capacitor. Mater. Lett. 2017, 186, 338–340. [Google Scholar] [CrossRef]
- Usui, H.; Suzuki, S.; Domi, Y.; Sakaguchi, H. TiO2/MnO2 composite electrode enabling photoelectric conversion and energy storage as photoelectrochemical capacitor. Mater. Today Energy 2018, 9, 229–234. [Google Scholar] [CrossRef]
- Usui, H.; Suzuki, S.; Domi, Y.; Sakaguchi, H. Impacts of MnO2 Crystal Structures and Fe Doping in Those on Photoelectrochemical Charge-Discharge Properties of TiO2/MnO2 Composite Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 9165–9173. [Google Scholar] [CrossRef]
- Usui, H.; Toriu, S.; Domi, Y.; Tanaka, T.; Sakaguchi, H. Material design based on impurity element doping for photoelectrochemical capacitor composite electrodes using metal oxides. J. Energy Storage 2021, 44, 103497. [Google Scholar] [CrossRef]
- Tong, Z.; Lyu, T.; Zheng, K.G.; Zhang, J.; Lv, C.; Zhou, Y.; Lu, M.; Li, J.T. A bifunctional hierarchical heterostructured integrated cathode based on lignin-derived carbon fiber microfilms for high performance photo-assisted Li-O2 battery. J. Colloid Interface Sci. 2025, 695, 137782. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.D.; Wang, Y.T. A review of the α-Fe2O3(hematite) nanotube structure: Recent advances in synthesis, characterization, and applications. Nanoscale 2020, 12, 10912–10932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.R.; Chiu, H.C.; Paolella, A.; Gauvin, R.; Zaghib, K.; Demopoulos, G.P. A sustainable light-chargeable two-electrode energy storage system based on aqueous sodium-ion photo-intercalation. Sustain. Energ. Fuels 2020, 4, 4789–4799. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.R.; Tang, D.M.; Zhou, H.S. Integrating a Photocatalyst into a Hybrid Lithium-Sulfur Battery for Direct Storage of Solar Energy. Angew. Chem.-Int. Edit. 2015, 54, 9271–9274. [Google Scholar] [CrossRef]
- Wang, P.; Chen, X.T.; Sun, G.Z.; Wang, C.; Luo, J.; Yang, L.Q.; Lv, J.; Yao, Y.F.; Luo, W.J.; Zou, Z.G. A Capacitor-type Faradaic Junction for Direct Solar Energy Conversion and Storage. Angew. Chem.-Int. Edit. 2021, 60, 1390–1395. [Google Scholar] [CrossRef]
- Chen, P.; Li, T.T.; Li, G.R.; Gao, X.P. Quasi-solid-state solar rechargeable capacitors based on in-situ Janus modified electrode for solar energy multiplication effect. Sci. China-Mater. 2020, 63, 1693–1702. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, B.M.; Lee, Y.; Han, H.G.; Cho, M.; Kwon, T.H.; Song, H.K. Electrochemically Induced Crystallite Alignment of Lithium Manganese Oxide to Improve Lithium Insertion Kinetics for Dye-Sensitized Photorechargeable Batteries. ACS Energy Lett. 2021, 6, 1198–1204. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Duan, L.F.; Zhang, X.Y.; Li, X.S.; Lü, W. A photo-assisted rechargeable battery: Synergy, compatibility, and stability of a TiO2/dye/Cu2S bifunctional composite electrode. Nanoscale 2020, 12, 530–537. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, K.; Wang, B.J.; Peng, H.S. Light-Assisted Metal-Air Batteries: Progress, Challenges, and Perspectives. Angew. Chem.-Int. Edit. 2022, 61, e202213026. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zhao, Q.C.; Fan, G.L.; Zhao, S.; Wang, L.B.; Li, F.J.; Chen, J. Photoinduced Oxygen Reduction Reaction Boosts the Output Voltage of a Zinc-Air Battery. Angew. Chem.-Int. Edit. 2019, 58, 12460–12464. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.L.; Fang, Z.S.; Yang, M.J.; Zhu, F.M.; Yu, D.S. Harvesting Air and Light Energy via “All-in-One” Polymer Cathodes for High-Capacity, Self-Chargeable, and Multimode-Switching Zinc Batteries. Adv. Funct. Mater. 2021, 31, 2007942. [Google Scholar] [CrossRef]
