Synthesis, Structure, and Investigation of Terbium(III) Luminescent Metal-Organic Framework Based on (N-Morpholyl)-Functionalized 1,10-Phenanthroline
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
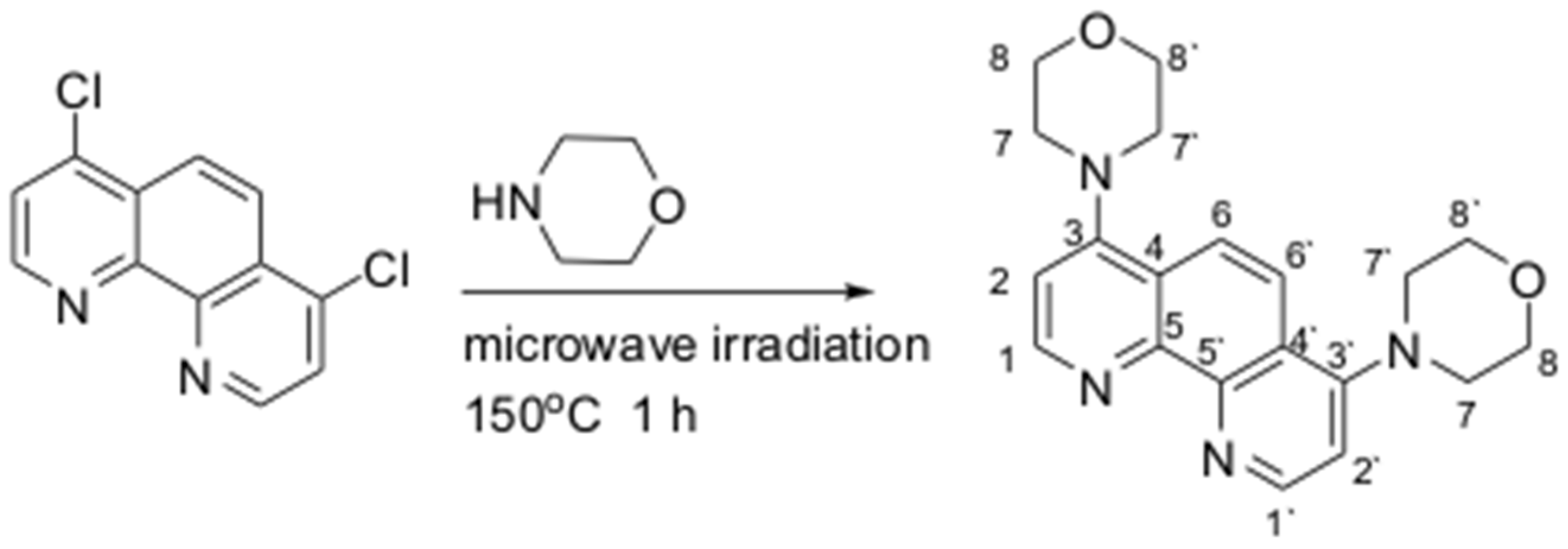
2.3. Synthetic Methods
2.4. Single-Crystal X-Ray Diffraction Details
3. Results and Discussion
3.1. Synthesis and Similar Compounds
3.2. Crystal Structure Description
3.3. Characterization and Stability
3.4. Luminescent Properties
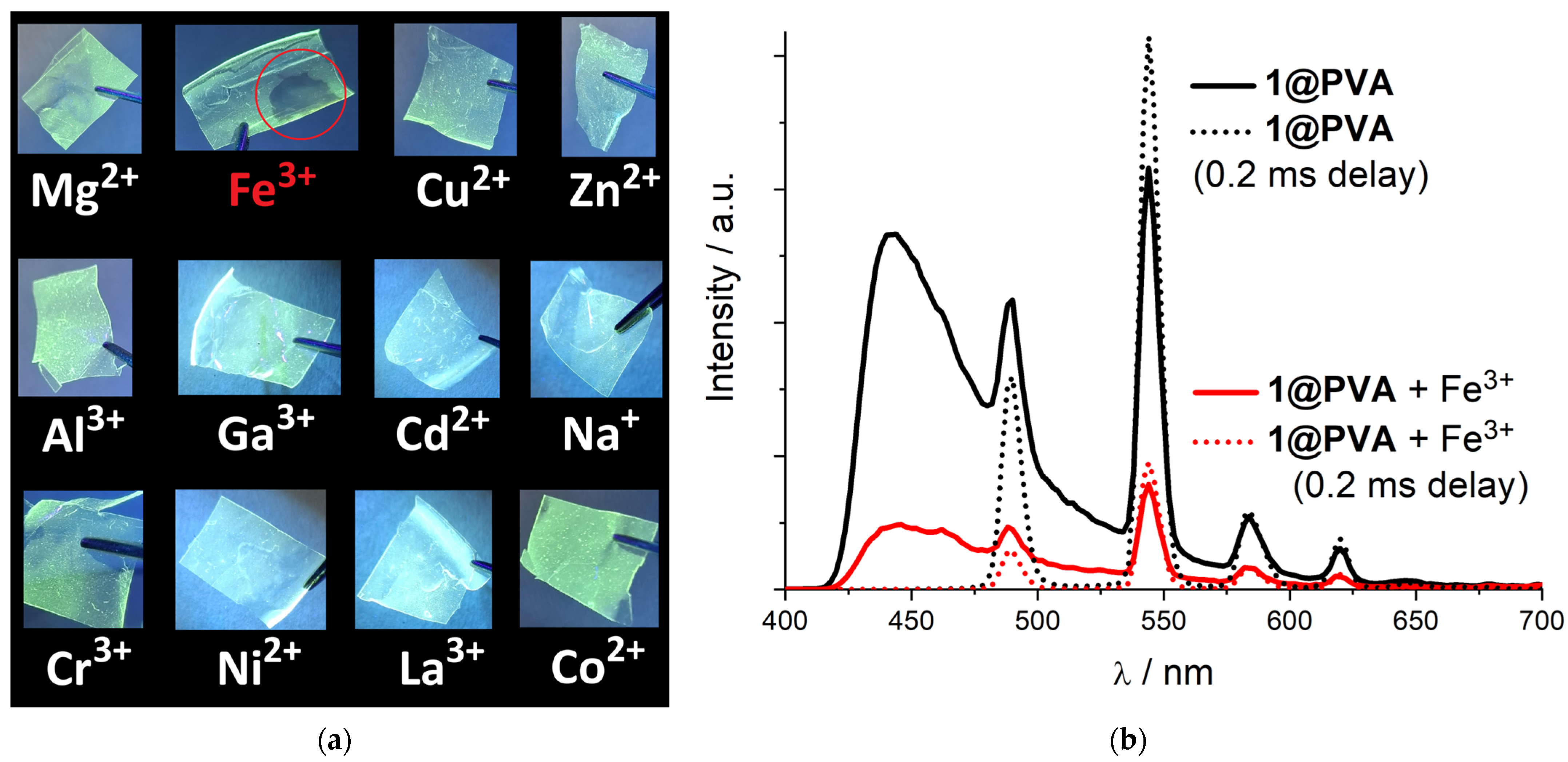
3.5. Film Preparation and Ion Sensing Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Crystal Data
| Parameter | 1 |
|---|---|
| Chemical formula | C56H64Br2N8O12Tb2 |
| Mr, g/mol | 1518.81 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a, Å | 13.546(3) |
| b, Å | 16.975(5) |
| c, Å | 13.121(3) |
| Temperature, K | 150 |
| α, ° | 90 |
| β, ° | 112.510(6) |
| γ, ° | 90 |
| V, Å3 | 2787.2(12) |
| Z | 2 |
| D(calc.), g·cm−3 | 1.810 |
| μ, mm−1 | 4.02 |
| F(000) | 1504 |
| Crystal size, mm | 0.06 × 0.02 × 0.02 |
| θ range for data collection, ° | 2.02 ≤ θ ≤ 25.24 |
| Index ranges | −15 ≤ h ≤ 14; −20 ≤ k ≤ 20; −16 ≤ l ≤ 16 |
| No. of reflections: measured/independent/ observed[I > 2σ(I)] | 4797/4797/3058 |
| Rint | 0.123 |
| Goodness-of-fit on F2 | 0.944 |
| Final R indices [I > 2σ(I)] | R1 = 0.0673; wR2 = 0.1503 |
| Final R indices [all data] | R1 = 0.1016; wR2 = 0.1663 |
| Largest diff. peak, hole, e/Å3 | 2.17, −1.32 |
| Polyhedron | 1 |
|---|---|
| Heptagonal pyramid (HPY-8) | 21.622 |
| Hexagonal bipyramid (HBPY-8) | 16.485 |
| Square antiprism (SAPR-8) | 2.898 |
| Triangulardodecahedron (TDD-8) | 2.148 |
| Johnson gyrobifastigium J26 (JGBF-8) | 14.773 |
| Johnson elongated triangular bipyramid J14 (JETBPY-8) | 26.205 |
| Biaugmented trigonal prism J50 (JBTPR-8) | 3.254 |
| Biaugmented trigonal prism (BTPR-8) | 2.386 |
| Snub disphenoid J84 (JSD-8) | 4.481 |
| Triakistetrahedron (TT-8) | 12.822 |
| Elongated trigonal bipyramid (ETBPY-8) | 24.261 |
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–organic frameworks for separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Ranocchiari, M.; Van Bokhoven, J.A. Catalysis by metal–organic frameworks: Fundamentals and opportunities. Phys. Chem. Chem. Phys. 2011, 13, 6388–6396. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 301–381. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.; Pal, H. Lanthanide-based metal–organic frameworks (Ln-MOFs): Synthesis, properties and applications. RSC Sustain. 2025, 3, 629–660. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Tissot, A.; Serre, C. Luminescent sensing platforms based on lanthanide metal-organic frameworks: Current strategies and perspectives. Coord. Chem. Rev. 2023, 497, 215454. [Google Scholar] [CrossRef]
