Synthesis of Multidoped Zirconia by Hydrothermal Method with Sequential Annealing
Abstract
1. Introduction
2. Materials and Methods
- —Flexural strength;
- P—Load in N;
- υ—Poisson’s ratio (0.305);
- t—Disk thickness;
- a—Radius of the circle on which the spherical supports are located;
- b—Radius of the pin pressing on the disk;
- R—Radius of ceramic disk.
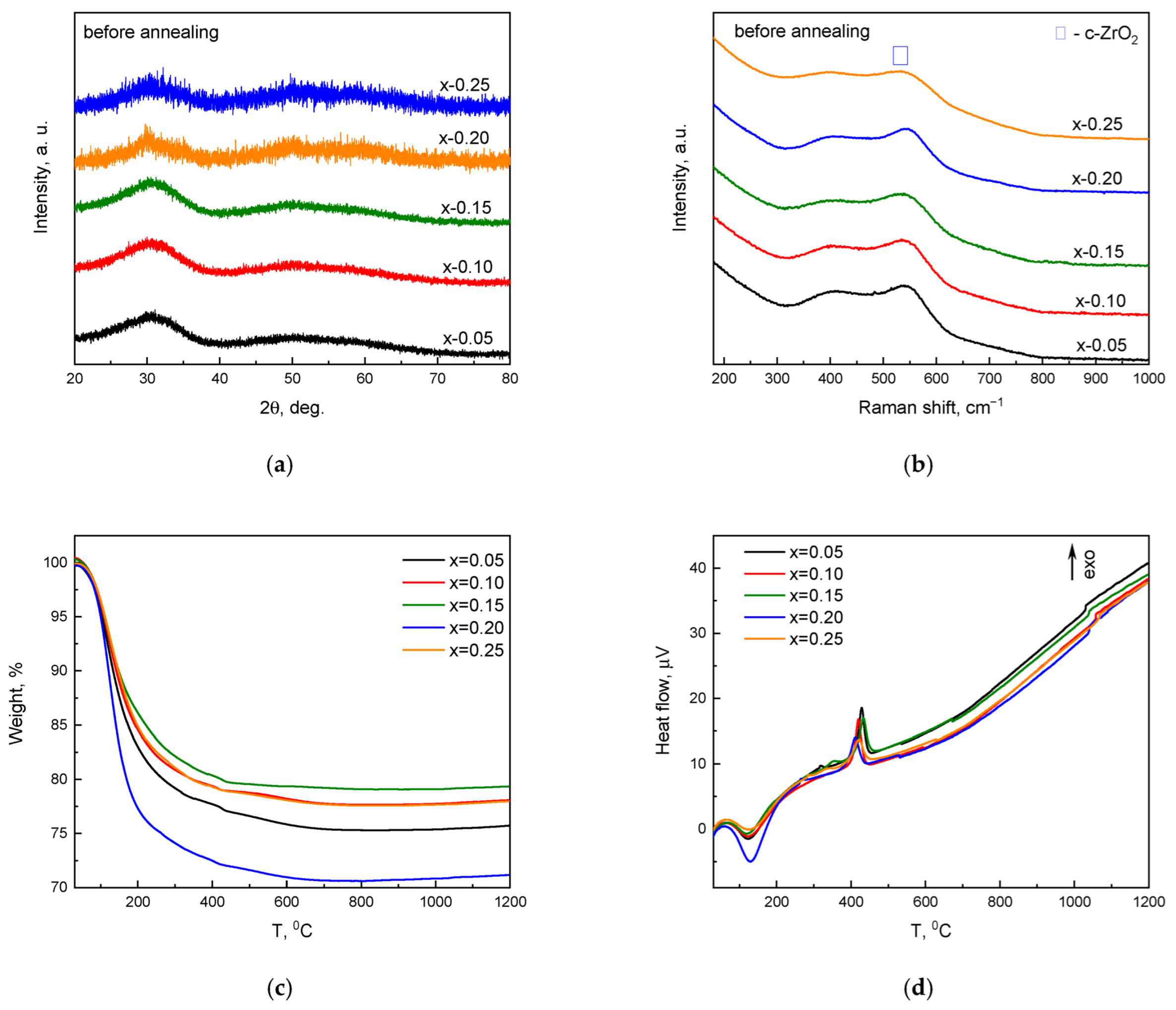
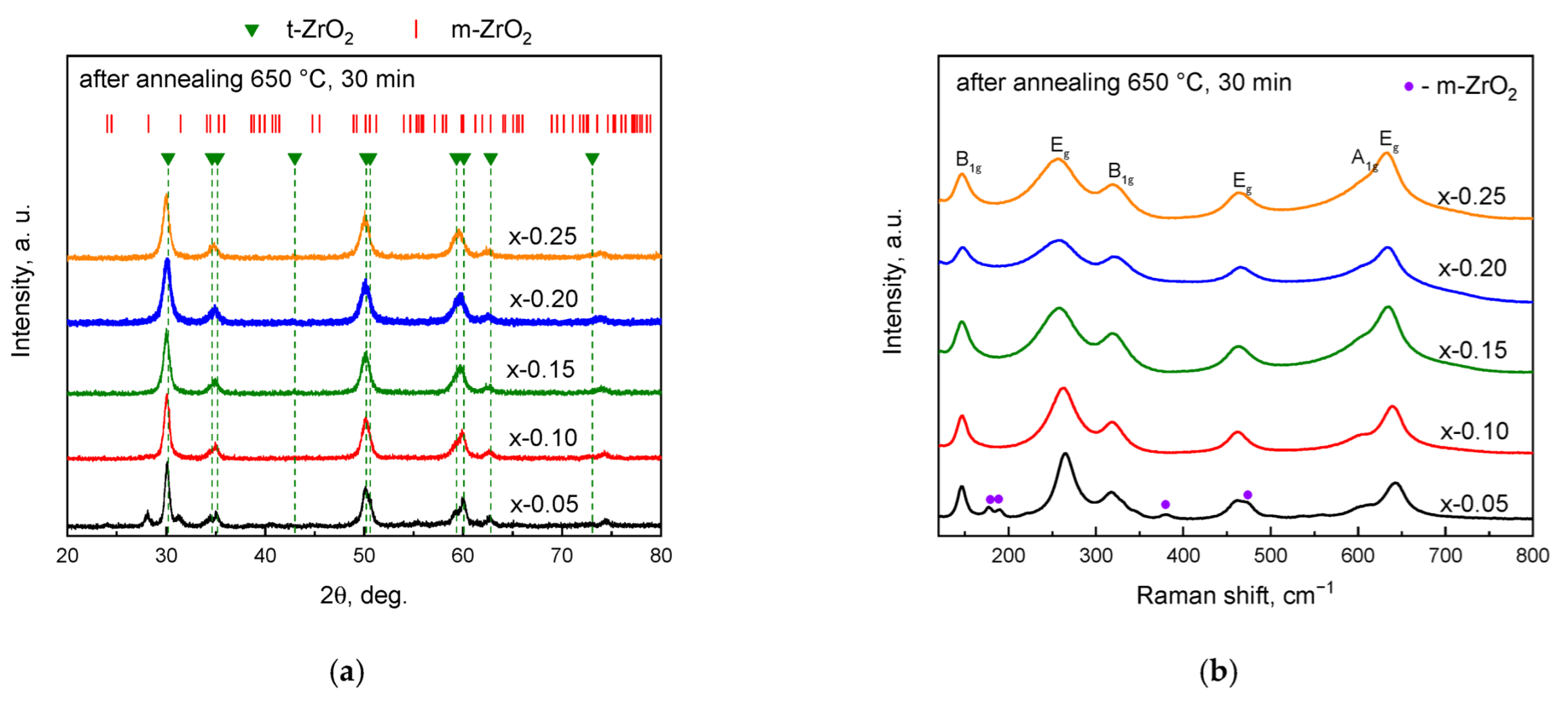
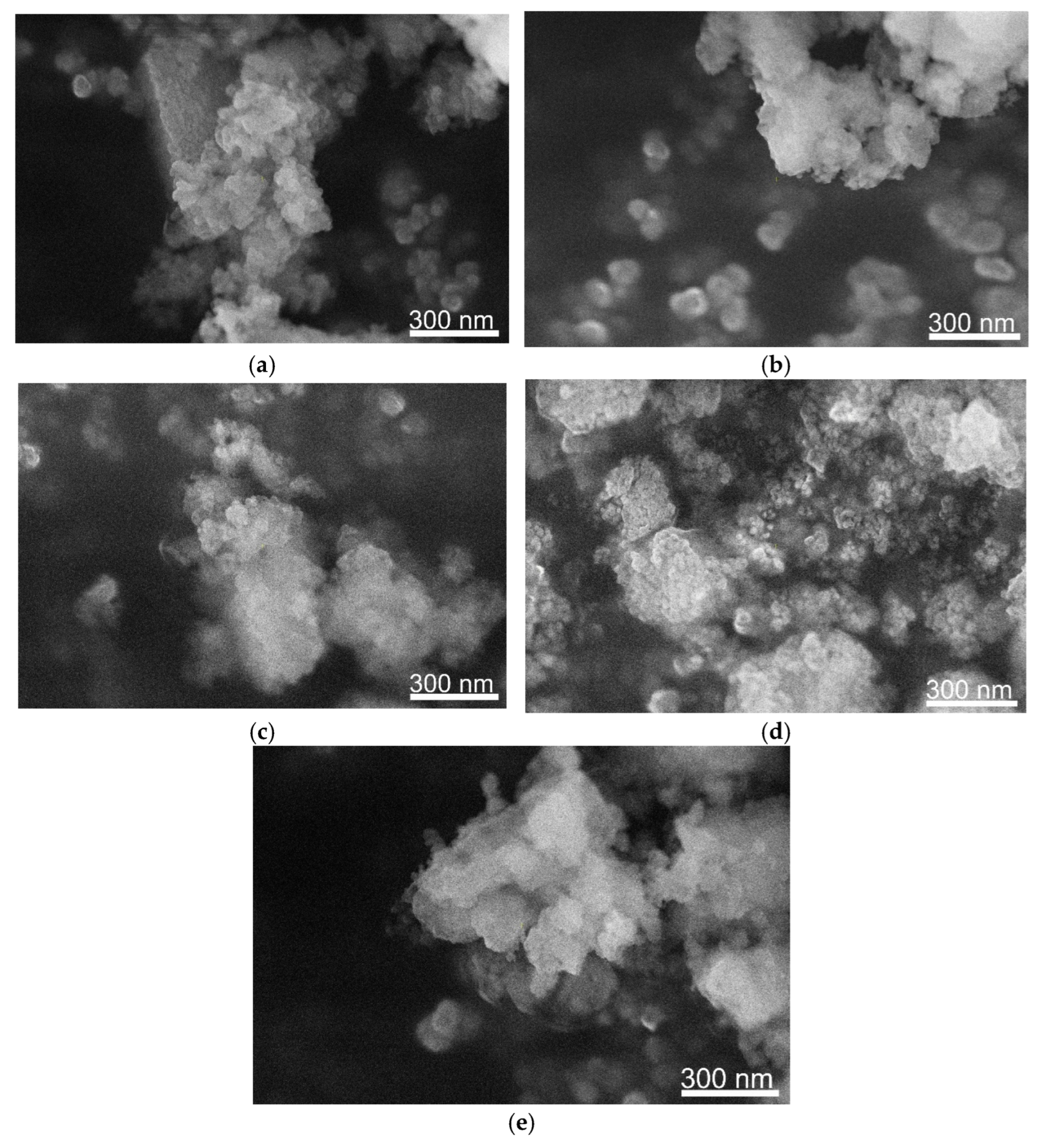
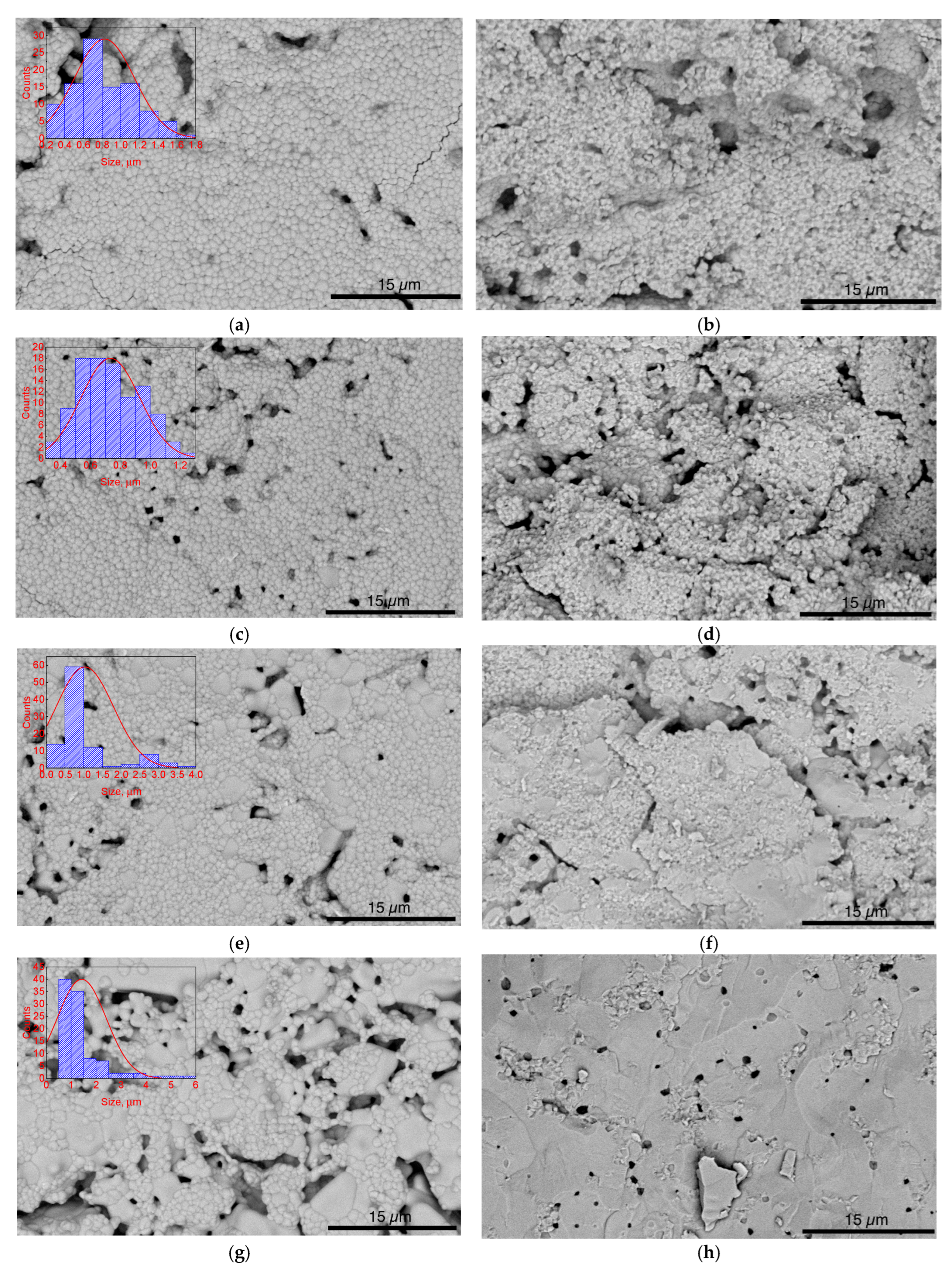
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bi, F.; Ma, S.; Gao, B.; Liu, B.; Huang, Y.; Qiao, R.; Zhang, X. Boosting Toluene Deep Oxidation by Tuning Metal-Support Interaction in MOF-Derived Pd@ZrO2 Catalysts: The Role of Interfacial Interaction between Pd and ZrO2. Fuel 2024, 357, 129833. [Google Scholar] [CrossRef]
- Peuchert, U.; Okano, Y.; Menke, Y.; Reichel, S.; Ikesue, A. Transparent Cubic-ZrO2 Ceramics for Application as Optical Lenses. J. Eur. Ceram. Soc. 2009, 29, 283–291. [Google Scholar] [CrossRef]
- Abrashov, A.A.; Grigoryan, N.S.; Vagramyan, T.A.; Meshalkin, V.P.; Kotel’nikova, A.V.; Gribanova, A.A. Protective Adhesive Zirconium Oxide Coatings. Prot. Met. Phys. Chem. Surf. 2016, 52, 1170–1174. [Google Scholar] [CrossRef]
- Fang, H.; Wang, W.; Deng, S.; Yang, T.; Zhu, H.; Huang, J.; Ye, D.; Guo, X. Interaction between Yb2O3-Y2O3 Co-Stabilized ZrO2 Ceramic Powder and Molten Silicate Deposition, and Its Implication on Thermal Barrier Coating Application. Mater. Charact. 2021, 180, 111418. [Google Scholar] [CrossRef]