- Wang, Q.Y.; O’Carroll, T.; Shi, F.C.; Huang, Y.F.; Chen, G.R.; Yang, X.X.; Nevar, A.; Dudko, N.; Tarasenko, N.; Xie, J.Y.; et al. Designing Organic Material Electrodes for Lithium-Ion Batteries: Progress, Challenges, and Perspectives. Electrochem. Energy Rev. 2024, 7, 15. [Google Scholar] [CrossRef]
- Tang, X.J.; Feng, M.Q.; Lv, W.H.; Lv, S. Applications of All-Solid-State Lithium-Ion Batteries Across Wide Temperature Ranges: Challenges, Progress, and Perspectives. Adv. Energy Mater. 2025, 15, 2500479. [Google Scholar] [CrossRef]
- Niu, X.W.; Liu, R.X.; Zhang, S.M.; Li, J.S.; Lei, X.F.; Liu, X.W. Optimization strategies for g-C3N4-based photoelectrodes and progress in their application to photo-assisted zinc-air batteries. J. Alloy. Compd. 2025, 1039, 183038. [Google Scholar] [CrossRef]
- He, S.L.; Shi, J.W.; Wang, B.L.; Hu, S.J. Decoupled cathode with light assistance for rechargeable zinc-air batteries: Achieving high energy density and wide temperature adaptability. Compos. Pt. B-Eng. 2025, 292, 112099. [Google Scholar] [CrossRef]
- Chen, Y.H.; Xu, J.J.; He, P.; Qiao, Y.; Guo, S.H.; Yang, H.J.; Zhou, H.S. Metal-air batteries: Progress and perspective. Sci. Bull. 2022, 67, 2449–2486. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Lee, J.H.; Kim, C.H.; Gautam, J.; Heo, K.; Hussain, S.; Ikram, M.; Alobaid, A.A.; Lee, S.Y.; et al. A Review of Rechargeable Zinc-Air Batteries: Recent Progress and Future Perspectives. Nano-Micro Lett. 2024, 16, 138. [Google Scholar] [CrossRef]
- Liu, M.; Ao, H.; Jin, Y.; Hou, Z.; Zhang, X.; Zhu, Y.; Qian, Y. Aqueous rechargeable sodium ion batteries: Developments and prospects. Mater. Today Energy 2020, 17, 100432. [Google Scholar] [CrossRef]
- Feng, H.E.; Zhang, C.M.; Luo, M.H.; Hu, Y.C.; Dong, Z.B.; Xue, S.L.; Chu, P.K. Photo Energy-Enhanced Oxygen Reduction and Evolution Kinetics in Zn-Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 6788–6796. [Google Scholar] [CrossRef]
- Guan, D.H.; Wang, X.X.; Li, M.L.; Li, F.; Zheng, L.J.; Huang, X.L.; Xu, J.J. Light/Electricity Energy Conversion and Storage for a Hierarchical Porous In2S3@CNT/SS Cathode towards a Flexible Li-CO2 Battery. Angew. Chem.-Int. Edit. 2020, 59, 19518–19524. [Google Scholar] [CrossRef]
- Sarawutanukul, S.; Tomon, C.; Duangdangchote, S.; Phattharasupakun, N.; Sawangphruk, M. Rechargeable Photoactive Zn-Air Batteries Using NiCo2S4 as an Efficient Bifunctional Photocatalyst towards OER/ORR at the Cathode. Batter. Supercaps 2020, 3, 541–547. [Google Scholar] [CrossRef]
- Hu, S.J.; Shi, J.W.; Yan, R.; Pang, S.C.; Zhang, Z.X.; Wang, J.J.; Zhu, M.S. Flexible rechargeable photo-assisted zinc-air batteries based on photo-active pTTh bifunctional oxygen electrocatalyst. Energy Storage Mater. 2024, 65, 103139. [Google Scholar] [CrossRef]