- Saraci, F.; Quezada-Novoa, V.; Donnarumma, P.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, P.; Li, Z.; Sun, Y.; Liu, Y.; Huang, S. Advancements in rare earth metal-organic frameworks: Harnessing the power of photonics and beyond. Coord. Chem. Rev. 2024, 514, 215905. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Utochnikova, V.V. The use of luminescent spectroscopy to obtain information about the composition and the structure of lanthanide coordination compounds. Coord. Chem. Rev. 2019, 398, 113006. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Kuzmina, N.P. Photoluminescence of lanthanide aromatic carboxylates. Russ. J. Coord. Chem. 2016, 42, 679–694. [Google Scholar] [CrossRef]
- Rothfuss, H.; Knöfel, N.D.; Tzvetkova, P.; Michenfelder, N.C.; Baraban, S.; Unterreiner, A.N.; Roesky, P.W.; Barner-Kowollik, C. Phenanthroline—A Versatile Ligand for Advanced Functional Polymeric Materials. Chem. Eur. J. 2018, 24, 17475–17486. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1, 10-Phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Takeda, H.; Irimajiri, M.; Mizutani, T.; Nozawa, S.; Matsuura, Y.; Kurosu, M.; Ishitani, O. Photocatalytic CO2 Reduction Using Mixed Catalytic Systems Comprising an Iron Cation with Bulky Phenanthroline Ligands. Inorg. Chem. 2024, 63, 7343–7355. [Google Scholar] [CrossRef]
- Hendrich, J.M.; White, F.D.; Sykora, R.E. Lanthanide dicyanoaurate coordination polymers containing 1, 10-phenanthroline: Synthesis, structure, and luminescence. Inorg. Chim. Acta 2021, 527, 120562. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Fan, C.; Pu, S. A novel fluorescent sensor for Al3+ and Zn2+ based on a new europium complex with a 1, 10-phenanthroline ligand. J. Rare Earths 2021, 39, 460–468. [Google Scholar] [CrossRef]
- Zahariev, T.; Shandurkov, D.; Gutzov, S.; Trendafilova, N.; Enseling, D.; Jüstel, T.; Georgieva, I. Phenanthroline chromophore as efficient antenna for Tb3+ green luminescence: A theoretical study. Dyes Pigm. 2021, 185, 108890. [Google Scholar] [CrossRef]
