Abstract
A new polymorph modification of SrB2O4 was obtained from melt containing Sr2CO3, H3BO3 and CuCl2·2H2O with a molar ratio of 2:2:1. The growth was carried out by cooling the melt from 1180 to 860 °C. The obtained material looks like a green bulk mass at the edges, of which grew transparent single crystals of SrB2O4 (approximately 0.1 × 0.2 × 0.1 mm in size). The crystals were studied by scanning electron microscopy, single crystal, powder X-ray diffraction, DTA/TG and FTIR spectroscopy. The single crystal structure data of SrB2O4 shows orthorhombic Pna21 symmetry. The structure is built up of linked BO4 and BO3 units and the charge is compensated by strontium cations.
1. Introduction
Borate materials are intensively and extensively studied due to their various properties and applications [1,2,3,4,5,6,7,8,9,10,11,12]. The variety of borates arise, in part, from the unique structural characteristics of boron–oxygen groups, e.g., BO3, BO4 and boroxol, etc., building units and the low melting temperature of B2O3—making it suitable for crystal growth—and conversely its tendency to act as glass former. Amongst those, crystal borates are known for their nonlinear optical (NLO) properties [13,14]. Strontium borate crystals have also been subject of investigations [15,16,17] principally related to its NLO properties, second harmonic generation (SHG) and luminescent properties [18,19,20,21,22,23,24,25,26].
Borate materials continue to attract intensive interest, owing to their structural diversity and broad applications, encompassing nonlinear optics (NLOs), photonics, luminescence, energy storage and bioactive glasses [1,2,3,4,5,6,7,8,9,10,11,12]. Structural richness originates from the versatile boron–oxygen polyhedra (e.g., planar BO3, BO4 tetrahedra and boroxol rings B3O6) and their combinatorial assembly into anion frameworks. Additionally, the relatively low melting temperature of anhydrous boron oxide (B2O3) and boric acid (B(OH)3) facilitates crystal growth, while its strong glass-forming tendency enables compositional design of glasses and glass ceramics [2,3,4,7,13]. In NLO science, recent reviews and reports highlight rapid progress in short-wavelength/deep-ultraviolet borate systems (including fluoro-oxoborates and borophosphates), where boron–oxygen skeleton modifications and mixed-anionic layers are leveraged to balance cutoff edge, birefringence and SHG response [2,3,4,14,15,16].
Within alkaline-earth borates, strontium borates remain a productive family, linking crystal chemistry and optical functionality. Strontium borates have been studied for NLO and second harmonic generation (SHG) behavior and as hosts for rare-earth (RE) luminescence, with new compositions and doping strategies emerging in recent years [8,9,11,17,18,19]. Classical Sr-borates (e.g., SrB4O7, SrB2O4) continue to serve as structural standards, while recent work explores RE3+ activators and energy-transfer pathways in Sr-borate hosts and related oxyborates, targeting color-tunable or UV-excited emissions [20,21,22,23,24,25,26]. Two crystal structures with chemical formula containing SrB2O4 can be located in the databases ICSD or ICDD: the high-pressure SrB2O4(IV) polymorph structure, space group Pa-3 [27] and the high-pressure SrB2O4(III) space group Pna21 [28]. Dernier et al. [28] suggested the existence of a SrB2O4(II) polymorph, but such a polymorph has not been reported. Here, we report the crystal structure of a new SrB2O4 polymorph obtained in ambient conditions (Pna21 space group, from powder film). As suggested, the space group and unit cell parameters are similar to the CaB2O4 high-pressure form [29]. Although, the comparison of the two structures with COMPSTRU [30] does not show significant differences, an in-depth analysis shows that four boron atoms are tetrahedrally coordinated and two are with triangular coordination in CaB2O4 form while in the new SrB2O4 form three with tetrahedral and three with triangular coordination.
2. Materials and Methods
2.1. Crystal Growth
The starting materials were SrCO3 (99.9%, Aldrich), H3BO3 (99.5%, Aldrich) and CuCl2·2H2O (99+%, AlfaAesar) mixed in 2:2:1 molar proportion (Samples 1 and 2) under ambient conditions. In this case, CuCl2·2H2O acts as a flux component that lowers the melting temperature of the SrO–B2O3 system and promotes the growth of SrB2O4 single crystals. It is not incorporated into the final structure. For Sample 1, firstly all reagents were homogenized by grinding manually in an agate mortar, then the mixture was transferred to a platinum crucible (covered by a platinum cap) and finally the crucible was placed in a preheated electric furnace at 1180 °C for 60 min. The furnace was cooled down to 860 °C at a speed of 1 °C·min−1 and the crucible was taken out of the furnace to rapidly cool down from 860 °C to room temperature. A visual inspection showed that the obtained material looked like a dark green crystalline mass. Elongated single crystals, up to 1 mm in size (Figure 1a) were observed at the edges. An analogous grinding and mixing procedure was applied for the preparation of the second sample. However, the thermal treatment included rapid heating of the mixture to 860 °C followed by a retention time of 4 h at this temperature and finally the crucible was taken out of the furnace to rapidly cool down from 860 °C to room temperature. Similarly to Sample 1, a visual inspection of Sample 2 showed that the obtained material was a green crystalline mass (Figure 1b). Because the employed temperature of 860 °C is considerably lower than that used for melt growth, the mixture is expected to remain largely below the complete melting point. The presence of a CuCl2-derived chloride species may create only a limited viscous flux, insufficient to produce well-developed single crystals. Consequently, the reaction proceeds mainly in the solid state, leading to a polycrystalline product.
