Abstract
Graphene Nano-Carbons (GNCs) have a huge potential, but current production methods limit their exploration and use. Many GNCs will be explored here with a focus on CNTs (Carbon NanoTubes) (which have some of the highest strengths of any known material, conductivity, EMF absorptivity, and many other useful properties. Manufacturing them abundantly, inexpensively, and in eco-friendly ways remains a significant challenge. Two CNT/GNCs production methods are compared and reviewed. Traditional Chemical Vapor Deposition (CVD) production heats organic reactants with metal catalysts to form GNC/CNTs. As of now, the CVD CNT production has been limited by the high-energy costs, costs per weight comparable to precious metals, and a high CO2-footprint. C2CNT is an electrochemical methodology that overcomes the constraints of CVD, while producing high-quality CNT/GNCs. C2CNT is a molten carbonate CO2-electrolysis that makes GNCs. The C2CNT process also selectively produces a wider variety of CNTs (including helical, magnetic, and doped) and GNCs with higher product specificity than CVD by fine-tuning electrolysis parameters. The wide variety of CNTs/GNCs that can be produced by each of these methods is reviewed and discussed. The goal of this perspective is to compare GNC production methods.
1. GNC and CNT Overview and Environmental Impact
GNCs (graphene nanocarbons) are a series of carbon allotropes, derived from 0D, 1D, 2D or 3D arrangements of graphene. One of the most widely explored of these GNCs are CNTs (carbon nanotubes), with one dimensional symmetry consisting of one or more concentric graphene cylinders. CNTs, due to their layered cylindrical morphology and graphene-based structure, exhibit high strength, flexibility, efficient charge storage, great thermal conductivity, and catalytic functionality [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. These properties have resulted in CNT applications in a growing number of charge storage, polymers, medical, electronic and composite uses. Commercially. CNTs are mass produced by traditional Chemical Vapor Deposition (CVD). The high energy use and high materials consumption in the production of CNTs by CVD, along with the high carbon footprint of CVD production, has led to a high market cost for commercial CNTs, which has limited the rate of market growth of these materials. Nevertheless, these unique one-dimensional graphene allotropes of carbon have a burgeoning global 2025 market of $US 3 billion, which is projected to more than double by 2030 [15].
To facilitate global use of these highly functional GNC materials, including CNTs, there is a need for more cost effective and more environmentally benign GNC production methodologies. The goal of this perspective is to compare and review two GNC production methods, with a focus on the production of CNTs. Traditional Chemical Vapor Deposition (CVD) GNC production is compared to an alternative, large scale electrochemical production methodology, C2CNT (CO2 to Carbon Nanomaterial Technology). C2CNT produces high-quality CNT/GNCs by the electrolysis of CO2 in a molten carbonate bath.
The selective fabrication of tailored GNCs, such as doped CNTs, Helical CNTs (HCNTs), Magnetic CNTs, Nano-bamboo or Nano-pearl morphology CNTs, or macro assemblies of CNTs, is relatively straightforward within the electrochemical system, while it can be complex in the CVD process [16,17,18,19,20,21,22]. Switching between the molten carbonate production of various GNCs is straightforward and does not incur additional costs. CNT Doping is accomplished by introducing the appropriate inorganic dopant salts into the electrolyte for C2CNT or in the precursor stream for CVD. Among the various dopants investigated, boron, nitrogen and phosphorous have been studied more extensively than sulfur [1,20]. Replacing carbon atoms with nitrogen or phosphorus induces curvature in the graphene structure through the formation of pentagonal rings and facilitates cross-linking between graphene layers. The curvature and interconnection of graphene layers enhance the mechanical strength of the planar sp2-bonded network by extending it into three dimensions. Energy calculations indicate with sulfur doping in CSₓ structures, pentagon and double-pentagon defects, exhibit notable stability. This stability arises from the sulfur atom’s ability to utilize its expanded d-orbitals, which helps stabilize the curvature in S-doped, pentagon-rich graphene-like layers [23,24].
B-doping has been shown to enhance the CNT’s electronic conductivity by about an order of magnitude [1,20]. Magnetic CNTs (MCNTs), with Ni or Fe in them, which are recoverable for recycling, have been synthesized by C2CNT and, to a lesser degree, CVD. Other forms of CNT synthesized include nano-bamboo; HCNTs from under high growth rate conditions, particularly in the presence of oxide species that promote sp3 defect-induced curvature; porous CNT networks, and nano-pearl CNT morphologies [16,17,18,19]. GNCs in composite materials can further contribute to greenhouse gas (GHG) reduction by decreasing the GHG-footprint of conventional products such as metals, cement, and plastics [25,26,27,28]. In composites of metals or cements, GNCs incorporated at a dosage of 0.05–3 wt%, can increase tensile strength >35% and durability, while additions of 0.5–3 wt% to polymers increase strength by 50%, and hardness by 4% [26]. Buckypaper, produced purely with CNTs, exhibits a low electrical resistivity of 0.9 × 10−11 Ohm meters, which is expected to decrease further with B-doping [29]. Beyond their established applications, such as in energy storage and structural composites, CNTs have been successfully used in a variety of functional materials, including buckypaper, polymers/plastics, and plasma-based systems [25,26,27,28,29,30,31,32,33,34,35,36,37,38].
CO2 induced global warming is of grave concern. CO2 emissions globally in 2024 reached 37.8 Gt annually and continue to increase [39,40,41,42]. Despite renewable energy growth, the United States of America has increased fossil fuel infrastructure, and total energy-related CO2 emissions increased by 0.8% in 2024, hitting an all-time high of 37.8 Gt CO2 [43]. Accelerated GHG-emissions are driving global temperature increases, rising sea levels, and extreme climate events at rates faster than predicted, posing an existential threat to Earth. CO2 removal is crucial to mitigate climate-driven biodiversity loss and economic disruption; a transition to renewable power alone will not suffice to curb the catastrophic pace of global warming [39,40,41,42].
CVD production of carbon nanotubes is a significant CO2 emitter [1,2]. Alternatively, GNCs such as CNTs could trap carbon if formed from CO2, as they have stability on the order of 100s of millions of years, as demonstrated by the stability of graphite, which is composed of many imperfect layers of graphene [26,27]. CNTs offer greater stability than graphene as they have fewer exposed edges. This may make it ideal to store captured carbon, if it does not release more than it captures during formation, and may store other pollutants, such as sulfur, through doping and adsorption/absorption. Matrixes of other materials surrounding the GNC and intermetallic compounds may provide further stabilization, acting as a catalyst to repair damage, as a physical barrier, or as a sacrificial element instead. This matrix may also increase their safety.
One common carbon feed source for CVD production is methane [44]. Some emissions from CVD have a higher ability to warm the Earth than CO2. For example, CH4 is 27–30× more potent, N2O is 273× more potent, and fluorine-based compounds are even more [45,46,47].
Large-scale pathways to produce GNCs include:
CVD (Chemical Vapor Deposition): CVD is currently used in the commercial production of GNCs. CVD functions by depositing a chemical vapor on a substrate, generally by introducing a feed gas or gases at high temperature and reacting them on a substrate. CVD is energy intensive, limited by Carnot Cycle (heat engine) and secondary energy losses. Carnot cycle limited efficiencies are expressed by:
EFC = (TH − TC)/TH
EFC is Carnot Efficiency, TH is the hot cycle temperature, and Tc the cold cycle.
Chemical vapor deposition has existed for arguably millennia and has been used to manufacture a wide variety of substances, such as silicon wafers, germanium wafers, specialized ceramics and glasses, and more beyond GNCs [2]. CVD is known for producing high-purity, uniform, and large crystal structures with few or no defects. Variants of CVD that are used in the production of CNTs are summarized in Figure 1.
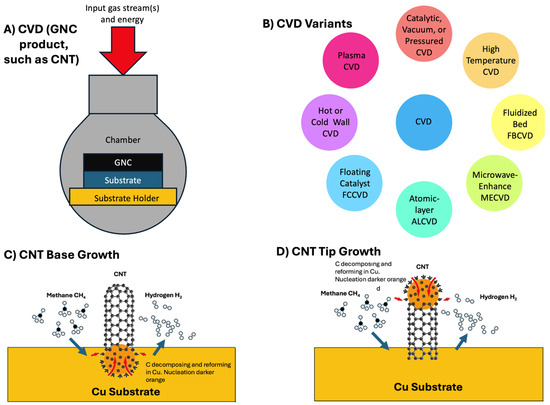
Figure 1.
(A) A general diagram of CVD. (B) Types of CVD commonly used to explore CNT, graphene, and carbon nano-onion production. (C) CVD Base CNT growth example. (D) CVD Tip CNT growth example.
C2CNT, uses high-temperature molten carbonate electrolysis of CO2. CO2 is split into a high purity GNC carbon product at the cathode and O2 at the anode. The specific GNC product, such various CNTs, is selected for, and controlled by, the electrochemical conditions (such as electrolyte and electrode composition, current density). The High temperatures of the electrolysis increases efficiencies beyond that of heat engines.
C2CNT is an electrochemical process that increases efficiency, which as with the increased efficiency of batteries and fuel cells compared to heat engines, is not limited by Carnot Efficiency losses by rather by the Gibbs free Energy:
where G = Gibbs free energy, H = enthalpy, T = temperature in kelvin, and S = entropy.