- Bolot’ko, A.E.; Shmelev, M.A.; Chistyakov, A.S.; Voronina, J.K.; Varaksina, E.A.; Gogoleva, N.V.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L. Luminescenceenhancementbymixingcarboxylatebenzoate–pentafluorobenzoateligandsinpolynuclear {Eu2Zn2} and {Tb2Zn2} complexes. Dalton Trans. 2025, 54, 5708–5720. [Google Scholar] [CrossRef]
- Kot, K.; Oczko, G.; Puchalska, M.; Starynowicz, P. Structural and spectroscopic studies of heavy lanthanide complexes with o-phenanthroline and isothiocyanate. Polyhedron 2019, 173, 114119. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, Z.-M.; Wang, X.-B.; Guan, W.-S.; Liu, L.-H.; Liu, B.; Wang, J.-K.; Che, G.-B.; Liu, C.-B.; Lin, X. Thermal behaviors and adsorption properties of two Europium (III) complexes based on 2-(4-carboxyphenyl) imidazo [4, 5-f]-1, 10-phenanthroline. Inorg. Chim. Acta 2018, 471, 397–403. [Google Scholar] [CrossRef]
- D’Vries, R.F.; Gomez, G.E.; Hodak, J.H.; Soler-Illia, G.J.A.A.; Ellena, J. Tuning the structure, dimensionality and luminescent properties of lanthanide metal–organic frameworks under ancillary ligand influence. Dalton Trans. 2016, 45, 646–656. [Google Scholar] [CrossRef]
- Wang, S.-J.; Li, Q.; Xiu, G.-L.; You, L.-X.; Fu Ding, F.; Van Deun, R.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. New Ln-MOFs based on mixed organic ligands: Synthesis, structure and efficient luminescence sensing of the Hg2+ ions in aqueous solutions. Dalton Trans. 2021, 50, 15612–15619. [Google Scholar] [CrossRef]
- Luo, A.-Y.; Lan, B.-L.; Shao, B.; Lu, X.-M.; Lan, Y.-F.; Liao, Y.-Z.; Zhang, Z. A 2D mixed-lanthanide metal–organic framework as dual-emitting luminescent sensor for ratiometric detection of tetracycline and nitrophenols. J. Mol. Struct. 2024, 1295, 136734. [Google Scholar] [CrossRef]
- Bryleva, Y.A.; Mikheylis, A.V.; Agafontsev, A.M.; Glinskaya, L.A.; Tkachev, A.V. Antenna effect of 1,10-phenanthroline derivative bearing (−)-borneol moieties in luminescent lanthanide(III) complexes. J. Lumin. 2025, 281, 121144. [Google Scholar] [CrossRef]
- Pettinari, C.; Drozdov, A.; Belousov, Y. Coordination Compounds of Lanthanides as Materials for Luminescent Turn Off Sensors. In Rare Earth Elements—Emerging Advances, Technology Utilization, and Resource Procurement; IntechOpen: London, UK, 2022; Chapter 1; pp. 1–31. [Google Scholar] [CrossRef]
- Zarubin, D.N.; Bushkov, N.S.; Lavrov, H.V.; Dolgushin, F.M.; Ustynyuk, N.A.; Ustynyuk, Y.A. 4,7-Di-n-butoxy-1,10-phenanthroline-2,9-dicarboxamide: A Tetradentate Ligand Featuring Excellent Solubility in Nonpolar Media. INEOS Open 2019, 2, 130–133. [Google Scholar] [CrossRef]