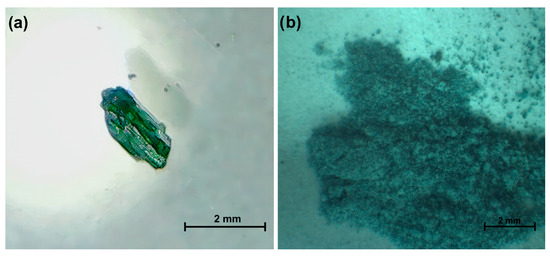
Figure 1.
Visualization of (a) Sample 1 and (b) Sample 2.
2.2. Scanning Electron Microscopy (SEM), Energy-Dispersive X-Ray Spectroscopy Analyses (EDS)
SEM EDS analyses of Samples 1 and 2 were performed on a JSM 6390 electron microscope (Japan) in conjunction with energy-dispersive X-ray spectroscopy (EDS, Oxford INCA Energy 350) equipped with an ultrahigh resolution scanning system (ASID-3D) in regimes of secondary electron images (SEIs) and backscattered electrons (BEC) images. The sample is mounted on a double coated conductive carbon tape that holds the sample firmly to the stage surface and can be used as a ground strap from the sample surface to sample holder. The samples were carbon coated (coated for ~20 s). Carbon at that thickness will have little or no effect on elemental analysis. The accelerating voltage was 20 kV, I ~65 mA. The pressure was of the order of 10−4 Pa.
2.3. Single-Crystal X-Ray Diffraction
The obtained SrB2O4 single crystals from Sample 1 were characterized by single-crystal X-ray diffraction on an Agilent, SuperNovaDual four-circle diffractometer equipped with an Atlas CCD detector using mirror-monochromatized MoKα (λ = 0.7107 Å) radiation from a micro-focus source. The determination of unit cell parameters, data integration, scaling and absorption correction were carried out using the CrysAlisPro program package [31]. The structures were solved by direct methods using ShelxS [32] and refined by full-matrix least-squares procedures on F2 with ShelxL [32].
2.4. Powder X-Ray Diffraction (PXRD)
The PXRD investigations of Samples 1 and 2 were performed on an X-ray powder diffractometer D2 Phaser (Bruker AXS) using CuKα radiation, with a step size 0.04° 2θ and a collection time of 1 s per step.
2.5. Differential Thermal Analysis (DTA) and Thermogravimetric Analysis (TGA)
The TGA and DTA curves for Samples 1 and 2 were obtained from grounded samples of single crystals (sample weight 12 ± 0.2 mg) placed in alumina crucibles and the temperature was ramped from 20 to 1150 °C at a constant heating rate of 10 °C min−1, under an air flow of 40 mL·min−1 on a Stanton Redcroft thermo-analyzer.
2.6. Fourier-Transform Infrared (FT-IR) Spectroscopy
The FT-IR spectra were collected on a Tensor 37 (Bruker, Berlin, Germany) spectrometer in the region of 4000–400 cm−1 (128 scans) using KBr pellets (sample: KBr of 1:50 w/w).
2.7. Fluorescence Analysis
The room temperature fluorescence analyses of Samples 1 and 2 were performed for crushed and grinded single crystals, e.g., powders on a PerkinElmer LS50 fluorescence spectrometer using a powder holder with a quartz window.
3. Results and Discussion
SEM EDS analyses were used in order to qualitatively determine the chemical composition (as boron can be assessed only qualitatively by this method). EDS analyses of single crystals from the edges and the green mass (Sample 1) were conducted separately and showed that the single crystals and green bulk mass have different chemical compositions (Table 1). The analyses of the bulk mass detected the presence of Cu, Sr, B, Cl and O, which are part of the employed starting compounds. In contrast, the analyses of the single crystals from the edges showed that only Sr, B and O elements were present, suggesting that strontium borate has grown from the bulk. Carbon is not listed as the samples were carbon coated.

Table 1.
Qualitative chemical composition of single crystals and bulk mass from Sample 1.
