Activated Carbon–Geopolymer Composites: Influence of Particle Size and Content on CO2 Adsorption and Mechanical and Thermal Properties
Abstract
1. Introduction
2. Experimental
2.1. Characterization Methods
2.2. Materials
2.3. Sample Preparation
3. Results and Discussion
3.1. Verification of Geopolymer Formation
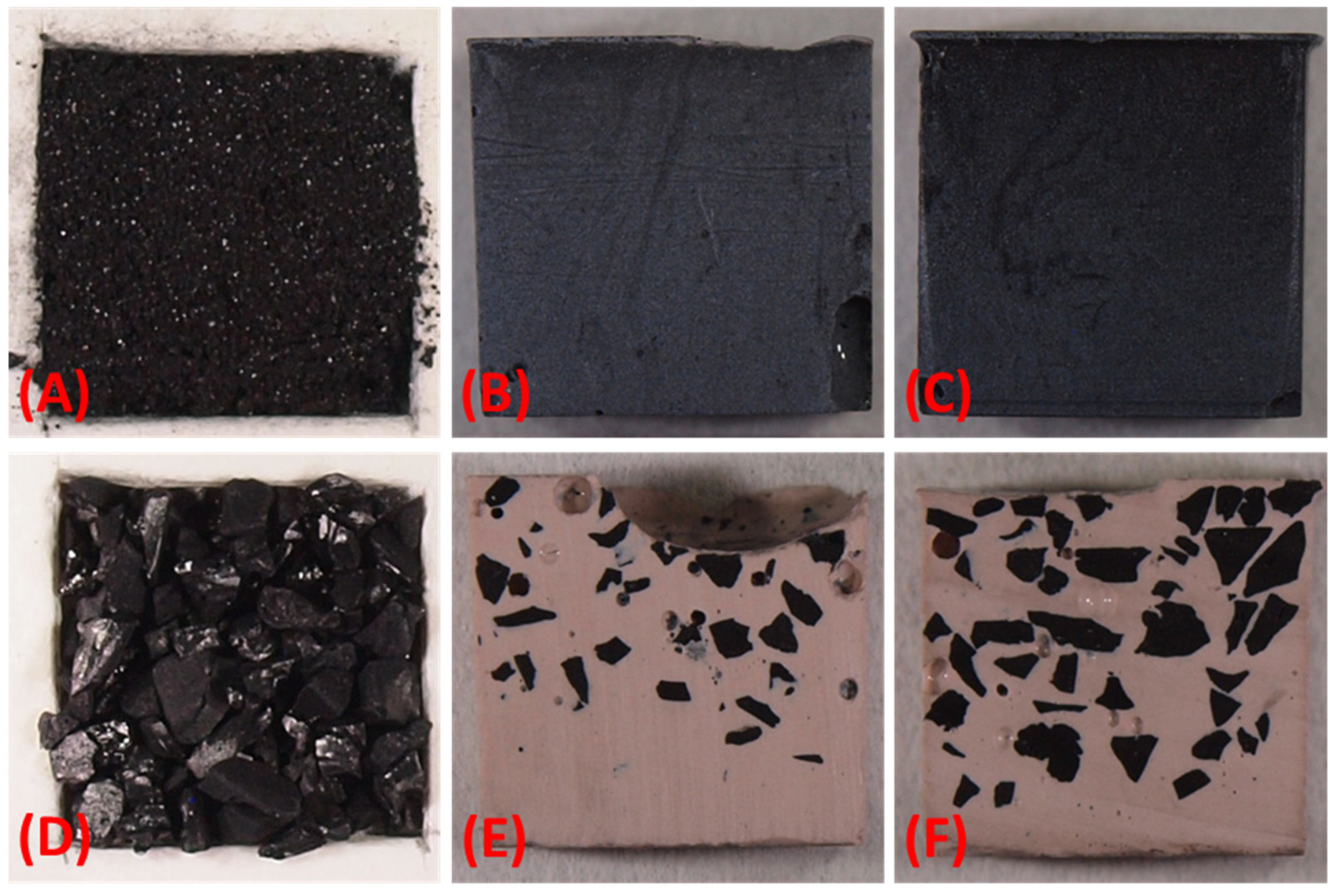
3.2. Characterization of Activated Carbon and Geopolymer Composites
3.2.1. Compressive Strength
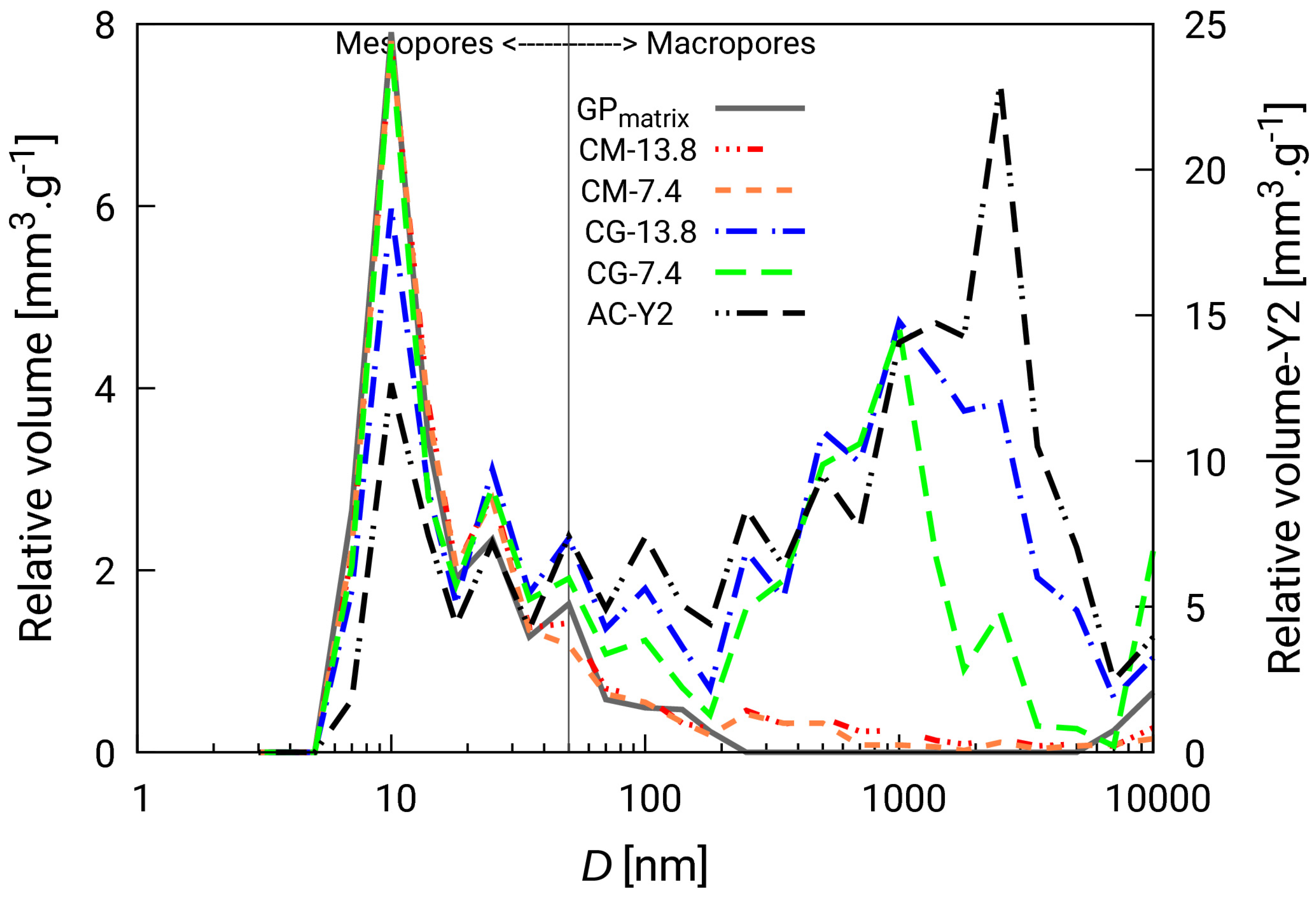
3.2.2. Textural and Adsorption Properties
3.2.3. Thermogravimetric Analyses
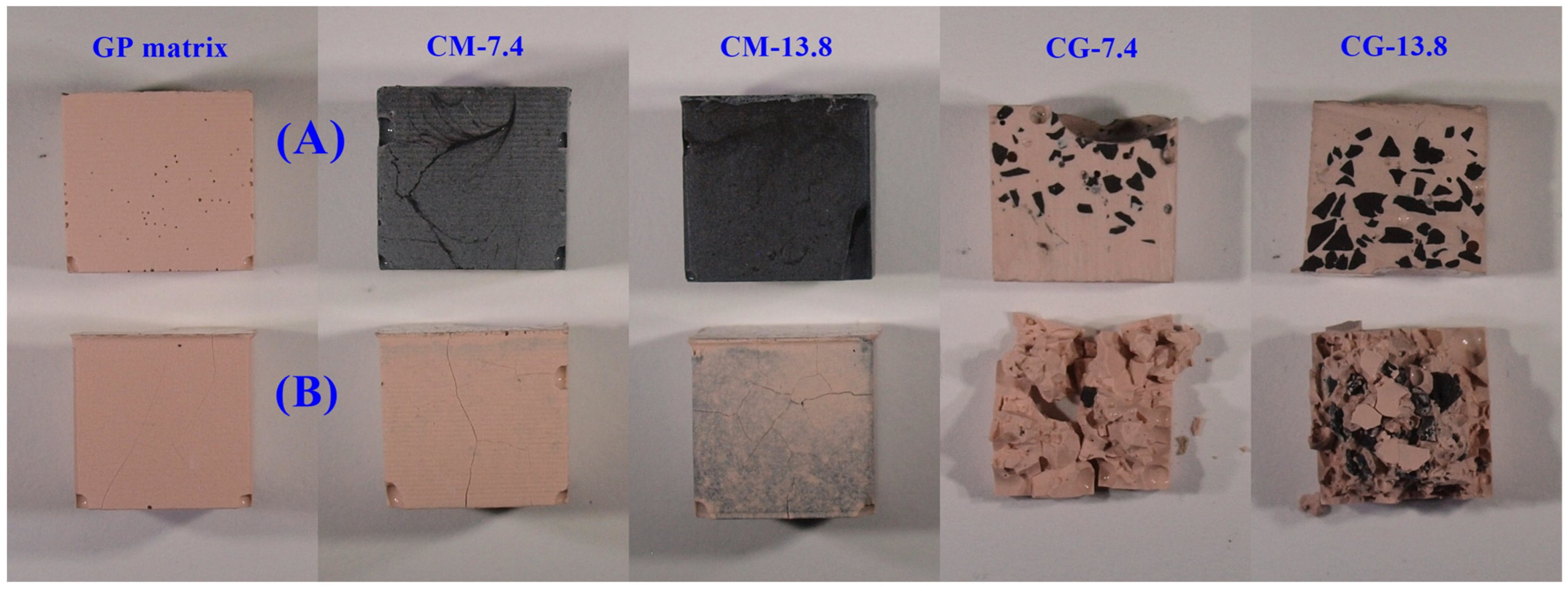
3.2.4. Microscopic Analyses
4. Conclusions
- Activated carbon K835 was successfully used as an additive in the preparation of geopolymer composites. The resulting materials were solid, mechanically resistant, and insoluble in water;
- FTIR analysis confirmed that the addition of activated carbon to the geopolymer matrix did not negatively affect the geopolymerization process, regardless of the added concentration or granulometry of activated carbon;
- The mechanical properties of the composites remained comparable to the pure geopolymer matrix, indicating that activated carbon does not compromise structural integrity;
- The incorporation of both milled and granular forms of activated carbon enhanced the adsorption capacity of the composites, with the granular form showing superior performance due to better preservation of pore openness;
- Activated carbon significantly influenced the textural properties of the composites, including pore volumes and surfaces and micropore content. The addition of granular form slightly increased total porosity and adsorption capacity;
- The addition of a granular form caused structural inhomogeneity, which led to earlier thermal degradation of the composite at around 500 °C, unlike the milled form, which showed greater thermal resilience;