- Borisova, N.E.; Kostin, A.A.; Reshetova, M.D.; Lyssenko, K.A.; Belova, E.V.; Myasoedov, B.F. The structurally rigid tetradentate N,N′,O,O′-ligands based on phenanthroline for binding of f-elements: The substituents vs. structures of the complexes. Inorg. Chim. Acta 2018, 478, 148–154. [Google Scholar] [CrossRef]
- Evsiunina, M.V.; Khult, E.K.; Matveev, P.I.; Kalle, P.; Lemport, P.S.; Petrov, V.S.; Aksenova, S.A.; Nelyubina, Y.V.; Koshelev, D.S.; Utochnikova, V.V.; et al. Unravelling the mechanism of f-element extraction by phenanthroline-diamides: A case of 4,7-substituted 1,10-phenanthroline-2,9-diamides. Sep. Purif. Technol. 2024, 339, 126621. [Google Scholar] [CrossRef]
- Zucchi, G.; Murugesan, V.; Tondelier, D.; Aldakov, D.; Jeon, T.; Yang, F.; Thuéry, P.; Ephritikhine, M.; Geffroy, B. Solution, Solid State, and Film Properties of a Structurally Characterized Highly Luminescent Molecular Europium Plastic Material Excitable with Visible Light. Inorg. Chem. 2011, 50, 4851–4856. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Cao, H.-T.; Zhu, D.-X.; Su, Z.-M.; Liao, Y. Synthesis, structure and photophysical properties of cationic Ir(III) complexes with functionalized 1,10-phenanthroline ancillary ligands. J. Organomet. Chem. 2012, 713, 20–26. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, X.; Hou, C.; Wei, S.; Lei, W.; Chen, X.-L. TADF-emitting copper(I) and silver(I) complexes featuring intra-ligand charge transfer based on a donor–acceptor–donor ligand. New J. Chem. 2025, 49, 755–760. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Zhan, L.; Zhong, C.; Gong, S.; Lu, Z.-H.; Yang, C. Tailoring Optoelectronic Properties of Phenanthroline-Based Thermally Activated Delayed Fluorescence Emitters through Isomer Engineering. Adv. Opt. Mater. 2016, 4, 1558–1566. [Google Scholar] [CrossRef]
- Palion-Gazda, J.; Choroba, K.; Penkala, M.; Rawicka, P.; Machura, B. Further Insights into the Impact of Ligand-Localized Excited States on the Photophysics of Phenanthroline-Based Rhenium(I) Tricarbonyl Complexes. Inorg. Chem. 2024, 63, 1356–1366. [Google Scholar] [CrossRef]
- Palion-Gazda, J.; Machura, B.; Szłapa-Kula, A.; Maroń, A.M.; Nycz, J.E.; Ledwon, P.; Schab-Balcerzak, E.; Siwy, M.; Grzelak, J.; Maćkowski, S. Effect of carbazole and pyrrolidine functionalization of phenanthroline ligand on ground- and excited-state properties of rhenium(I) complexes. Interplay between 3MLCT and 3IL/3ILCT. Dyes Pigm. 2022, 200, 110113. [Google Scholar] [CrossRef]