ΔG = ΔH − TΔS
As illustrated in Figure 2, C2CNT, from introduction of the feedstock CO2, to production of the GNC product at the cathode, is a single step process. In C2CNT, the high concentration of carbonate at the cathode surface minimizes mass transport losses and ensures low electrolysis potentials. Variations of C2CNT that have been developed include, the use of renewable electricity to drive the electrolysis process, the specific use of split solar (termed STEP Carbon Capture, in which solar visible generate PV electricity, and solar IR heat) [16], CCUS C2CNT for decarbonization of concentrated (such as industrial) CO2 sources [48,49], and DAC C2CNT, for direct air decarbonization [50].
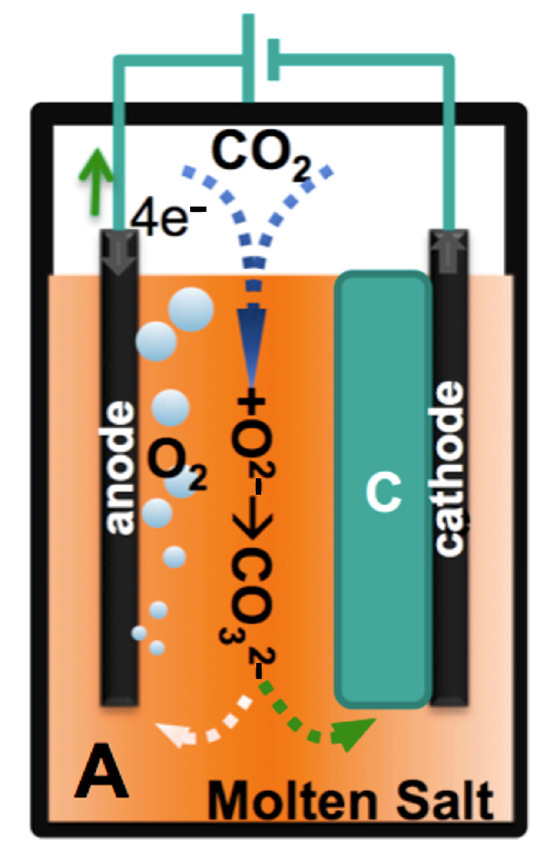
Figure 2.
CO2 electrolysis in molten carbonate producing “C”, GNC products. From reference [19].
The goal of this perspective is to compare GNC CVD and C2CNT production methods, with a focus on the production of CNTs.
2. CVD History
The first use of CVD may have originated in the Syrian cultures ~300 B.C. [51,52]. These cultures had priests who deposited CuS and CuAs by using vapor reactions on copper or copper alloy surfaces for artistic reasons. Later in the 1880s, industrially, carbon was deposited on metallic surfaces to make filaments for carbon lamps, and for the Mond process, which was used to make high-purity nickel deposits [1,51,52]. Atmospheric-pressure CVD with precursor hydrides is one of the oldest and most common methods for thin film forming, utilizing PH3, silane gas, B2H6, and O2 diluted with nitrogen at 510–770 k [51,52,53]. CVD in the 1950s was used to coat graphite susceptors (carriers and heaters for substrates used in other CVD methods) for the zone-refining of InSb and Ge, as well as direct growth of Ge. During the 1960s, epitaxial films of III-IV semiconducting compounds were obtained by CVD. They opened the way for Si-based electronics devices and production of integrated circuitry [51,52].
3. Introduction to CVD
3.1. General CVD
CVD, as illustrated in Figure 1A, is the most used process to make GNCs, mostly CNT and graphene [53]. It functions by heating a vapor, sometimes ionizing, and then depositing it on a substrate [52,53]. The substrate often acts as a catalyst that induces the correct chemical reactions and selects the correct products. For example, in CVD for making carbon products, the substrate may be numerous metallic (Cu, Cu-alloys, Ni, and iron alloys are widely used) or semi-metallic (usually silicon and, to a lesser extent, Mn or, 79Ge) [51], alternatively, a metal catalyst can be incorporated as part of the feed gas (es), such as in metallo-organics. The main reactions in CVD can be simple or complex, with various organic or metallo-organics being reduced to carbon with waste products of either H2, water, CO2, CO, metal nanoparticles, metal carbides, or metal oxides [51,52,53]. The input gas can be as simple as methane to much more complex organometallics such as Fe(C5H5)2, or even more complex substances [45,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Thermal chemical vapor deposition (CVD) is now a well-established and efficient method widely used in industry, such as for producing vertical graphene nanosheets, as shown in Figure 3 [65]. It offers advantages like compatibility with substrates of various shapes and sizes, high production yield, and relatively low equipment costs, for large-scale manufacturing [66].
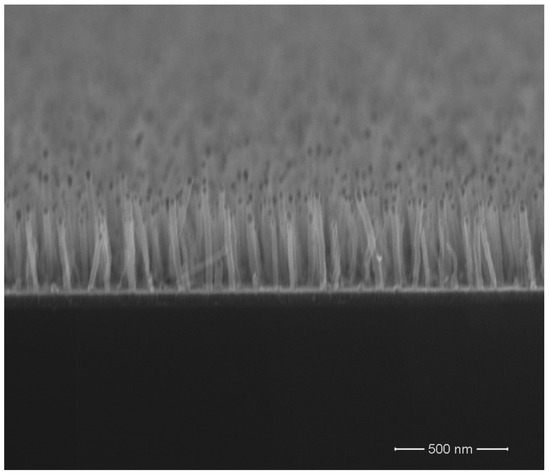
Figure 3.
SEM of CVD CNT grown with a fixed CH4:NH3 = 70:50 sccm gas flow ratio 800 °C, for 1 h. From reference [65]. Open access by CC 3.0.
As illustrated in Figure 1C,D CNT CVD growth can occur either from catalyst remaining at the base of the substrate (1C), or from catalyst which remains at the tip of the growing CNT (tip growth), which often leads to higher product purity and can produce longer CNT. Both types of growth occur in most systems, and the substrate may get poisoned or disrupted after repeated use [46,53,54,55,56,57,58,59].
Organometallics CVD inputs have the advantages of metallic components acting as a catalyst for principal reactions and for correcting defects that might arise. However, they may increase waste due to several factors. For example, in the case of ferrocene it is not widely produced for any applications, and current ferrocene production methods often generate a lot of reactive or environmentally unfriendly waste; other metallo-organic compounds are similar [51,53]. Other metallo-organics do not fare better. Scale-up in the future may lead to improved syntheses with organometallics; however, ferrocene and related compounds used for CVD do not yet have many uses [54,55]. Additional downsides include that the metallic component reacts and produces waste for certain CVD input streams; and after or during production, metal and metal-element-containing waste needs to be purified out or disposed of, or could lead to the risk of the product being contaminated, clog up the system, or excess can inhibit the desired chemical reactions from happening [45,46].
To prevent undesired interactions of more complex gas mixtures, higher temperatures [67,68,69], and certain pressure regimes may be used. Sometimes, input gas contains functional groups that promote reaction at lower temperatures, and/or remove defects by reacting with less stable carbon bonds. Input gas may also contain rings to act as larger blocks to build graphene nano-carbons, and constituents that do not react directly to form GNC but instead to get rid of defects (such as hydrogen gas to remove less stable carbon compounds) [44,45,46]. However, all of this may reduce energy efficiency or create environmental problems in similar ways to using organo-metallic compounds. Some of the input gas may be used as a carrier to make pumping and directional control of the incoming gas stream better, control pressure, and absorb excess. Some commonly used gases as additions: hydrogen; CO2; CO; Ar and, to a lesser extent, other noble gases; nitrogen; oxygen; organometallic compounds; atmospheric feed gases; dopant gases; cleaning gases in between cycles; seed material; fluoride/fluorocompounds and other halogen-based compounds; dust or particles for actively cleaning CVD surfaces during or after making the product.; and ammonia [68].
Lower temperatures can lead to more undesirable products, partly due to higher temperatures selecting for often more stable graphene nano-carbon allotropes than the less stable amorphous carbon compounds, or organic compounds, and taking away waste better. However, higher temperatures limit which gases can be used stably [46,54,67,68]. Researchers have explored facilitating CVD production using several processes, including microwave irradiation, photon, radio waves and electron beams to selectively heat and reduce certain bonds, including inducing high or low temperature plasmas localized along carbon nanostructures [54,56,69,70,71,72,73,74,75,76]. Coupled with CVD, molecular, atomic, ion, EM or electron beams selectively deposit atoms with higher energy in a select direction along a certain direction. Feed gas modification: Pulses or phased gas sequences provide quicker depositing, a cleaning phase, and a phase for fixing or getting rid of defects, and prevent gas scattering [46,53,54,55]. Finally, the CVD feed gas has been changed to improve initiation, growth, repairing defects in products, and controlling the amount of catalyst involved [46,53,54,55].