- Fan, J.T.; Chen, M.; Zhao, Z.L.; Zhang, Z.; Ye, S.Y.; Xu, S.Y.; Wang, H.J.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Iwata, R.C.; Zhang, L.N.; Wilke, K.L.; Gong, S.; He, M.F.; Gallant, B.M.; Wang, E.N. Bubble growth and departure modes on wettable/non-wettable porous foams in alkaline water splitting. Joule 2021, 5, 887–900. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, J.W.; Mu, X.Q.; Cheng, R.L.; Li, W.Q.; Liu, S.L.; Pu, Z.H.; Lin, C.; Mu, S.C. Nitrogen-Doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and zn-air batteries. Appl. Catal. B-Environ. Energy 2020, 268, 118729. [Google Scholar] [CrossRef]
- Tang, W.Q.; Mai, J.R.; Liu, L.L.; Yu, N.F.; Fu, L.J.; Chen, Y.H.; Liu, Y.K.; Wu, Y.P.; van Ree, T. Recent advances of bifunctional catalysts for zinc air batteries with stability considerations: From selecting materials to reconstruction. Nanoscale Adv. 2023, 5, 4368–4401. [Google Scholar] [CrossRef]
- Fan, W.J.; Zhang, B.Q.; Wang, X.Y.; Ma, W.G.; Li, D.; Wang, Z.L.; Dupuis, M.; Shi, J.Y.; Liao, S.J.; Li, C. Efficient hydrogen peroxide synthesis by metal-free polyterthiophene via photoelectrocatalytic dioxygen reduction. Energy Environ. Sci. 2020, 13, 238–245. [Google Scholar] [CrossRef]
- Li, M.Y.; Ye, K.H.; Qiu, W.T.; Wang, Y.F.; Ren, H. Heterogeneity between and within Single Hematite Nanorods as Electrocatalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2022, 144, 5247–5252. [Google Scholar] [CrossRef]
- Kong, L.Q.; Ruan, Q.S.; Qiao, J.Y.; Chen, P.Y.; Yan, B.Z.; He, W.; Zhang, W.; Jiang, C.R.; Lu, C.J.; Sun, Z.M. Realizing Unassisted Photo-Charging of Zinc-Air Batteries by Anisotropic Charge Separation in Photoelectrodes. Adv. Mater. 2023, 35, 2304669. [Google Scholar] [CrossRef]
- Song, L.X.; Fan, Y.B.; Fan, H.Q.; Yang, X.Y.; Yan, K.; Wang, X.Y.; Ma, L.T. Photo-assisted rechargeable metal batteries. Nano Energy 2024, 125, 109538. [Google Scholar] [CrossRef]
- Zhang, B.B.; Yu, S.Q.; Dai, Y.; Huang, X.J.; Chou, L.J.; Lu, G.X.; Dong, G.J.; Bi, Y.P. Nitrogen-incorporation activates NiFeOx catalysts for efficiently boosting oxygen evolution activity and stability of BiVO4 photoanodes. Nat. Commun. 2021, 12, 6969. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ye, X.Y.; Chen, S.; Ma, L.; Wang, Z.P.; Wang, Q.T.; Hua, N.B.; Xiao, X.Q.; Cai, S.G.; Liu, X.H. Ti3C2 MXene nanosheet/TiO2 composites for efficient visible light photocatalytic activity. Ceram. Int. 2020, 46, 25895–25904. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chu, W.B.; Zheng, Q.J.; Benderskii, A.V.; Prezhdo, O.V.; Zhao, J. Suppression of Electron-Hole Recombination by Intrinsic Defects in 2D Monoelemental Material. J. Phys. Chem. Lett. 2019, 10, 6151–6158. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, X.M.; Zhao, G.Y.; Shen, X.J.; Liu, Y.F.; Bao, X.Y.; Luo, J.; Yu, L.P.; Zhang, N.Q. A visible light illumination assistant Li-O2 battery based on an oxygen vacancy doped TiO2 catalyst. Electrochim. Acta 2022, 405, 139794. [Google Scholar] [CrossRef]
- Huang, Z.X.; Qin, X.P.; Gu, X.F.; Li, G.Z.; Mu, Y.C.; Wang, N.G.; Ithisuphalap, K.; Wang, H.X.; Guo, Z.P.; Shi, Z.C.; et al. Mn3O4 Quantum Dots Supported on Nitrogen-Doped Partially Exfoliated Multiwall Carbon Nanotubes as Oxygen Reduction Electrocatalysts for High-Performance Zn-Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 23900–23909. [Google Scholar] [CrossRef]