- CO2 adsorption experiments revealed that the composites achieved capacities ranging from 48.8 to 60.0 mg.g−1 at 25 °C and up to 0.1 MPa, with the highest value observed for the lower content of granular form of activated carbon. For comparison, commercial pure activated carbon reached a CO2 adsorption capacity of 120.8 mg.g−1;
- Despite the reduced thermal stability and compressive strength in composites with granular activated carbon, both types of composites show promising potential for environmental applications, such as gas and pollutant capture, especially under medium-temperature and mechanically demanding conditions.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouna, L.; Ait El Fakir, A.; Benlhachemi, A.; Draoui, K.; Ezahri, M.; Bakiz, B.; Villain, S.; Guinneton, F.; Elalem, N. Synthesis and Characterization of Mesoporous Geopolymer Based on Moroccan Kaolinite Rich Clay. Appl. Clay Sci. 2020, 196, 105764. [Google Scholar] [CrossRef]
- Ghani, U.; Hussain, S.; Noor-ul-Amin, M.I.; Imtiaz, M.; Ali Khan, S. Laterite Clay-Based Geopolymer as a Potential Adsorbent for the Heavy Metals Removal from Aqueous Solutions. J. Saudi Chem. Soc. 2020, 24, 874–884. [Google Scholar] [CrossRef]
- Strnadová, N.; Matějková, D. Adsorption of Copper and Zinc from Aqueous Solution on Mg(OH)2. Chem. List. 2006, 100, 803–808. [Google Scholar]
- Řimnáčová, D.; Bičáková, O.; Moško, J.; Straka, P.; Čimová, N. The Effect of Carbonization Temperature on Textural Properties of Sewage Sludge-Derived Biochars as Potential Adsorbents. J. Environ. Manag. 2024, 359, 120947. [Google Scholar] [CrossRef] [PubMed]