- Malode, S.J.; Shetti, N.P. ZrO2 in Biomedical Applications. In Metal Oxides for Biomedical and Biosensor Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 471–501. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Magnacca, G.; Boffa, V.; Candelario, V.M.; Luiten-Olieman, M.; Zhang, W. From Ultra to Nanofiltration: A Review on the Fabrication of ZrO2 Membranes. Ceram. Int. 2023, 49, 8683–8708. [Google Scholar] [CrossRef]
- Agarkov, D.; Borik, M.; Buzaeva, E.; Korableva, G.; Kulebyakin, A.; Kuritsyna, I.; Larina, N.; Kyashkin, V.; Lomonova, E.; Milovich, F.; et al. Structure and Physical Properties of Ceramic Materials Based on ZrO2-Sc2O3 for SOFC Electrolytic Membranes Obtained from Powders of Melted Solid Solutions with a Similar Composition. Membranes 2023, 13, 717. [Google Scholar] [CrossRef] [PubMed]
- KERAMIDAS, V.G.; WHITE, W.B. Raman Scattering Study of the Crystallization and Phase Transformations of ZrO2. J. Am. Ceram. Soc. 1974, 57, 22–24. [Google Scholar] [CrossRef]
- Chen, B.; Wu, M.; Liu, Q.; He, C.; Yang, Y.; Ye, X.; Gao, C.; Huang, Z.; Min, X.; Zheng, X.; et al. One-Step Synthesis of t-ZrO2 from Zircon Using Magnesium-Calcium Minerals as Stabilizers. Ceram. Int. 2022, 48, 29997–30004. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Zhu, Y.; Li, F.; Duan, R.; Zhang, M.; Wang, X. Preparations and Characterizations of New Mesoporous ZrO2 and Y2O3-Stabilized ZrO2 Spherical Powders. Powder Technol. 2012, 227, 9–16. [Google Scholar] [CrossRef]
- Giniyatova, S.G.; Shakirzyanov, R.I.; Garanin, Y.A.; Sailaukhanov, N.A.; Kozlovskiy, A.L.; Volodina, N.O.; Shlimas, D.I.; Borgekov, D.B. Investigation of the Phase Composition, Structural, Mechanical, and Dielectric Properties of (1 − x)∙ZrO2-X∙CeO2 Ceramics Synthesized by the Solid-State Method. Appl. Sci. 2024, 14, 2663. [Google Scholar] [CrossRef]