- Chen, B.H.; Jiang, Z.Q.; Huang, J.L.; Deng, B.L.; Zhou, L.S.; Jiang, Z.J.; Liu, M.L. Cation exchange synthesis of NixCo(3-x)O4 (x = 1.25) nanoparticles on aminated carbon nanotubes with high catalytic bifunctionality for the oxygen reduction/evolution reaction toward efficient Zn-air batteries. J. Mater. Chem. A 2018, 6, 9517–9527. [Google Scholar] [CrossRef]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Yang, S.; Liu, C.; Li, H.G.; Wang, S.L.; Choi, J.; Li, L. Quantum dot heterostructure with directional charge transfer channels for high sodium storage. Energy Storage Mater. 2021, 39, 278–286. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, B.; Gao, Y.H.; Zhu, H.; Chen, Y.T.; Li, X.K.; Zhang, Q. Mott-Schottky Effect in Core-Shell W@WxC Heterostructure: Boosting Both Electronic/Ionic Kinetics for Lithium Storage. Small 2023, 9, 2300955. [Google Scholar] [CrossRef]
- Haghighat-Shishavan, S.; Nazarian-Samani, M.; Nazarian-Samani, M.; Hosseini-Shokouh, S.H.; Maschmeyer, T.; Kim, K.B. Realization of Sn2P2S6-carbon nanotube anode with high K+/Na+ storage performance via rational interface manipulation-induced shuttle-effect inhibition and self-healing. Chem. Eng. J. 2022, 435, 134965. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, M.F.; Tao, Z.; He, L.H.; Guo, C.P.; Liu, B.Z.; Zhang, Z.H. Photo-assisted Zn-air battery-driven self-powered aptasensor based on the 2D/2D Schottky heterojunction of cadmium-doped molybdenum disulfide and Ti3C2Tx nanosheets for the sensitive detection of penicillin G. Anal. Chim. Acta 2023, 1270, 341396. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Li, Y.; Zhang, G.K. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Shen, R.C.; Lu, X.Y.; Zheng, Q.Q.; Chen, Q.; Ng, Y.H.; Zhang, P.; Li, X. Tracking S-Scheme Charge Transfer Pathways in Mo2C/CdS H2-Evolution Photocatalysts. Sol. RRL 2021, 5, 2100177. [Google Scholar] [CrossRef]
- Li, J.F.; Li, Z.Y.; Liu, X.M.; Li, C.Y.; Zheng, Y.F.; Yeung, K.W.K.; Cui, Z.D.; Liang, Y.Q.; Zhu, S.L.; Hu, W.B.; et al. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing. Nat. Commun. 2021, 12, 1224. [Google Scholar] [CrossRef]
- Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K. Reduced graphene oxide-CdS heterostructure: An efficient fluorescent probe for the sensing of Ag(I) and sunset yellow and a visible-light responsive photocatalyst for the degradation of levofloxacin drug in aqueous phase. Appl. Catal. B-Environ. 2019, 245, 143–158. [Google Scholar] [CrossRef]
- Low, J.X.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.G. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Li, X.; Garlisi, C.; Guan, Q.S.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L.X. A review of material aspects in developing direct Z-scheme photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]
- Wang, X.T.; Li, Y.; Yang, M.J.; Liu, C.; Li, J.; Yu, D.S. Unique Step-Scheme Heterojunction Photoelectrodes for Dual-Utilization of Light and Chemical Neutralization Energy in Switchable Dual-Mode Batteries. Adv. Funct. Mater. 2022, 32, 2205518. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ji, S.F.; Chen, C.; Peng, Q.; Wang, D.S.; Li, Y.D. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ji, S.F.; Zhao, S.; Chen, W.X.; Dong, J.C.; Cheong, W.C.; Shen, R.A.; Wen, X.D.; Zheng, L.R.; Rykov, A.I.; et al. Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air battery and hydrogen-air fuel cell. Nat. Commun. 2018, 9, 5422. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.X.; Fan, L.; Li, L.L.; Peng, S.J.; Yu, D.S.; Song, J.N.; Ramakrishna, S.; Guo, S.J. Atomically Transition Metals on Self-Supported Porous Carbon Flake Arrays as Binder-Free Air Cathode for Wearable Zinc-Air Batteries. Adv. Mater. 2019, 31, 1808267. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, B.Z.; Chen, S.W. Carbon-Supported Single Atom Catalysts for Electrochemical Energy Conversion and Storage. Adv. Mater. 2018, 30, 1801995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.G.; Wang, S.M.; Zhao, Z.Y.; Shi, Z.; Yan, S.C.; Zou, Z.G. A Facet-Dependent Schottky-Junction Electron Shuttle in a BiVO4-Au-Cu2O Z-Scheme Photocatalyst for Efficient Charge Separation. Adv. Funct. Mater. 2018, 28, 1801214. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Wen, M.; Wang, N.; Wu, Q.S.; Wu, Q.N.; Cheng, L.Y. Highly active NiCo alloy hexagonal nanoplates with crystal plane selective dehydrogenation and visible-light photocatalysis. J. Mater. Chem. 2012, 22, 16858–16864. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.W.; Liu, W.C.; Liang, Z.H.; Liao, B.; Yang, F.; Zhao, M.; Yan, B.; Gui, X.C.; Yang, H.B.; et al. Engineering Bipolar Doping in a Janus Dual-Atom Catalyst for Photo-Enhanced Rechargeable Zn-Air Battery. Nano-Micro Lett. 2025, 17, 203. [Google Scholar] [CrossRef]
- Xue, Z.H.; Luan, D.Y.; Zhang, H.B.; Lou, X.W. Single-atom catalysts for photocatalytic energy conversion. Joule 2022, 6, 92–133. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, X.Y.; Liu, J.N.; Wang, J.; Wan, X.; Li, X.Y.; Tang, C.; Wang, C.D.; Song, L.; Shui, J.L.; et al. Inductive Effect on Single-Atom Sites. J. Am. Chem. Soc. 2023, 145, 27531–27538. [Google Scholar] [CrossRef]
- He, K.L.; Huang, Z.M.; Chen, C.; Qiu, C.T.; Zhong, Y.L.; Zhang, Q.T. Exploring the Roles of Single Atom in Hydrogen Peroxide Photosynthesis. Nano-Micro Lett. 2024, 16, 23. [Google Scholar] [CrossRef]
- Liu, R.Y.; Chen, Y.Z.; Yu, H.D.; Polozij, M.; Guo, Y.Y.; Sum, T.C.; Heine, T.; Jiang, D.L. Linkage-engineered donor-acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 2024, 16, 195–206. [Google Scholar] [CrossRef]
- Janeta, M.; Heidlas, J.X.; Daugulis, O.; Brookhart, M. 2,4,6-Triphenylpyridinium: A Bulky, Highly Electron-Withdrawing Substituent That Enhances Properties of Nickel (II) Ethylene Polymerization Catalysts. Angew. Chem.-Int. Edit. 2021, 60, 4566–4569. [Google Scholar] [CrossRef]
- Ding, J.; Teng, Z.Y.; Su, X.Z.; Kato, K.; Liu, Y.H.; Xiao, T.; Liu, W.; Liu, L.Y.; Zhang, Q.; Ren, X.Y.; et al. Asymmetrically coordinated cobalt single atom on carbon nitride for highly selective photocatalytic oxidation of CH4 to CH3OH. Chem 2023, 9, 1017–1035. [Google Scholar] [CrossRef]
- Li, D.; Li, H.M.; Wen, Q.; Gao, C.Y.; Song, F.; Zhou, J. Investigation on Photo-Assisted Fenton-like Mechanism of Single-Atom Mn-N-Fe-N-Ni Charge Transfer Bridge Across Six-Membered Cavity of Graphitic Carbon Nitride. Adv. Funct. Mater. 2024, 34, 2313631. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.L.; Cheng, W.R.; Cao, Y.J.; Liu, X.K.; Zhang, W.; Mou, X.L.; Jin, L.L.; Zheng, X.S.; Che, W.; et al. Single-Site Active Cobalt-Based Photocatalyst with a Long Carrier Lifetime for Spontaneous Overall Water Splitting. Angew. Chem.-Int. Edit. 2017, 56, 9312–9317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Su, X.Z.; Ding, J.; Zhou, J.; Liu, Z.; Wei, X.J.; Yang, H.B.; Liu, B. Progress and challenges in structural, in situ and operando characterization of single-atom catalysts by X-ray based synchrotron radiation techniques. Chem. Soc. Rev. 2024, 53, 11850–11887. [Google Scholar] [CrossRef]
