Synthesis, Crystal Structure and Optical Properties of Novel 1,10-Phenanthroline Derivatives Containing 2,6-Diisopropylphenoxy Substituents
Abstract
1. Introduction
- (i)
- Single-crystal X-ray diffraction and Hirshfeld surface analysis to determine molecular geometry and intermolecular interactions;
- (ii)
- UV–Vis absorption spectra in different solvents to assess band energies and molar absorptivities;
- (iii)
- Fluorescence emission spectra and quantum yields (Φ) to evaluate solvatochromic shifts and efficiencies, complemented by fluorescence lifetimes (τ) to distinguish between radiative and non-radiative decay channels.
2. Materials and Methods
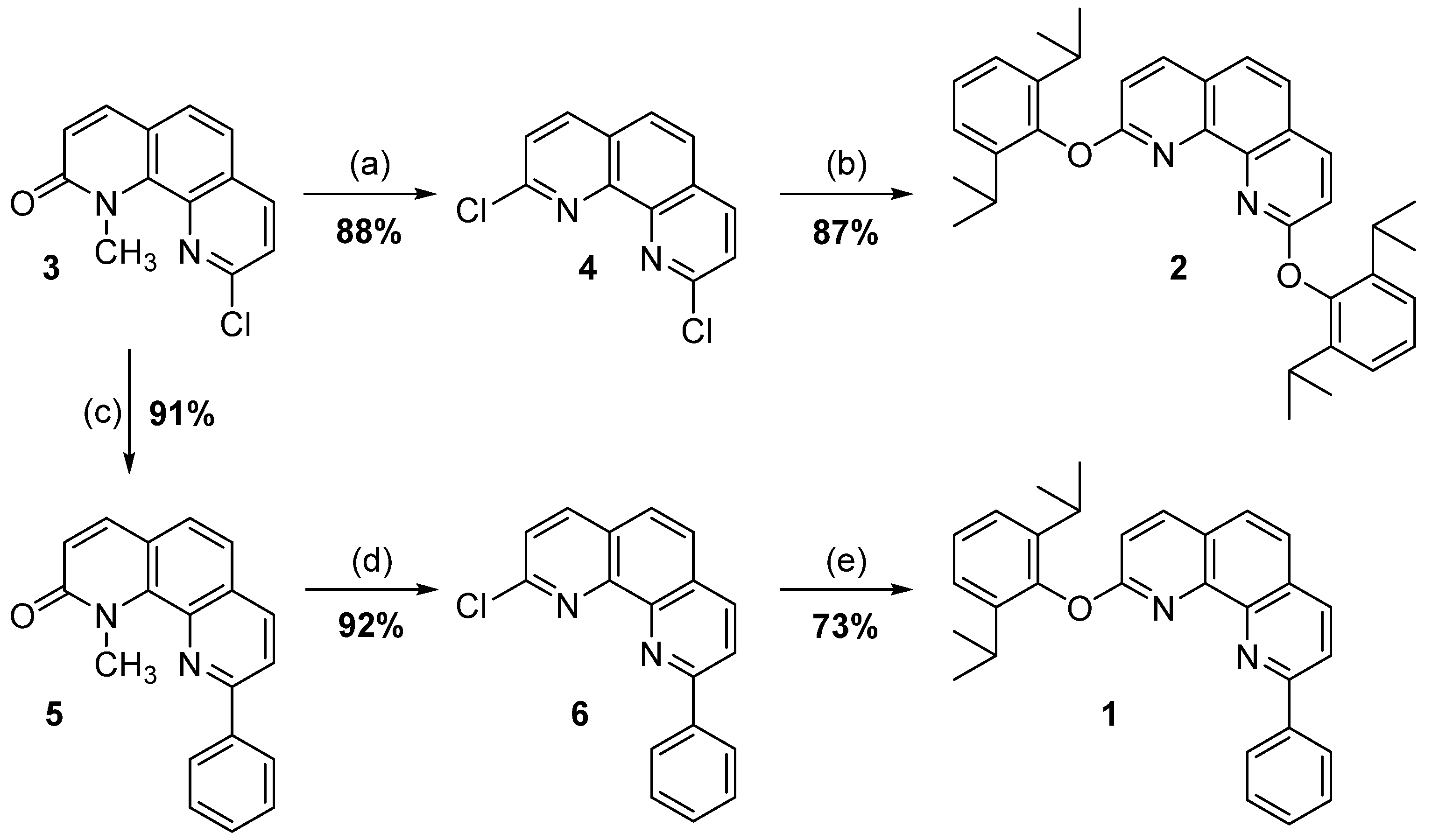
Synthesis of the 1,10-Phenanthroline Derivates
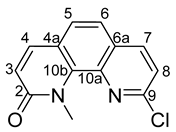
- 9-chloro-1-methyl-1,10-phenanthrolin-2(1H)-one (3)
- 2,9-dichloro-1,10-phenanthroline (4)

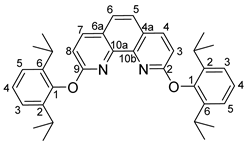
- 2,9-bis(2,6-diisopropylphenoxy)-1,10-phenanthroline (2)
- 1-methyl-9-phenyl-1,10-phenanthrolin-2(1H)-one (5)

- 2-chloro-9-phenyl-1,10-phenanthroline (6)

- 2-(2,6-diisopropylphenoxy)-9-phenyl-1,10-phenanthroline (1)

3. Results and Discussion
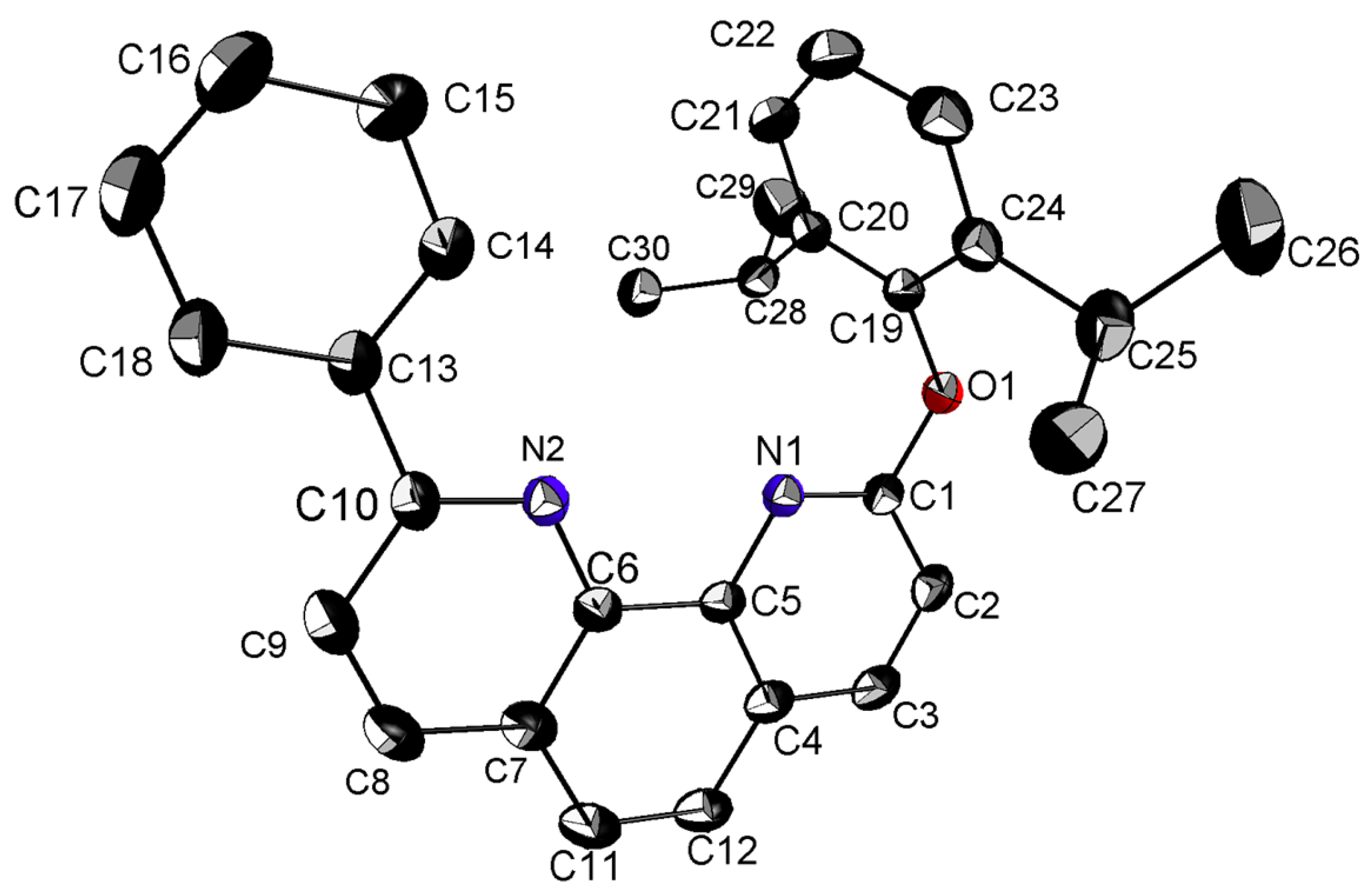
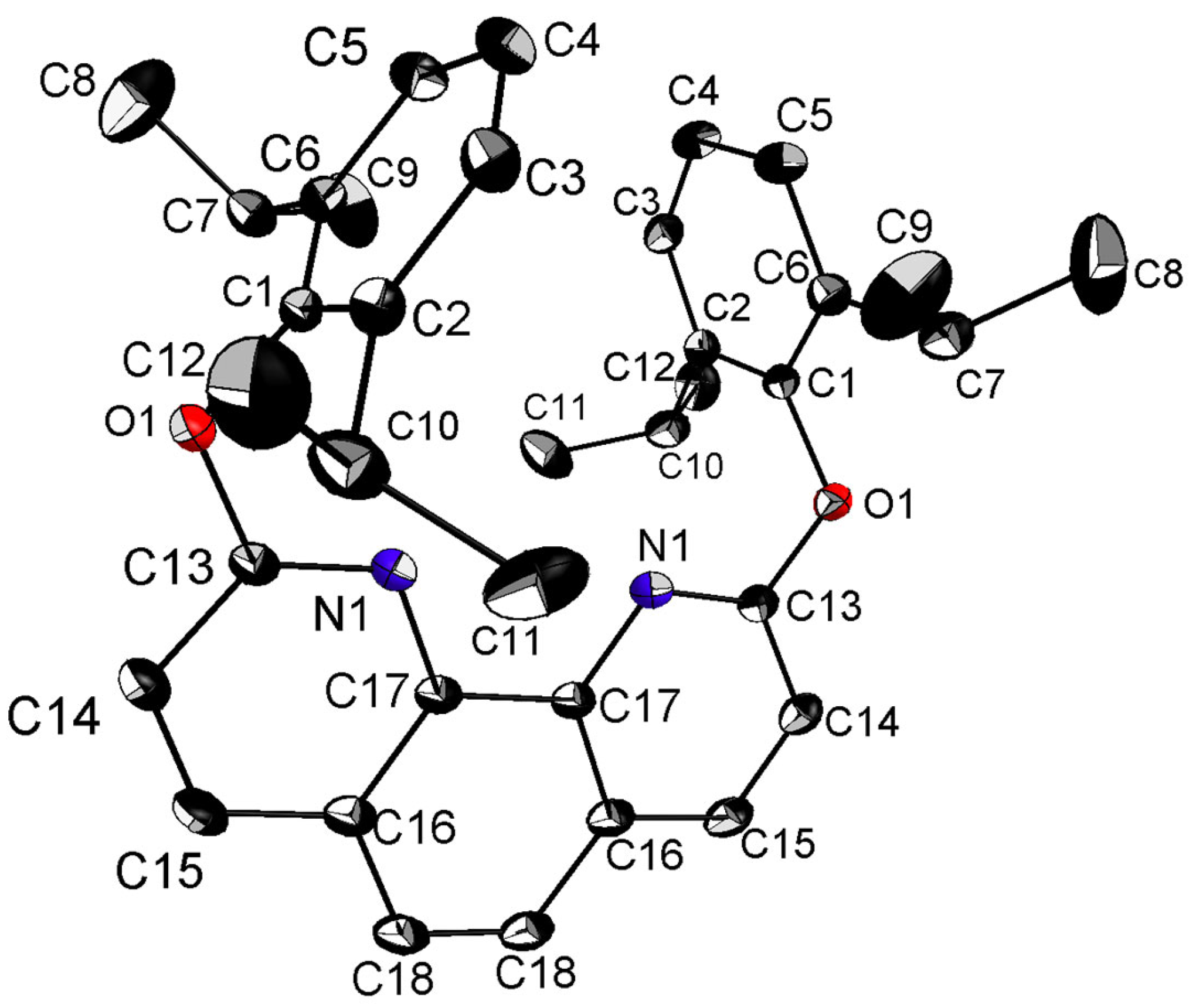
3.1. Crystal Structure
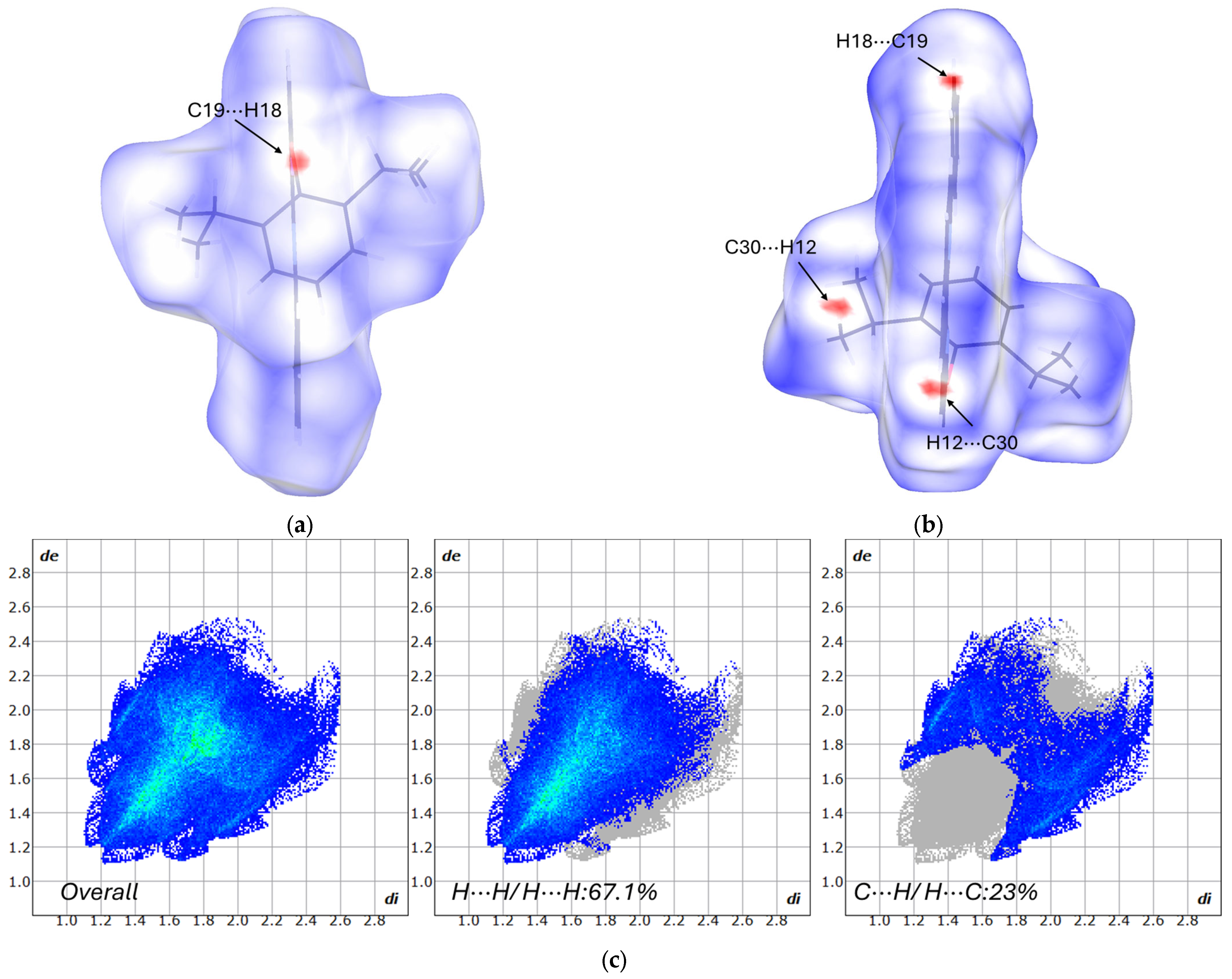
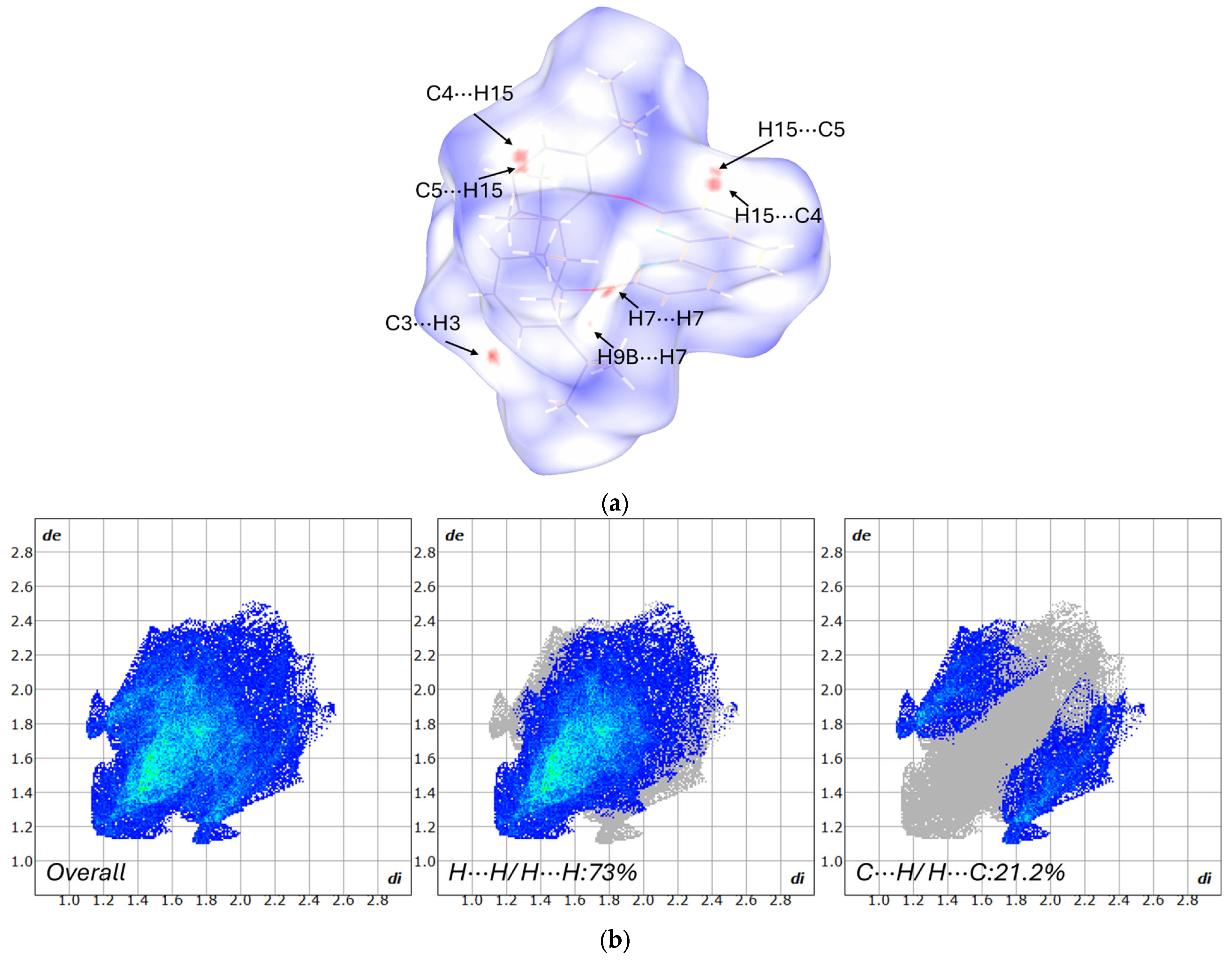
3.2. Hirshfield Surface Analysis
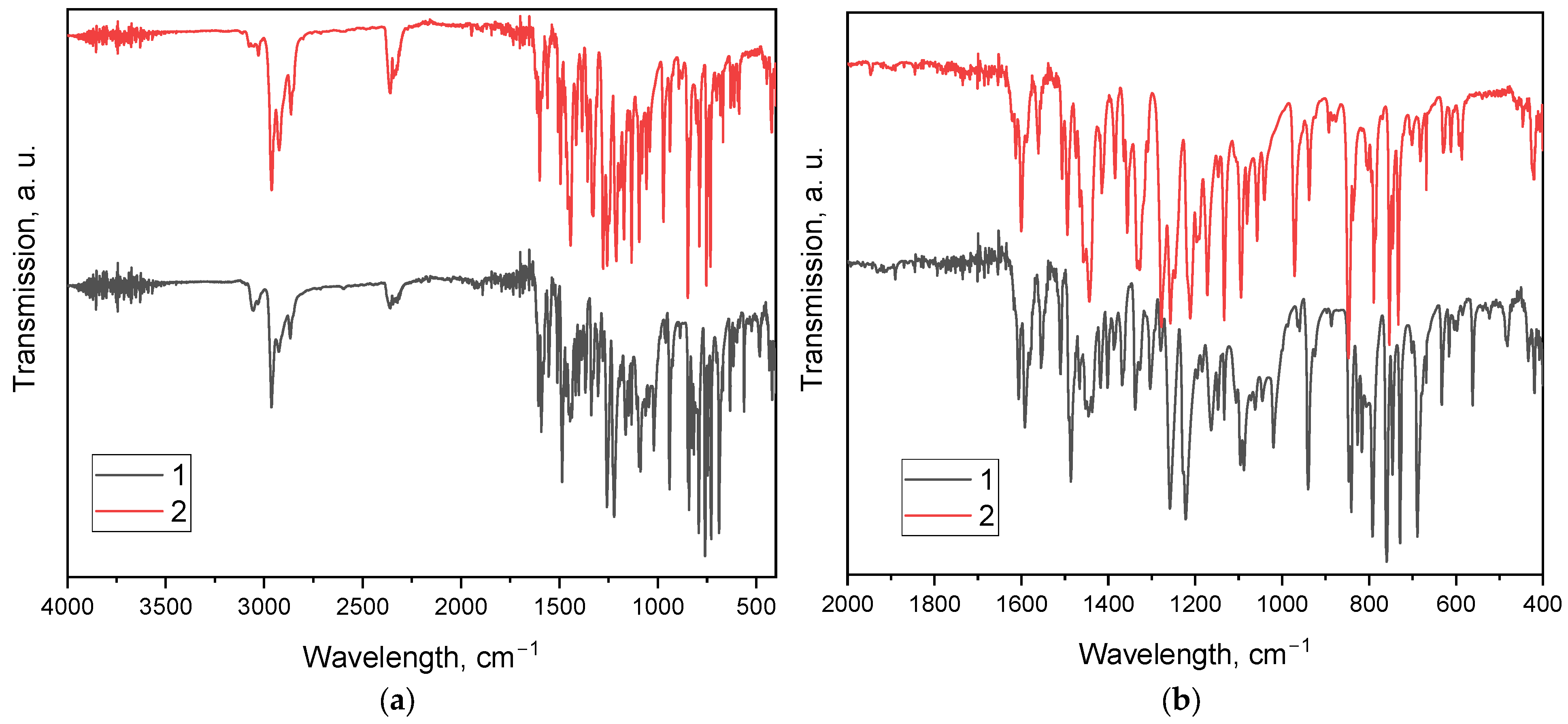
3.3. Structural Characterization by FTIR
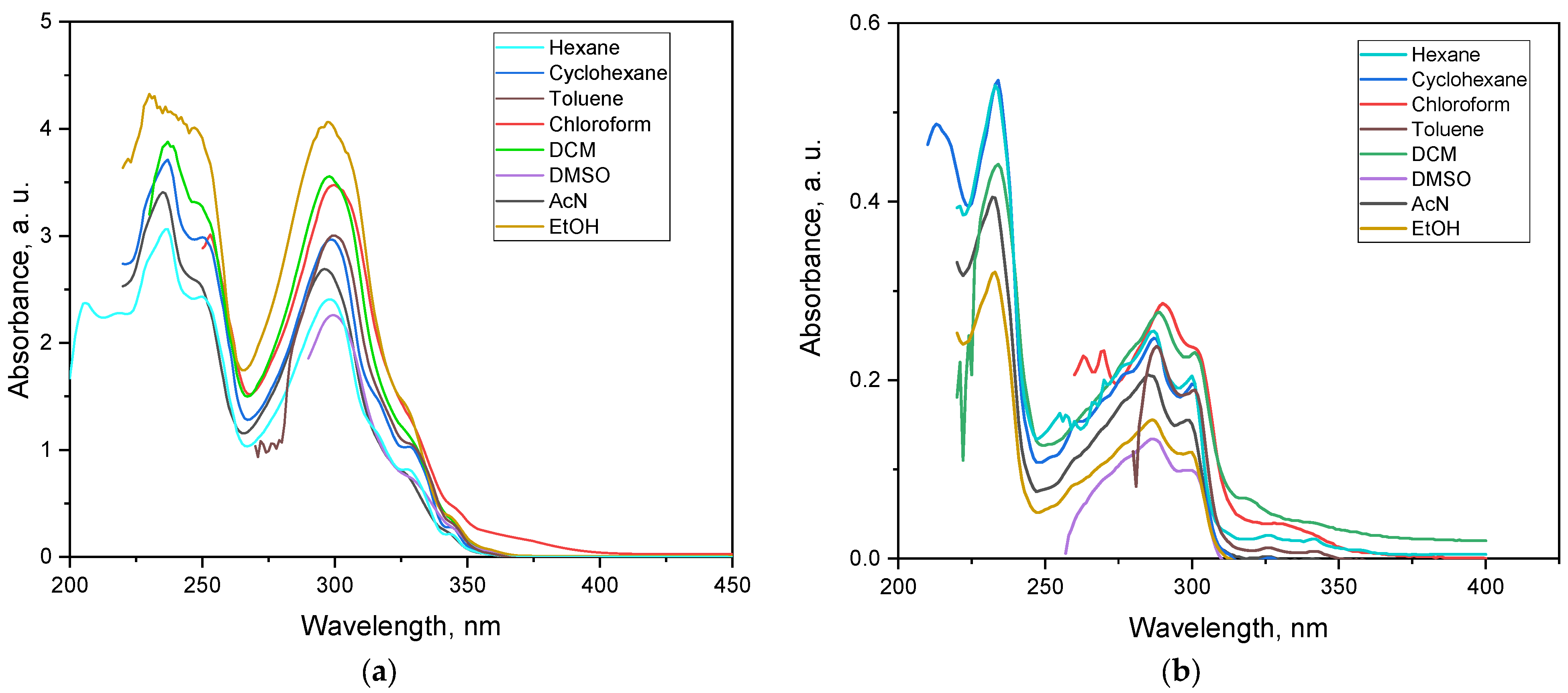
3.4. Electronic Properties
3.5. Fluorescence Properties
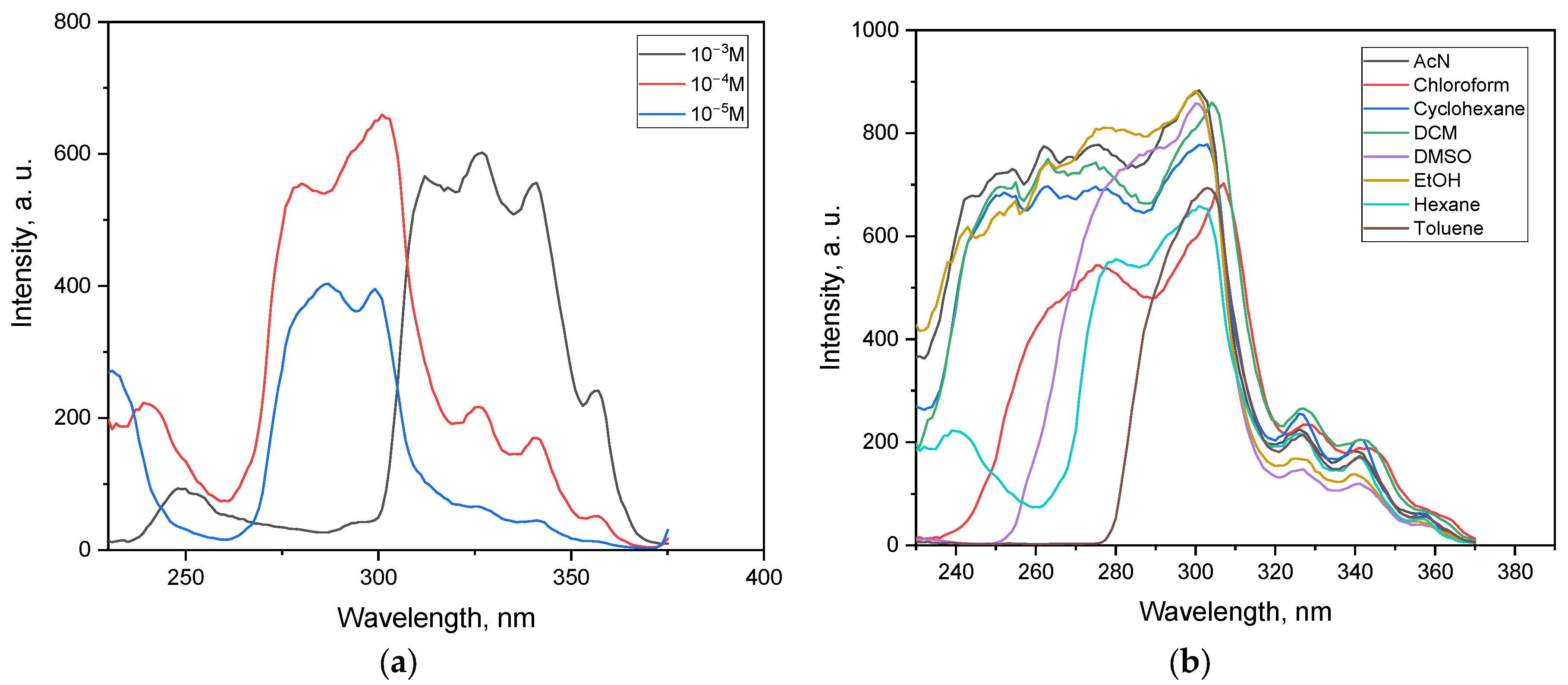
3.5.1. Excitation Spectra