3.2. CVD Methodologies and Modifications
The CVD process offers numerous tunable parameters to enhance nanocarbon product selectivity, but these also increase complexity and energy demands. Variants of CVD, summarized in Figure 1B and detailed in Table 1. Examples of Plasma-enhanced CVD and Atomic-layer CVD are shown respectively in Figure 4 and Figure 5. The dynamic reaction environment of CVD variants complicates chemical understanding, modeling, and control of selectivity and energy use. Multiple simultaneous reactions at varied interfaces and energy levels lead to local fluctuations in temperature, pressure, and energy. Excess input energy, often inefficiently used, can disrupt catalytic processes or degrade products. Additives, while potentially enhancing properties (e.g., conductivity in doped CNTs), may hinder product formation. Prolonged growth can reduce substrate contact, sometimes aiding catalysis. Due to high costs, optimization is typically limited to select products [53]. Additionally, graphene detachment during growth may obstruct CNT formation and disrupt gas flow, impairing the overall CVD process.

Table 1.
Variants of the CVD process [2,3,4,5,6,7,8,9,10,11,12,13,14,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
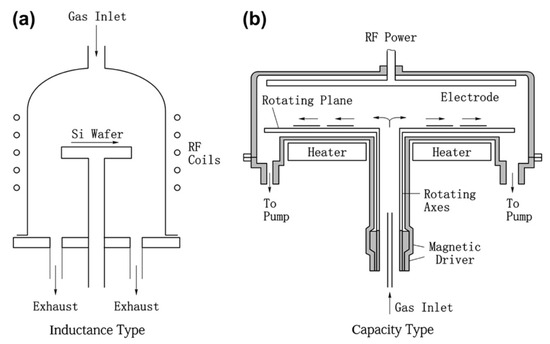
Figure 4.
Simple (a,b) parallel plate Plasma-enhanced CVD from reference [69]. Note, substrates other than Si wafer are also useful.
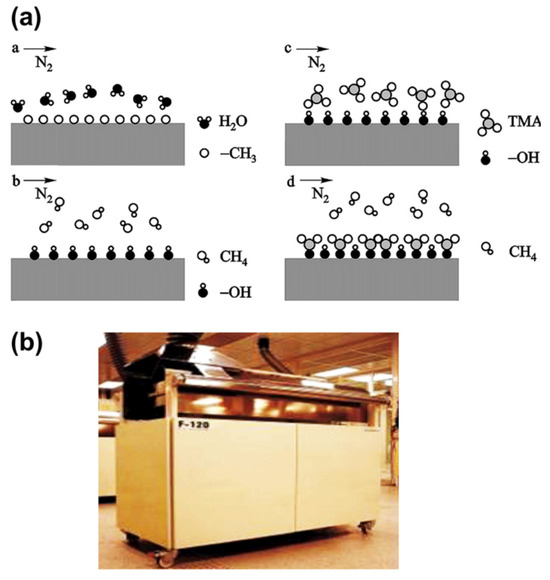
Figure 5.
Principle (a) and equipment (b) of Atomic-layer CVD from reference [69].
3.3. CVD Alternative Precursors and Products
To reduce CVD environmental impacts, decrease price, and increase production capacity, attempts have been made to include solid precursor, sometimes sourced from biomass or recycled material [46,54,55,56,57,58,59,60]. For example, reducing polymers in the CVD chamber with a copper substrate or reducing biomaterials, such as rice husks, in the CVD chamber with a copper substrate [62]. A widening body of precursors is being used to optimize the CVD CNT process [70,71,72]. Mixed biological-derived compounds can be used in the CVD process [45,54,55,56,57,58,59,60,61,62]. However, this usually requires higher temperatures to produce the desired products and still give a greater variety of often lower-quality products [30,31,32,33,34,35,36]. However, the defect-ridden product, porous product, may have some select usages due to its higher surface area and reactivity [46,54,55,56,57,58,59,60]. This may also lead to better doping of the final product if the dopant is mixed in a solid precursor, easier separation from the substrate, and more fine-tuned micro and macrostructures [6,54,55,56,57,58,59,62].
Additional environmental costs may arise from the difficulty of removing the product from the CVD chamber, cool it, detach it from the substrate, and purify the product, and renewing chamber conditions (appropriate atmosphere, pressure, remaking substrate, etc.) [46,54,55,56,57,62]. Attachment to substrate can also be beneficial in some cases, as it may make handling the product easier, improve the metal or semiconductor or other interface, or allow for a tighter and more specific assembly of nanocarbons, such as nano-forests of vertically aligned nano-carbons that excel at absorbing light due to overall structure, absorbing certain chemicals, and emitting electrons [46,54,55,56,57,62,63].
In addition to CNTs and graphene, CVD can make nano-diamond surfaces or nano-diamonds, but not inexpensive or larger diamonds [64]. CVD has also been used for other chemical processes, such as depositing of molybdenum and other metallic sulfides, arsenides, carbides, silicides, and nitrides and metals as coatings including tungsten, molybdenum, tantalum, titanium, and nickel [87,88,89,90,91,92]. A particular use of these CVD processes is depositing thin films directly on the desired substrate, which may serve as the back or front side of the final product, such as an electrical contact for a thin-film solar cell or an electrical contact for a radiation sensor. These processes differ greatly from the production of GNCs as structural materials, which are consumed at a much higher rate than materials used for advanced electronics and solar cells. For example, silicon for films, had a production level of 4.6 metric Mt in 2024 [78], while production levels of the structural material cement were 3 orders of magnitude higher [79]. Large-scale CVD production techniques are not readily adaptable to changing parameters for different outputs to adjust to different markets.
Ongoing research explores the production of materials other than GNCs, usually produced by CVD, for production alternatives, for example, the electrochemical deposition of perovskites and thin-film semiconductors to reduce energy use. CVD systems face limitations beyond chemistry; designing chambers capable of withstanding and maintaining reaction conditions is technically challenging, costly, and often difficult to scale. This is especially true for systems requiring uniform or precisely controlled environments, extreme conditions, or high-energy beams. Maintenance and operational expertise are also significant barriers, particularly for highly specialized setups. While advances in non-GNC CVD may offer some benefits, their relevance is limited due to the unique scaling and customization challenges in GNC production. Nonetheless, developing processes that overcome CVD’s fundamental constraints could accelerate broader research, especially with support from adjacent fields
4. CVD Pricing
CNT pricing depends on purity, length, quantity, width, and how many walls. CVD CNTs currently costs around $60–1000 per gram for over 98% purity, high quality CNT and $100 to $200 per kg for low quality CNT under 95% purity when purchased in bulk [82,83,84]. This does not include the cost of more exotic CNT structures, and the cost of CNT CVD production is not expected to decrease significantly due to intrinsic energy, materials and equipment costs, even if capacity dramatically increases. Even at these high costs, scale up continues and production levels continue to grow. For graphene and CNOs produced by CVD, costs are several orders of magnitude higher [82,83,84,85,86].
5. CVD Scale-Up
Maintaining an isolated chamber to conduct CVD is challenging, but a plasma window, which is a technology that fills a space with an electrically or magnetically confined plasma, can be utilized. A plasma window’s viscosity, which increases with temperature, allows it to separate a vacuum from gas at standard atmospheric pressure and can reportedly handle a pressure difference of up to 9 atmospheres. At the same time, the plasma window will allow radiation to pass through. Currently, this window is very small [73,74,75,76,77]. Electron-beam welding is a major application of plasma windows [76]. Other ideas beyond plasma window include better protective gases and their usage, higher-pressure CVD process, better repair correcting agents for defective GNCs, better use of seed agents, cleaning of chamber, pumps, better substrates, and high-energy controlled and directed beams from EUV to radio frequency to electron beams may all help with scale-up.
6. C2CNT Processes Introduction
From 2009–2010, carbon capture by CO2-splitting through molten carbonate electrolysis was introduced as a potential climate change solution. By 2015, it was shown that the transition metal nucleated growth process could directly turn CO2 into CNTs [16]. This C2CNT method of transforming the carbon dioxide into valuable GNCs offers a promising way to address climate change [93]. This procedure has a 4-e redox reaction of carbonate to GNCs and O2 (Equation (3)):
CO32− ⟶ CGNC + O2 + O2−
CO2 exothermally reacts with oxide, to continuously renew the carbonate, such as for the carbonate, Li2CO3 (Equations (4) and (5))
CO2 + O2− ⟶ CO32−
CO2 + Li2O ⟶ Li2CO3 ∆H (770 °C) = −158,000 J/mol.
C2CNT is illustrated in Figure 2, and shown as deployed at a large-scale in Figure 6. Hot O2 byproducts can be used to power more efficient combustion for power plants, pollution filtering, ozone generators, used in chemical production plants, or released [93].
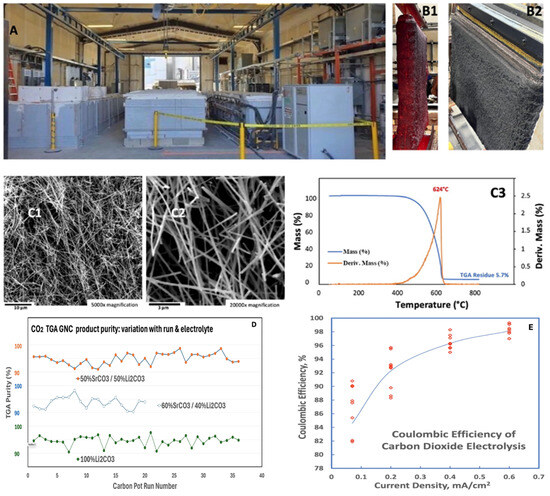
Figure 6.