The PXRD analyses of the bulk mass present in Sample 1 (Figure 2) reveals the presence of at least three phases, the predominant one being Sr2B5O9Cl with a Hilgardite-type structure [33] (ICDD 00-027-0835). The additional peaks observed in the diffractogram are not as intensive and disclose the presence of smaller amounts of CuBO2 and Cu3B7ClO13 (ICDD Reference codes: 00-028-1256 and 04-002-6119, respectively). The phase diagrams of the systems containing B2O3-Cu2O [34], B2O3-CuO [35] and SrO-B2O3 [36] have been previously studied. For the SrO-B2O3 system with molar content around 50%, normally SrB2O4 crystallization occurs above 1100 °C, while below that temperature, SrB4O7 (930, 970 or 994 °C [37]) or Sr4B14O25 [38] may be formed. The congruently melting compound CuBO2 was found only as a fine-grained mass [34], and its melting temperature varied from 1005 to 1060 °C [39]. Sr2B5O9Cl was obtained due to the combustion of SrCO3 and CuCl2. Thus, the phase analysis of the greenish mass is consistent with the used starting compounds and employed temperature regime.
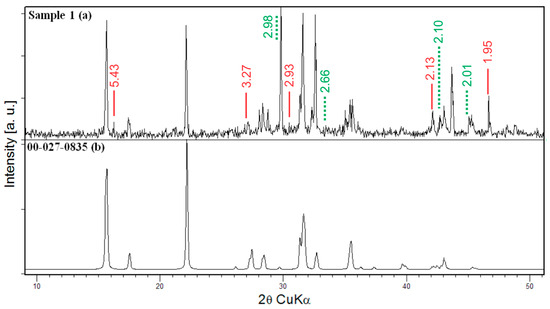
Figure 2.
PXRD pattern and phase analysis of Sample 1 (a) the crystalline bulk obtained after rapid cooling from 860 °C to room temperature and (b) the simulated powder pattern of the main phase: Sr2B5O9Cl with a Hilgardite-type structure; red thick line—CuBO2 (00-028-1256), dashed green line—Cu3B7ClO13 (04-002-6119).
If we use the same starting composition but perform the synthesis at lower temperatures (Sample 2 was heated to 860 °C and kept at that temperature for four hours), the diffraction pattern also reveals the presence of several phases—at least three. However, in that case, the formation of single crystals is not observed. Most of the diffraction present in the diffractogram (Figure 3) belongs to Sr4B14O25 [38] (00-056-0923). The less intensive peaks are consistent with previously observed CuBO2 phase (00-028-1256) and Cu3B7ClO13 (04-002-6119) phase. Both PXRD phase analyses of Samples 1 and 2 suggest that SrCO3 and CuCl2 decompose and that the presence of CuCl2.2H2O lowers the melting point of the SrO-B2O3 system. The single crystal X-ray diffraction experiment for the obtained SrB2O4 crystals of Sample 1 showed that they crystallize in the orthorhombic Pna21 space group (Table 2).
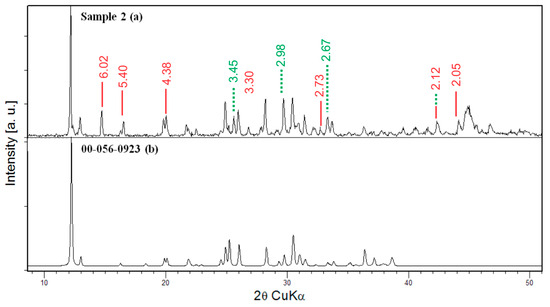
Figure 3.
PXRD pattern and phase analysis of (a) Sample 2 of the crystalline bulk obtained after heating to 860 °C and (b) the simulated powder pattern of the main phase Sr4B14O25; thick line—CuBO2 (00-028-1256), dashed line—Cu3B7ClO13 (04-002-6119).

Table 2.
Most relevant crystallographic data and refinement parameters for SrB2O4.
A search in the ICSD database revealed two structures with the same composition [27,40] but with Pa-3 and Pbcn space groups and a high-pressure calcium borate, CaB2O4 [29], with similar unit cell parameters. “Artificially” removing the Sr2+ from structures shows that the Pbcn [40] structure is built up by only BO3 units, the Pa-3 [27] structure comprises only BO4 units (and is obtained under high pressure), while, according to the single-crystal refinement, the present structure contains both BO3 and BO4 units. Due to the different topology of the building units present in the structures, group and subgroup relation analyses are not appropriate.
Indeed, the Pna21 structure is build up by interconnected BO4 and BO3 units and contains Sr2+ that compensates the negative charge. Boron atoms are located in six positions named B1 to B6 (Table 3). Boron atoms in positions B1, B2 and B3 are tetrahedrally coordinated by oxygen atoms and construct infinite zig-zag chains along c axis and alternate along b and c (Figure 4).

Table 3.
Atom coordinates and atom pairings of SrB2O4 to CaB2O4 * structures.
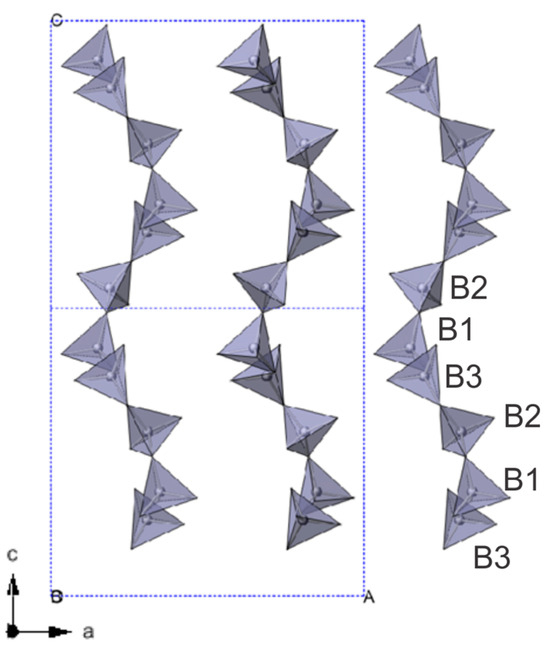
Figure 4.
