1. Introduction
The paradigm shift from batch to continuous crystallization equipment marks a significant advancement in small-scale production and process development, particularly within the pharmaceutical and specialty chemistry industries [
1,
2,
3,
4,
5,
6,
7,
8]. This transition is driven by the need for increased flexibility and efficiency, allowing for rapid adjustments to market demands [
9,
10,
11].
Crystallization is a separation and purification process that is widely used in the process industry [
12,
13]. For many industrial crystallization processes, a draft tube baffle crystallizer (DTBC) is utilized. The most common application of this type of crystallizer is as an evaporative crystallizer for substances whose solubility depends only slightly on temperature [
14,
15,
16,
17]. However, it is also employed for antisolvent [
18] or cooling crystallization [
19]. Among various crystallizer types, the DTBC is prominent for its characteristic hydrodynamic behavior, which enables effective particle separation in the DTBC and thus a narrow crystal size distribution (CSD) in the product [
15,
17,
20]. To create this hydrodynamic behavior, a stirrer at the bottom of the DTBC generates a circulation flow by conveying the suspension from the body of the DTBC through the tube upward into the head, where it then flows back down between the tube and the outer wall in the annular gap. Due to the discontinuous cross-sectional jump as it flows back into the DTBC body, a classifier zone is formed that enables particle separation. Within the classifier zone, the particles separate based on their specific settling velocity, with smaller particles being drawn into the fine grain dissolution (FGD) for complete dissolution, while larger particles settle down with the gravitational force [
21]. While the DTBC in an industrial scale has been investigated in many aspects such as the hydrodynamic behavior with Computational Fluid Dynamics (CFD) simulations [
22,
23,
24,
25,
26], the influence of the dissolution of fine grain in the FGD [
27,
28,
29] and scale-up studies [
30,
31], the research on lab-scale DTBC has been limited on the startup behavior and residence time distribution during cooling crystallization [
19,
32]. The implementation of evaporation crystallization in a lab-scale DTBC is hampered by the FGD functionality. Further heating of the suspension in the FGD through the heat exchanger can cause unwanted evaporation of the solution in the FGD, potentially leading to uncontrolled crystallization. Adding geodetic height to the FGD increases the local pressure in the FGD and, consequently, the boiling point. This prevents evaporation in the FGD and creates a small operating window for the dissolution of fine grain in the FGD in a lab-scale DTBC [
33]. However, during continuous cooling crystallization blockages occurred [
19], particularly affecting the product removal due to high crystal content. Therefore, a reliable product removal system is important, ensuring that crystals are extracted without dead zones and with minimal stress to avoid mechanical strain and abrasion that could compromise quality [
34]. Since the DTBC is also used for vacuum evaporation crystallization, the product removal must be vacuum-tight as well.
Peristaltic pumps are typically used for the continuous conveying of crystallization suspensions due to their affordability and adjustable flow rate [
35]. However, this approach is not suitable for lab-scale vacuum applications, since collapsing prevents the use of sufficiently large tubing inner diameters to avoid clogging in the product tube. Gravimetric transfer via an overflow tube does not affect crystal size, but it requires a relatively homogeneous suspension in the crystallizer, making it particularly suitable for stirred tank and ideally mixed crystallizers [
36]. Schmalenberg et al. [
32] introduced a novel particle screw for efficient product removal, showing promising results for cooling crystallization. However, vacuum tightness is only achievable with a peristaltic pump, limiting its use under vacuum conditions.
For the enhancement of crystallization process performance, larger crystals can be selectively removed from the product. By using a so-called external classification, a partial stream is drawn from the crystallizer and large product crystals are separated using a hydrocyclone. In this method, part of the feed stream is fed through an inlet pipe positioned centrally at the bottom of the DTBC. In this pipe, only large crystals sediment against the upward flow generated in this way and are discharged at the bottom of the pipe [
26,
37]. However, these approaches are not applicable on a laboratory scale. The required upward flows are too high to be achieved by the feed flow, due to the small diameter. An alternative approach is the adjustment of the process parameters to influence the hydrodynamic behavior in such a way that large crystals with a narrow particle size distribution are preferentially discharged [
24,
25,
38,
39,
40,
41].
In this study a gate system for product removal from a DTBC with minimal impact on the crystallization process is presented. Similar to Acevedo et al. [
42], a transfer zone is created between two valves where the suspension flows in by gravity when the upper valve is opened. By evacuating the transfer zone, the influence on vacuum evaporation during crystallization can be minimized. The fully automated product removal system allows for semi-continuous product removal with a defined product removal rate through adjustable opening intervals.
The objective of this work is to investigate the influence of the volume flow in the FGD, the product removal rate, the stirring speed, and the solid content in suspension on the sedimentation behavior in a DTBC using the developed gate system. Therefore, the process parameters are systematically changed and the CSD in the FGD, and in the product, are examined. The aim is to enable process optimization through a selective product removal, which will ensure a narrow CSD on the product and compliance with the operating window of the FGD for evaporation crystallization. In addition, a reliable approach for continuous product removal in lab-scale application is demonstrated through the developed gate system, with its design and automation detailed in this work.