C2CNT decarbonization has been scaled to an industrial scale at Carbon Corp (a subsidiary of C2CNT LLC, in Calgary, CA, USA. (A): In the medium and large-scale C2CNT processes, CNTs are grown from CO2 in a 304 Stainless Steel (304 SS) electrolysis pots on planar Muntz brass cathodes. Modern large-scale industrial electrolysis at the site utilizes cathodes with > m2 area. (B): An extracted cathode containing the deposited cathode product grown during a 16-h electrolysis of 0.05 CO2 concentration in 770 °C Li2CO3 electrolyte. (C1,C2), SEM and (C3), TGA of the product and (D): the TGA GNC purity over multiple runs in various electrolytes [66,67,68]. Bottom (E): The C2CNT coulombic efficiency versus the applied electrolysis current density for medium-scale C2CNT® electrolyses. 10 electrolyses are done at each current density. From reference [93]. Open access by CC 3.0.
The planned C2CNT decarbonization process scale-up to capture from 0.1–1 kt of this O2 annually involves increasing kiln dimensions from 3.3 × 1.8 m by either 1.8 m or 2.4 m tall for the 0.1 or 1 kt unit [93]. This scale-up includes carbon pots and electrodes [66] (Figure 6 and Figure 7). In the 1kt Genesis kiln, each cathode sheet is 3× larger, with additional cathodes. The kilotonne module unit is significant because current aluminum production already employs electrolyzers with a similar throughput (kt/module annually), with 10 s connected in series for aluminum output at the MT-scale. The C2CNT electrolysis operates at 350 kA (Figure 7). The C2CNT process shares similarities with aluminum smelting, both molten electrolysis and non-precious materials. In Al-smelting, alumina is reduced to aluminum metal at 960 °C via electrolysis in a cryolite, unlike at a colder temperature (770 °C). Another key difference is that the C2CNT process uses an inert O2 anode, typically 304SS, which does not consume carbon, unlike the aluminum process that consumes a carbon electrode that releases CO2. Also, C2CNT has a lower capital costs, as it works at colder temperatures, does not require coke production, and avoid bauxite refinement. The top-right image in Figure 7. shows a typical aluminum smelter potline producing 1 Mt of Al annually. Each of the three potlines operates at ~350 kA and consists of 100 s of pots arranged in series with consumable coke anodes, replaced periodically. The molten aluminum (mp 660 °C) is heavier than the electrolyte and is collected using a mobile vacuum crucible.
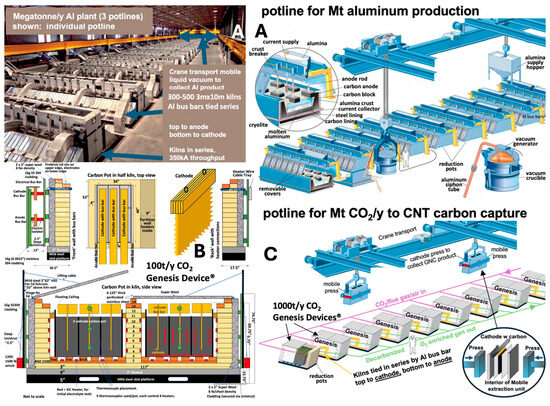
Figure 7.
(A) (left and right): Commercially operable aluminum smelting facility. (B): Design of the current 100 t/y (tonne/y) CO2 Genesis Device® for decarbonization and production of GNCs such as CNTs. (C): Planned design of the Genesis Device to deliver 1 Mt/yr CO2 decarbonization (and produce ¼ Mt GNCs) based on the analogous Mt Al facility using a ten-fold increase (kt/y) from the current module Genesis Device used in series.
The aluminum and C2CNT processes proceed at high current densities of 100’s of mA/cm2. Both processes’ cells are constructed from readily available components [86]. Al-electrolysis operates at ~4 V, compared to the C2CNT process 0.8–2 V, depending on current density.
The Genesis Device is made for bolt-on, modular deployment. C2CNT nodes can be serially connected, enabling scalability from initial installations capturing kilo to mega-tonnes of carbon dioxide annually. Similar to Al-plants, the 1 Mt carbon dioxide yearly C2CNT facility consists of 3 potlines, each comprising several hundred kt CO2 annually Genesis Devices in series. The right-hand sections of Figure 7 offer a view of an Al-facility compared to the proposed MT-scale C2CNT decarbonization plant. Interestingly, higher current densities promote increased efficiencies, which is another positive sign for scale-up as shown in Figure 6E.
As shown in Figure 6C3, compared to amorphous C (oxidation ~300 °C), these GNCs resist oxidation up to 600 °C (Figure 5) [93]. Under N2, CNTs (whether produced by CVD or C2CNT) are stable and can retain their viscoelasticity to 1000 °C [94,95]. CNTs under vacuum or inert environment have been reported as thermally stable and providing twice the thermal conductivity of diamond at temperatures up to 2800 °C for use in the aerospace industry [96]. The resistance of GNCs to oxidation and their high thermal conductivity is useful for applications including aerospace components, high temperature lubricants, flame retardants, tire and rubber durability, coatings and paintings, crucibles, and semiconductor manufacture.
The modular design of C2CNT facilitates localized decarbonization and production of CNTs and other CNTs Local production, reduces long-distance shipping and environmental costs. Over 18% of NOx emissions and 3% of greenhouse gas originate from shipping [96,97,98]. GNCs might partly mitigate this by allowing for fewer materials to be used; for example, stronger materials may mean fewer materials used in construction [26,27,29]. GNCs produced locally may also make for less sensitivity to supply chain disruptions, shorter delivery time, and the use of less energy-intensive methods of transport.
7. IC2CNT (Insulation Facilitated C2CNT)
In IC2CNT, the carbon dioxide in the feed gas passes through membranes into a headspace and then into molten electrolyte, which act as a carbon sink. Industrial feed and atmospheric gases principal components (N2, O2, and H2O) are insoluble in molten carbonates [49]. The membrane insulates the feed gas from the hot molten carbonate chamber, mitigating the need to heat the (non-CO2) majority of the feed gas to high temperature. The C2CNT is highly impervious to ppm-levels of SO2, NOx, or CO found in a typical flue gas [38]. Older designs of C2CNT brought room-temperature gases in direct contact with the electrolysis chamber, causing heating of the complete gases, which is not done in the new IC2CNT [49]. This chemistry avoids heat transfer to the non-CO2 flue gas components by implementing a CO2-diffusion zone [The gas output that is not O2 gas produced contains less CO2 and may contain fewer organic compounds, making it easier to clean].
8. Beyond Pure Lithium Electrolyte C2CNT
Li2CO3 is expensive, partly due to the competitive demand for Li2CO3 for batteries. Global Li2CO3 prices for 2022–2024 vacillate in the range of $10–75 k/t [99,100]. With these prices and competition, an effective, lower cost alternative electrolyte would be useful for the C2CNT process.
Pure lithium, sodium, and potassium carbonate melt respectively at 723, 851, and 891 °C [48]. Eutectic ternary mixes of these salts have been well characterized as molten carbonate electrolytes and do not produce significant amounts of CNTs. The synthesized CNTs are increasingly defect-ridden at contents of 20% potassium carbonate or higher, making it not an ideal for electrolyte mixtures. Upon electrolysis, a binary mixture of sodium and lithium carbonate produces CNTs up to 20 wt% Na2CO3, but above that, the product is increasingly deformed. Interestingly, colder 670 °C electrolysis, rather than 770 °C, 50 wt% sodium carbonate and 50 wt% Li2CO3 form another GNC, other than CNTs, which we have termed carbon nano-scaffolds. However, adding Na2CO3 to Li2CO3 considerably increases the electrolysis potential [100].
The basis of cement production is CaCO3 to lime thermal decomposition (>840 °C). Barium carbonate (mp. 811 °C) with lithium carbonate has a eutectic at 609 °C. CNTs can be grown in lithium carbonate with a maximum of ~20 wt% Ca and Ba carbonate. CO2-electro-splitting in a mixed calcium/lithium carbonate electrolyte proceeds differently from that in other Li2CO3-based electrolytes [100]. In Li or Li/Ba electrolytes, the respective oxide is highly soluble, whereas lime only dissolves at 0.2 m in Li2CO3. Hence, during electrolysis, rather than reacting with CO2, lime precipitates out, consuming calcium carbonate. Using Ba, Mg, or Ca carbonate additives with lithium carbonate was observed to harmfully increase the electrolysis potential. Despite a high melting point, SrCO3 (mp. 1494 °C) was shown to be unusually soluble in lithium carbonate [100]. Strontium carbonate is the only carbonate with a similar thermodynamic affinity for CO2 to that of Li2CO3, and as with Li2CO3, supports low-energy CO2-electro-splitting to make useful CNT products [90]. Concentrated strontium carbonate electrolytes are demonstrated to form high-purity CNT products, but SrCO3 is available at a more stable global price of ~$1700/t, a cost that is 10 times less than lithium carbonate [100,101,102]. Strontium is the 10th most abundant metal, while lithium is the 24th most abundant metal in the Earth’s core. SrCO3 is widely mined and refined to form strontium carbonate or sulfate.