- Thakur, M.; Vij, A.; Singh, F.; Rangra, V.S. Spectroscopic Studies of Metastable Tetragonal ZrO2 Nanocrystals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 305, 123495. [Google Scholar] [CrossRef] [PubMed]
- Garanin, Y.; Shakirziyanov, R.; Borgekov, D.; Volodina, N.; Shlimas, D.; Zdorovets, M. Influence of Precursors and Mineralizers on Phase Formation in ZrO2 Nanoparticles Synthesized by the Hydrothermal Method. Sci. Rep. 2025, 15, 26165. [Google Scholar] [CrossRef]
- Matsui, K.; Nakamura, K.; Saito, M.; Kuwabara, A.; Yoshida, H.; Ikuhara, Y. Low-Temperature Degradation in Yttria-Stabilized Tetragonal Zirconia Polycrystal: Effect of Y3+ Distribution in Grain Interiors. Acta Mater. 2022, 227, 117659. [Google Scholar] [CrossRef]
- Czeppe, T.; Zieba, P.; Pawlowski, A. Crystallographic and Microchemical Characterization of the Early Stages of Eutectoid Decomposition in MgO-Partially Stabilized ZrO2. J. Eur. Ceram. Soc. 2002, 22, 35–40. [Google Scholar] [CrossRef]
- Petriceanu, M.; Ioniță, F.G.; Piticescu, R.R.; Nicoară, A.I.; Matei, A.C.; Ioța, M.A.; Tudor, I.A.; Caramarin, Ș.; Ciobota, C.F. Effect of Doping ZrO2 on Structural and Thermal Properties. Inorganics 2024, 12, 290. [Google Scholar] [CrossRef]
- Ma, X.; Gong, J.; Wang, J.; Li, A.; Gao, P.; Wang, X.; Yang, B. Microstructure and High-Temperature Phase Stability of Co-Precipitation (Mg0.2Al0.2Ce0.2Y0.2Zr0.2)O1.6 High Entropy Ceramics Powders. Ceram. Int. 2024, 50, 40181–40184. [Google Scholar] [CrossRef]
- Sathya, A.; Anburaj, D.B.; Porkalai, V.; Muthuvel, A.; Al-Zaqri, N. Hydrothermal Synthesis of ZrO2 Nanoparticles: Study on Structural, Optical, Morphology Properties and Photocatalyst Activity. Phys. Solid. State 2025, 67, 196–206. [Google Scholar] [CrossRef]