2. Materials and Methods
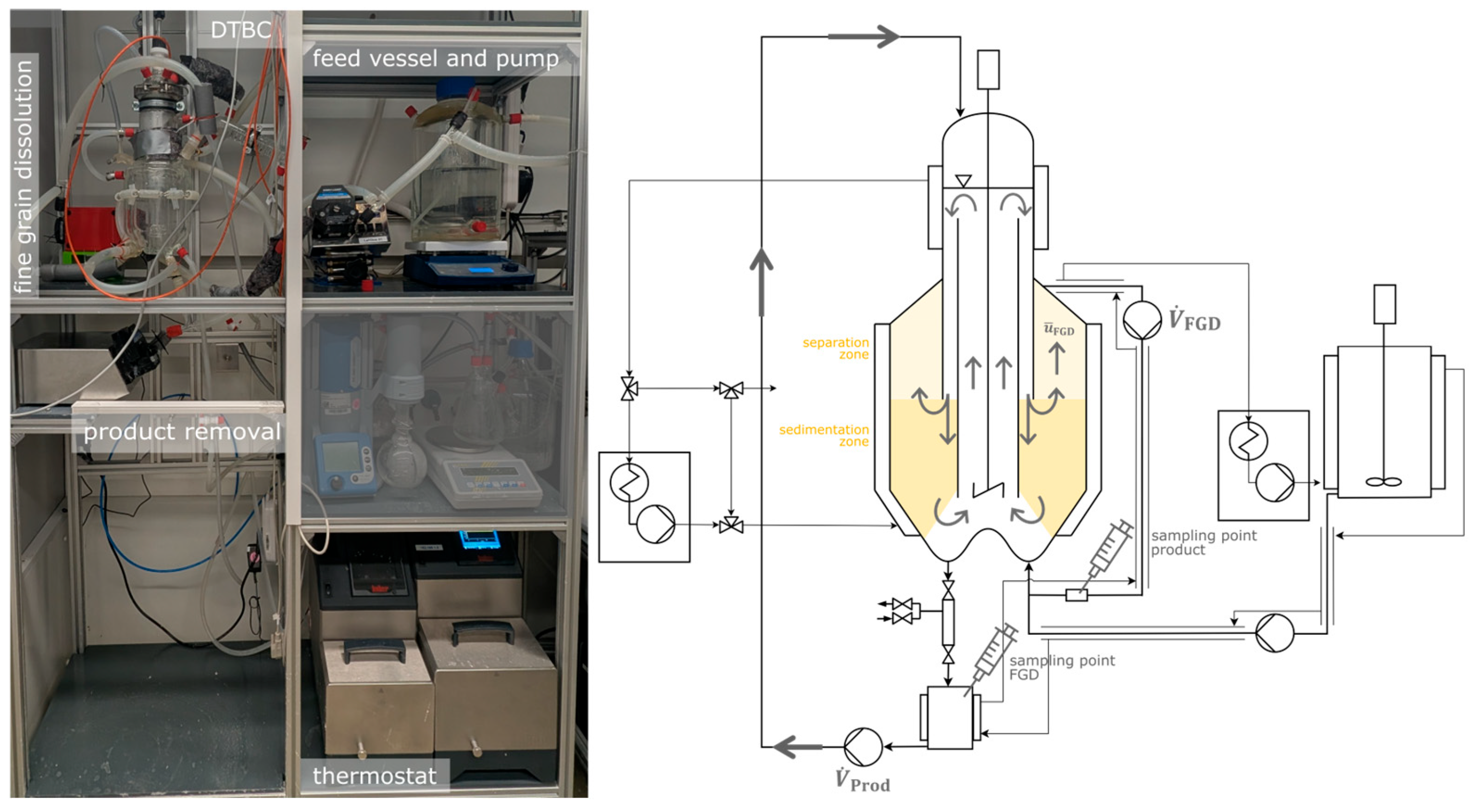
The classification experiments are conducted in a lab-scale DTBC made out of glass (glassblower workshop, TU Dortmund University, Dortmund, Germany) with an inner volume of 2.1 L. As test system, a binary model system of deionized water (<10 μS cm
−1) and the amino acid
l-alanine was used. The density difference between
l-alanine and water at 45.8 °C is 375 kg m
−3 [
43] and the experimentally determined correlation of solubility according to Wohlgemuth and Schembecker [
44] was applied to determine the solubility. Further properties of the test system can be found in the
Supporting Information (SI), Section S1. The experiments were carried out at a temperature of 45.9 °C.
2.1. Experimental Setup
This study investigates the classification within a DTBC by analyzing the CSD at various locations. A defined CSD is added to the solution instead of being grown in the DTBC, allowing the impact of hydrodynamic behavior to be assessed independently.