- Minelli, M.; Medri, V.; Papa, E.; Miccio, F.; Landi, E.; Doghieri, F. Geopolymers as Solid Adsorbent for CO2 Capture. Chem. Eng. Sci. 2016, 148, 267–274. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and Properties of Clay-Based Geopolymer Cements: A Review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-Activated Metakaolin: A Short Guide for Civil Engineer-An Overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A Review on Properties of Fresh and Hardened Geopolymer Mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of Fresh and Hardened Fly Ash/Slag Based Geopolymer Concrete: A Review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Hanzlíček, T.; Perná, I.; Uličná, K.; Římal, V.; Štěpánková, H. The Evaluation of Clay Suitability for Geopolymer Technology. Minerals 2020, 10, 852. [Google Scholar] [CrossRef]
- Lyu, X.; Robinson, N.; Elchalakani, M.; Johns, M.L.; Dong, M.; Nie, S. Sea Sand Seawater Geopolymer Concrete. J. Build. Eng. 2022, 50, 104141. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Straka, P.; Steinerová, M. Acoustic Absorption of Geopolymer/Sand Mixture. Ceram. Silikáty 2009, 53, 48–51. [Google Scholar]
- Sui, J.; Li, X.; Zhang, H.; Xu, F.; Deng, J.; Hu, R.; Chen, M. Effect of Different Fibers on Shrinkage Properties and Bonding Properties of Geopolymer Mortar Repair Materials and Analysis of the Mechanism. Coatings 2023, 13, 1542. [Google Scholar] [CrossRef]
- Novotná, M.; Perná, I.; Hanzlíček, T. Review of Possible Fillers and Additives for Geopolymer Materials. Waste Forum 2020, 2, 78–89. [Google Scholar]
- Perná, I.; Novotná, M.; Řimnáčová, D.; Šupová, M. New Metakaolin-Based Geopolymers with the Addition of Different Types of Waste Stone Powder. Crystals 2021, 11, 983. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T. The Solidification of Aluminum Production Waste in Geopolymer Matrix. J. Clean. Prod. 2014, 84, 657–662. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Cempa, M.; Król, M.; Kiełbasa, K.; Zhang, L.; Liu, R.; Smolinski, A. A Modified Fly Ash-Based Geopolymer as a Sustainable Solution for Ammonia Storage by Sorption. Ind. Crops Prod. 2025, 230, 121057. [Google Scholar] [CrossRef]
