Calcium Hexaboride Synthesis from Anhydrous Colemanite by Mechanochemical Method
Abstract
1. Introduction
2. Material and Method
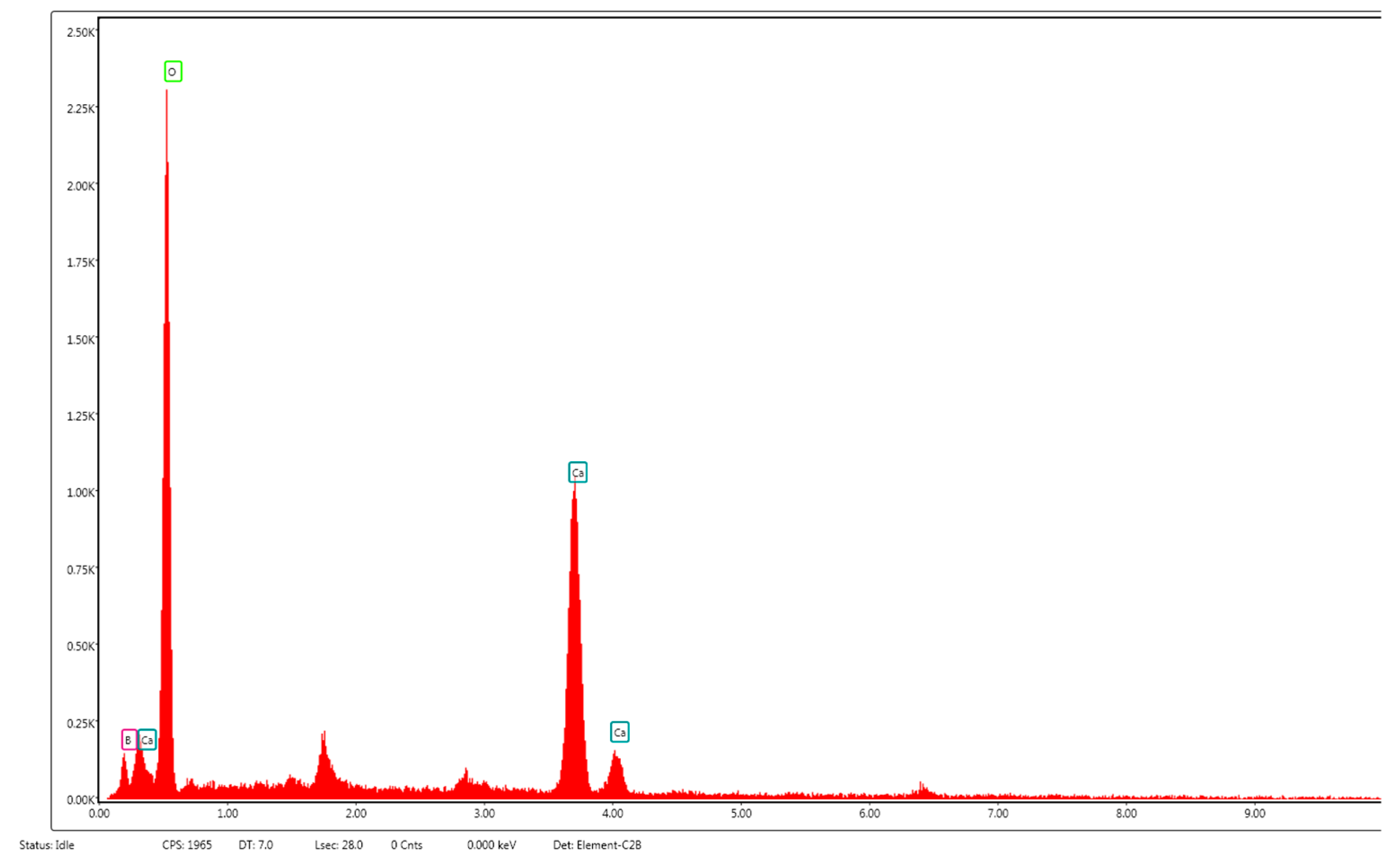
3. Results and Discussion
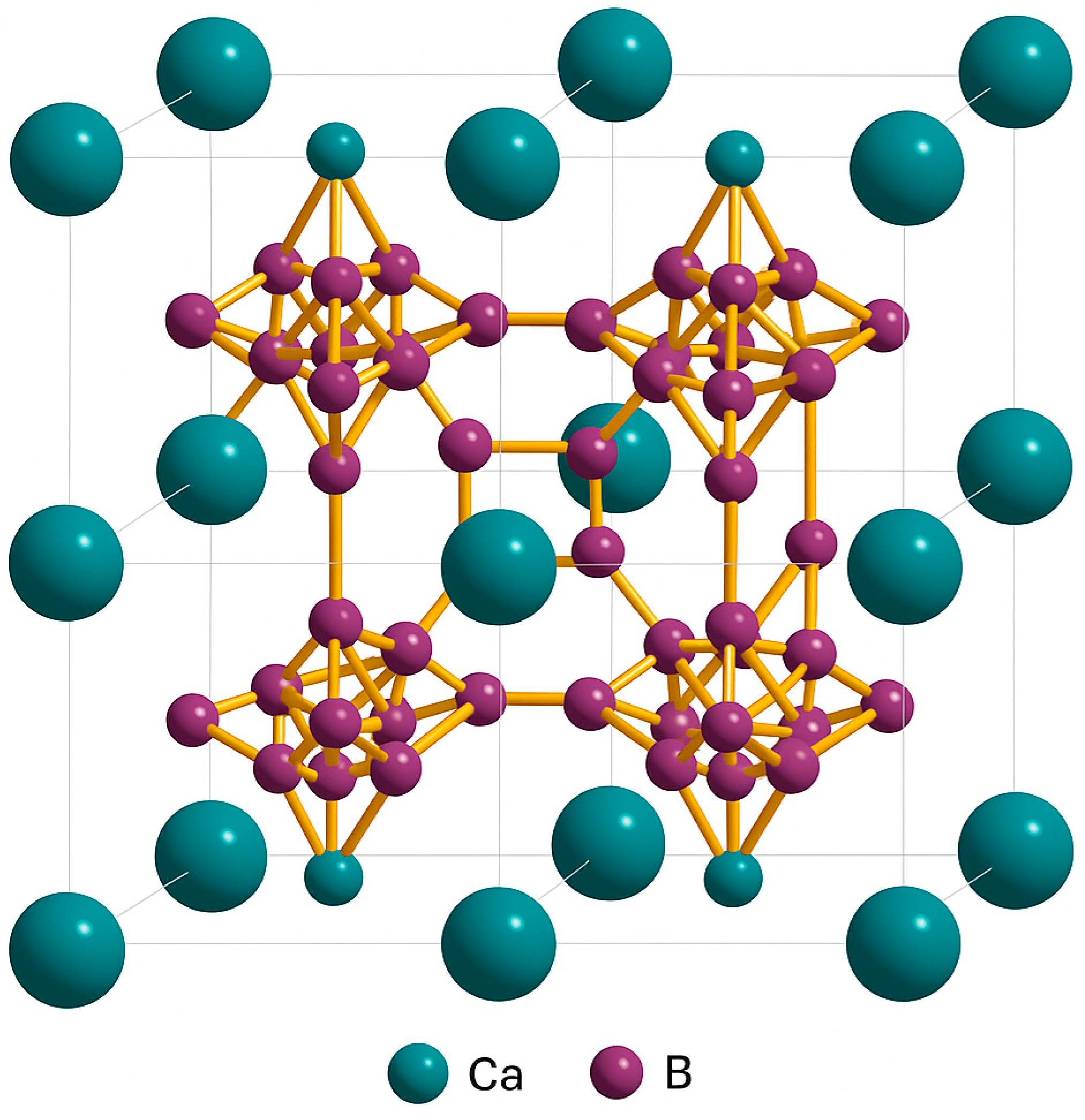
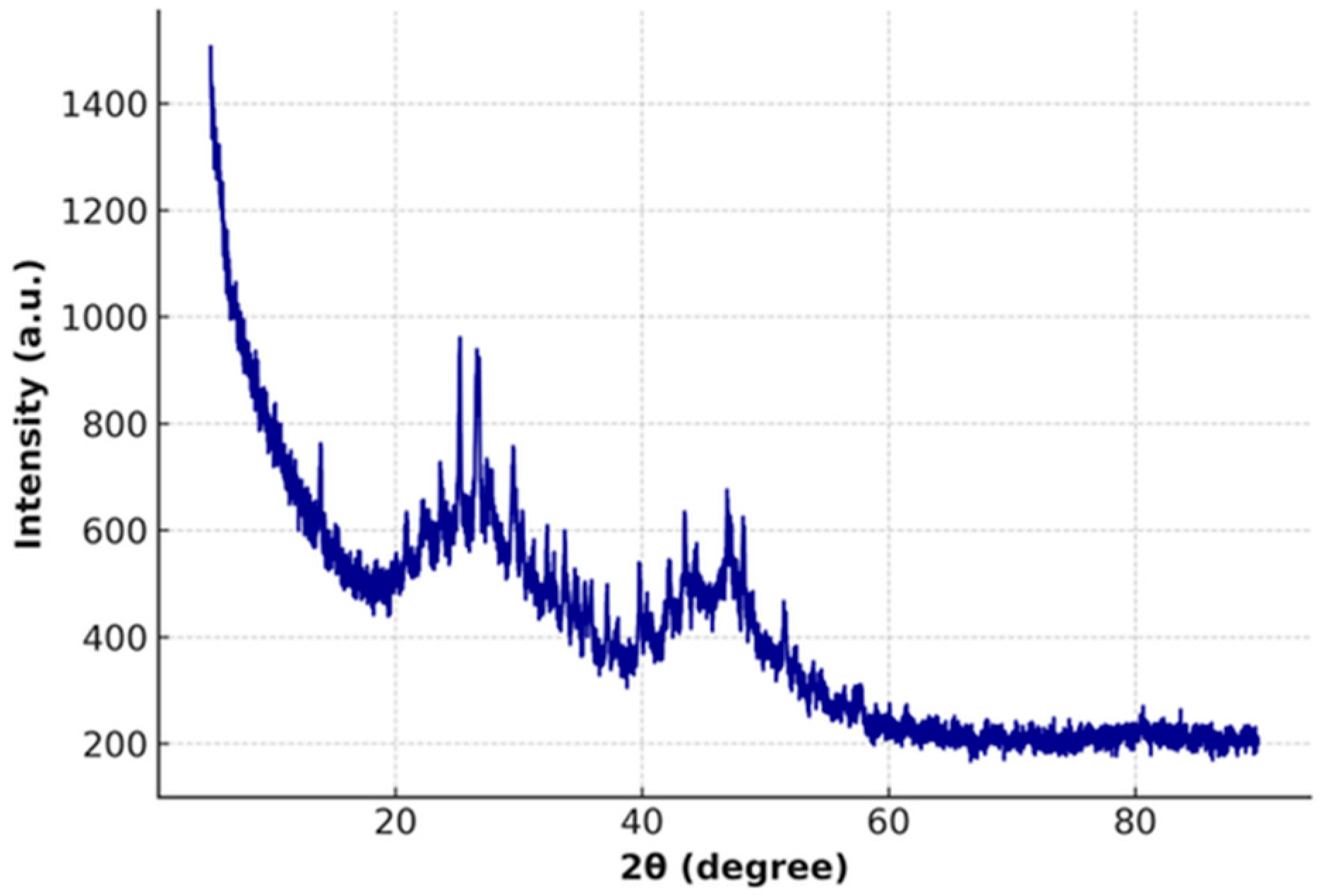
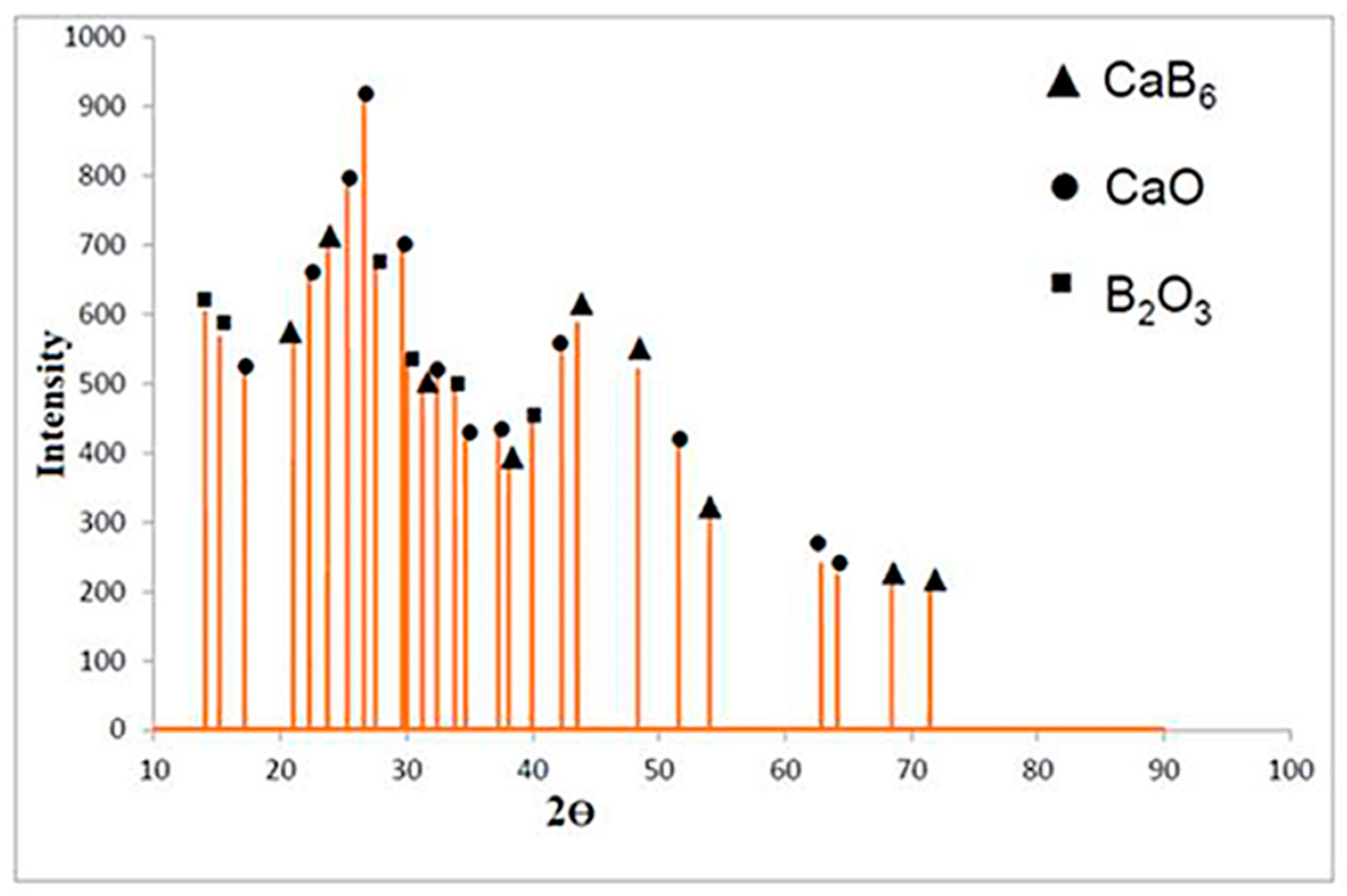
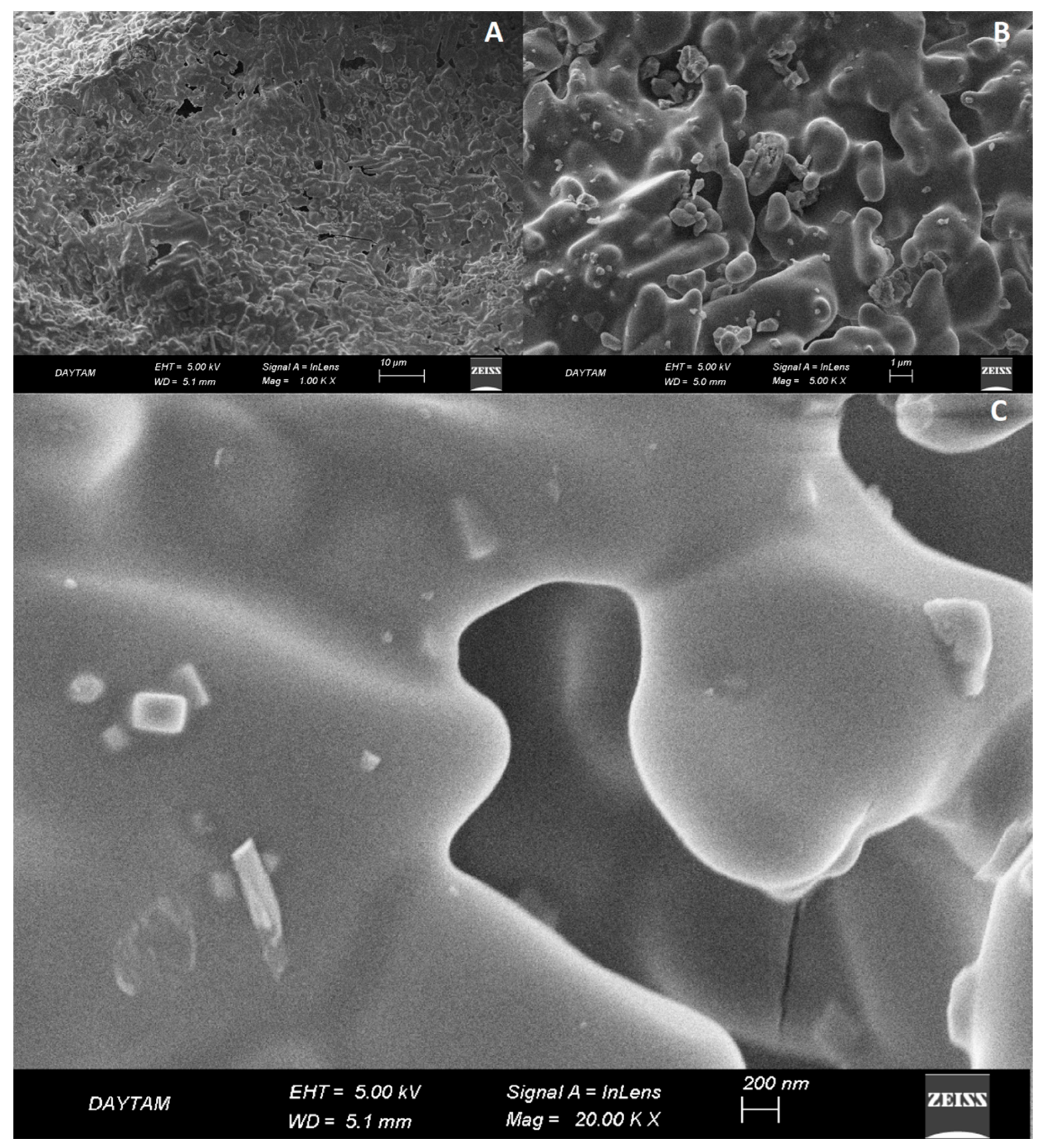
3.1. XRD Analysis
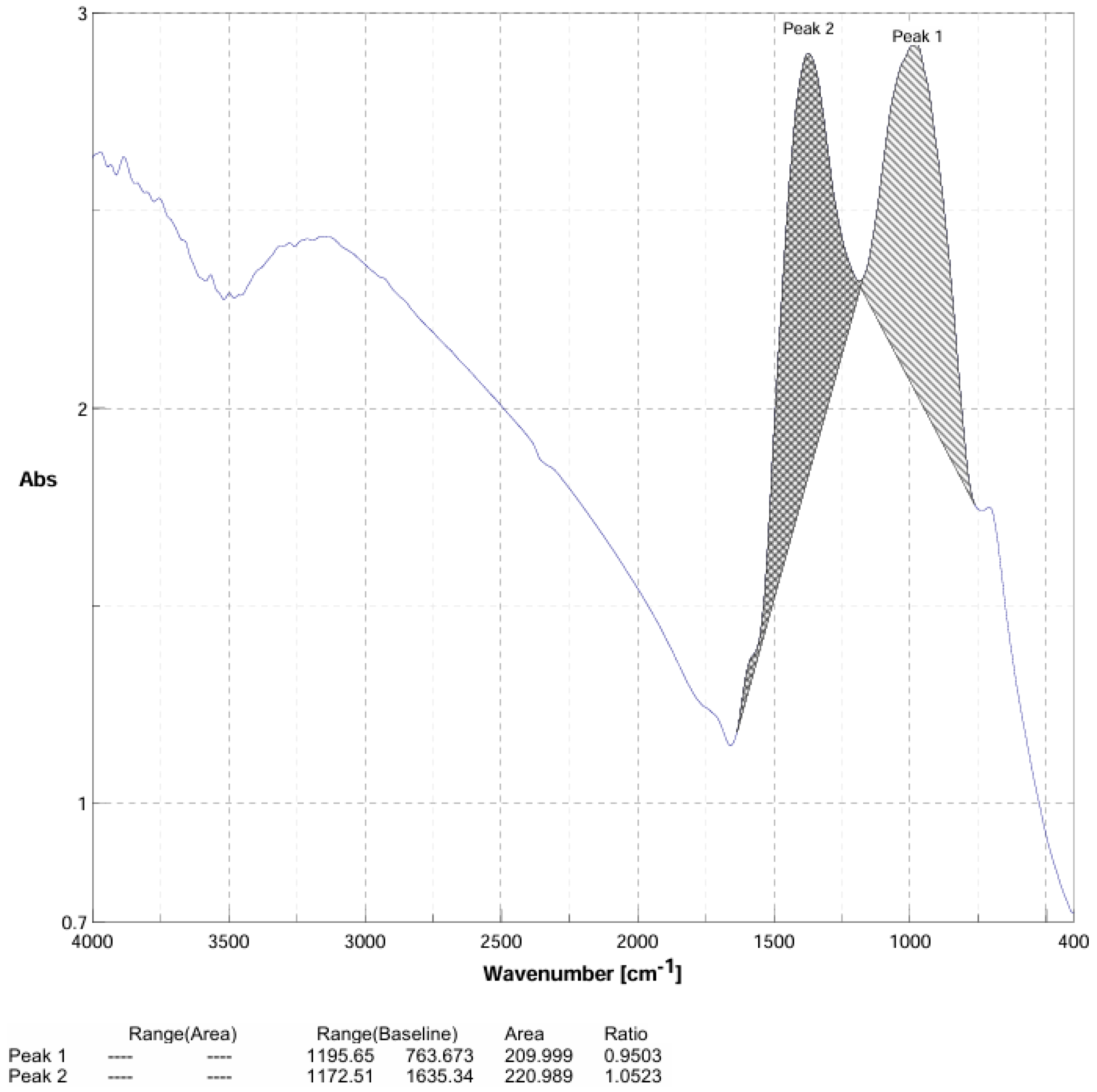
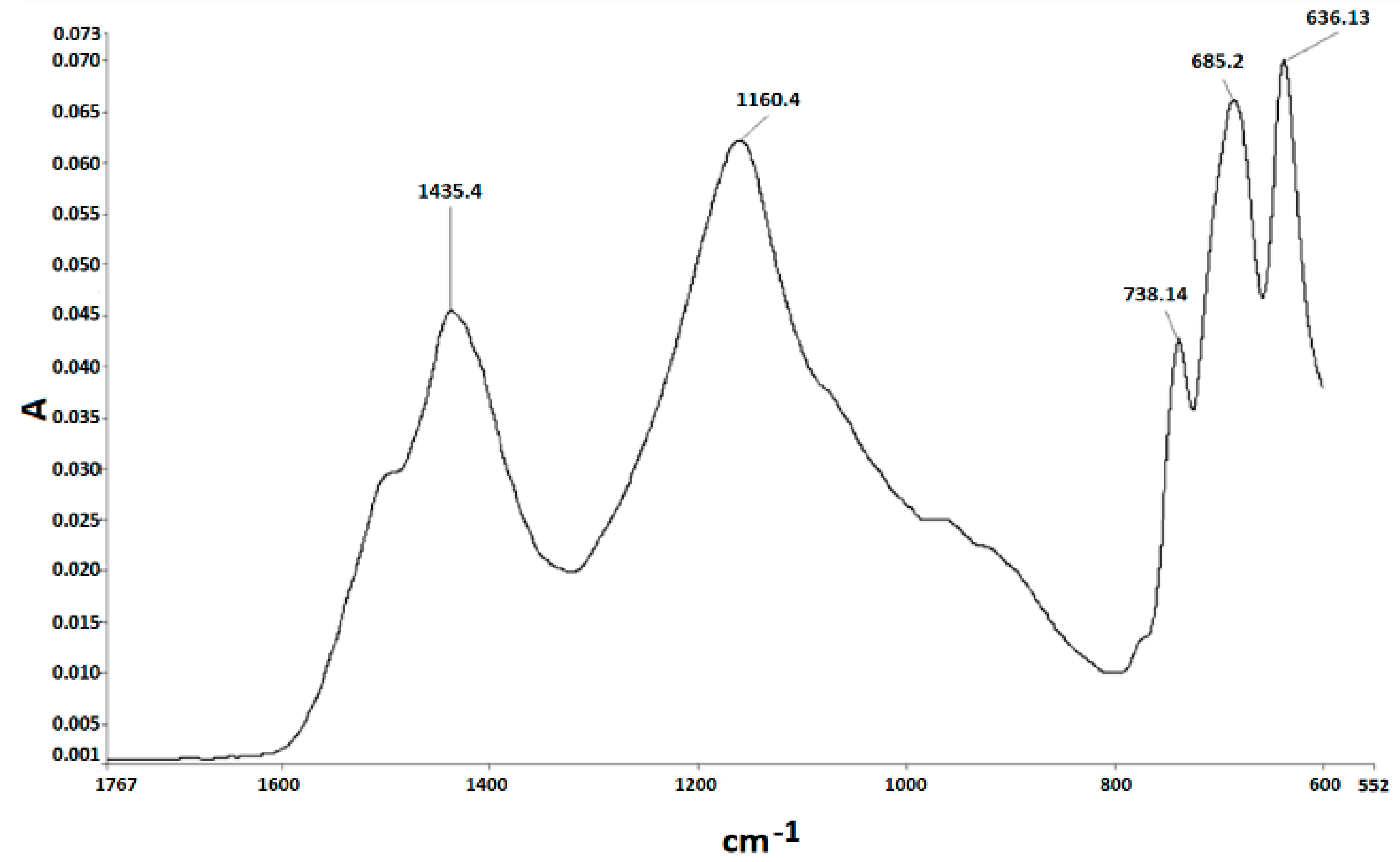
3.2. FT-IR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vepřek, S.; Reiprich, S. A concept for the design of novel superhard coatings. Thin Solid Films 1995, 268, 64–71. [Google Scholar] [CrossRef]