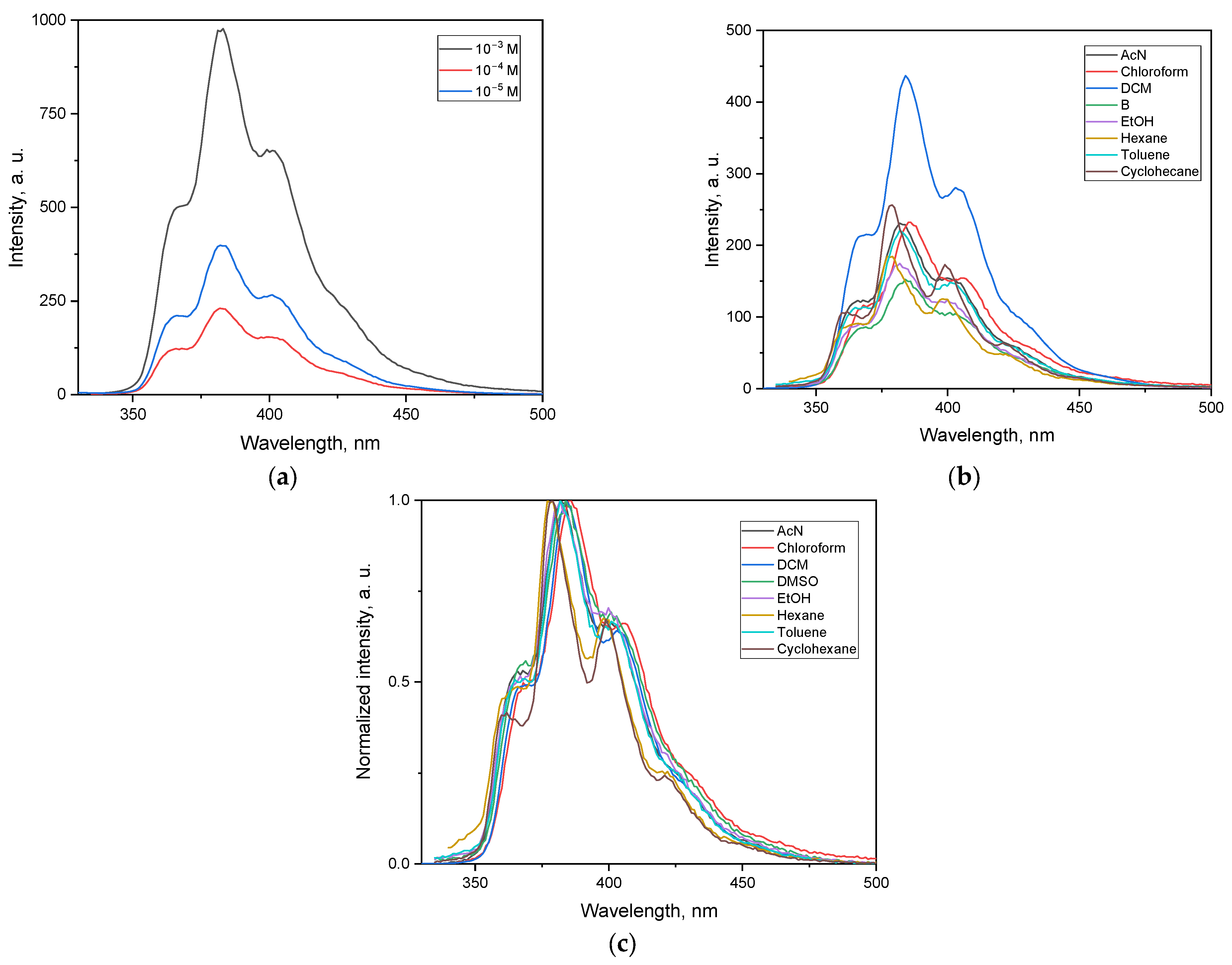
3.5.2. Emission Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alreja, P.; Kaur, N. Recent Advances in 1,10-Phenanthroline Ligands for Chemosensing of Cations and Anions. RSC Adv. 2016, 6, 23169–23217. [Google Scholar] [CrossRef]
- Rillema, D.P.; Cruz, A.J.; Tasset, B.J.; Moore, C.; Siam, K.; Huang, W. Structural Properties of Platinum(II) Biphenyl Complexes Containing 1,10-Phenanthroline Derivatives. J. Mol. Struct. 2013, 1041, 82–91. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Cesaretti, A.; Elisei, F.; Mencaroni, L.; Spalletti, A.; Fortuna, C.G. New Styryl Phenanthroline Derivatives as Model D−π−A−π−D Materials for Non-Linear Optics. ChemPhysChem 2018, 19, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Peñaloza, F.; Guajardo, K.; Arce, R.; Pizarro, N.; Vega, A. Synthesis, electronic and photophysical properties of 3,8-diaromatic-1,10-phenanthroline molecules. J. Chil. Chem. Soc. 2024, 69, 6188–6194. [Google Scholar] [CrossRef]
- Nycz, J.E.; Kolińska, J.; Karaush-Karmazin, N.; Chen, T.; Książek, M.; Kusz, J. New Tools in Heavy Metal Detection: Synthesis, Spectroscopic, and Quantum Chemical Characterization of Selected Water-Soluble Styryl Derivatives of Quinoline and 1,10-Phenanthroline. Molecules 2025, 30, 2659. [Google Scholar] [CrossRef]
- Wildervanck, M.J.; Hecht, R.; Nowak-Król, A. Synthesis and Strong Solvatochromism of Push-Pull Thienylthiazole Boron Complexes. Molecules 2022, 27, 5510. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Kumar, Y.; Kumar, S.; Singh, R.S. Competitive ICT in Asymmetric D−A Scaffolds Showing Visible Solvatochromism, Temperature-Induced Emission Enhancement and AIE Based Acidochromism. J. Lumin. 2022, 250, 119072. [Google Scholar] [CrossRef]