- Mohsen, Q.; Al-Gethami, W.S.; Zaki, Z.; Alotaibi, S.H.; Ibrahim, M.M.; Ezzat, M.; Amin, M.A.; Kamel, M.M.; Mostafa, N.Y. Effect of PH on Hydrothermal Synthesis of ZrO2 Nanoparticles and Their Electrocatalytic Activity for Hydrogen Production. Int. J. Electrochem. Sci. 2022, 17, 22073. [Google Scholar] [CrossRef]
- Tsukada, T.; Venigalla, S.; Morrone, A.A.; Adair, J.H. Low-Temperature Hydrothermal Synthesis of Yttrium-Doped Zirconia Powders. J. Am. Ceram. Soc. 1999, 82, 1169–1174. [Google Scholar] [CrossRef]
- Zhi, H.; Gao, L.; Zhang, S.; Liu, S.; Zhao, J. Hydrothermal Synthesized F Doped ZrO2 Powders with Novel Photocatalytic Activities. Inorg. Chem. Commun. 2024, 162, 112170. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Wang, Z.; Wang, X. Continuous Hydrothermal Flow Synthesis and Characterization of ZrO2 Nanoparticles Doped with CeO2 in Supercritical Water. Nanomaterials 2022, 12, 668. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Shim, J.; Kim, D.; Yoo, K. Synthesis and Structural, Optical, Photocatalytic, and Electrochemical Properties of Undoped and Yttrium-Doped Tetragonal ZrO2 Nanoparticles. Ceram. Int. 2018, 44, 12329–12339. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.; Kim, S.W.; Lee, H.W.; Song, H.S. Characterization of the Electrical Properties of Y2O3-Doped CeO2-Rich CeO2–ZrO2 Solid Solutions. Solid. State Ion. 2004, 166, 45–52. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, L.; Yan, Z.; Jin, Y.; Liu, Z.; Yu, J. Enhancement of the Electrochemical Performance in MgO Stabilized ZrO2 Oxygen Sensors by Co-Doping Trivalent Metal Oxides. Curr. Appl. Phys. 2022, 39, 133–139. [Google Scholar] [CrossRef]