- Maroń, A.M.; Szlapa-Kula, A.; Matussek, M.; Kruszynski, R.; Siwy, M.; Janeczek, H.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E.; Machura, B. Photoluminescence enhancement of Re(I) carbonyl complexes bearing D–A and D–π–A ligands. Dalton Trans. 2020, 49, 4441–4453. [Google Scholar] [CrossRef]
- Sen, B.; Patra, S.K.; Khatua, S. Ruthenium(II) Polypyridine Complex-Based Aggregation-Induced Emission Luminogen for Rapid and Selective Detection of Phosgene in Solution and in the Gas Phase. Inorg. Chem. 2021, 60, 19175–19188. [Google Scholar] [CrossRef]
- Patra, S.K.; Rabha, M.; Sen, B.; Aguan, K.; Khatua, S. An aggregation induced emission active bis-heterolepticruthenium(II) complex for luminescence light-up detection of pyrophosphate ions. Dalton Trans. 2023, 52, 2592–2602. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Dorovatovskii, P.V.; Lazarenko, V.A.; Samsonenko, D.G.; Brylev, K.A.; Fedin, V.P.; Dybtsev, D.N. Intense multi-colored luminescence in a series of rare-earth metal–organic frameworks with aliphatic linkers. Dalton Trans. 2021, 50, 11899–11908. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Vasileva, A.A.; Lazarenko, V.A.; Ryadun, A.A.; Fedin, V.P. Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks. Crystals 2021, 11, 1375. [Google Scholar] [CrossRef]
- Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. [Google Scholar] [CrossRef]
- Vasileva, A.A.; Demakov, P.A.; Guselnikova, T.Y.; Ryadun, A.A.; Fedin, V.P.; Dybtsev, D.N. Solvatomorphic phase transitions and tunable luminescence emission in lanthanide metal–organic frameworks. Dalton Trans. 2025, 54, 641–648. [Google Scholar] [CrossRef]
- Ovchinnikova, A.A.; Demakov, P.A.; Ryadun, A.A.; Fedin, V.P.; Dybtsev, D.N. Structures and Luminescent Sensing Properties of Terbium Metal–Organic Frameworks with Methyl-Decorated Phenanthroline Ligand. Crystals 2024, 14, 1026. [Google Scholar] [CrossRef]
- Graf, G.I.; Hastreiter, D.; da Silva, L.E.; Rebelo, R.A.; Montalban, A.G.; McKillop, A. The synthesis of aromatic diazatricycles from phenylenediamine-bis(methylene Meldrum’s acid) derivatives. Tetrahedron 2002, 58, 9095–9100. [Google Scholar] [CrossRef]
- Bruker AXS, Inc. APEX3, Version 3.0. Bruker Advanced X-ray Solutions. Bruker AXS Inc.: Madison, WI, USA, 2012.
- SAINT, Version 8.40a. Bruker Advanced X-ray Solutions. Bruker AXS Inc.: Madison, WI, USA, 2012.