The characteristic vertical flow in the DTBC is generated by a five-blade diagonal stirrer made of glass (glassblower workshop, TU Dortmund, Dortmund, Germany) with a diameter of 35 mm and a height of 20 mm. The stirrer blade is positioned directly at the end of the DT over the W-shaped bottom and is driven by a laboratory motor RZR 2102 Control from Heidolph Scientific Products GmbH (Schwabach, Germany). The heat is supplied to the suspension in the DTBC by a two-stage heating jacket, which is circulated with deionized water from a thermostat (Thermostat 01, Pilot One Ministat 125, Huber Kältemaschinen GmbH, Offenburg, Germany). The inlet into the FGD is located on the upper right side of the DTBC body and the volume flow rate in the FGD () is adjusted using a Watson peristaltic pump (120S/DV, Watson-Marlow Pumps Group, Wilmington, DE, USA). A sampling point for the FGD is placed behind the pump. The polyvinyl chloride tubes of the FGD with an inner diameter of 3.2 mm (Tygon®, Saint-Gobain Corporation, Malvern, PA, USA) were insulated with a tube heater due to the temperature dependence of the l-alanine solubility in water. The water temperature in the tube heater was maintained at 45 °C using a thermostat (Ministat 230, Huber Kältemaschinen GmbH, Offenburg, Germany). In order to avoid any impacts on the added CSD, no heat exchanger is implemented in the FGD.
The product removal is positioned at the bottom of the DTBC, opposite the FGD. In order to ensure that the least amount of product is withdrawn and to maintain a constant CSD during the tests, the volume flow of the product (
) is recirculated back into the DTBC. For this purpose, a heated, double-walled glass vessel is used to collect the product and the suspension is pumped back into the head of the DTBC by a peristaltic pump (LabDos easy-load, HiTec Zang GmbH, Herzogenrath, Germany) with a volume flow of 200 mL min
−1. To prevent blockages, a tube with an inner diameter of 6.35 mm (TY6,35AG9,53, Norprene
® A-60-G, Saint-Gobain Performance Plastics Corporation, La Défense Cedex, France) is used. The entire setup is shown schematically in
Figure 1. A list of all equipment used can be found in the
SI, Table S1 and detailed information to the geometry of the DTBC is given in
Figure S1.
2.2. Product Removal System
A reliable product removal system is crucial for the realization of continuous crystallization. However, only a few solutions are available, especially for vacuum applications in a laboratory scale. For this reason, a product removal system has been developed that is suitable for removing solid products from laboratory equipment under vacuum, in particular from a lab-scale DTBC. The developed product removal system is based on the concept of a gate system, in which a gate volume is created between two valves that are opened and closed sequentially. By opening the upper valve, the suspension enters the previously evacuated gate volume. This minimizes the influence of product removal on the vacuum inside the DTBC. After the upper valve is closed, the gate volume is opened to ambient pressure so that the product suspension can be drained by opening the lower valve. In addition, the gate volume can be pressurized with 2 bar compressed air to flush out any remaining suspension from the gate volume. A flow sheet of the automated gate system is shown in
Figure 2.
Two pinch valves with an inner diameter of 10 mm (VMP010.03XK.72, AKO Armaturen & Separationstechnik GmbH, Trebur, Germany) are used for the gate system, as the inner diameter is only minimally reduced and no dead spaces occur. Due to the pneumatic control, a short setting time of approx. 0.05 s is achieved, enabling a defined product removal per valve opening. By quickly repeating the valve openings, an almost continuous product removal is possible. The pressurized air supply for opening the pinch valves is controlled by two 3/2-way solenoid valves (M07310HN/24DC, AKO Armaturen & Separationstechnik GmbH, Trebur, Germany). Further information about the developed gate system and the automation and the calibration is given in the
SI, Figures S4–S7 and Section S3.
Although the product removal system was specifically designed for application in a DTBC, it is equally suitable for other lab-scale operations under vacuum. The connection via DIN G3/8 fittings (Bohlender GmbH, Grünsfeld, Germany) enables straightforward integration with standard process equipment. The product removal rate is limited only by the valve switching time of approximately 0.05 s and the time required for evacuation of the gate volume. The calibration of the pinch valves with respect to the opening time of the upper valve is provided in the
SI, Figure S6. Furthermore, the automation concept is readily adaptable, as the control function is simple and can be implemented in other programming languages as well.
2.3. Experimental Procedure
For characterizing the classification in lab-scale DTBC, a suspension with a defined CSD is used. Therefore, six crystal classes were defined and added to the solution in equal weight proportions: 0–45 μm, 45–90 μm, 90–180 μm, 180–250 μm, 250–355 μm, and 355–500 μm.
The crystals are prepared through grinding and sieving and three samples are taken to determine the initial CSD (CSDinit). The saturated solution is prepared at least 12 h before the experiments at 55 °C in a feed vessel and subsequently pumped into the DTBC before the experiments. Before setting the desired stirring speed, the crystals are seeded into the saturated solution within the DTBC. The flow rate of is set by the peristaltic pump in the FGD, while the flow rate for is set through the opening intervals of the gate system.