- Park, M.S.; Jo, K.; Lee, H.; Lee, H. Lu2O3–Y2O3–ZrO2: A Lu3+ Co-Doped YSZ System—Oxygen-Ion Conductor with High Electrical Conductivity and Improved Mechanical Properties. J. Mater. Res. Technol. 2023, 27, 8403–8411. [Google Scholar] [CrossRef]
- Turon-Vinas, M.; Zhang, F.; Vleugels, J.; Anglada, M. Effect of Calcia Co-Doping on Ceria-Stabilized Zirconia. J. Eur. Ceram. Soc. 2018, 38, 2621–2631. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, L.; Liu, T.; Sun, Q.; Jin, E.; Tian, C.; Yu, J. Enhanced Ionic Conductivity and Thermal Shock Resistance of MgO Stabilized ZrO2 Doped with Y2O3. Ceram. Int. 2020, 46, 19835–19842. [Google Scholar] [CrossRef]
- Jin, E.; Yuan, L.; Yu, J.; Ding, D.; Xiao, G. Enhancement of Thermal Shock and Slag Corrosion Resistance of MgO–ZrO2 Ceramics by Doping CeO2. Ceram. Int. 2022, 48, 13987–13995. [Google Scholar] [CrossRef]
- Zeeshan, N.; Rafiuddin. Solid Electrolytes Based on {1 − (X + y)}ZrO2-(x)MgO-(y)CaO Ternary System: Preparation, Characterization, Ionic Conductivity, and Dielectric Properties. J. Adv. Res. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Yeh, T.H.; Chou, C.C. Doping Effect and Vacancy Formation on Ionic Conductivity of Zirconia Ceramics. J. Phys. Chem. Solids 2008, 69, 386–392. [Google Scholar] [CrossRef]
- Lang, J.; Ren, K.; Wang, Y. Probing the Long-Term Thermal Stability Mechanism of Multi-Rare-Earth Oxide-Doped Zirconia for Solid Oxide Fuel Cell Electrolyte. J. Eur. Ceram. Soc. 2024, 44, 116681. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Dhaliwal, A.S. Phase Transformation Behavior of Ca-Doped Zirconia Sintered at Different Temperatures. J. Korean Ceram. Soc. 2022, 59, 370–382. [Google Scholar] [CrossRef]