9. Unique Properties of C2CNT GNCs Compared to CVD
To date, CNCs, such as CNTs, produced by C2CNT, offer a greater range of selective carbon nano-allotrope products and properties than those currently produced by CVD. CO2 molten carbonate electrolysis conditions, including electrolyte composition, current density, and electrode materials, influence GNC properties [17,18,19,20,21,22,26,27,28,29,30,49,50,93,100]. Varying conditions yield doped, magnetic, thin, long, bamboo, pearl, or helical CNTs. A different composition, such as 54/41/5 wt% SrCO3/Li2CO3/Na2CO3, produces CNOs, some of which are seen in Figure 8. Graphene and carbon nano-scaffolds have also been synthesized. Morphological variations to the basic graphene structure bring additional properties to these GNC variants, as summarized in Table 2.
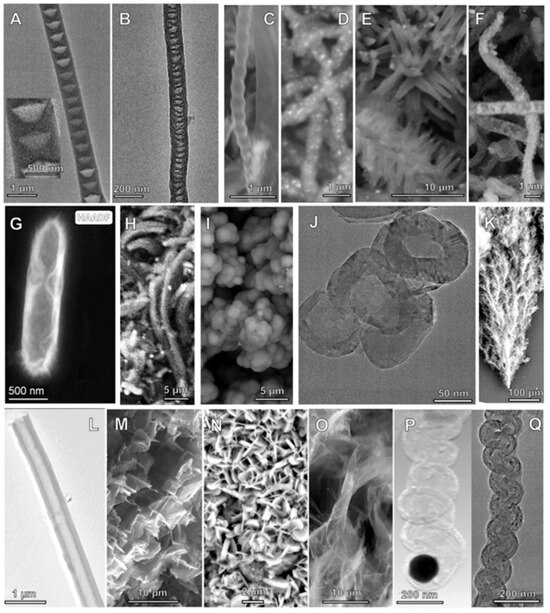
Figure 8.
SEM of nanocarbon allotropes synthesized by C2CNT. Top row (from left to right): (A): conical CNF, (B): nano-bamboo, (C): nano-pearl, (D): Ni-coated CNT, (E): nano-flower, (F): nano-dragon. Middle row: (G): nano-rod, (H): nano-belt, (I): nano-onion (J): hollow nano-onion, and (K): nano-tree. Bottom row (from left to right) (L): Carbon nanotube, (M): nano-scaffold, (N): nano-platelet, (O): graphene, (P,Q): nano-helices. From reference [18]. Open access by CC 3.0.

Table 2.
Variants of the C2CNT GNC products [17,18,19,20,21,22,26,27,28,29,30,49,50,93,100].
Multi-walled carbon nanotubes (MWCNTs) synthesized through the C2CNT method utilize microwave energy to form localized hotspots that can directly generate microwave plasma—something not observed with CNTs produced via chemical vapor deposition (CVD) [30]. This prompts an important question: what are the key physicochemical distinctions between CVD-grown CNTs and those made using the C2CNT approach?
While all CNTs are known to absorb microwaves effectively due to their high dielectric properties (both dielectric constant and loss factor), C2CNTs offer additional advantages. These include: (i) strong ferromagnetic behavior, and (ii) enhanced electrical conductivity, which contribute to better microwave absorption and the ability to maintain higher operational temperatures.
The narrower electromagnetic emission spectra of MWCNTs may support more efficient constructive interference, potentially boosting plasma generation—though this remains to be confirmed. Furthermore, because nanotubes with varying diameters interact with different wavelengths, MWCNTs can absorb a broader microwave spectrum [30]. This broader absorption is largely attributed to the narrow emission bands and the redistribution of electromagnetic energy across their surface through the skin effect.
The structural rigidity of MWCNTs helps them maintain their form under extreme conditions. Their layered construction also protects internal walls, resulting in longer lifespan. Unlike single-walled CNTs (SWCNTs), which only exhibit metallic behavior at certain larger diameters [30], MWCNTs are generally metallic. This metallic nature allows for more efficient movement of electrons and holes both between tubes and within tube bundles.
Interactions between the concentric layers in MWCNTs further enhance the transport of charge carriers, improving microwave coupling. Additionally, when plasma strips away electrons or holes, MWCNTs are more resilient due to their capacity to conduct higher currents. Their robustness, higher carbon density, and current tolerance contribute to improved self-repair during plasma exposure.
Moreover, MWCNTs are less likely to fail at defect points and are more resistant to physical displacement thanks to their greater mass and density. Their thermal properties—such as more vibrational modes and higher heat capacity—also enable more effective radiation and dissipation of surface energy.
C2CNT-based graphene-nanotube composites (GNCs) are magnetically active and enriched with metals or metal carbides, which improves charge mobility and overall conductivity. In general, longer CNTs enhance mechanical strength, electrical performance, and accommodate more vibrational modes. The wider structures of MWCNTs made through C2CNT can carry higher currents, display improved skin effects, absorb microwaves more efficiently, and remain more chemically and physically stable.
These characteristics also support better charge transport (electrons and holes) and thermal conductivity. Additionally, the unique hierarchical structure of C2CNTs—from nanoscale to macroscale—originating from carbanogel material directly deposited on the cathode, contributes to better ion capture, microwave absorption, and heat retention [30].
Metal-based impurities present in C2CNT GNCs can increase the density of charge carriers, enhance electrical pathways, catalyze molecular splitting reactions, promote CNT regeneration, and intensify magnetic interactions with electromagnetic fields, all of which improve absorption efficiency. Their high thermal conductivity also aids in effectively transferring microwave heat to plasma-facing substrates and any ejected material [30,31].
10. Future Tech for C2CNT
In the future, custom or different feedstock gases may lead to more customizable products, such as prepared doped products or CO2 gas feed rate (dependent on concentration and gas flow rate), compared to the current input density affecting structures grown [16,17,18]. Custom structures may be grown by reducing a solid organic substrate and perhaps turning organic waste into GNC Heating solid or waste carbon products to CO2, which is unlikely to lead to custom structures. This may further be enhanced with custom metals for electrodes, electrical connections (including one GNC to make metal more conductive by order of magnitude–need to delete or state what GNC), etc., that may allow for even higher efficiencies or better control of what allotropes are produced, which also might come about through more experimentation. Better electrical connections, transformers, and renewable energy might all lower the environmental impact, along with economies of scale. Better modular designs may allow for more widespread adoption, development, advantages that come with local production, and better adaptability. Some designs could be transported if they cannot be built locally. Automation may greatly reduce cost and waste. Better ability to change electrolysis parameters, and perhaps even electrolyte, may lead to more control of the allotrope expanding market and flexibility. Better heat recovery and usage may occur with things like heat pumps or counterflow devices, along with better insulation and better bricks to build the outside structure of the device. Ultrasonicating or vibrating device while in use may produce fluffier scaffolds, or dislodge platelets, or other thin layers. Energy could be stored in molten salts to be used later, like a heat battery. Finally, direct nano-gel and buckypaper-like products could be refined from the electrode.
11. Comparative Process and Future Economics Speculation
Processes for the CVD production of carbon nanotubes (CNTs) are energy-intensive and can require extensive purification. CVD production of CNT and other carbon nanoparticles are on the order of 2 to 100 times more energy-intensive than aluminum, even with production idealized production models, a principal factor and the present high cost of commercial CNTs [103,104]. C2CNT uses substantially less energy from 2–4 GJ/t CO2, with the added benefits of being a complete carbon capture process, and of producing graphene nanocarbons and oxygen as useful, valuable byproducts. The C2CNT energy requirement is half that of aluminum, and ranges from a low of 2 GJ by (i) recovering the heat from the carbon product, (ii) looping the hot O2 product as an oxy-fuel reactant, and using low voltage carbonate electrolytes, up to the 4 GJ to drive high current density electrolyses [105].
Carbon taxes and carbon-free future grants or promotions will decrease demand for CVD and support demand for the C2CNT decarbonization process. This carbon exchange, tax, and grant market is currently evaluated at over 100 billion USD annually and expected to grow, increasing funding, which will mean more money for green technology and penalization for other technologies [106]. More accessibility of the C2CNT process through incentives will mean more experimentation with it, leading to faster adoption and better development. More usage of it will lead to a feedback cycle.
CVD emits greenhouse gases with a CO2-equivalent ranging from 480 to 34,000 g per gram of CNT (not all gases CO2, but global warming potential is equivalent to that many grams of CO2, some gases such as NOx or hydrocarbons may have other deleterious effects to human and environmental health), while the C2CNT process consumes approximately 3.7 g of CO2 per gram of CNT produced [90,104] Water use in CVD ranges from 0.1 to 1 g per gram of CNT, whereas C2CNT does not consume water (excluding purification/device-related use in both). CVD also requires 5–9 g of hydrogen, acetylene, or ethylene, and 600–650 g of argon per gram of CNT, none of which are consumed in the C2CNT process [105]. Though C2CNT uses lithium carbonate, it is fully recycled—unlike newer methods using strontium carbonate—and avoids the material consumption inherent in CVD. Recycling solids or liquids, as in C2CNT, is also simpler than handling the gases and high-temperature plasmas used in CVD. CVD consumes 0.2 to 10 kWh per gram of CNT, while C2CNT’s energy use is 0.007 kWh per gram of CNT. Additionally, substrate detachment is more challenging in CVD, which also poses greater safety and cleanliness concerns compared to C2CNT [93,107].