- SADABS, Version 2016-2. Bruker Advanced X-ray Solutions. Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Cryst 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013.
- Schmittel, M.; Ammon, H.; Wöhrle, C. Tris(1,10-phenanthroline)iron(II) Complexes—Broad Variation of the Redox Potential by 4,7-Substitution at the Phenanthroline Ligands. Chem. Ber. 1995, 128, 845–850. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiza, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Tiwari, G.; Khanna, A.; Mishraa, V.K.; Sagar, R. Recent developments on microwave-assisted organic synthesis of nitrogen- and oxygen-containing preferred heterocyclic scaffolds. RSC Adv. 2023, 13, 32858–32892. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-J.; Wang, Y.-L.; Cao, H.-Y.; Liu, Q.-Y.; Chen, L.-L. Ionothermal Syntheses, Crystal Structures and Luminescence of Two Lanthanide-Carboxylate Frameworks based on the 1, 4-Naphthalenedicarboxylate and Oxalate Mixed Ligands. Z. Anorg. Allg. Chem. 2014, 640, 2472–2476. [Google Scholar] [CrossRef]
- Wang, R.; Selby, H.D.; Liu, H.; Carducci, M.D.; Jin, T.; Zheng, Z.; Anthis, J.W.; Staples, R.J. Halide-Templated Assembly of Polynuclear Lanthanide-Hydroxo Complexes. Inorg. Chem. 2002, 41, 278–286. [Google Scholar] [CrossRef]
- Zhong, S.; Yin, Q.; Diao, Y.; Yang, F.; He, X.; Liu, S.; Wang, Y. Optimization of synthesis conditions, characterization and magnetic properties of lanthanide metal organic frameworks from Brønsted acidic ionic liquid. J. Mol. Struct. 2023, 1278, 134974. [Google Scholar] [CrossRef]
- Chen, W.-X.; Ren, Y.-P.; Long, L.-S.; Huang, R.-B.; Zheng, L.-S. Ionothermal synthesis of 3d–4f and 4f layered anionic metal–organic frameworks. CrystEngComm 2009, 11, 1522–1525. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Wu, Y.; Chen, W.; Rotsch, D.A.; Messerle, L.; Zheng, Z. A systematic study of halide-template effects in the assembly of lanthanide hydroxide cluster complexes with histidine. Inorg. Chem. Front. 2021, 8, 26–34. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Ibrayev, N.; Seliverstova, E.; Valiev, R.; Aymagambetova, A.; Sundholm, D. The effect of heavy atoms on the deactivation of electronic excited states of dye molecules near the surface of metal nanoparticles. Phys. Chem. Chem. Phys. 2024, 26, 25986–25993. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Pekareva, I.S.; Bünzli, J.-C.G. Intermolecular interactions as actors in energy-transfer processes in lanthanide complexes with 2,2′-bipyridine. Intermolecular Interactions as Actors in Energy-Transfer Processes in Lanthanide Complexes with 2,2′-Bipyridine. J. Phys. Chem. B 2009, 113, 9265–9277. [Google Scholar] [CrossRef] [PubMed]