- Karoui, O.; Andrejkovičová, S.; Pato, P.; Patinha, C.; Řimnáčová, D.; Perná, I.; Hajjaji, W.; Rocha, F.; Mlayah, A. Foamed Phosphate By-Product Based Geopolymers and Dye Adsorption Efficiency. Appl. Clay Sci. 2024, 257, 107446. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Boura, P.; Lučaník, A. The Manufacture of the Grinding Wheels Based on the Ca–K Geopolymer Matrix. Mater. Manuf. Process. 2016, 31, 667–672. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Lučaník, A.; Šupová, M. Geopolymer-Based Grinding Stones Utilizable in Metal Machining. Constr. Build. Mater. 2023, 363, 129869. [Google Scholar] [CrossRef]
- Perná, I.; Novotná, M.; Hanzlíček, T.; Šupová, M.; Řimnáčová, D. Metakaolin-Based Geopolymer Formation and Properties: The Influence of the Maturation Period and Environment (Air, Demineralized and Sea Water). J. Ind. Eng. Chem. 2024, 134, 415–424. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Law, D. A State-of-the-Art Review on the Durability of Geopolymer Concrete for Sustainable Structures and Infrastructure. Constr. Build. Mater. 2021, 291, 123381. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential Applications of Geopolymer Concrete in Construction: A Review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-Ash Based Geopolymer Mortar for High-Temperature Application—Effect of Slag Addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Růžek, V.; Dostayeva, A.M.; Walter, J.; Grab, T.; Korniejenko, K. Carbon Fiber-Reinforced Geopolymer Composites: A Review. Fibers 2023, 11, 17. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous Geopolymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the Chemical Foaming Reaction to Control the Porosity of Geopolymer Foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Bai, C.; Shao, J.; Li, X.; Zhang, Z.; Qiao, Y.; Hao, J.; Li, H.; Zheng, T.; Colombo, P. Fabrication and Properties of Slag-Based Geopolymer Syntactic Foams Containing Hollow Glass Microspheres. Mater. Lett. 2022, 308, 131158. [Google Scholar] [CrossRef]