- Lang, Z.Q.; Wang, X.X.; Jabeen, S.; Cheng, Y.Y.; Liu, N.Y.; Liu, Z.H.; Gan, T.; Zhuang, Z.C.; Li, H.T.; Wang, D.S. Destabilization of Single-Atom Catalysts: Characterization, Mechanisms, and Regeneration Strategies. Adv. Mater. 2025, 37, 2418942. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Pan, Z.C.; Wu, Z.L.; Huang, B.K.; Lai, B.; Liu, W. Advanced Characterization Techniques and Theoretical Calculation for Single Atom Catalysts in Fenton-like Chemistry. Molecules 2024, 29, 3719. [Google Scholar] [CrossRef]




| Battery Type | PES Materials | Charge Potential Under Dark/Light | Capacity Under Dark/Light | Cycle Number | ηc | Ref. |
|---|---|---|---|---|---|---|
| LIB | TiO2 | 2.04/1.37 | / | 30 | / | [52] |
| N719 + LiFePO4 | Photocharge | 40/340 F g−1 | 115 | 0.06–0.08% | [53] | |
| V2O5/P3HT/rGO | Photocharge | 118/161 F g−1 | 35 | ~0.22% | [54] | |
| NT-COF | 2.53/2.02 | 129/200 F g−1 | 5 | / | [55] | |
| ZIB | V2O5/P3HT/rGO | Photocharge | 190/370 F g−1 | 25 | 1.2% | [56] |
| VO2/rGO | Photocharge | 282/315 F g−1 | 250 | 0.18% | [57] | |
| MoS2/ZnO | Photocharge | 245/340 F g−1 | 40 | ~0.2% | [58] | |
| LOB | g-C3N4 | 3.61/1.96 | / | 70 | / | [59] |
| ZnS@CNT | 4.09/2.08 | / | 150 | / | [60] | |
| TiO2-Fe2O3 | 4.2/3.2 | / | 100 | / | [61] | |
| Siloxene nanosheets | 3.80/1.90 | --/1170 F g−1 | 100 | / | [62] | |
| Co-TABQ | 4.31/3.32 | / | 50 | / | [63] | |
| ZAB | a-Fe2O3 | 1.97/1.43 | 598.7/--F g−1 | 50 h | / | [64] |
| C4N | ~ 1.65/1.34 | / | 50 | / | [65] | |
| PDTB and TiO2 | 1.88/0.59 | / | ~ 33 | / | [66] | |
| SC | Co3O4 | Photocharge | 80.8/35.9 F cm−3 | 5000 | / | [67] |
| Ag@V2O5 | Photocharge | 52/92 F g−1 | 4000 | ~0.05% | [56] | |
| g-C3N4 | 1.00/0.68 | 6.65/11.4 F g−1 | 500 | ~0.01% | [68] | |
| ITO/P3HT | 0.56/0.26 | --/2.44 mF cm−2 | ~0.0017% | [69] | ||
| Ti3C2Tx-NCDs | Photocharge | 464/630 F g−1 | 900 | / | [70] |
| Comparison Items | Photo-Assisted Zinc–Air Batteries [45,95,96] | Lithium-Ion Batteries [93,94] |
|---|---|---|
| Working Principle | Combines light energy and electrical energy; photocatalysts accelerate ORR/OER; relies on air for oxygen supply | Relies solely on electrical energy; Li+ intercalation/deintercalation between positive and negative electrodes; requires internal Li+ storage |
| Cost | Low raw material cost (zinc, low-cost catalysts), low preparation cost, and low system cost | Scarce lithium resources, positive electrodes contain precious metals, complex preparation of all-solid-state systems, and high cost |
| Efficiency | Round-trip efficiency of 60–87.7% with light assistance; maximum energy density of 1021.42 mWh g−1 | Round-trip efficiency of 80–90% for traditional liquid systems and 75–85% for all-solid-state systems; energy density of 400–600 Wh/kg |
| Lifespan | Decoupled cathode system: 1064 h of cycling (1596 cycles); two-electrode system: 1580 h of cycling | Traditional liquid system: 1000 cycles (70% capacity retention); all-solid-state Si-based system: 5000 cycles (61.5% capacity retention) |