- Begantsova, Y.E.; Baranov, E.V.; Chesnokov, S.A. 4-(1H-Imidazo [4,5-f][1,10]Phenanthrolin-2-Yl)Benzaldehyde as a Probe in Pure Solvents: Solvatochromism, Electric Dipole Moment and PH Influence. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121480. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Q.; Yu, Z.; Yang, M.; Zhou, H.; Wu, J.; Tian, Y. Four New Two-Photon Absorbing Imidazo [4,5-f]1,10-Phenanthroline Dye Derivatives with Different Dipole Moment Orientation Based on Different Groups: Synthesis, Optical Characterization and Bioimaging. J. Mater. Chem. C 2013, 1, 822–830. [Google Scholar] [CrossRef]
- Liu, X.; Shen, B.; Zuo, R.; Hong, S.; Xiao, Y. 1,10-Phenanthroline-Based Hexacatenar LCs with Complex Self-Assembly, Photophysical and Binding Selectivity Behaviors. J. Mol. Liq. 2021, 340, 116892. [Google Scholar] [CrossRef]
- Aldeen, A.W.; Chen, Z.; Disher, I.A.; Yan, K.; Zhu, Y. Study of Initial β-Zr Formation in β-Quenched N36 Zirconium Alloy Using Dynamic and Metallographic Methods. Crystals 2022, 12, 1535. [Google Scholar] [CrossRef]
- Bin, Z.; Shi, D.; Su, R.; Han, W.; Zhang, D.; Duan, L. Hydrogen Bond Modulation in 1,10-Phenanthroline Derivatives for Versatile Electron Transport Materials with High Thermal Stability, Large Electron Mobility and Excellent n-Doping Ability. Sci. Bull. 2020, 65, 153–160. [Google Scholar] [CrossRef]