- Rogl, P.; Schobinger-Papamantellos, P.; Yvon, K. Crystal chemistry and stability of metal borides. In Handbook on the Physics and Chemistry of Rare Earth; Gschneidner, K.A., Jr., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 18, pp. 153–236. [Google Scholar]
- Okada, S.; Atoda, N. Thermoelectric properties of hexaborides. J. Appl. Phys. 1990, 67, 3653–3657. [Google Scholar]
- Hayashi, K. Neutron absorption and metallurgical use of borides. J. Nucl. Mater. 1995, 223, 50–58. [Google Scholar]
- Gourdon, O. Structure of alkaline earth hexaborides. J. Solid State Chem. 2004, 177, 500–505. [Google Scholar]
- Lafferty, J.M. Boron Compounds; Academic Press: New York, NY, USA, 1958. [Google Scholar]
- Emin, D.; Aselage, T.L. Electrical properties of CaB6. Phys. Rev. B 2000, 61, 13454–13460. [Google Scholar]
- Jha, A. Protective coatings based on borides. Surf. Coat. Technol. 2005, 200, 293–298. [Google Scholar]
- Suryanarayana, C. Hexaborides as electrode materials. J. Mater. Sci. 2002, 37, 399–407. [Google Scholar]
- Chen, Z. High-temperature behavior of CaB6. J. Alloys Compd. 2008, 460, 268–273. [Google Scholar] [CrossRef]
- Çakanyıldırım, Ç.; Gürü, M. Supported CoCl2 catalyst for NaBH4 dehydrogenation. Renew. Energy 2010, 35, 839–844. [Google Scholar] [CrossRef]