| Temperature Adaptability | Operating range of −25 °C–60 °C; photothermal effect mitigates low-temperature issues | All-solid-state system: −60 °C–120 °C; traditional liquid system: −20 °C–60 °C |
| Safety | No flammability risk, no internal oxygen storage explosion hazard; high-concentration KOH is corrosive | All-solid-state systems: no electrolyte leakage; traditional liquid systems: flammable; lithium dendrites may cause short circuits |
| Application Scenarios | Portable electronics, outdoor emergency power supplies, low-power devices | Electric vehicles, large-scale energy storage, extreme environment equipment |
| Test Category | Test Item | Specific Requirements | Description |
|---|---|---|---|
| Light Intensity/ Spectroscopy | Light Source | A 300 W xenon lamp is used. An AM 1.5 G filter must be equipped for simulating sunlight; if no filter is used, full-spectrum irradiation should be clearly specified. | |
| Measurement Position and Light Intensity | The measurement point is 1 cm away from the electrode surface; the light intensity is approximately 90 mW cm−2 with a fluctuation range of ±5 mW cm−2. | ||
| UV-Vis Spectrophotometer Parameters | Test wavelength range: 300–800 nm (visible light absorption range); scanning rate: 200 nm min−1; BaSO4 is used as the reference. | ||
| Battery Structure/ Active Area | Liquid Battery Structure (Taking Zinc–Air Battery as an Example) | Anode: zinc foil; cathode: sample catalyst; electrolyte: liquid 6 M KOH + 0.2 M Zn(Ac)2; encapsulation uses a glass mold with an electrode spacing of 1 cm; continuous O2 purging (flow rate: 10 mL min−1) is maintained. | Stable and continuous O2 supply must be ensured to avoid interference from other components in the air with the electrolyte. |
| Active Area Calibration | The edge sealing area and lead connection area must be excluded; cross-validation is required using the ImageJ (ImageJ 1.x series or ImageJ2) visual calibration method (error < 2%) and the weight back-calculation method (combined with catalyst loading, deviation < 5%). | Both calibration methods must be used simultaneously to ensure the accuracy of active area data. | |
| Electrode Mass Loading (Taking Zinc-Air Battery as an Example) | Cathode Catalyst Loading | 0.5–1 mg cm−2 | The loading amount must be precisely controlled to avoid affecting the battery performance test results. |
| Zinc Foil Parameters | Thickness: 50 μm; the mass change in the zinc foil before and after pretreatment must be recorded. | The mass change before and after pretreatment is a key indicator for evaluating the initial state of the zinc foil. | |
| Basic Formula of Liquid Electrolyte Test | Preparation Method | Prepare 6 M KOH + 0.2 M Zn(Ac)2 using deionized water as the solvent. Stir at room temperature for 30 min until completely dissolved, then purge with O2 for 30 min to saturate the electrolyte. | Sufficient stirring is required to ensure complete dissolution of the solute, and O2 purging must last for an adequate time to ensure electrolyte saturation. |
| Routine Test Temperature | 25 °C, using a constant temperature chamber (fluctuation ± 0.5 °C). | Temperature stability must be maintained to reduce the impact of temperature fluctuations on test results. | |
| Temperature Range | Low-Temperature Test | Conducted in a low-temperature constant temperature chamber at a test temperature of −10 °C. | |