The operating ranges for
and
are defined using the fines-removal rate
in Equation (1) [
13]. The fines removal rate relates the dissolution of the fine grain to the product removal rate, providing a measure to define a stable crystallization process, typically within the range of 2.62 to 6.55 [
29,
45].
Due to the potential evaporation of the solution in the FGD, only a narrow operating window with a limited temperature range is feasible. Preliminary experiments were conducted to determine the maximum
, at which complete dissolution were achieved for fine particles with a crystal size of 90 μm and a solid content of 0.5 wt%. Therefore, 25 mL min
−1 ± 5 mL min
−1 is chosen as the operating range for
. By applying the recommended
= 2.76 for a stable evaporation crystallization proposed by Sutradhar and Randolph [
27], an operating point for
of 15 mL min
−1 was calculated. For defining an operating range for
, the operating points at
± 5 mL min
−1 were also examined. Prior to the classification experiments, it was experimentally determined that complete suspension of the crystals in the lab-scale DTBC is achieved at a stirring speed of 800 rpm. To enable sedimentation of the product crystals, stirring speeds of 500, 600, and 700 rpm were investigated. The impact of
,
, and stirring speed on classification is examined by considering all parameter combinations, resulting in a total of 27 operating points. The investigated parameters are summarized in
Table 1.
The experiments are carried out at a constant solid content of 1 wt% and a constant stirring speed, whereas a variation of and led to a total of nine operating points per experiment. In order to investigate the classification for different points in time during the crystallization process, experiments are carried out at higher solid contents of 3 wt% and 5 wt% at a = 20 mL min−1 and = 30 mL min−1. All experiments are conducted three times.
2.4. Image Analysis and Determination of the CSD
The classification within the DTBC is evaluated based on the CSD in the product (CSD
Prod) and the FGD (CSD
FGD). For this purpose, two samples are taken with a preheated syringe for each operating point: one from the sampling point of the FGD and the other from the product vessel. To counteract potential changes in crystal size, crystals are directly separated from the saturated solution by filtration, dried, and resuspended in 5 mL of ethanol, in which
l-alanine has low solubility. The workflow for sample preparation was adapted from the method described by Höving et al. [
46] and is illustrated in
Figure S2. The crystals in the resuspended samples are photographed in a Petri dish at 10× magnification (DIN lens 10×, Bresser GmbH, Rhede, Germany) using a Nikon Z6 II camera (Nikon Corporation, Tokyo, Japan), as shown in
Figure S3 in the SI. To ensure that no crystal is photographed twice, squares of cm
2 are defined using graph paper, which are subsequently focused on in the photographs.
For a representative CSD, the crystal size of at least 200 crystals per operating point are determined. The Feret diameter was determined according to [
47]. In
Figure 3, the marking of the crystals with polygons and ovals for determining the Feret diameter is exemplarily illustrated.
From the total number of particle sizes determined according to the Feret diameter, the CSD was calculated according to Equation (2) and density distribution
according to Equation (3). For
r = 0, 1, or 2, the distribution is characterized either by the number, length, or area of the crystals.
The relative frequency
is calculated by normalizing
over the interval width
. The variable
defines a crystal size threshold for a certain percentage
i, which describes percentiles at different levels with a range of
i = 1, 2, …,
n. Accordingly,
represents the maximum crystal size below which 100% of the crystal population is contained. For an intuitive understanding, the CSD are evaluated based on the resulting
distributions. Additionally, the distributions are visualized using boxplots, which allow estimation of statistical values such as median (
). The central crystal size in the distribution is characterized by
, indicating the crystal size above which 50% of the crystals are larger. The 25th and 75th percentiles (
and
) define lower and upper edges of the boxplots, corresponding to the particle sizes below which 25% and 75% of the crystals fall, respectively. To assess the homogeneity of the particle size distribution, the interquartile range (IQR) as a difference between
and
is calculated. Since the focus of this work is on the quantitative comparison of the distributions rather than on exact statistical values, the percentiles are approximated from the boxplots [
12,
48,
49].
2.5. Description of the Suspension Behavior
The classification in the DTBC depends mainly on particle separation in the separation zone in the upper half of the DTBC body (see
Figure 1). Within this zone, the particle separation is influenced by gravity, the flow regime, and the inflow velocity into the FGD
. To describe particle separation,
is calculated using Equation (4), while the specific particle settling velocity
is described by Equation (5), assuming perfectly spherical particles [
50].
Since the diameter of the FGD inlet connector () cannot be modified during the experiments, any change of can only be achieved by adjusting , according to Equation (4). In addition to the crystal density and the solution density , the settling velocity depends on gravity and the particle size . Furthermore, is influenced by the drag coefficient , which is a function of the Reynolds number and thus of the prevailing flow regime. For crystal separation, the criterion < must be fulfilled. The crystal size at which both velocities are equal is referred to as the cut-off particle size. Crystals larger than this threshold remain in the DTBC and settle down in the sedimentation zone in the lower half of the DTBC body, whereas smaller ones are withdrawn into the FGD and dissolved.