Representation of the alternating chains (along b) built by BO4 units.
The B atoms in positions B4, B5 and B6 are triangularly coordinated by oxygen atoms to produce BO3 units. The BO4 and BO3 units interact with compose layers perpendicular to a (Figure 5a). Namely, the boron triangularly coordinated B4 shares its oxygens with tetrahedrally coordinated B1, B2 and B3 atoms. This interaction produces a void (ring) created from seven BO4 and two BO3 units (Figure 5b), a feature also observed in the Hilgardite structure. The B5 shares oxygens (O3 and O5) with B1 and B3 units and coordinates Sr1. The third oxygen of B5 unit is O6 and it is involved only in charge compensation, as it participates in the coordination of all three strontium cations. B6 shares O1 with B2 while the remaining two oxygens (O2 and O8) are shared only with cations (Sr1, Sr2 and Sr3). Details about the coordination of Sr2+ cations are shown in Figure 6. Sr3 is located in the center of the ring created by the seven BO4 and two BO3 units. It is coordinated by oxygens O4, O7, O9, O10, O11 and O12 from the “ring” and Sr3 coordination is completed by oxygens O6, O8 and O2 (external to the ring). Strontium Sr1 and Sr2 are located in between the layers (not inside the layers) and are involved in the three-dimensional stabilization of the crystal structure. The performed bond valence analysis [41] shows that the observed arrangement of the building units in the structure is extremely close to the reference values for Sr and B (Table 4).
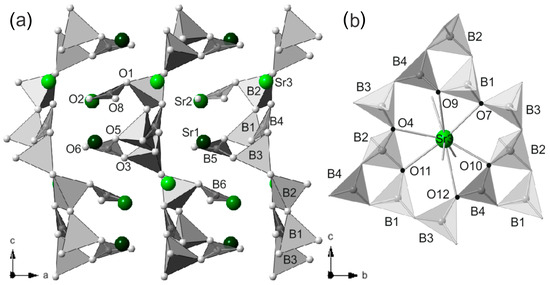
Figure 5.
A view of (a) the of Sr1, Sr2 positioning in between the layers of BO3/BO4 units and Sr3 located inside the BO3/BO4 layers (b) rings (voids) created from seven BO4 and two BO3 units along with Sr3 in the center of the ring.
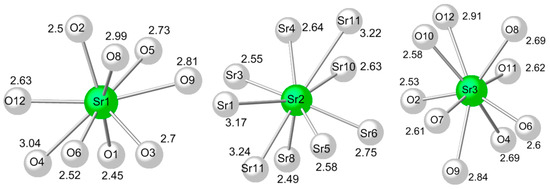
Figure 6.
Representation of Sr2+ coordination; bond distances are given in Å.

Table 4.
Bond valence calculations [41] for SrB2O4 Pna21.
The comparison of SrB2O4 with CaB2O4 structures performed using the Bilbao crystallographic server COMPSTRU [30] does not show huge dissimilarities: the degree of lattice distortion or strain (S) is 0.0271, the maximal displacement between the atomic positions of the paired atoms (dmax) 0.6287 Å (Table 5) and the arithmetic mean (dav) of the distances is 0.3863 Å with a measure of similarity of 0.232(Δ) [42]. The differences in the atom mapping (Table 3) of the two structures, although not drastic, suggested that some specifics may be present. The conducted manual inspection and comparison of the two structures showed that four boron atoms are tetrahedrally coordinated and two are with triangular coordination in CaB2O4 form while here, SrB2O4 forms three with tetrahedral and three with triangular coordination. Indeed, this change in the coordination of triangular to tetrahedral (BO4 < > BO3), (Figure 7) is clearly expressed by the a parameter of the two structures: 11.38 vs. 12.40 Å for the Ca and Sr forms, respectively. This elongation of the a parameter is due to the lack of interaction between B4 and B5 in the SrB2O4 structure (distance B4–B5 of 3.938 Å vs. 2.672 Å in CaB2O4). Actually, this may explain the effect of the applied high pressure on the production of the Pna21 CaB2O4 form. The CaB2O4 (Pna21) structure reported by Marezio et al. was obtained under approximately 4 GPa at 1000 °C [29]; no pressure was applied in our synthesis. The discussion refers only to structural analogy, where the elongation of the a parameter in SrB2O4 reflects the absence of compression present in the high-pressure Ca form.

Table 5.
Differences in coordinates of CaB2O4 and SrB2O4 structures.