12. Conclusions
This review presents a comprehensive analysis of Graphene Nanocarbon and Carbon Nanotube production methods, comparing traditional Chemical Vapor Deposition (CVD) with the emerging CO2 to Carbon Nanotechnology (C2CNT) process. Unlike CVD, C2CNT is both a GNC production and decarbonization technology. While CVD remains the dominant method for synthesizing high-quality carbon nanomaterials, it suffers from significant environmental, economic, and scalability limitations. In contrast, the C2CNT approach introduces a paradigm shift-transforming CO2 emissions into valuable nanomaterials through molten carbonate electrolysis, offering a carbon-negative, energy-efficient, and economically scalable alternative. The C2CNT process produces a wider portfolio of CNT and GNC product types, and the production is more energy and cost effective.
This perspective is timely and necessary, as current literature lacks integrated evaluations of both the technical and environmental dimensions of these competing processes. It is particularly novel in its detailed comparison of the structural, electromagnetic, and thermal properties of CVD- versus C2CNT-derived GNCs, alongside an exploration of emerging electrolytes and insulation strategies (e.g., IC2CNT). By highlighting the potential of C2CNT to simultaneously address material demand and climate change mitigation, this work provides critical insight for researchers, policymakers, and industry leaders aiming to accelerate sustainable nanomaterial production at scale.
Author Contributions
Conceptualization; methodology, validation, formal analysis; investigation, writing—original draft preparation; writing—review and editing, visualization, project administration, G.L. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors directly participate in the scale-up of the C2CNT process and declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| C2CNT | CO2 to Carbon NanoTube |
| CNT | Carbon NanoTube |
| CNO | Carbon Nano-Onions |
| CVD | Chemical Vapor Deposition |
| GHG | GreenHouse Gas |
| GNC | Graphene Nano-Carbon |
| IC2CNT | Insulated CO2 to Carbon NanoTube |
| MWCNT | Multi-Walled Carbon NanoTube |
| SWCNT | Single-Walled Carbon NanoTube |
References
- Islam, M.A.; Hasan, M.; Rahman, M.; Mobarak, M.H.; Mimona, M.A.; Hossain, N. Advances and Significances of Carbon Nanotube Applications: A Comprehensive Review. Eur. Polym. J. 2024, 220, 113443. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Jafarkhani, P.; Moztarzadeh, F. High-Yield Synthesis of Carbon Nanotubes Using a Water-Soluble Catalyst Support in Catalytic Chemical Vapor Deposition. Carbon 2006, 44, 1343–1345. [Google Scholar] [CrossRef]
- Ren, Z.F.; Huang, Z.P.; Wu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- NanoLab. SEM Images & TEM Images of Carbon Nanotubes, Aligned Carbon Nanotube Arrays, and Nanoparticles. Available online: http://www.nano-lab.com/imagegallery.html (accessed on 9 September 2025).
- Neupane, S.; Lastres, M.; Chiarella, M.; Li, W.; Su, Q.; Du, G. Synthesis and Field Emission Properties of Vertically Aligned Carbon Nanotube Arrays on Copper. Carbon 2012, 50, 2641–2650. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology. J. Phys. Conf. Ser. 2007, 61, 643–646. [Google Scholar] [CrossRef]
- Smalley, R.E.; Li, Y.; Moore, V.C.; Price, B.K.; Colorado, R.; Schmidt, H.K.; Hauge, R.H.; Barron, A.R.; Tour, J.M. Single Wall Carbon Nanotube Amplification: En Route to a Type-Specific Growth Mechanism. J. Am. Chem. Soc. 2006, 128, 15824–15829. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Mizuno, K.; Yumura, M.; Iijima, S. Kinetics of Water-Assisted Single-Walled Carbon Nanotube Synthesis Revealed by a Time-Evolution Analysis. Phys. Rev. Lett. 2005, 95, 056104. [Google Scholar] [CrossRef]
- Hiraoka, T.; Izadi-Najafabadi, A.; Yamada, T.; Futaba, D.N.; Yasuda, S.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S.; Hata, K. Compact and Light Supercapacitor Electrodes from a Surface-Only Solid by Opened Carbon Nanotubes with 2200 m2 g−1 Surface Area. Adv. Funct. Mater. 2010, 20, 422–428. [Google Scholar] [CrossRef]
- Unidym. UnidymTM Product Sheet: Single-Walled Carbon Nanotubes (SWNT); Unidym, Inc.: Menlo Park, CA, USA; Available online: https://web.archive.org/web/20110717162816/http://www.unidym.com/files/Unidym_Product_Sheet_SWNT.pdf (accessed on 13 October 2025).
- Yamada, T.; Namai, T.; Hata, K.; Futaba, D.N.; Mizuno, K.; Fan, J.; Yudasaka, M.; Yumura, M.; Iijima, S. Size-Selective Growth of Double-Walled Carbon Nanotube Forests from Engineered Iron Catalysts. Nat. Nanotechnol. 2006, 1, 131–136. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-Engineerable and Highly Densely Packed Single-Walled Carbon Nanotubes and Their Application as Super-Capacitor Electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Carbon Nanotubes Market (2025–2030). Available online: https://www.grandviewresearch.com/industry-analysis/carbon-nanotubes-cnt-market (accessed on 3 October 2025).
- Ren, J.; Yu, A.; Peng, P.; Lefler, M.; Li, F.-F.; Licht, S. Recent Advances in Solar Thermal Electrochemical Process (STEP) for Carbon Neutral Products and High Value Nanocarbons. Acc. Chem. Res. 2019, 52, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Licht, G.; Wang, X.; Licht, S. Controlled Transition Metal Nucleated Growth of Carbon Nanotubes by Molten Electrolysis of CO2. Catalysts 2022, 12, 137. [Google Scholar] [CrossRef]
- Liu, X.; Licht, G.; Wang, X.; Licht, S. Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2. Catalysts 2022, 12, 125. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Licht, G.; Wang, X.; Licht, S. Carbon Nano-Onions Made Directly from CO2 by Molten Electrolysis for Greenhouse Gas Mitigation. Adv. Sustain. Syst. 2019, 3, 1900056. [Google Scholar] [CrossRef]
- Johnson, M.; Ren, J.; Lefler, M.; Licht, G.; Vicini, J.; Licht, S. Data on SEM, TEM and Raman Spectra of Doped, and Wool Carbon Nanotubes Made Directly from CO2 by Molten Electrolysis. Data Brief 2017, 14, 592–606. [Google Scholar] [CrossRef]
- Wang, X.; Sharif, F.; Liu, X.; Licht, G.; Lefler, M.; Licht, S. Magnetic Carbon Nanotubes: Carbide Nucleated Electrochemical Growth of Ferromagnetic CNTs from CO2. J. CO2 Util. 2020, 40, 101218. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Licht, G.; Licht, S. Transformation of the Greenhouse Gas Carbon Dioxide to Graphene. J. CO2 Util. 2020, 36, 288–294. [Google Scholar] [CrossRef]
- Lai, C.-C.; Goyenola, C.; Broitman, E.; Naslund, L.-A.; Hogberg, H.; Hulman, L.; Gueorguiev, G.K.; Rosen, J. Synthesis and properties of CSxFy thin films deposited by reactive magnetron sputtering in an Ar/SF6 discharge. Chem. Phys. Lett. 2017, 506, 86–91. [Google Scholar]
- Gueorguiev, G.K.; Stafström, S.; Hultman, L. Fullerene-like CSx: A first-principles study of synthetic growth. Chem. Phys. Lett. 2011, 506, 86–91. [Google Scholar]
- Megatonne-Scale Plants Planned to Turn Captured CO2 Into Industrial Nanocarbons—Nanowerk. Available online: https://www.nanowerk.com/spotlight/spotid=67373.php#google_vignette (accessed on 11 October 2025).
- Licht, S.; Liu, X.; Licht, G.; Wang, X.; Swesi, A.; Chan, Y. Amplified CO2 Reduction of Greenhouse Gas Emissions with C2CNT Carbon Nanotube Composites. Mater. Today Sustain. 2019, 6, 100023. [Google Scholar] [CrossRef]
- Licht, G.; Hofstetter, H.; Licht, S. Polymer Composites with Carbon Nanotubes Made from CO2. RSC Sustain. 2024, 2, 2496–2504. [Google Scholar] [CrossRef]
- Licht, S. Co-Production of Cement and Carbon Nanotubes with a Carbon-Negative Footprint. J. CO2 Util. 2017, 18, 378–389. [Google Scholar] [CrossRef]
- Licht, G.; Hofstetter, H.; Licht, S. Buckypaper Made with Carbon Nanotubes Derived from CO2. RSC Adv. 2024, 14, 27195–27197. [Google Scholar] [CrossRef] [PubMed]
- Licht, G.; Hofstetter, K.; Licht, S. Intense, Self-Induced Sustainable Microwave Plasma Using Carbon Nanotubes Made from CO2. Nanoscale 2024, 17, 9279–9296. [Google Scholar] [CrossRef] [PubMed]
- Berger, M. Scientists Discover Unexpected Plasma Formation When Microwaving CO2-Derived Carbon Nanotubes (w/Video). 2025. Available online: https://www.nanowerk.com/spotlight/spotid=66357.php (accessed on 19 September 2025).