In the sedimentation zone, interactions between particles lead to a kind of collective hindrance effect, in which the upward flow of fluid between the settling particles slows down the sinking of the neighboring particles. To account for the influence of the volume fraction of particles in the suspension on the cut-off particle size, the approach of Richardson-Zaki in Equation (6) is used to calculate the swarm settling velocity
. This approach considers the particle volume fraction
and the flow regime through the exponent
n.
According to Equation (6), particles in suspensions with high solid concentrations exhibit lower settling velocities than those in dilute suspensions [
49]. The energy introduced by stirring counteracts the sedimentation of the particles. Due to its influence on the flow regime, the stirring speed has a direct effect on
and thus on the cut-off particle size.
3. Results and Discussion
The presented setup and methodology are designed to investigate the characteristic classification of crystals along the DTBC body. The objective is to characterize how changes in process parameters affect the hydrodynamic behavior within the DTBC and the suspension behavior of the crystals. This allows assessment of how the CSD in the product and the FGD can be influenced by targeted adjustment of
and
, and the stirring speed for different solid contents in the suspension. The experiments are based on the added CSD
init, which contains equal weight proportions of six crystal size classes. To evaluate classification, CSD
FGD and CSD
Prod are compared to CSD
init. The relative frequency q
0 and the cumulative distribution Q
0 of CSD
init are shown in
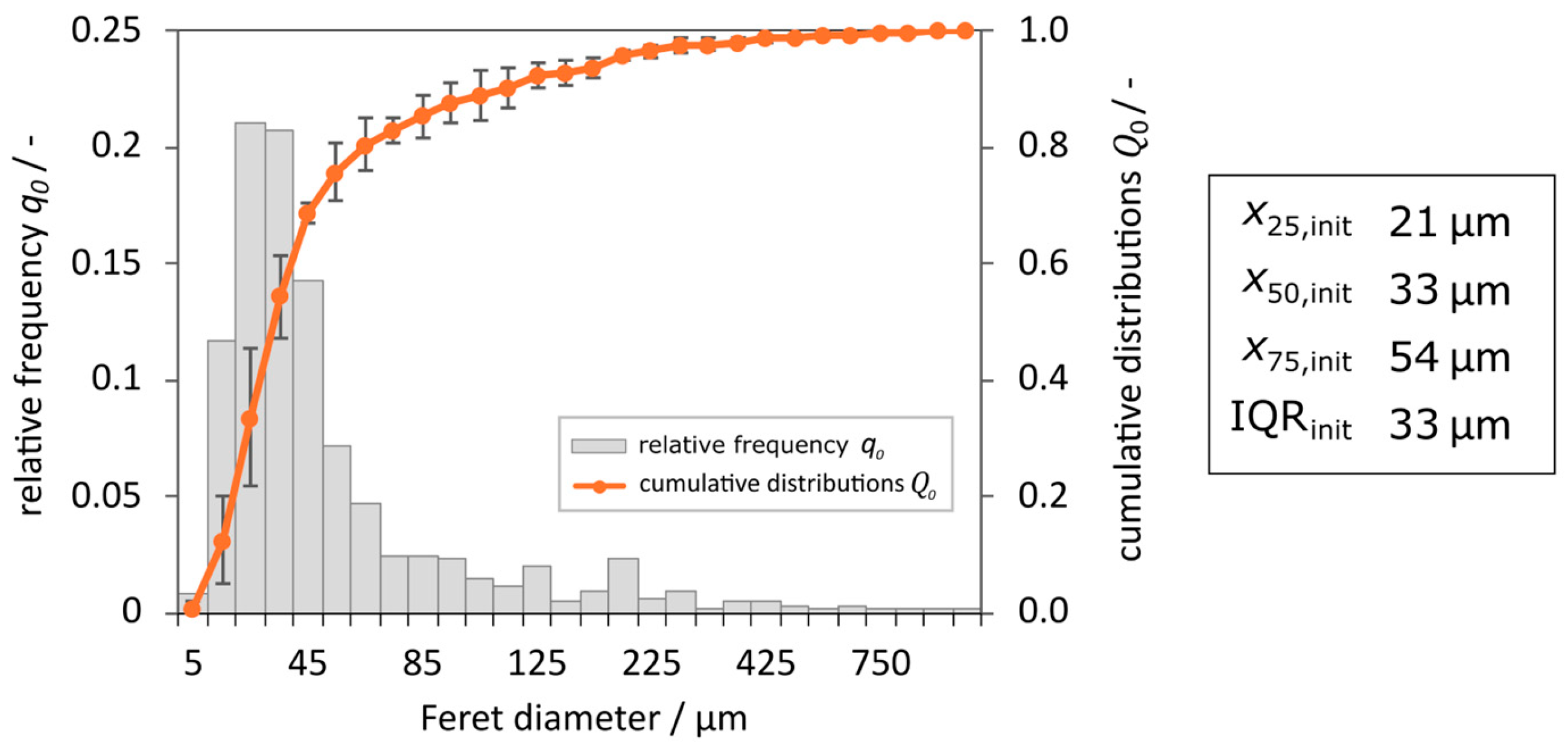
Figure 4.