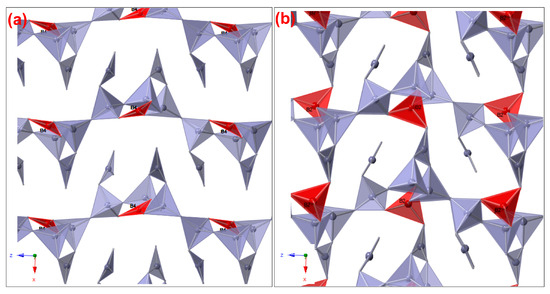
Figure 7.
Depiction of the observed differences related to BO3/BO4 availability in (a) a SrB2O4 polymorph and (b) high pressure CaB2O4; the marked differences (B4 and B2) are shown in red (online version).
The FT-IR spectrum (Figure 8) also endorses the simultaneous existence of BO3 and BO4 groups in the structure. The interaction between BO3 and BO4 groups results in frequency splitting in the vibrational spectra of the SrB2O4 [43]. A tentative interpretation of the spectral lines [43] results in the assignment of the frequency range 1491–1300 cm−1 and bands at 909 and 870 cm−1 to the asymmetric and symmetric stretching vibrations of the BO3 group, respectively. The asymmetric and symmetric stretching vibrations of the BO4 group are presented as bands in the frequency range 1097–977 cm−1 and bands at 812 and 770 cm−1. The bands in the frequency range 770–606 cm−1 respond to the out-of-plane bending vibrations of BO3 group and those below that range are owed to the bending of the BO3 and BO4 groups.
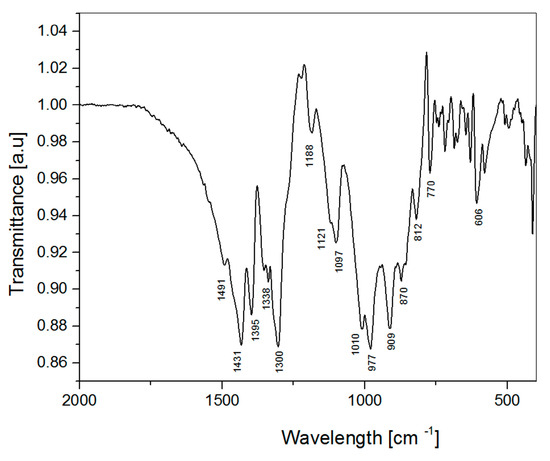
Figure 8.
FTIR spectra of Pna21 SrB2O4 polymorph.
The DTA experiment conducted on the single crystals obtained in Sample 1 shows an exothermic effect at 640 °C and probably a combination of two endothermic effects at 944 and 987 °C (Figure 9). As the TGA curve does not display any mass variations, presumably the exothermic effect is related to a phase transition while the endothermic effect(s) are linked with the melting of the sample.
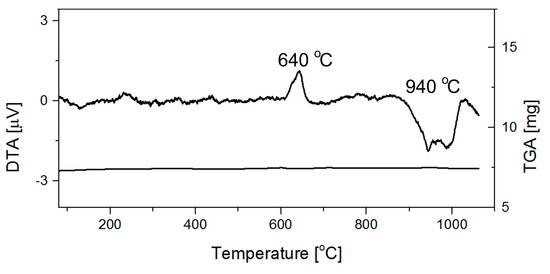
Figure 9.
DTA/TGA analyses of Pna21 SrB2O4 crystals.
The room temperature fluorescence spectrum of SrB2O4 is illustrated in Figure 10 and shows a broad emission band with a maximum around 440 nm.
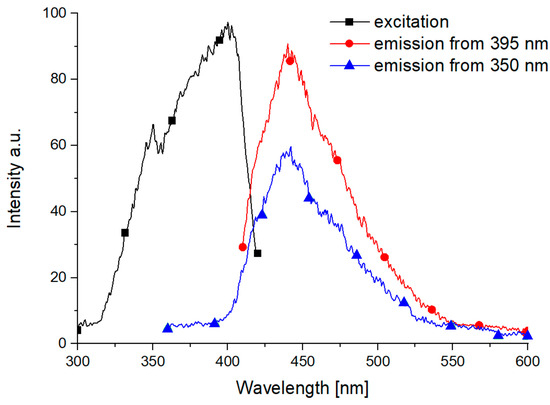
Figure 10.
Fluorescence (excitation and emission) spectra of SrB2O4. The excitation spectra are shown as black squares ( ), the emissions using 395 nm excitation are shown as a red circle (
), the emissions using 395 nm excitation are shown as a red circle ( ) and the emissions using 350 nm excitation are shown as blue triangles (
) and the emissions using 350 nm excitation are shown as blue triangles ( ).
).
 ), the emissions using 395 nm excitation are shown as a red circle (
), the emissions using 395 nm excitation are shown as a red circle ( ) and the emissions using 350 nm excitation are shown as blue triangles (
) and the emissions using 350 nm excitation are shown as blue triangles ( ).
).