- Liu, J.; Gao, W.; Kityk, I.V.; Liu, X.; Zhen, Z. Optimization of Polycyclic Electron-Donors Based on Julolidinyl Structure in Push–Pull Chromophores for Second Order NLO Effects. Dye. Pigment. 2015, 122, 74–84. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S.; Etzbach, K.-H.; Krämer, P.; Lukaszuk, K.; Matschiner, R.; Schmidt, A.J.; Schuhmacher, P.; Sens, R.; Seybold, G.; Wortmann, R.; et al. Electrooptical Chromophores for Nonlinear Optical and Photorefractive Applications. Adv. Mater. 1999, 11, 536–541. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Lanza, J.B. Improved synthesis of 2-chloro- and 2, 9-dichloro-1, 10-phenanthrolines. Org. Prep. Proced. Int. 2007, 39, 603–608. [Google Scholar] [CrossRef]
- Romanova, J.; Lyapchev, R.; Kolarski, M.; Tsvetkov, M.; Elenkova, D.; Morgenstern, B.; Zaharieva, J. Molecular Design of Luminescent Complexes of Eu(III): What Can We Learn from the Ligands. Molecules 2023, 28, 4113. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Elenkova, D.; Kolarski, M.; Lyapchev, R.; Morgenstern, B.; Videva, V.; Zaharieva, J.; Milanova, M. Synthesis, Crystal Structure and Luminescence Properties of Two Novel Tb(III) Complexes with 1,10-Phenanthroline Derivatives as Ligands. J. Mol. Struct. 2024, 1314, 138768. [Google Scholar] [CrossRef]
- Brandl, T.; Kerzig, C.; Le Pleux, L.; Prescimone, A.; Wenger, O.S.; Mayor, M. Improved Photostability of a CuI Complex by Macrocyclization of the Phenanthroline Ligands. Chem. –A Eur. J. 2020, 26, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Cen, S.; Li, S.; Zhao, Y.; Zhao, M.; Zhang, Z. Catalytic Asymmetric Synthesis of Unnatural Axially Chiral Biaryl Δ-Amino Acid Derivatives via a Chiral Phenanthroline-Potassium Catalyst-Enabled Dynamic Kinetic Resolution. Angew. Chem. Int. Ed. 2024, 63, e202407920. [Google Scholar] [CrossRef] [PubMed]