As shown by the relative frequency
q0 in
Figure 4, the peak, and thus the majority of particles, occurs at approximately 30 µm. Around this value the particle distribution is narrow. For particle sizes above 30 µm, the distribution decreases sharply with increasing crystal size, so that only scattered particles were counted for crystal sizes > 500 µm. This is also apparent in the cumulative distribution function, which shows a steep rise at the beginning and then flattens out, indicating that the initial distribution contains many smaller crystals with a size above 100 µm but few larger ones. These observations are confirmed by the statistical values. The median value of CSD
init is
x50,init = 33 µm, while
x25,init = 21 µm, indicating the narrow distribution of small particles characterized by an IQR
init of 33 µm. Furthermore, a strong decrease in the relative frequency
q0 for particles larger than 50 µm is observed, which is also evident by a value of
x50,init = 54 µm.
Since a higher number of smaller particles is required to achieve the same weight as larger crystals, the shift towards smaller particles in CSDinit can be explained by using equal masses of each crystal class. Maintaining a clear classification effect in the product requires minimizing the sedimentation of smaller crystals into the product stream to achieve a high x25,Prod. For the CSDFGD, a clear classification is not expected, since the initial distribution is already close to the expected CSDFGD with x75,FGD < 100 µm. Instead, the focus is on minimizing the number of large crystals entering the FGD, keeping x75,FGD as low as possible.
3.1. Impact of Different Flow Rates
The particle separation and classification in the DTBC is based on the particle velocity in the separation zone. As described in
Section 2.5, the flow rate of
has a direct impact on the cut-off particle size, thereby influencing the classification within the DTBC. To characterize the actual influence of the flow rates of
and
on the classification, CSD
Prod and CSD
FGD will be examined at a solid content of 1 wt% and a stirrer speed of 500 rpm. Additionally, to account for potential correlations between the volume flow rates, every possible combination of
and
was considered. The corresponding boxplots of the resulting CSD
FGD are shown in
Figure 5.
Figure 5 shows the boxplots of CSD
FGD and CSD
Prod in three blocks with constant
per block, in which
increases from left to right. The IQR and median values for CSD
FGD (
x50,FGD) and CSD
Prod (
x50,Prod) are higher than those for CSD
init for all operating points. For
= 10 mL min
−1 and
= 20 mL min
−1, the highest values of approximately
x25,FGD = 70 µm,
x50,FGD = 90 µm, and
x75,FGD = 130 µm are reached. Additionally, the whiskers and outliers of the boxplots show that crystals with a crystal size > 400 µm were counted in CSD
FGD. From this, it can be concluded that there is no sharp separation in the cut-off particle size, or that the cut-off particle size must be >400 µm.
For CSDProd, the expected classification effect of an DTBC is recognizable in the significant increase in statistical values across all operation points compared to CSDinit. The values of x25,Prod are above 100 μm and thus significantly above all values of x75,init. The values of x50,Prod are above 170 μm and for x75,Prod at least above 300 μm.
A direct comparison of the statistical values for the different flow rates of at the respective flow rate of only shows a small increasing of the values of x25,FGD, x50,FGD, and x75,FGD. When the influence of different on CSDProd is compared, it becomes evident that the values of x75,Prod and x50,Prod for = 15 and 20 mL min−1 are only slightly higher than for = 10 mL min−1. Therefore, the influence of on the suspension behavior was considered to be minor.
However, when comparing the CSD
FGD for different
values, a particular increase in
x75,FGD and IQR
FGD is observed with increasing
. The highest statistical values are consistently achieved at the maximum flow rate of
= 30 mL min
−1. The influence of
on CSD
Prod is evident from the increase in
x25,Prod with increasing
. Similar to the observations for CSD
FGD, the enhanced classification at higher
can be explained by an increasing
, and consequently, a larger cut-off particle size, as described in
Section 2.5. As a higher
leads to a higher likelihood of smaller crystals being entrained into the FGD instead of settling into the product removal, a higher product quality with higher flow rates of
were expected.
3.2. Impact of Different Stirring Speeds
The characteristic hydrodynamic behavior in the DTBC requires energy input from a stirrer. While ensuring circulation through the tube, the stirrer simultaneously generates turbulence in the crystallizer body, which counteracts sedimentation. To evaluate the influence of stirring speed on CSD
Prod and CSD
FGD, and to assess whether the effects observed for varying flow rates are also applicable at different stirring speeds, experiments were conducted at several rpm values for
= 30 mL min
−1. Since increasing the stirring speed affects the sedimentation behavior in the opposite way to the flow rate in the product removal, the CSDs are analyzed for different flow rates of
in
Figure 6.