- Yu, H.; Xu, M.; Chen, C.; He, Y.; Cui, X. A Review on the Porous Geopolymer Preparation for Structural and Functional Materials Applications. Int. J. Appl. Ceram. Technol. 2022, 19, 1793–1813. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer Foam Concrete: An Emerging Material for Sustainable Construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Liu, L.C. Synthesis, Characterization, and Selective CO2 Capture Performance of a New Type of Activated Carbon-Geopolymer Composite Adsorbent. J. Clean. Prod. 2021, 325, 129271. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for Use in Heavy Metals Adsorption, and Advanced Oxidative Processes: A Critical Review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Ariffin, N.; Mustafa, M.; Bakri, A.; Remy, M.; Mohd, R.; Zaino, A.; Murshed, M.F.; Faris, M.A. Review on Adsorption of Heavy Metal in Wastewater by Using Geopolymer. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 97, p. 01023. [Google Scholar]
- Ji, Z.; Su, L.; Pei, Y. Characterization and Adsorption Performance of Waste-Based Porous Open-Cell Geopolymer with One-Pot Preparation. Ceram. Int. 2021, 47, 12153–12162. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Mor, M.; Vaccari, A.; Natali Murri, A.; Piotte, L.; Melandri, C.; Landi, E. Mechanical Strength and Cationic Dye Adsorption Ability of Metakaolin-Based Geopolymer Spheres. Appl. Clay Sci. 2020, 193, 105678. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From Theory to Practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Řimnáčová, D.; Malhocká, A.; Havelcová, M.; Hendrych, J.; Weishauptová, Z. Physicochemical Characteristics of Natural and Anthropogenic Inorganic and Organic Solid Porous Materials: Comprehensive View. Materialia 2024, 33, 101973. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Garnier, C.; Finqueneisel, G.; Zimny, T.; Pokryszka, Z.; Lafortune, S.; Défossez, P.D.C.; Gaucher, E.C. Selection of Coals of Different Maturities for CO2 Storage by Modelling of CH4 and CO2 Adsorption Isotherms. Int. J. Coal Geol. 2011, 87, 80–86. [Google Scholar] [CrossRef]
- Dubinin, M. Adsorption in Micropores. J. Colloid Interface Sci. 1967, 23, 487–499. [Google Scholar] [CrossRef]
- Medek, J. Possibility of Micropore Analysis of Coal and Coke from the Carbon Dioxide Isotherm. Fuel 1977, 56, 131–133. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Bernabé, Y.; Maineult, A. Physics of Porous Media: Fluid Flow Through Porous Media. In Treatise on Geophysics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 11, pp. 19–41. [Google Scholar]
- Scheidegger, A.E. The Physics of Flow Through Porous Media. Soil Sci. 1958, 86, 355. [Google Scholar] [CrossRef]
- Košek, F.; Dudák, J.; Tymlová, V.; Žemlička, J.; Řimnáčová, D.; Jehlička, J. Evaluation of Pore-Fracture Microstructure of Gypsum Rock Fragments Using Micro-CT. Micron 2024, 181, 103633. [Google Scholar] [CrossRef]
- ASTM D4607-14(2021); Standard Test Method for Determination of Iodine Number of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/d4607-14r21.html (accessed on 20 March 2023).
- Ehrlich, R.; Crabtree, S.J.; Horkowitz, K.O.; Horkowitz, J.P. Petrography and Reservoir Physics I: Objective Classification of Reservoir Porosity (1). Am. Assoc. Pet. Geol. Bull. 1991, 75, 1547–1562. [Google Scholar] [CrossRef]
- České Lupkové Závody, A.S. Mefisto L05. Available online: https://www.cluz.cz/en/metakaolin-general-information (accessed on 12 October 2025).
- Chemap K835. Available online: http://www.chemap.cz/silcarbon-k835/ (accessed on 12 October 2025).