- Reck, L.M.; Haberhauer, G.; Lüning, U. Enantiopure Chiral Concave 1,10-Phenanthrolines. Eur. J. Org. Chem. 2016, 2016, 1119–1131. [Google Scholar] [CrossRef]
- Huang, Q.; Su, Y.-X.; Sun, W.; Hu, M.-Y.; Wang, W.-N.; Zhu, S.-F. Iron-Catalyzed Vinylzincation of Terminal Alkynes. J. Am. Chem. Soc. 2022, 144, 515–526. [Google Scholar] [CrossRef]
- Yen, F.W.; Chang, C.H.; Teng, C.M.; Lin, I.F. The Phenanthroline-Based Compound and Use Thereof 2016. US Patent No. 9698357, 4 July 2017. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Fiedor, L.; Heriyanto; Fiedor, J.; Pilch, M. Effects of Molecular Symmetry on the Electronic Transitions in Carotenoids. J. Phys. Chem. Lett. 2016, 7, 1821–1829. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Geetha, D.V.; Harisha, A.S.; Karthik, V.; Chandana, S.N.; Kavitha, H.D.; Lakshminarayana, B.N.; Sridhar, M.A. X-Ray Structural Analysis, Quantum Chemical Computations, Molecular Docking, and Molecular Dynamics Simulations of Diethyl 5′-Amino-3,3-Dibromo-2,6-Dicyano1,2,3,4-Tetrahydro-[1,1.3,1-Terphenyl] 2,4-Dicarboxylate. J. Mol. Struct. 2026, 1351, 144142. [Google Scholar] [CrossRef]
- Rösel, S.; Quanz, H.; Logemann, C.; Becker, J.; Mossou, E.; Cañadillas-Delgado, L.; Caldeweyher, E.; Grimme, S.; Schreiner, P.R. London Dispersion Enables the Shortest Intermolecular Hydrocarbon H⋯H Contact. J. Am. Chem. Soc. 2017, 139, 7428–7431. [Google Scholar] [CrossRef]