In this study, a single broad emission band at 438 nm was observed in undoped SrB2O4 under 350 and 395 nm excitation, attributed to intrinsic lattice or defect-related transitions. In contrast, the previously reported SrB2O4:Eu2+ system [44] exhibited two distinct emission bands at 374 nm and 458 nm, originating from Eu2+ ions occupying substitutional and interstitial sites, respectively. Although both systems emit in the near-UV to blue region, the luminescence in this study is matrix-dependent rather than activator-induced.
4. Conclusions
The SrB2O4 crystals have been successfully obtained from melts containing Sr2CO3, H3BO3 and CuCl2·2H2O in a 2:2:1 ratio. The crystallization process involves the consumption of Cl atoms through the formation of a Hilgardite (Sr2B5O9Cl) phase, the Cu atoms produce CuBO2 and several other crystal phases with poor crystallinity formed by the reaction between SrO and B2O3 units. The same starting composition heated only to 860 °C and kept for 4 h at that temperature, revealed the formation of Sr4B14O25, Cu3B7ClO13 and CuBO2 phases. FT-IR results show that there are trigonal BO3 and tetrahedral BO4 groups in the as-prepared new SrB2O4 polymorph. Single crystal refinement of SrB2O4 revealed that the structure contains three tetrahedrally coordinated B atoms and three triangularly coordinates ones. The combination of borate building units is complemented by three Sr2+ acting as a charge compensating agent. Although the unit cell parameters are close to the CaB2O4 form, SrB2O4 has three BO4 and three BO3 units vs. four and two in CaB2O4. Owing to its non-centrosymmetric orthorhombic Pna21 symmetry, the SrB2O4 polymorph is expected to exhibit nonlinear optical behavior such as second harmonic generation, which will be examined in future work.
Further details of the crystal structure investigation may be obtained from FIZ Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49)7247-808-666; e-mail: crysdata@fiz-karlsruhe.de, on quoting the deposition number CSD-431439.
Author Contributions
Conceptualization, B.S. and R.N.; methodology, B.S.; formal analysis, B.S. and R.N.; investigation, M.A.; writing—original draft preparation, B.S., M.A., R.N. and R.R.; writing—review and editing, B.S. and R.N.; visualization, B.S., R.N. and M.A.; supervision, B.S.; project administration, B.S.; funding acquisition, R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Fund under “Research Innovation and Digitization for Smart Transformation” program 2021–2027 under the Project BG16RFPR002-1.014-0006 “National Centre of Excellence Mechatronics and Clean Technologies”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Research equipment of the project № BG16RFPR002-1.014-0006 “National Centre of Excellence Mechatronics and Clean Technologies” was used for experimental work, financially supported by the European Regional Development Fund under “Research Innovation and Digitization for Smart Transformation” program 2021–2027.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DTA | Differential Thermal Analysis |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| ICDD | International Centre for Diffraction Data |
| ICSD | Inorganic Crystal Structure Database |
| NLO | Nonlinear optical |
| PXRD | Powder X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| SHG | Second harmonic generation |
| TGA | Thermogravimetric Analysis |
References
- Mutailipu, M.; Poeppelmeier, K.R.; Pan, S. Borates: A rich source for optical materials. Chem. Rev. 2020, 121, 1130–1202. [Google Scholar] [CrossRef]
- Cheng, M.; Hou, X.; Yang, Z.; Pan, S. Recent Progress in Borate-Based Short-Wavelength Nonlinear Optical Crystals with Boron–Oxygen Skeleton Modification. Mater. Chem. Front. 2023, 7, 4683–4692. [Google Scholar] [CrossRef]