- Su, Z.; Hou, W.; Sun, Z. Recent Advances in Carbon Nanotube-Geopolymer Composite. Constr. Build. Mater. 2020, 252, 118940. [Google Scholar] [CrossRef]
- Zheng, J.; Li, X.; Bai, C.; Zheng, K.; Wang, X.; Sun, G.; Zheng, T.; Zhang, X.; Colombo, P. Rapid Fabrication of Porous Metakaolin-Based Geopolymer via Microwave Foaming. Appl. Clay Sci. 2024, 249, 107238. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Palomo, J.G.; Puertas, F. Sodium Silicate Solutions from Dissolution of Glasswastes. Statistical Analysis. Mater. Constr. 2014, 64, 05213. [Google Scholar] [CrossRef]
- ElBatal, H.A.; Hassaan, M.Y.; Fanny, M.A.; Ibrahim, M.M. Optical and FT Infrared Absorption Spectra of Soda Lime Silicate Glasses Containing Nano Fe2O3 and Effects of Gamma Irradiation. Silicon 2017, 9, 511–517. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Attenuated Total Reflectance Fourier Transform Infrared Analysis of Fly Ash Geopolymer Gel Aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Sitarz, M.; Mozgawa, W.; Handke, M. Vibrational Spectra of Complex Ring Silicate Anions—Method of Recognition. J. Mol. Struct. 1997, 404, 193–197. [Google Scholar] [CrossRef]
- Jankowska, H.; Świątkowski, A.; Choma, J. Active Carbon; Kemp, T.J., Horwood, E., Eds.; Cornell University: Ithaca, NY, USA, 1991; ISBN 9780130049124. [Google Scholar]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Światkoski, A. The Characterization of Activated Carbons with Oxygen and Nitrogen Surface Groups. Carbon N. Y. 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Rychlicki, G. The Influence of Activated Carbon Surface Chemical Composition on the Adsorption of Acetaminophen (Paracetamol) in Vitro: The Temperature Dependence of Adsorption at the Neutral PH. Colloids Surf. A Physicochem. Eng. Asp. 2000, 163, 135–150. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic Carbons Activated with Phosphoric—Acid I. Surface Chemistry and Ion Binding Properties. Carbon N. Y. 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, E.R.; Bagane, M. CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Allen, T.; Burevski, D. Adsorption of Gases on Microporous Carbons. Powder Technol. 1977, 18, 139–148. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Cai, Y.; Wang, Y.; Teng, J. Adsorption Pore Structure and Its Fractal Characteristics of Coals by N2 Adsorption/Desorption and FESEM Image Analyses. Fuel 2019, 257, 116031. [Google Scholar] [CrossRef]
- Everett, D.H. International Union of Pure and Applied Chemistry (IUPAC) Manuals of Symbols and Terminology for Physico Chemical Quantities and Units. In Pure and Applied Chemistry; Butterworths: London, UK, 1972; pp. 579–638. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 Capture by Solid Adsorbents and Their Applications: Current Status and New Trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Kundu, S.; Khandaker, T.; Anik, M.A.-A.M.; Hasan, M.K.; Dhar, P.K.; Dutta, S.K.; Latif, M.A.; Hossain, M.S. A Comprehensive Review of Enhanced CO2 Capture Using Activated Carbon Derived from Biomass Feedstock. RSC Adv. 2024, 14, 29693–29736. [Google Scholar] [CrossRef]