- Zaharieva, J.; Videva, V.; Kolarski, M.; Lyapchev, R.; Morgenstern, B.; Tsvetkov, M. A Para-Substituted 2-Phenoxy-1,10-Phenanthroline Ligand for Lanthanide Sensitization: Asymmetric Coordination and Enhanced Emission from Eu3+, Tb3+, Sm3+ and Dy3+ Complexes. Molecules 2025, 30, 3548. [Google Scholar] [CrossRef]
- Mazumdar, P.; Das, D.; Sahoo, G.P.; Salgado-Morán, G.; Misra, A. Aggregation Induced Emission Enhancement from Bathophenanthroline Microstructures and Its Potential Use as Sensor of Mercury Ions in Water. Phys. Chem. Chem. Phys. 2014, 16, 6283. [Google Scholar] [CrossRef]


| Compound 1 | Compound 2 | |
|---|---|---|
| CCDC numbers | 2486309 | 2486310 |
| Crystal data | ||
| Empirical formula | C30H28N2O | C36H40N2O2 |
| Molecular weight, (g/mol) | 432.54 | 921.17 |
| Crystal system, space group | Triclinic, P | Monoclinic, C2/c |
| Temperature (K) | 143(2) | 143(2) |
| a, b, c (Å) | 7.7988(3), 10.0248(4), 16.6885(7) | 17.9825(4), 11.8411(3), 15.5954(6) |
| α, β, γ (°) | 73.3110(10), 77.2500(10), 72.8410(10) | 90.0, 115.3900(10), 90.0 |
| V, (Å3) | 1180.89(8) | 3000.02(16) |
| Z | 2 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ, (mm−1) | 0.074 | 0.072 |
| Crystal size (mm) | 0.200 × 0.180 × 0.080 | 0.200 × 0.200 × 0.080 |
| Data collection | ||
| Diffractometer | Bruker D8 Venture | Bruker D8 Venture |
| Absorption correction | Multi-scan, SADABS [27] | Multi-scan, SADABS [27] |
| Tmin, Tmax | 0.6944, 0.7455 | 0.7224. 0.7455 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 32,237, 4364, 3663 | 31,989, 3319, 2889 |
| Rint | 0.0306 | 0.0356 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.0427, 0.1013, 1.061 | 0.0396, 0.1010, 1.014 |
| No. of reflections | 4364 | 3319 |
| No. of restrains | 0 | 0 |