- Basheer, B.V.; George, J.; Siengchin, S.; Parameswaranpillai, J. Polymer Grafted Carbon Nanotubes—Synthesis, Properties, and Applications: A Review. Nano-Struct. Nano-Objects 2022, 22, 100429. [Google Scholar] [CrossRef]
- Imtiaza, S.; Siddiqa, M.; Kausara, A.; Muntha, S.J.; Ambreenb, I.B. A Review Featuring Fabrication, Properties, and Applications of Carbon Nanotubes (CNTs) Reinforced Polymer and Epoxy Nanocomposites. Chin. J. Polym. Sci. 2018, 36, 445–461. [Google Scholar] [CrossRef]
- Gantayata, S.; Routb, D.; Swain, S.K. Carbon Nanomaterial–Reinforced Epoxy Composites: A Review. Polym. Plast. Technol. Eng. 2018, 57, 1–16. [Google Scholar] [CrossRef]
- Rafiquea, I.; Kausara, A.; Anwara, Z.; Muhammad, B. Exploration of Epoxy Resins, Hardening Systems, and Epoxy/Carbon Nanotube Composite Designed for High Performance Materials: A Review. Polym.-Plast. Technol. Eng. 2016, 55, 312–333. [Google Scholar] [CrossRef]
- Kausara, A.; Rafiquea, I.; Muhammad, B. Review of Applications of Polymer/Carbon Nanotubes and Epoxy/CNT Composites. Polym.-Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Singh, B.; Gorji, Z.E.; Singh, R.; Sharma, V.; Repo, T. Silica Gel Supported Solid Amine Sorbents for CO2 Capture. Energy Environ. Mater. 2025, 8, e12832. [Google Scholar] [CrossRef]
- Waseem, M.; Al-Marzouqi, M.; Ghasem, N. A Review of Catalytically Enhanced CO2-Rich Amine Solutions Regeneration. J. Environ. Chem. Eng. 2023, 11, 110188. [Google Scholar] [CrossRef]
- CO2.Earth. Latest Daily CO2. Available online: https://www.co2.earth/daily-co2 (accessed on 9 September 2025).
- NASA. The Relentless Rise of Carbon Dioxide. Available online: https://science.nasa.gov/resource/graphic-the-relentless-rise-of-carbon-dioxide/ (accessed on 9 September 2025).
- Arrhenius, S. Worlds in the Making; Borns, H., Translator; Harper and Brothers Publishers: London, UK, 1908; Volume 53, p. 61. [Google Scholar]
- Urban, M.C. Accelerating Extinction Risk from Climate Change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- CO2 Emissions—Global Energy Review 2025—Analysis, International Energy Agency (IEA). Available online: https://www.iea.org/reports/global-energy-review-2025/co2-emissions (accessed on 3 October 2025).
- Dong, Z.; Li, B.; Cui, C.; Qian, W.; Jin, Y.; Wei, F. Catalytic methane technology for carbon nanotubes and graphene. React. Chem. Eng. 2020, 5, 991–1004. [Google Scholar] [CrossRef]
- Plata, D.L.; Hart, A.J.; Reddy, C.M.; Gschwend, P.M. Early Evaluation of Potential Environmental Impacts of Carbon Nanotube Synthesis by Chemical Vapor Deposition. Environ. Sci. Technol. 2009, 43, 8367–8837. [Google Scholar] [CrossRef]
- Saputri, D.D.; Jan’ah, A.M.; Saraswati, T.E. Synthesis of Carbon Nanotubes (CNT) by Chemical Vapor Deposition (CVD) Using a Biogas-Based Carbon Precursor: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 959, 012019. [Google Scholar] [CrossRef]
- EPA. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 10 September 2025).
- Licht, S. Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach. Adv. Mater. 2011, 23, 5592–5612. [Google Scholar] [CrossRef]
- Licht, G.; Peltier, E.; Gee, S.; Licht, S. Eliminating Active CO2 Concentration in Carbon Capture and Storage (CCUS): Molten Carbonate Decarbonization through an Insulation/Diffusion Membrane. DeCarbon 2025, 7, 100094. [Google Scholar] [CrossRef]
- Licht, G.; Peltier, E.; Gee, S.; Licht, S. Direct Air Capture (DAC): Molten Carbonate direct transformation of airborne CO2 to durable, useful carbon nanotubes and nano-onions. RSC Sustain. 2025, 3, 1330. [Google Scholar] [CrossRef]
- Haubner, R. The History of Hard CVD Coatings for Tool Applications at the University of Technology Vienna. Int. J. Refract. Met. Hard Mater. 2013, 41, 22–34. [Google Scholar] [CrossRef]
- Greene, J.E. Tracing the 5000-Year Recorded History of Inorganic Thin Films from ∼3000 BC to the Early 1900s AD. Appl. Phys. Rev. 2014, 1, 041302. [Google Scholar] [CrossRef]
- Yuriansyah Barmaki, M.J. An Introduction to Engineering Materials: Synthesis, Properties, and Application of Carbon Nanotubes. Academia. Available online: https://www.academia.edu/9792032/An_Introduction_To_Engineering_Materials_Synthesis_Properties_And_Application_Of_Carbon_Nanotubes?email_work_card=view-paper (accessed on 9 September 2025).
- Assouar, M.B.; Dossot, M.; Rizk, S.; Tiusan, C.; Bougdira, J. The Use of Microwave Plasma-Assisted CVD on Nanostructured Iron Catalysts to Grow Isolated Bundles of Carbon Nanotubes. Nanotechnology 2010, 21, 065708. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, N.M.; Abdullah, E.C.; Jayakumar, N.S.; Sahu, J.N. An Overview on Methods for the Production of Carbon Nanotubes. J. Ind. Eng. Chem. 2014, 20, 1186–1197. [Google Scholar] [CrossRef]
- Li, Y. Chemical Vapor Deposition of Carbon Nanotubes. Available online: https://www.photon.t.u-tokyo.ac.jp/~maruyama/visitors/16_CVD_CNT.pdf (accessed on 9 September 2025).
- Cadence PCB Solutions. Exploring Chemical Vapor Deposition (CVD) for Electronics, 2023, Cadence. Available online: https://resources.pcb.cadence.com/blog/2023-exploring-chemical-vapor-deposition-cvd-for-electronics (accessed on 10 September 2025).
- Chemical Vapour Deposition: CVD. 2025. Available online: https://www.sfu.ca/~gchapman/e495/e495l10j.pdf (accessed on 10 September 2025).
- Kermani, A.; Ku, Y.H.; Wong, F.; Kim, K.B.; Maillot, P.; Morgan, A.E.; Hahn, S. The Application of Rapid Thermal Chemical Vapor Deposition of Doped-Thin Single Crystal Silicon for MOS and Bipolar Technologies. In Proceedings Volume 1189, Rapid Isothermal Processing; SPIE: St Bellingham, WA, USA, 1990; p. 121. [Google Scholar] [CrossRef]
- Mattox, D.M. The “Good” Vacuum (Low Pressure) Processing Environment; Elsevier: Amsterdam, The Netherlands, 2010; pp. 73–145. [Google Scholar] [CrossRef]
- Vacuum Pump. ScienceDirect Topics in Agricultural and Biological Sciences; Elsevier: Amsterdam, The Netherlands, 2011; Chapter 6; Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/vacuum-pump (accessed on 10 September 2025).
- Fathy, N.A. Carbon Nanotubes Synthesis Using Carbonization of Pretreated Rice Straw through Chemical Vapor Deposition of Camphor. RSC Adv. 2017, 7, 28535–28541. [Google Scholar] [CrossRef]
- Azqandi, O.R.; Kalantarian, A.; Mollaee, M.; Sabeghenia, E. Growth of Vertical Forest-like Arrangement Carbon Nanotube Using CVD Technique. Int. J. Nanosci. 2025, 24, 2550001-58. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, B.; Zhang, J.; Wu, G.; Zhao, H.; Jia, J.; Meng, Q.; Zhao, Q. CVD Diamond Processing Tools: A Review. J. Adv. Res. 2024, 74, 333–358. [Google Scholar] [CrossRef]
- Simionescu, O.-G.; Brîncoveanu, O.; Romaniţan, C.; Vulpe, S.; Avram, A. AStep-By-Step Development of Vertically Aligned Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition. Coatings 2022, 12, 943. [Google Scholar] [CrossRef]
- Wu, Q.; Ji, X.; Yu, P.; Cao, Y.; Li, Z.; Huang, Y. Scalable growth of vertical graphene nanosheets by thermal chemical vapor deposition. Nat. Protoc. 2025. [Google Scholar] [CrossRef]
- Schropp, I.; Stannowski, B.; Brockhoff, A.M.; Veenendaal, P.A.; Rath, J.K. Hot Wire CVD of Heterogeneous and Polycrystalline Silicon Semiconducting Thin Films for Application in Thin Film Transistors and Solar Cells. Mater. Phys. Mech. 2000, 2, 73–78. [Google Scholar]
- Lau, K.K.S.; Caulfield, J.A.; Gleason, K.K. Structure and Morphology of Fluorocarbon Films Grown by Hot Filament Chemical Vapor Deposition. Chem. Mater. 2000, 12, 3032–3037. [Google Scholar] [CrossRef]
- Wang, J.-T. CVD and Its Related Theories in Inorganic Synthesis and Materials Preparations. In Modern Inorganic Synthetic Chemistry; Xu, R., Pang, W., Huo, Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 151–171. [Google Scholar] [CrossRef]
- Li, Q.; Hao, H.; Zhang, J.; Liu, L. Effect of hydrocarbons precursors on the formation of carbon nanotubes in chemical vapor deposition. Carbon 2004, 42, 829–835. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Windle, A.H. Effect of Carbon Precursors on the Structure and Properties of Continuously Spun Carbon Nanotube Fibers. Sci. Adv. Mater. 2015, 7, 643–653. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Shiga, D. Plasma Bubble Could Protect Astronauts on Mars Trip. New Scientist. Available online: https://www.newscientist.com/article/dn9567-plasma-bubble-could-protect-astronauts-on-mars-trip.html (accessed on 10 September 2025).