- Kettlewell, B.; Boyd, D. Inside the Borate Anomaly: Leveraging a Predictive Modelling Approach to Navigate Complex Composition–Structure–Property Relationships in Oxyhalide Borate Glasses. Materials 2024, 17, 2073. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Hu, Z.; Wang, J.; Wu, Y.; Yu, H. Rational Design of a Deep-Ultraviolet Nonlinear Optical Crystal. J. Am. Chem. Soc. 2025, 147, 33023–33030. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y. Second Generation Tris(2-Pyridyl)Borate Ligands: Toward Supramolecular Polymeric Materials. Master’s Thesis, Rutgers, The State University of New Jersey, Newark, NJ, USA, October 2014. [Google Scholar]
- Cao, J.; Wang, Y.F.; Song, X.G.; Feng, J.C. One-Dimensional Nickel Borate Nanowhiskers: Characterization, Properties, and a Novel Application in Materials Bonding. RSC Adv. 2014, 4, 19221–19225. [Google Scholar] [CrossRef]
- Sanglay, G.D.D.; Garcia, J.S.; Palaganas, M.S.; Sorolla, M.; See, S.; Limjuco, L.A.; Ocon, J.D. Borate-Based Compounds as Mixed Polyanion Cathode Materials for Advanced Batteries. Molecules 2022, 27, 8047. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Chu, Y.; Tudi, A.; Mutailipu, M.; Zhang, F.; Yang, Z.; Pan, S. (NH4)3B11PO19F3: A Deep-UV Nonlinear Optical Crystal with Unique [B5PO10F]∞ Layers. Nat. Sci. Rev. 2022, 9, nwac110. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Sun, M.; Liu, W.; Meng, X.; Yao, J. Synthesis and Characterization of a New Rare-Earth Borate Nonlinear Optical Crystal K7PbLu2B15O30. Dalton Trans. 2023, 52, 10109–10114. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Chen, X.; Chen, S.; Li, Y.; Shang, X.; Bai, Z.; Jiang, N.; Yan, Z.; Luo, J.; Zhao, S. A Nonlinear Optical Crystal with Deep-Ultraviolet Transparency and Appropriate Birefringence Achieved Using π-Conjugated Confined [B3O3F4(OH)]2−. New J. Chem. 2024, 48, 15281–15286. [Google Scholar] [CrossRef]
- Princy, A.; Kennedy, S.M.M.; Sayyed, M.I.; Hanafy, T.A.; Kamath, S.D. Structural, Optical, and Thermal Traits of Sm3+-Doped SrB2O4 Phosphors for Solid-State Lighting Applications. Solid State Sci. 2024, 157, 107724. [Google Scholar]
- Kumar, R.A.; Arivanandhan, M.; Hayakawa, Y. Rare Earth-Based Borate Single Crystals: Potential Materials for Nonlinear Optical and Laser Applications. Prog. Cryst. Growth Charact. Mater. 2013, 59, 113–132. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, X.; Xiao, W.; Song, X. Two New Rare-Earth Oxyborates Ba4BiTbO(BO3)4 and Ba1.54Sr2.46BiTbO(BO3)4 and Luminescence Properties of the Ba4BiTb1−xEuxO(BO3)4 Phosphors. RSC Adv. 2024, 14, 6270–6284. [Google Scholar] [CrossRef]
- Kang, L.; Lin, Z. Deep-Ultraviolet Nonlinear Optical Crystals: Concept Development and Materials Discovery. Light Sci. Appl. 2022, 11, 201. [Google Scholar] [CrossRef]
- Liu, M.; Kong, X.; Li, S.; Ye, N.; Hu, Z.; Wu, Y.; Li, C. Designing Rare-Earth Borates as UV Nonlinear Optical Crystals Exhibiting Strong Second-Harmonic Generation Responses. Chem. Commun. 2025, 61, 3155–3158. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, W.J.; Wang, H.; Lin, H.; Wu, L.M. Mixed-Anion Inorganic Compounds: A Favorable Candidate for Infrared Nonlinear Optical Materials. Cryst. Growth Des. 2019, 19, 4172–4192. [Google Scholar] [CrossRef]
- Vasiliev, A.D.; Cherepakhin, A.V.; Zaitsev, A.I. The Trigonal Polymorph of Strontium Tetraborate, β-SrB4O7. Acta Crystallogr. Sect. E 2010, 66, i48. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Chen, X.; Li, M. Synthesis and Crystal Structure of a New Strontium Borate, Sr2B16O26. Solid State Sci. 2008, 10, 894–900. [Google Scholar] [CrossRef]
- Wei, Z.F.; Chen, X.L.; Wang, F.M.; Li, W.C.; He, M.; Zhang, Y. Phase Relations in the Ternary System SrO–TiO2–B2O3. J. Alloys Compd. 2001, 327, L10–L13. [Google Scholar] [CrossRef]
- O’Connell, K.; Hanson, M.; O’Shea, H.; Boyd, D. Linear Release of Strontium Ions from High Borate Glasses via Lanthanide/Alkali Substitutions. J. Non-Cryst. Solids 2015, 430, 1–8. [Google Scholar] [CrossRef]
- Rajesh, D.; Naidu, M.D.; Ratnakaram, Y.C.; Balakrishna, A. Ho3+-Doped Strontium–Aluminium–Bismuth–Borate Glasses for Green Light Emission. Luminescence 2014, 29, 854–860. [Google Scholar] [CrossRef]
- Zhang, X.M.; Lian, Q.; Pan, Q.; Yuan, G.M.; Seo, H.J. Unusual Ce3+ Luminescence and Ce3+ → Tb3+ Energy Transfer Behavior in Strontium Lithium Borate SrLiB9O15. J. Alloys Compd. 2014, 607, 44–47. [Google Scholar] [CrossRef]