- Yang, S.; Ba, R.; Hong, J.; Li, J.; Guo, J.; He, X.; Zhang, H.; Yi, N.; Ma, W. Modification of LaB6 with ZrO2–Al2O3–TiO2 for Improvement of Density and Mechanical and Electrical Properties. Crystals 2024, 14, 452. [Google Scholar] [CrossRef]
- Auvray, T.; Friščić, T. Shaking Things from the Ground-Up: A Systematic Overview of the Mechanochemistry of Hard and High-Melting Inorganic Materials. Molecules 2023, 28, 897. [Google Scholar] [CrossRef] [PubMed]
- Balcı, Ö. Mechanochemical synthesis of CaB6 from Ca/B2O3. J. Alloys Compd. 2012, 520, 109–115. [Google Scholar]
- Kalaycıoğlu, H. Sintering and high-temperature stability of nano-CaB6. Ceram. Int. 2010, 36, 1793–1798. [Google Scholar] [CrossRef]
- Yıldız, R.; Telle, R.; Schmalzried, C.; Kaiser, A. Phase transformation of transient B4C to CaB6 during production of CaB6 from colemanite. J. Eur. Ceram. Soc. 2005, 25, 3375–3381. [Google Scholar] [CrossRef]
- Higashi, I. Carbothermal synthesis of CaB6. J. Am. Ceram. Soc. 1986, 69, 740–746. [Google Scholar]
- Kakiage, M. Carbothermal reduction of CaCO3–H3BO3 for CaB6 synthesis. J. Ceram. Soc. Japan 2015, 123, 234–240. [Google Scholar]
- Xu, C.N. Electrochemical synthesis of CaB6. J. Electrochem. Soc. 2009, 156, E1–E7. [Google Scholar]
- Otani, S. Floating-zone growth of CaB6. J. Cryst. Growth 1993, 128, 1081–1084. [Google Scholar]