In
Figure 6, the higher distributions of CSD
FGD and CSD
Prod compared to CSD
init indicates that a classification effect also occurs at higher stirrer speeds. Regardless of the stirring speed, it is observable, that an increase in
is associated with a decrease in the statistical values within the CSD
FGD. This suggests that fewer larger particles are being drawn into the FGD. Additionally, an increase of
x50,Prod and
x25,Prod is evident for higher flow rates in
in the CSD
Prod. Since
remains unchanged, this effect cannot be attributed to an increase in
but must instead result from the increase in
. A possible explanation for this observation is that opening the valve causes a sudden increase in the fluid velocity in the DTBC, which accelerates the settling of the particles in the sedimentation zone. Since smaller particles have already been separated in the separation zone and drawn into the FDG, they are less affected by the acceleration, which explains the decrease in
x50,FGD and
x75,FGD. The diameter of the DTBC is ten-times larger than that of the product outlet, hence, according to mass conservation, this results in only a slight increase in fluid velocity. Nevertheless, this acceleration may be sufficient at
= 15 and 20 mL min
−1 to increase
and thereby enhance sedimentation.
By comparing the influence of the stirring speed on the classification, it can be seen, that for a stirring speed of 700 rpm, the values of x50,Prod are between 150–170 μm and x75,Prod between 250–350 μm, which is significantly lower than the values at a stirring speed of 500 rpm, where x50,Prod is between 180–200 μm and x75,Prod are between 380–440 μm. However, the most pronounced decrease in statistical values is observed at 600 rpm. For this stirring speed, the values of x50,Prod are between 100–120 μm and for x75,Prod between 170–240 μm. One possible explanation for the decrease in CSDProd at higher stirring speeds is a change in the height of the zones within the DTBC.
Figure S8 in the SI provides images of the separation and sedimentation zones at 600 rpm and 700 rpm. The separation zone can be identified as the region with less pronounced turbidity, which is attributable to a lower suspension density in this zone. As shown in
Figure S8, with increasing rpm, the separation zone becomes progressively smaller, resulting in a lower suspension density in the sedimentation zone. According to Sha et al. [
38], suspension density significantly influences classification, because
decreases when the solid content is higher. This explains the improved classification observed at 700 rpm compared to 600 rpm. In contrast to 700 rpm, suspension density at 600 rpm is highest at the center of the DTBC, which most strongly restricts sedimentation in this region. At 500 rpm, the lower energy input from the stirrer is expected to result in an increased suspension density in the lower half of the DTBC, which means that particle separation in the classifier is less affected and large particles settle. Due to the enabled sedimentation, a higher proportion of larger particles is discharged during product removal.
3.3. Impact of Different Solid Fractions
In a continuous crystallization process, varying solid contents in the suspension are achieved depending on flow rates, energy input, and residence time. The particle settling velocity and the suspension behavior are influenced by these solid content variations. To investigate the impact of solid content on classification, CSD
FGD and CSD
Prod at solid contents of 1 wt%, 3 wt%, and 5 wt% are compared in
Figure 7. Similar to the analysis of stirring speed effects, the experiments are conducted at flow rates of
= 30 mL min
−1 and
= 20 mL min
−1. To ensure sufficient suspension even at higher solid contents, the experiments are performed at stirring speeds of 600, 700, and 800 rpm.
For the influence of the stirring speed on CSD
FGD at higher solid fractions,
Figure 7 shows an increase in
x50,FGD,
x25,FGD,
x75,FGD, and IQR
FGD. This observation is consistent with the results for increased stirring speed at a solid content of 1 wt%, where the statistical values also increased with higher stirring speed. This confirms that an increase in stirring speed, regardless of solid content, leads to better suspension, allowing larger particles to enter the FGD.
The combined consideration of the impacts of stirring speed and solid content shows that the solid content has an even greater impact on the classification than the stirring speed. For a solid content of 1 wt%, the smallest values of x50,Prod and x25,Prod are observed. For the solid contents of 3 wt% and 5 wt%, the best classification with the highest values for x50,Prod and x75,Prod and therefore the highest product quality are achieved. In contrast to 3 wt%, the values at 5 wt% are not constant and increase for higher stirring speeds. These results can be explained by a balance between suspension and sedimentation that has to be found for the optimal operation of a DTBC. The stirring speed has a direct impact on the suspension state in the DTBC and thus, on the particle density in the sedimentation zone. However, a high energy input by the stirrer can also impairs classification performance. With an almost homogeneous suspension, the cut-off particle size can increase so much that too many larger particles enter the FGD, where they can no longer be dissolved. Additionally, sedimentation into the product removal is minimized, which results in suspension is drawn into the product removal without classification and therefore in lower product quality. Therefore, the stirring speed must be adjusted according to the solid content.