| No. of parameters | 302 | 185 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.187, −0.203 | 0.241, −0.245 |
| Wavenumber (cm−1) | Assignment | Compound 1 | Compound 2 |
|---|---|---|---|
| ≈3050 | Aromatic C–H stretching | √ (strong) | √ (weak) |
| ≈2960 | Aliphatic C–H (iPr, asym) | √ (strong) | √ (strong) |
| ≈2870 | Aliphatic C–H (iPr, sym) | √ (strong) | √ (strong) |
| ≈1600 | C=C/C=N aromatic ring stretching | √ (strong) | √ (strong) |
| ≈1500 | C=C ring stretching/phenanthroline | √ (strong) | √ (strong) |
| ≈1460 | C–H bending (iPr, CH3) | √ (strong) | √ (medium) |
| ≈1380 | C–H bending (iPr, CH(CH3)2) | √ (strong) | √ (strong) |
| ≈1250, ≈1180, ≈1080 | C–O–C (aryl–O stretching) | √ (strong, medium, strong) | √ (weak, strong, strong) |
| ≈880, ≈690 | Aromatic C–H out-of-plane bending | √ (medium, strong) | √ (medium, strong) |
| ≈750 | Aromatic C–H out-of-plane bending (1,2-disubst.) | √ (strong) | √ (strong) |
| Solvent | Compound 1 | Compound 2 | ||||
|---|---|---|---|---|---|---|
| λmax (nm) | A | ε (L·mol−1·cm−1) | λmax (nm) | A | ε (L·mol−1·cm−1) | |
| Hexane | 236.0 | 3.061 | 30,610 | 233.0 | 3.253 | 32,530 |
| Cyclohexane | 237.0 | 3.712 | 37,120 | 233.0 | 3.551 | 35,510 |
| DCM | 237.0 | 3.878 | 38,780 | 235.0 | 3.087 | 30,870 |
| AcN | 235.0 | 3.409 | 34,090 | 232.0 | 3.153 | 31,530 |
| Ethanol | 230.0 | 4.325 | 43,250 | 233.0 | 2.364 | 23,640 |
| Solvent | Compound 1 | Compound 2 | ||||
|---|---|---|---|---|---|---|
| λmax (nm) | A | ε (L·mol−1·cm−1) | λmax (nm) | A | ε (L·mol−1·cm−1) | |
| Hexane | 298.0 | 2.406 | 24,060 | 287.0 | 1.664 | 16,640 |