- Hot Mettle. New Scientist. Available online: https://www.newscientist.com/article/mg17823904.800-hot-mettle.html?full=true (accessed on 10 September 2025).
- Hershcovitch, A. Plasma Window Technology for Propagating Particle Beams and Radiation from Vacuum to Atmosphere. Brookhaven National Laboratory. Available online: http://www.techbriefs.com/content/view/1834/32/ (accessed on 10 September 2025).
- Crystec Technology Trading GmbH. Plasma Enhanced Chemical Vapor Deposition—Technology and Equipment. Crystec. Available online: http://www.crystec.com/tridepe.htm (accessed on 10 September 2025).
- Tavares, J.; Swanson, E.J.; Coulombe, S. Plasma Synthesis of Coated Metal Nanoparticles with Surface Properties Tailored for Dispersion. Plasma Process. Polym. 2008, 5, 759–769. [Google Scholar] [CrossRef]
- Schnebele, E.K. Silicon; U.S. Geological Survey: Reston, VA, USA, 2025; pp. 160–161. Available online: https://pubs.usgs.gov/periodicals/mcs2025/mcs2025-silicon.pdf (accessed on 10 September 2025).
- Hatfield, A.K. Cement; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 54–55. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-cement.pdf (accessed on 10 September 2025).
- Dorval Dion, C.A.; Tavares, J.R. Photo-Initiated Chemical Vapor Deposition as a Scalable Particle Functionalization Technology (a Practical Review). Powder Technol. 2013, 239, 484–491. [Google Scholar] [CrossRef]
- LPCVD. Crystec. Available online: http://www.crystec.com/klllpcvde.htm (accessed on 10 September 2025).
- Single Walled Carbon Nanotubes. Cheap Tubes. Available online: https://www.cheaptubes.com/product-category/single-walled-carbon-nanotubes/ (accessed on 10 September 2025).
- Carbon Nanotubes. Nanografi. Available online: https://shop.nanografi.com/carbon-nanotubes/ (accessed on 10 September 2025).
- wenwen@0506. How Much Do Carbon Nanotubes Cost? GrapheneRich. Available online: https://graphenerich.com/how-much-do-carbon-nanotubes-cost/ (accessed on 10 September 2025).
- Spasenovic, M. The Price of Graphene. Graphenea. Available online: https://www.graphenea.com/pages/graphene-price (accessed on 10 September 2025).
- Pathak, A.D.; Potphode, D.; Sharma, C.S. Graphitization Induced Structural Transformation of Candle Soot Carbon into Carbon Nano-Onion as a Functional Anode for Metal-Ion Batteries. Mater. Adv. 2022, 3, 3610–3619. [Google Scholar] [CrossRef]
- Hamzan, N.B.; Yi, C.; Sadri, R.; Lee, M.K.; Chang, L.; Tripathi, M.; Dalton, A.B.; Goh, B.T. Controlled Physical Properties and Growth Mechanism of Manganese Silicide Nanorods. J. Alloys Compd. 2021, 851, 156693. [Google Scholar] [CrossRef]
- Shah, V.A.; Dobbie, A.; Myronov, M.; Leadley, D.R. Reverse Graded SiGe/Ge/Si Buffers for High-Composition Virtual Substrates. J. Appl. Phys. 2010, 107, 064304. [Google Scholar] [CrossRef]
- Shareef, I.A.; Rubloff, G.W.; Anderle, M.; Gill, W.N.; Cotte, J.; Kim, D.H. Subatmospheric Chemical Vapor Deposition Ozone/TEOS Process for SiO2 Trench Filling. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1995, 13, 1888–1892. [Google Scholar] [CrossRef]
- Wahl, G.; Davies, P.B.; Bunshah, R.F.; Joyce, B.A.; Bain, C.D.; Wegner, G.; Remmers, M.; Walsh, F.C.; Hieber, K.; Sundgren, J.; et al. Thin Films in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Lau, K.K.S.; Tenhaeff, W.; Xu, J.; Gleason, K.K. Designing Polymer Surfaces via Vapor Deposition. Mater. Today 2010, 13, 26–33. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Styles, M.J.; Grenci, G.; Gorp, H.V.; Vanderlinden, W.; De Feyter, S.; Falcaro, P.; De Vos, D.; Vereecken, P.M.; Ameloot, R. Chemical Vapour Deposition of Zeolitic Imidazolate Framework Thin Films. Nat. Mater. 2016, 15, 304–310. [Google Scholar] [CrossRef]
- Hofstetter, K.; Licht, G.; Licht, S. Industrial scaling of molten carbonate electrolytic carbon capture and production of graphene allotropes. DeCarbon 2025, 9, 100122. [Google Scholar] [CrossRef]
- Xu, M.; Futaba, D.N.; Yamada, T.; Yumura, M.; Hata, K. Carbon Nanotubes with Temperature-Invariant Viscoelasticity from −196° to 1000 °C. Science 2010, 330, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Saeed, A.; Ul-Hamid, A. A review featuring the fundamentals and advancements of polymer/CNT nanocomposite application in aerospace industry. Polym. Bull. 2021, 78, 539–557. [Google Scholar] [CrossRef]
- Kaminski, I. Climate Impact of Shipping under Growing Scrutiny Ahead of Key Meeting. The Guardian, 22 June 2023. Available online: https://www.theguardian.com/environment/2023/jun/22/climate-impact-shipping-under-growing-scrutiny-imo-meeting-seascape (accessed on 10 September 2025).
- Walker, T.R.; Adebambo, O.; Del Aguila Feijoo, M.C.; Elhaimer, E.; Hossain, T.; Edwards, S.J.; Morrison, C.E.; Romo, J.; Sharma, N.; Taylor, S.; et al. Environmental Effects of Marine Transportation. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 505–530. [Google Scholar] [CrossRef]
- Schrooten, L.; De Vlieger, I.; Panis, L.I.; Chiffi, C.; Pastori, E. Emissions of Maritime Transport: A European Reference System. Sci. Total Environ. 2009, 408, 318–323. [Google Scholar] [CrossRef]
- Trading Economics. Lithium. Available online: https://tradingeconomics.com/commodity/lithium (accessed on 22 February 2025).
- Licht, G.; Wang, X.; Hofstetter, K.; Licht, S. A new electrolyte for molten carbonate decarbonization. Commun. Chem. 2024, 7, 211. [Google Scholar] [CrossRef]
- IndexBox Inc. Carbonates; Strontium Carbonate Price in China—2025. Available online: https://www.indexbox.io/search/carbonates-strontium-carbonate-price-china/ (accessed on 22 February 2025).
- IndexBox Inc. Carbonates; Strontium Carbonate Price in the United States—2025. Available online: https://www.indexbox.io/search/carbonates-strontium-carbonate-price-the-united-states/ (accessed on 11 October 2024).
- Kusnir, D.; Sanden, B.A. Energy Requirements of Carbon Nanoparticle Production. J. Ind. Eco. 2008, 12, 360–375. [Google Scholar] [CrossRef]
- Zhai, P.; Isaacs, J.A.; Eckelman, M.J. Net energy benefits of carbon nanotube applications. Appl. Energy 2016, 173, 624–634. [Google Scholar] [CrossRef]
- Hofstetter, K.; Licht, G.; Licht, S. Comparative Analysis of Amine, Lime, and Molten Carbonate Electrolytic CO2 Carbon Capture. ECS Adv. 2025, 4, 031003. [Google Scholar] [CrossRef]
- World Bank. State and Trends of Carbon Pricing 2025. World Bank. Available online: https://www.worldbank.org/en/publication/state-and-trends-of-carbon-pricing (accessed on 11 October 2025).
- Teah, H.Y.; Sato, T.; Namiki, K.; Asaka, M.; Feng, K.; Noda, S. Life Cycle Greenhouse Gas Emissions of Long and Pure Carbon Nanotubes Synthesized via On-Substrate and Fluidized-Bed Chemical Vapor Deposition. ACS Sustain. Chem. Eng. 2020, 8, 1730–1740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).