For a solid content of 5 wt%, all three statistical values increase with rising stirring speed in the CSDProd. At 800 rpm, the maximum value of approximately x75,Prod = 750 µm, is reached. Consequently, for a solid content of 5 wt%, stirring speeds above 700 rpm are advantageous, as sufficient energy is introduced to increase the cut-off particle size for an optimized CSDProd. For a solid content of 1 wt%, the lowest product quality is observed at the considered stirring speeds. This can be attributed to an excessively high stirring speed, which results in an almost homogeneous suspension in the DTBC. For suspensions with a solids content of less than 3 wt%, a maximum stirring speed of 500 rpm should therefore be set to obtain a CSDProd with a high x50,Prod. Since the suspension behavior for a suspension with a solids content of 3 wt% has not yet been investigated at 500 rpm, further investigations are necessary to confirm this assumption.
The highest values for x50,Prod and x25,Prod, and thus the best classification performance, were obtained at a solid content of 3 wt%. Across all three stirring speeds, x25,Prod remained constant at approximately 200 µm. For x50,Prod, a decreasing trend with increasing stirring speed was observed, the values are declining from about 500 µm to 450 µm at 700 rpm and to 400 µm at 800 rpm. The operating point with the highest x50,Prod value and the narrowest CSDProd is therefore observed at 3 wt%, 600 rpm, = 20 mL min−1, and a flow rate in the FGD of = 30 mL min−1.
4. Conclusions
In this study a newly developed gate system is presented for a continuous product removal for laboratory-scale vacuum crystallization. Over continuous testing periods lasting up to 6 h per experiment and cumulatively exceeding for 30 h, no blockages occurred in the product removal. This demonstrates the reliability of the system as a removal system for solid products and also illustrates a potential approach to overcoming challenges in product removal for other lab-scale vacuum applications.
Furthermore, this study investigated the optimization of the crystallization process in a 2.1 L draft tube baffle crystallizer (DTBC) through selective product removal by adjusting process parameters. Systematic experiments were conducted to evaluate classification performance, with crystal size distributions (CSD) in the fine grain dissolution and product analyzed based on statistical values.
The results demonstrated a clear and expected effect of the flow rate in the fine grain dissolution on classification, as it directly influences the cut-off particle size. While previous studies on larger-scale DTBC systems have primarily linked flow rate effects to residence time, this work revealed that higher volume flow rates in the product removal directly impact classification within the DTBC. Specifically, higher product removal rates accelerate particle settling velocity, resulting in CSDs with larger particles in the product. In general, higher flow rates in the fine grain dissolution and higher product removal rates promoted improved classification outcomes for the CSD in the product.
The stirring speed also emerged as a critical parameter due to its influence on the suspension and the height of the sedimentation zone in the DTBC body. In order to achieve an optimal classification in the DTBC, a balance has to be maintained between suspension and sedimentation. The energy input from the stirrer needs to be sufficient to increase the cut-off particle size. However, the stirring speed should not be set too high to avoid an almost homogeneous suspension. This would minimize the sedimentation of larger particles in the product removal and thus reduce product quality. Therefore, the stirring speed must be adjusted according to solid fraction. For this DTBC setup, the optimal range was identified at 500–600 rpm for a solid fraction of 3 wt%, where the best operating point with the narrowest crystal size distribution in the product was achieved. Lower stirring speed of <400 rpm is recommended for lower solid fractions such as 1 wt%, while higher stirring speeds of >700 rpm are preferable for solid fractions of 5 wt%. Additional experiments are needed to validate these results across solid contents ranging from 3–7 wt% and stirring speeds between 400–800 rpm.
The results in this work establish a foundation for the operation of a lab-scale DTBC by enabling an assessment of how process parameter variations influence the CSD in the fine grain dissolution and the product. As the present study focused on the suspension behavior of crystals while neglecting crystal growth, additional investigations are required to translate these results to an actual crystallization process. In this context, it must be considered that, particularly during the start-up phase, the solid content increases dynamically until a balance between abrasion and nucleation is established in the DTBC.
For future work, additional analytical methods, such as X-ray diffraction, can be employed to study the crystallization process in more detail, particularly to monitor crystal form and size during nucleation and growth. Moreover, more complex interactions are expected when crystallization dynamics are included. In such cases, machine learning approaches, e.g., random forest models such as XGBoost, can be applied to evaluate multifactorial interdependencies. The results obtained here can thus serve as a basis for future studies and control strategies aimed at ensuring consistent product quality throughout the entire crystallization process.
Additionally, the shown operating ranges refer specifically to the used test system l-alanine in water. For substance systems with different density differences or crystal shapes, the trends discussed are generally transferable, as the effects described occur independently of these parameters. However, the specified operating ranges may differ, because for the suspension of particles with a higher density differences greater energy input from the stirrer is required. Therefore, repeating the experiments is necessary to define the operating ranges for other substance systems.