- Salikhodzha, Z.M.; Bairbayeva, G.B.; Popov, A.I.; Kassymkhanova, R.N.; Zhangylyssov, K.B.; Popova, E.; Konuhova, M. DFT Study of Oxygen Ion Migration in Mg-Doped Cubic Zirconia. Solids 2025, 6, 55. [Google Scholar] [CrossRef]
- Mahato, N.; Gupta, A.; Balani, K. Doped Zirconia and Ceria-Based Electrolytes for Solid Oxide Fuel Cells: A Review. Nanomater. Energy 2012, 1, 27–45. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized Zirconia as a Structural Ceramic: An Overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Toraya, H. Whole-Powder-Pattern Fitting without Reference to a Structural Model: Application to X-Ray Powder Diffraction Data. J. Appl. Crystallogr. 1986, 19, 440–447. [Google Scholar] [CrossRef]
- Szepesi, C.J.; Adair, J.H. High Yield Hydrothermal Synthesis of Nano-Scale Zirconia and YTZP. J. Am. Ceram. Soc. 2011, 94, 4239–4246. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Özcan, M.; Hallmann, L.; Ender, A.; Mehl, A.; Hämmerlet, C.H.F. The Effect of Zirconia Sintering Temperature on Flexural Strength, Grain Size, and Contrast Ratio. Clin. Oral. Investig. 2013, 17, 269–274. [Google Scholar] [CrossRef]
- Garanin, Y.; Shakirzyanov, R.; Borgekov, D.; Kozlovskiy, A.; Volodina, N.; Shlimas, D.; Zdorovets, M. Study of Morphology, Phase Composition, Optical Properties, and Thermal Stability of Hydrothermal Zirconium Dioxide Synthesized at Low Temperatures. Sci. Rep. 2024, 14, 29398. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, L.; Firoz, M.K.; Lakshya, A.K.; Roy, A.; Chowdhury, A. Correlation between Oxygen-Related Defects and Lattice Strain in Tetragonal Phase Stabilized Doped-Zirconia Systems. Materialia 2023, 32, 101895. [Google Scholar] [CrossRef]