- Matthes, P.R.; Nitsch, J.; Kuzmanoski, A.; Feldmann, C.; Steffen, A.; Marder, T.B.; Müller-Buschbaum, K. The series of rare earth complexes [Ln2Cl6(μ-4,4′-bipy)(py)6], Ln = Y, Pr, Nd, Sm-Yb: A molecular model system for luminescence properties in MOFs based on LnCl3 and 4,4′-bipyridine. Chem Eur. J 2013, 19, 17369–17378. [Google Scholar] [CrossRef]
- Bardonov, D.A.; Komarov, P.D.; Ovchinnikova, V.I.; Puntus, L.N.; Minyaev, M.E.; Nifant’ev, I.E.; Lyssenko, K.A.; Korshunov, V.M.; Taidakov, I.V.; Roitershtein, D.M. Accessing mononuclear triphenylcyclopentadienyl lanthanide complexes by using tridentate nitrogen ligands: Synthesis, structure, luminescence, and catalysis. Organometallics 2021, 40, 1235–1243. [Google Scholar] [CrossRef]
- Sheichenko, E.D.; Yapryntsev, A.D.; Gogoleva, N.V.; Kiskin, M.A.; Volykhov, A.A.; Breslavskaya, N.N.; Ananyev, I.V.; Efimov, N.N.; Baranchikov, A.E.; Ivanov, V.K. Layered Rare-Earth Hydroxides Intercalated with Metal Complexes: Copper Malonates Make a Difference. Inorg. Chem. 2025, 64, 16838–16855. [Google Scholar] [CrossRef]
- Petrova, A.S.; Butorlin, O.S.; Toikka, Y.N.; Kolesnikov, I.E.; Orlov, S.N.; Ryazantsev, M.N.; Bogachev, N.A.; Skripkin, M.Y.; Mereshchenko, A.S. The Structure and Optical Properties of Luminescent Terbium Terephthalate Metal–Organic Frameworks Doped with Yttrium, Gadolinium, and Lanthanum Ions. Crystals 2024, 14, 966. [Google Scholar] [CrossRef]
- Gogoleva, N.V.; Shmelev, M.A.; Chistyakov, A.S.; Razgonyaeva, G.A.; Korshunov, V.M.; Tsorieva, A.V.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L. Synthesis, structure, and photoluminescent properties of a mixed carboxylate pentafluorobenzoate–phenylacetate complex of terbium. Mend. Commun. 2024, 34, 484–487. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, S.; Li, M.; Wang, Y.; Mei, D. Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods. Membranes 2023, 13, 755. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Guo, Z.; Zhang, Y.; Wu, X.; Zhao, C.; Sun, X.; Yang, G.; Feng, Y.; Zhang, N. Stable artificial solid electrolyte interphase films for lithium metal anode via metal–organic frameworks cemented by polyvinyl alcohol. J. Mater. Chem. A 2020, 8, 251–258. [Google Scholar] [CrossRef]