| High-Temperature Test | Test temperatures: 45 °C, 55 °C; the battery performance changes under different high temperatures must be recorded. | Focus should be placed on the impact of high temperatures on battery stability and performance. | |
| Cycling Protocol | Cycling Stability Test | The structural stability of the battery after 50 cycles must be recorded. | 50 cycles is the minimum test standard, which is used to evaluate the long-term service potential of the battery. |
| Test Conditions | Test Equipment and Mode | Equipment: LAND-CT2001A battery testing system; test mode: constant current charge–discharge. | |
| Current Density Range for Liquid Battery | 0.1–2 mA cm−2 | An appropriate current density within this range should be selected for testing to cover different application scenarios. | |
| Cut-Off Voltage | Charging upper limit: 2.0 V vs. RHE; Discharging lower limit: 0.8 V vs. RHE. | The cut-off voltage requirements must be strictly followed to prevent battery damage caused by overcharging and over-discharging. | |
| Lighting Conditions | Equipped with an AM 1.5 G filter; Light intensity: 90 mW cm−2; the illuminated area must cover the entire active area. | The illuminated area must be completely matched with the active area to avoid uneven local illumination. | |
| Auxiliary Tests | A three-electrode system is used to evaluate ORR/OER performance; Linear Sweep Voltammetry (LSV) is performed at a scanning rate of 2 mV s−1; the Rotating Ring-Disk Electrode (RRDE) is operated at a rotation speed of 1600 rpm. | Auxiliary tests are used to conduct in-depth analysis of the electrode reaction process and supplement the overall battery performance data. | |
| Timing Modes | Continuous Lighting Mode | A light intensity of 90 mW cm−2 is maintained during both charging and discharging stages; turn on the light 5 min in advance and turn it off 3 min after discharging ends. | The light-on and light-off times must be precisely controlled to ensure synchronization between the lighting and the charge–discharge process. |
| Intermittent Lighting Mode | Cycle duration: 20 min (10 min of lighting/10 min of darkness) with a duty cycle of 50%; the charging voltage changes under different timings must be recorded. | Focus on the fluctuation of charging voltage when switching between darkness and lighting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, H.; Li, Y.; Liang, X. Mechanism and Air Cathode Materials of Photo-Assisted Zinc–Air Batteries for Photoelectrochemical Energy Storage. Crystals 2025, 15, 923. https://doi.org/10.3390/cryst15110923
Zhang M, Wang H, Li Y, Liang X. Mechanism and Air Cathode Materials of Photo-Assisted Zinc–Air Batteries for Photoelectrochemical Energy Storage. Crystals. 2025; 15(11):923. https://doi.org/10.3390/cryst15110923
Chicago/Turabian StyleZhang, Mengmeng, Haoxiang Wang, Yuanyuan Li, and Xiangyu Liang. 2025. "Mechanism and Air Cathode Materials of Photo-Assisted Zinc–Air Batteries for Photoelectrochemical Energy Storage" Crystals 15, no. 11: 923. https://doi.org/10.3390/cryst15110923
APA StyleZhang, M., Wang, H., Li, Y., & Liang, X. (2025). Mechanism and Air Cathode Materials of Photo-Assisted Zinc–Air Batteries for Photoelectrochemical Energy Storage. Crystals, 15(11), 923. https://doi.org/10.3390/cryst15110923