- Matsui, K.; Horikoshi, H.; Ohmichi, N.; Ohgai, M.; Yoshida, H.; Ikuhara, Y. Cubic-Formation and Grain-Growth Mechanisms in Tetragonal Zirconia Polycrystal. J. Am. Ceram. Soc. 2003, 86, 1401–1408. [Google Scholar] [CrossRef]
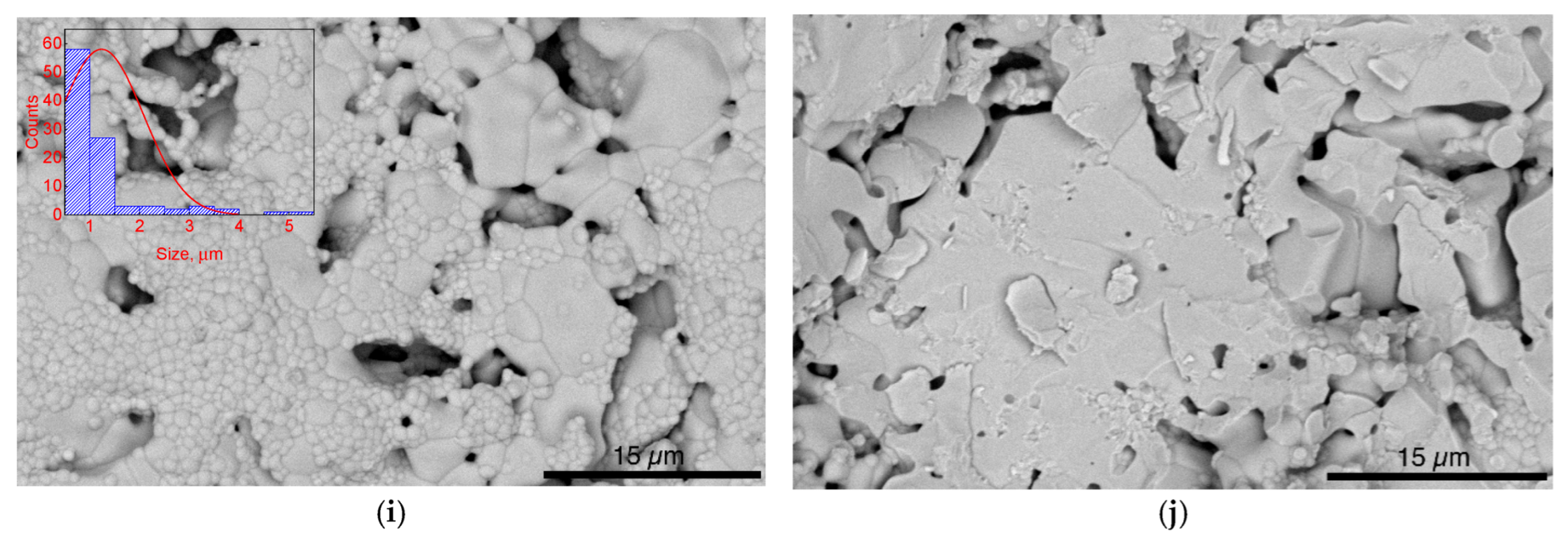
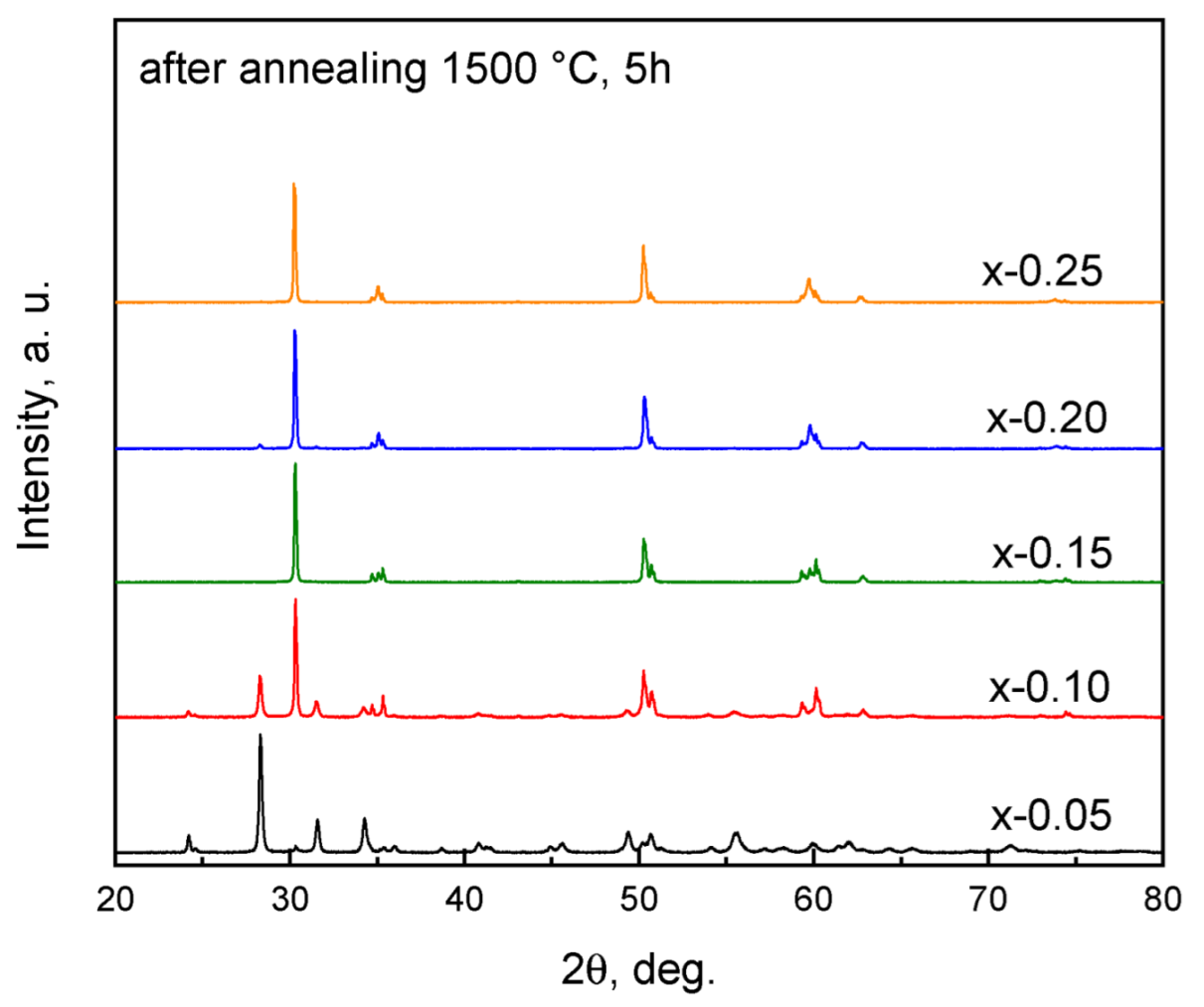
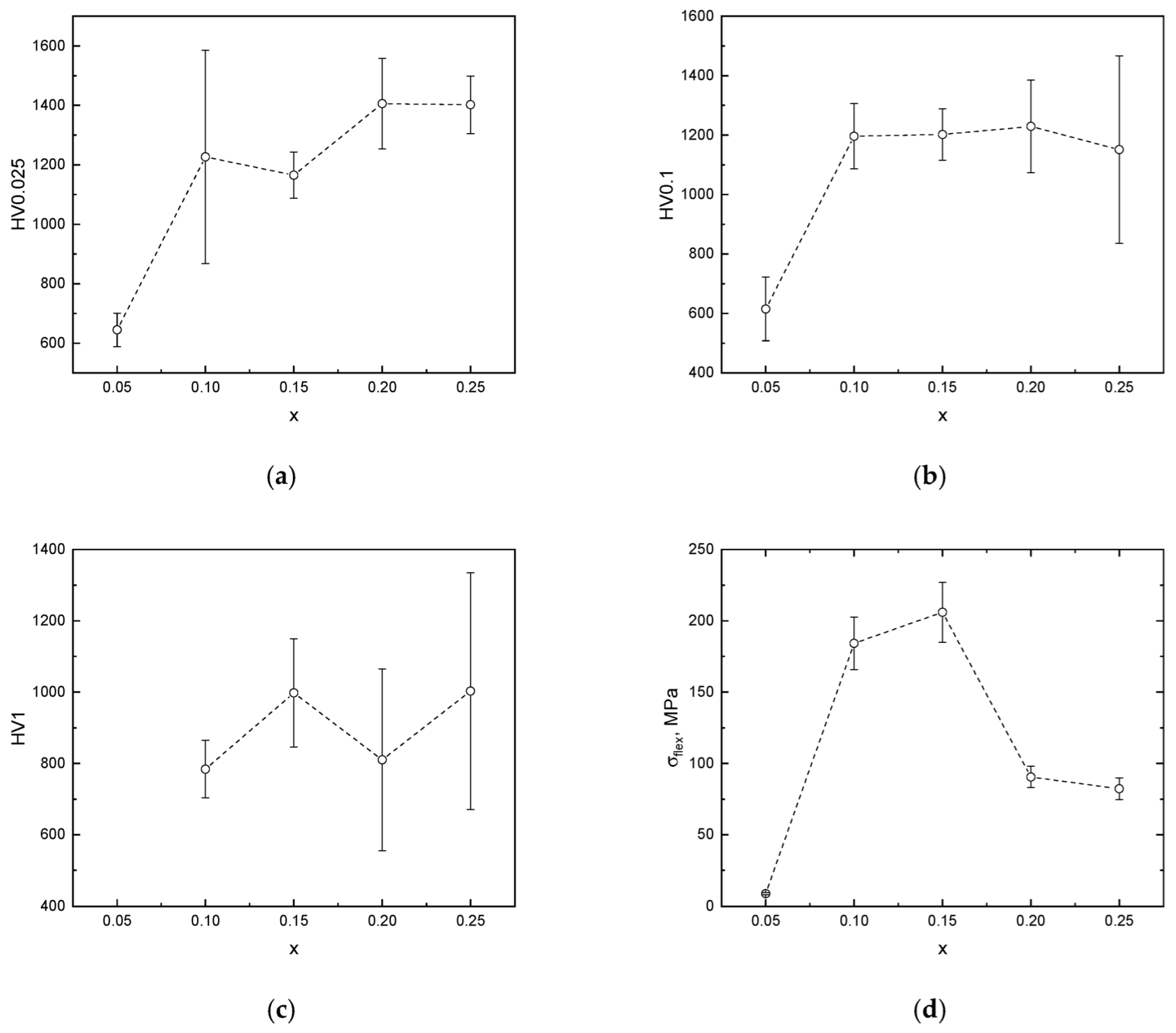
| x, mol.% | a, Å | c, Å | V, Å3 | Rwp, % | Rp, % |
|---|---|---|---|---|---|
| 0.05 | 3.6006 | 5.1857 | 67.229 | 17.05 | 12.90 |
| 0.10 | 3.6102 | 5.1884 | 67.625 | 17.69 | 13.29 |
| 0.15 | 3.6139 | 5.1806 | 67.662 | 17.79 | 13.48 |
| 0.20 | 3.6256 | 5.1841 | 68.146 | 16.96 | 12.75 |
| 0.25 | 3.6271 | 5.1955 | 68.350 | 17.88 | 13.49 |
| x | Phase Name | Phase Content, % | Statistic | V | Porosity, % | , g/cm2 | ||
|---|---|---|---|---|---|---|---|---|
| Rwp | Rp | Χ2 | ||||||
| 0.05 | m-ZrO2 | 94.46 | 9.84 | 7.06 | 2.0666 | 141.724 | 10 | 4.99 |
| t-ZrO2 | 5.54 | 67.357 | ||||||
| c-ZrO2 | 0 | – | ||||||
| 0.1 | m-ZrO2 | 46.28 | 9.23 | 7.00 | 1.7646 | 142.442 | 7 | 5.41 |
| t-ZrO2 | 44.59 | 67.645 | ||||||
| c-ZrO2 | 9.13 | 134.794 | ||||||
| 0.15 | m-ZrO2 | 0 | 7.66 | 5.85 | 1.1584 | - | 9 | 5.39 |
| t-ZrO2 | 72.4 | 67.686 | ||||||
| c-ZrO2 | 27.6 | 135.944 | ||||||
| 0.2 | m-ZrO2 | 5.6 | 8.42 | 6.51 | 1.3798 | 142.187 | 17 | 4.87 |
| t-ZrO2 | 35.4 | 67.659 | ||||||
| c-ZrO2 | 59.0 | 135.983 | ||||||
| 0.25 | m-ZrO2 | 0 | 9.35 | 7.03 | 1.7145 | - | 16 | 4.87 |
| t-ZrO2 | 27.2 | 67.764 | ||||||
| c-ZrO2 | 72.8 | 136.548 | ||||||
| x, mol.% | Zr, wt % | Y, wt % | Ce, wt % | Mg, wt % | Ca, wt % | O, wt % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | Theoretical | Experiment | Theoretical | Experiment | Theoretical | Experiment | Theoretical | Experiment | Theoretical | Experiment | Theoretical | |