- Zhang, J.; Han, B.; Zhang, Y.; Lv, Q. Luminescence Properties of Ce3+ in Strontium Borate SrB2O4. J. Mater. Sci. Mater. Electron. 2016, 27, 3906–3910. [Google Scholar] [CrossRef]
- Krushna, B.R.; Pruthviraj, I.S.; Sharma, S.C.; Premkumar, H.B.; Manjunatha, K.; Wu, S.Y.; Ganesan, L.; George, A.; Nagabhushana, H. Synergistic Ce3+, Tb3+ Activated LaCaAl3O7 Phosphor: Bridging Forensic Science with Advanced White LED Applications. J. Lumin. 2025, 280, 121080. [Google Scholar]
- Aleksandrovsky, A.S.; Krylov, A.S.; Malakhovskii, A.V.; Potseluyko, A.M.; Zaitsev, A.I.; Zamkov, A.V. Europium Doped Strontium Borate Glasses and Their Optical Properties. J. Phys. Chem. Solids 2005, 66, 75–79. [Google Scholar] [CrossRef]
- Demyanyshyn, N.M.; Mytsyk, B.G.; Sakharuk, O.M. Elasto-Optic Effect Anisotropy in Strontium Borate Crystals. Appl. Opt. 2014, 53, 1620–1628. [Google Scholar] [CrossRef]
- Ross, N.L.; Angel, R.J. Crystal Structure of High Pressure SrB2O4 (IV). J. Solid State Chem. 1991, 90, 27–30. [Google Scholar] [CrossRef]
- Dernier, P. Crystal Data of Two High Pressure Phases of SrB2O4. Acta Crystallogr. Sect. B 1969, 25, 1001–1003. [Google Scholar] [CrossRef]
- Marezio, M.; Remeika, J.P.; Dernier, P.D. The Crystal Structure of the High-Pressure Phase CaB2O4 (IV), and Polymorphism in CaB2O4. Acta Crystallogr. Sect. B 1969, 25, 965–970. [Google Scholar] [CrossRef]
- de la Flor, G.; Orobengoa, D.; Tasci, E.; Perez-Mato, J.M.; Aroyo, M.I. Comparison of Structures Applying the Tools Available at the Bilbao Crystallographic Server. J. Appl. Crystallogr. 2016, 49, 653–664. [Google Scholar] [CrossRef]
- Diffraction, Rigaku Oxford. Single crystal diffraction software: CrysAlisPro. Rigaku J. 2016, 32, 31–34. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Fouassier, C.; Levasseur, A.; Hagenmuller, P. Les Halogénoborates M2B5O9X (M = Ca, Sr, Ba, Eu, Pb; X = Cl, Br). J. Solid State Chem. 1971, 3, 206–208. [Google Scholar] [CrossRef]
- Rza-Zade, P.F.; Abdullaev, G.K.; Eyubova, N.A.; Samedov, F.R. Physicochemical Study of the Li2O–CuO–B2O3 System. Izv. Akad. Nauk SSSR Neorg. Mater. 1971, 7, 2098–2100. [Google Scholar]
- Abdullaev, G.K.; Rza-Zade, P.F.; Mamedov, K.S. Physicochemical Study of the Triple Li2O–CuO–B2O3 System. Zhurnal Neorg. Khim. 1982, 27, 1837–1841. [Google Scholar]
- Guertler, W. Über Die Schmelzpunkte Der Mischungen Der Alkalischen Erden Mit Borsäureanhydrid. Z. Anorg. Chem. 1904, 40, 337–354. [Google Scholar] [CrossRef]
- Oseledchik, Y.S.; Prosvirnin, A.L.; Starshenko, V.V.; Osadchuk, V.V.; Pisarevsky, A.I.; Belokrys, S.P.; Korol, A.S.; Svitanko, N.V.; Selevich, A.F.; Krikunov, S.A. Crystal Growth and Properties of Strontium Tetraborate. J. Cryst. Growth 1994, 135, 373–376. [Google Scholar] [CrossRef]
- Kudrjavtcev, D.P.; Oseledchik, Y.S.; Prosvirnin, A.L.; Svitanko, N.V. Growth of a New Strontium Borate Crystal Sr4B14O25. J. Cryst. Growth 2003, 254, 456–460. [Google Scholar] [CrossRef]
- Kaundal, R.S.; Kaur, S.; Singh, N.; Singh, K.J. Investigation of Structural Properties of Lead Strontium Borate Glasses for Gamma-Ray Shielding Applications. J. Phys. Chem. Solids 2010, 71, 1191–1195. [Google Scholar] [CrossRef]
- Kim, J.-B.; Lee, K.-S.; Suh, I.-H.; Lee, J.-H.; Park, J.-R.; Shin, Y.-H. Strontium Metaborate, SrB2O4. Acta Crystallogr. Sect. C 1996, 52, 498–500. [Google Scholar] [CrossRef]
- Brown, I. Valence: A Program for Calculating Bond Valences. J. Appl. Crystallogr. 1996, 29, 479–480. [Google Scholar] [CrossRef]
- Bergerhoff, G.; Berndt, M.; Brandenburg, K.; Degen, T. Concerning Inorganic Crystal Structure Types. Acta Crystallogr. Sect. B 1999, 55, 147–156. [Google Scholar] [CrossRef]
- Zhu, D.; Yun, S.; Nai, X.; Zhao, D.; Liu, X.; Li, W. Synthesis and Characterization of Strontium Chloroborate Whiskers. Cryst. Res. Technol. 2013, 48, 6–10. [Google Scholar] [CrossRef]
- Jin, Y.; Ran, R.; Lin, H.; Li, Y.; Chen, H.; Fang, F.; Lin, H.; Ren, H. The Luminescence Properties of the Interstitial Eu2+ in SrB2O4 for UV Light-Emitting Diode. Phys. Status Solidi A 2023, 220, 2200823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).