| Cyclohexane | 298.0 | 2.966 | 29,660 | 287.0 | 1.805 | 18,050 |
| Toluene | 299.0 | 2.250 | 30,030 | 288.0 | 1.680 | 16,800 |
| Chloroform | 300.0 | 3.474 | 34,740 | 290.0 | 2.210 | 22,100 |
| DCM | 298.0 | 3.557 | 35,570 | 288.0 | 1.967 | 19,670 |
| DMSO | 299.0 | 2.258 | 22,580 | 287.0 | 0.909 | 9090 |
| AcN | 296.0 | 2.689 | 26,890 | 285.0 | 1.683 | 16,830 |
| Ethanol | 297.0 | 4.063 | 40,630 | 286.0 | 1.192 | 11,920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetkov, M.; Lyapchev, R.; Kolarski, M.; Morgenstern, B.; Zaharieva, J. Synthesis, Crystal Structure and Optical Properties of Novel 1,10-Phenanthroline Derivatives Containing 2,6-Diisopropylphenoxy Substituents. Crystals 2025, 15, 883. https://doi.org/10.3390/cryst15100883
Tsvetkov M, Lyapchev R, Kolarski M, Morgenstern B, Zaharieva J. Synthesis, Crystal Structure and Optical Properties of Novel 1,10-Phenanthroline Derivatives Containing 2,6-Diisopropylphenoxy Substituents. Crystals. 2025; 15(10):883. https://doi.org/10.3390/cryst15100883
Chicago/Turabian StyleTsvetkov, Martin, Rumen Lyapchev, Mihail Kolarski, Bernd Morgenstern, and Joana Zaharieva. 2025. "Synthesis, Crystal Structure and Optical Properties of Novel 1,10-Phenanthroline Derivatives Containing 2,6-Diisopropylphenoxy Substituents" Crystals 15, no. 10: 883. https://doi.org/10.3390/cryst15100883
APA StyleTsvetkov, M., Lyapchev, R., Kolarski, M., Morgenstern, B., & Zaharieva, J. (2025). Synthesis, Crystal Structure and Optical Properties of Novel 1,10-Phenanthroline Derivatives Containing 2,6-Diisopropylphenoxy Substituents. Crystals, 15(10), 883. https://doi.org/10.3390/cryst15100883