| 0.05 | 70.5 | 70.84 | 0.2 | 0.91 | 0.4 | 1.43 | 0.0 | 0.25 | 0.0 | 0.41 | 28.9 | 26.16 |
| 0.10 | 72.3 | 67.61 | 1.1 | 1.83 | 3.9 | 2.88 | 0.0 | 0.50 | 0.0 | 0.83 | 22.7 | 26.35 |
| 0.15 | 69.7 | 64.33 | 2.1 | 2.77 | 5.4 | 4.36 | 0.0 | 0.76 | 0.0 | 1.25 | 22.8 | 26.55 |
| 0.20 | 66.5 | 60.99 | 3.5 | 3.72 | 6.9 | 5.86 | 0.0 | 1.02 | 0.0 | 1.67 | 23.1 | 26.74 |
| 0.25 | 62.5 | 57.61 | 4.2 | 4.68 | 7.6 | 7.37 | 0.0 | 1.28 | 0.0 | 2.11 | 25.7 | 26.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garanin, Y.A.; Shakirzyanov, R.I.; Shlimas, D.I.; Saidullayeva, M.A.; Borgekov, D.B.; Kaliyekperov, M.E. Synthesis of Multidoped Zirconia by Hydrothermal Method with Sequential Annealing. Crystals 2025, 15, 904. https://doi.org/10.3390/cryst15100904
Garanin YA, Shakirzyanov RI, Shlimas DI, Saidullayeva MA, Borgekov DB, Kaliyekperov ME. Synthesis of Multidoped Zirconia by Hydrothermal Method with Sequential Annealing. Crystals. 2025; 15(10):904. https://doi.org/10.3390/cryst15100904
Chicago/Turabian StyleGaranin, Yuriy Alexsandrovich, Rafael Iosifivich Shakirzyanov, Dmitriy Igorevich Shlimas, Milana Abasovna Saidullayeva, Daryn Boranbaevich Borgekov, and Malik Erlanovich Kaliyekperov. 2025. "Synthesis of Multidoped Zirconia by Hydrothermal Method with Sequential Annealing" Crystals 15, no. 10: 904. https://doi.org/10.3390/cryst15100904
APA StyleGaranin, Y. A., Shakirzyanov, R. I., Shlimas, D. I., Saidullayeva, M. A., Borgekov, D. B., & Kaliyekperov, M. E. (2025). Synthesis of Multidoped Zirconia by Hydrothermal Method with Sequential Annealing. Crystals, 15(10), 904. https://doi.org/10.3390/cryst15100904