- Schneider, M.; Rodríguez-Castellón, E.; Guerrero-Pérez, M.O.; Hotza, D.; De Noni Junior, A.; de Fátima Peralta Muniz Moreira, R. Hierarchically Porous Composites Containing Mining Tailings-Based Geopolymer and Zeolite 13X: Application for Carbon Dioxide Sequestration. Adsorption 2025, 31, 21. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical Evolution of Na-Geopolymer Derived from Metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- He, R.; Dai, N.; Wang, Z. Thermal and Mechanical Properties of Geopolymers Exposed to High Temperature: A Literature Review. Adv. Civ. Eng. 2020, 2020, 7532703. [Google Scholar] [CrossRef]
- Wang, R.; Meng, T.; Zhang, B.; Chen, C.; Li, D. Preparation and Characterization of Activated Carbon/Ultra-high Molecular Weight Polyethylene Composites. Polym. Compos. 2021, 42, 2728–2736. [Google Scholar] [CrossRef]








| L05 | Al2O3 | SiO2 | CaO | Na2O | K2O | MgO | Fe2O3 | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Before | 41.99 | 50.28 | 0.14 | <0.11 | 0.59 | 0.14 | 1.03 | 1.52 | 3.65 |
| After | 43.24 | 50.94 | 0.79 | <0.11 | 0.59 | 0.14 | 1.03 | 1.52 | 1.21 |
| Material/Parameter | Activated Carbon Content | Size of Particles |
|---|---|---|
| (wt.%) | (mm) | |
| K836 | 100 | 0.5–2.0 |
| CM-13.8 | 13.8 | <0.7 |
| CG-13.8 | 13.8 | 0.5–2.0 |
| CM-7.4 | 7.4 | <0.7 |
| CG-7.4 | 7.4 | 0.5–2.0 |
| Compressive Strength | Standard Deviation | |
|---|---|---|
| (MPa) | ||
| GP matrix | 72.8 | 7.5 |
| CM-13.8 | 105.4 | 16.4 |
| CG-13.8 | 40.1 | 13.1 |
| CM-7.4 | 62.9 | 17.9 |
| CG-7.4 | 37.7 | 7.3 |
| Sample | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| SBET | Vm | VHg | Por | n | IN | Esorp | |
| (m2.g−1) | (mm3.g−1) | (%) | (mg.g−1) | (mg.g−1) | (kJ.mol−1) | ||
| GP matrix | 120.3 | 5.7 | 25.8 | 4.0 | 54.4 | 25 | 16.9 |
| AC | 903.7 | 21.8 | 228.5 | 20.5 | 120.8 | 985 | 10.1 |
| CM-13.8 | 144.2 | 4.0 | 31.4 | 4.8 | 48.8 | 129 | 13.1 |
| CG-13.8 | 142.3 | 7.1 | 60.3 | 9.0 | 53.7 | 145 | 13.6 |
| CM-7.4 | 117.0 | 4.1 | 28.7 | 4.5 | 55.6 | 90 | 15.2 |
| CG-7.4 | 135.0 | 7.2 | 47.9 | 7.3 | 60.0 | 95 | 15.5 |
| Sample | ∆mcube | ∆mpowder |
|---|---|---|
| (wt.%) | ||
| GP matrix | 12.23 | 8.05 |
| AC | - | 1.95 |
| CM-13.8 | 19.48 | 7.80 |
| CG-13.8 | 20.13 | 7.76 |
| CM-7.4 | 15.14 | 7.98 |
| CG-7.4 | 19.11 | 7.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Řimnáčová, D.; Perná, I.; Novotná, M.; Šupová, M.; Nováková, M.; Bičáková, O. Activated Carbon–Geopolymer Composites: Influence of Particle Size and Content on CO2 Adsorption and Mechanical and Thermal Properties. Crystals 2025, 15, 892. https://doi.org/10.3390/cryst15100892
Řimnáčová D, Perná I, Novotná M, Šupová M, Nováková M, Bičáková O. Activated Carbon–Geopolymer Composites: Influence of Particle Size and Content on CO2 Adsorption and Mechanical and Thermal Properties. Crystals. 2025; 15(10):892. https://doi.org/10.3390/cryst15100892
Chicago/Turabian StyleŘimnáčová, Daniela, Ivana Perná, Martina Novotná, Monika Šupová, Martina Nováková, and Olga Bičáková. 2025. "Activated Carbon–Geopolymer Composites: Influence of Particle Size and Content on CO2 Adsorption and Mechanical and Thermal Properties" Crystals 15, no. 10: 892. https://doi.org/10.3390/cryst15100892
APA StyleŘimnáčová, D., Perná, I., Novotná, M., Šupová, M., Nováková, M., & Bičáková, O. (2025). Activated Carbon–Geopolymer Composites: Influence of Particle Size and Content on CO2 Adsorption and Mechanical and Thermal Properties. Crystals, 15(10), 892. https://doi.org/10.3390/cryst15100892