- Wen, J.; Guo, Y.; Li, X.; Wang, B.; Wang, H.; Gao, X.; Niu, B.; Li, W. Photocatalytic Ag-MOF confers efficient antimicrobial activity to modified polyvinyl alcohol films. Food Biosci. 2024, 61, 104959. [Google Scholar] [CrossRef]
- Noori, S.M.A.; Khezerlou, A.; Hashemi, M.; Alizadeh-Sani, M.; Firoozy, S.; Khodaian, F.; Adibi, S.; Naghashpour, M.; Tavassoli, M. Polyvinyl Alcohol/Chitosan Nanofiber-Based Films Incorporated with Barberry Anthocyanin-Loaded CO-MOF as Multifunctional Performance for Red Meat Sample Packaging. Food Bioprocess Technol. 2025, 18, 1498–1513. [Google Scholar] [CrossRef]
- Khan, N.A.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Noor, T.; Azhar, O.; Rashid, T.; Iqbal, N. Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications. Polymers 2021, 13, 2307. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Ünügül, T. Polyvinyl Alcohol/Zr-based Metal Organic Framework Mixed-matrix Membranes Synthesis and Application for Hydrogen Separation. J. Inorg. Organomet. Polym. 2024, 34, 4463–4476. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Zhong, S.; Yang, Y.; Feng, G.; Meng, Q.; Gao, Y.; Cui, X. Clustering-triggered emission of poly(vinyl) alcohol. Polym. Chem. 2021, 12, 7048–7055. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovchinnikova, A.A.; Demakov, P.A.; Ryadun, A.A.; Agafontsev, A.M.; Fedin, V.P.; Dybtsev, D.N. Synthesis, Structure, and Investigation of Terbium(III) Luminescent Metal-Organic Framework Based on (N-Morpholyl)-Functionalized 1,10-Phenanthroline. Crystals 2025, 15, 906. https://doi.org/10.3390/cryst15100906
Ovchinnikova AA, Demakov PA, Ryadun AA, Agafontsev AM, Fedin VP, Dybtsev DN. Synthesis, Structure, and Investigation of Terbium(III) Luminescent Metal-Organic Framework Based on (N-Morpholyl)-Functionalized 1,10-Phenanthroline. Crystals. 2025; 15(10):906. https://doi.org/10.3390/cryst15100906
Chicago/Turabian StyleOvchinnikova, Anna A., Pavel A. Demakov, Alexey A. Ryadun, Alexander M. Agafontsev, Vladimir P. Fedin, and Danil N. Dybtsev. 2025. "Synthesis, Structure, and Investigation of Terbium(III) Luminescent Metal-Organic Framework Based on (N-Morpholyl)-Functionalized 1,10-Phenanthroline" Crystals 15, no. 10: 906. https://doi.org/10.3390/cryst15100906
APA StyleOvchinnikova, A. A., Demakov, P. A., Ryadun, A. A., Agafontsev, A. M., Fedin, V. P., & Dybtsev, D. N. (2025). Synthesis, Structure, and Investigation of Terbium(III) Luminescent Metal-Organic Framework Based on (N-Morpholyl)-Functionalized 1,10-Phenanthroline. Crystals, 15(10), 906. https://doi.org/10.3390/cryst15100906