- Ciomaga Hatnean, M.; Ahmad, T.; Walker, M.; Lees, M.R.; Balakrishnan, G. Crystal Growth by the Floating Zone Method of Ce-Substituted Crystals of the Topological Kondo Insulator SmB6. Crystals 2020, 10, 827. [Google Scholar] [CrossRef]
- Emin, D. Thermal and electrical transport in CaB6 and related borides. J. Phys. Chem. Solids 2002, 63, 2045–2054. [Google Scholar]
- Balcı, Ö. Mechanochemical reaction kinetics of CaB6 formation. Mater. Chem. Phys. 2013, 138, 932–940. [Google Scholar]
- Chakta, S. Gel-derived precursors for CaB6 synthesis. J. Am. Ceram. Soc. 2016, 99, 2131–2139. [Google Scholar]
- Karabulut, A.F.; Guru, M.; Boynueğri, T.A.; Aydin, M.Y. Synthesis of Ca(BH4)2 from Synthetic Colemanite Used in Hydrogen Storage by Mechanochemical Reaction. J. Electron. Mater. 2016, 45, 3957–3963. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Wang, Z.; Ma, Y.; Liu, H. Rapid preparation of CaB6 powders via induction heating from low-cost colemanite and petroleum coke. Ceram. Int. 2018, 44, 14070–14075. [Google Scholar] [CrossRef]
- Yahia, Z. Spectroscopic investigation of lattice vacancies in hexaborides. J. Raman Spectrosc. 1993, 24, 207–212. [Google Scholar] [CrossRef]

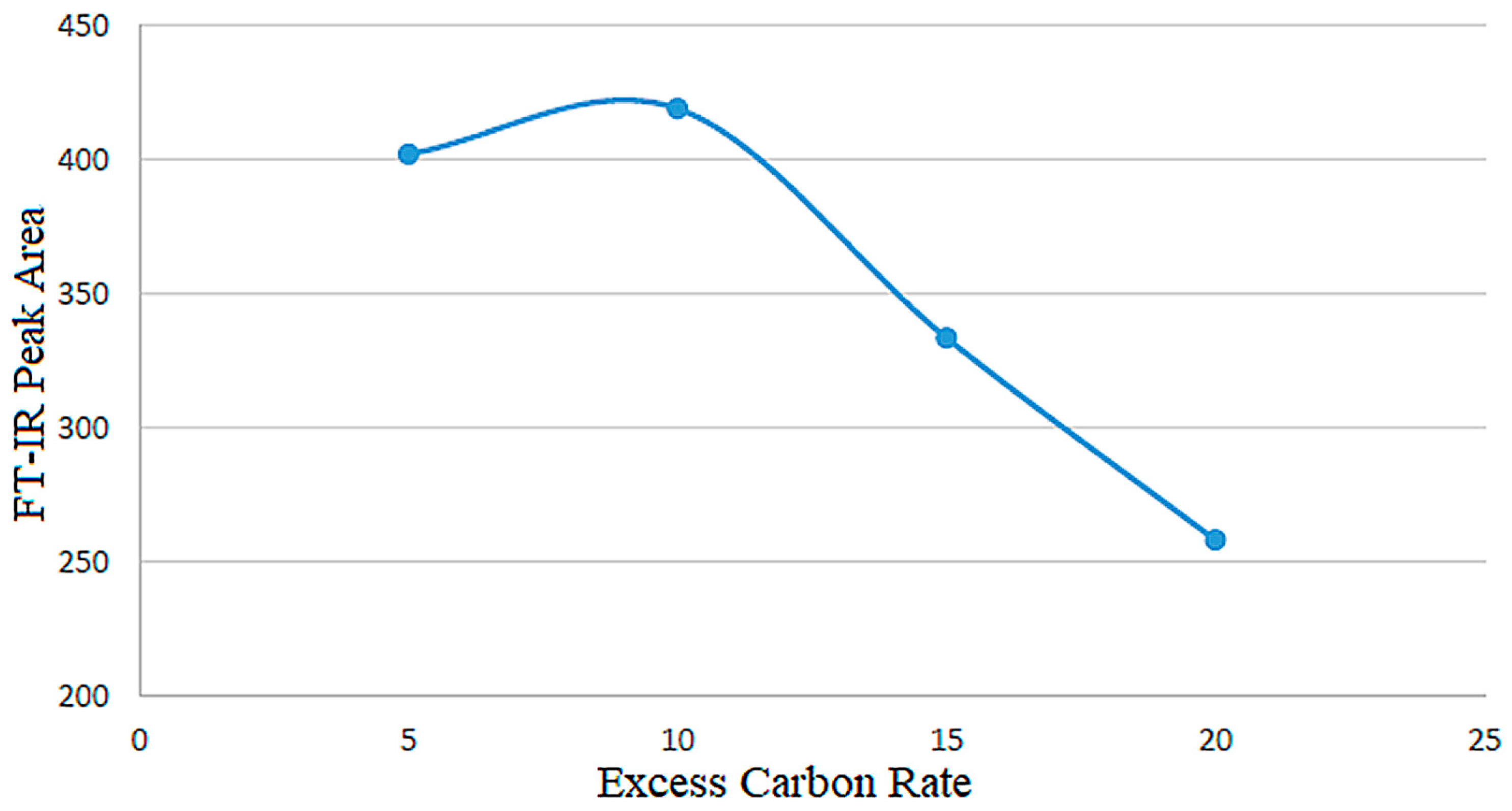
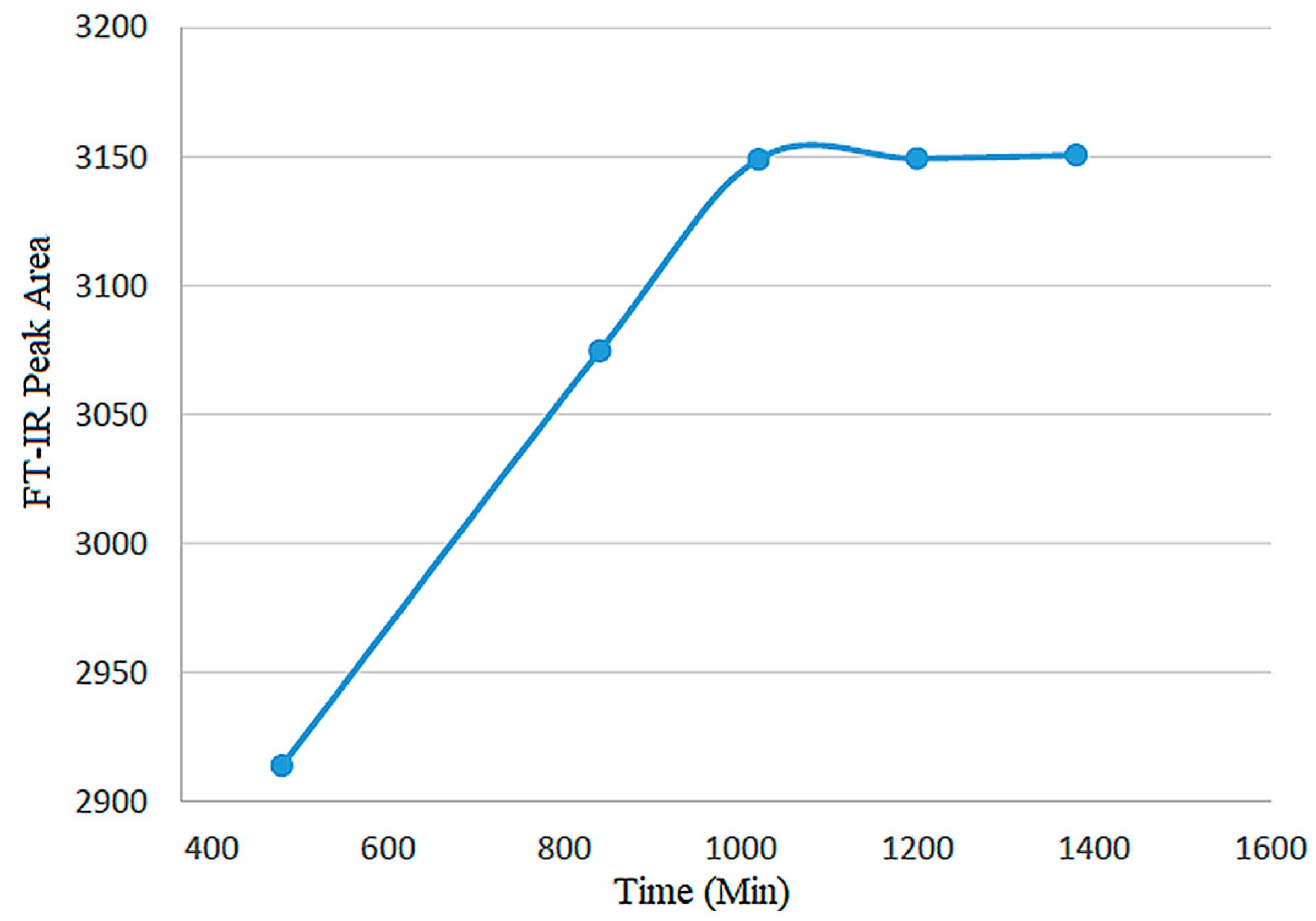
| Experiment | Carbon/Colemanite Ratio | Time (min) | FT-IR Peak Area (a.u.) |
|---|---|---|---|
| Set A | 5:1 (stoichiometric) | 840 | 402 |
| Set B | 10:1 (excess, 2× sto.) | 840 | 420 |
| Set C | 15:1 (excess, 3× sto.) | 840 | 335 |
| Set D | 20:1 (excess, 4× sto.) | 840 | 260 |
| Experiment | Time (min) | 10:1 (2× Stoichiometric, CO2 Basis) | FT-IR Peak Area (a.u.) |
|---|---|---|---|
| Set 1 | 600 | 10:1 | 2920 |
| Set 2 | 800 | 10:1 | 3090 |
| Set 3 | 960 | 10:1 | 3145 |
| Set 4 | 1020 | 10:1 | 3149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasemin, A.; Karabulut, A.F. Calcium Hexaboride Synthesis from Anhydrous Colemanite by Mechanochemical Method. Crystals 2025, 15, 837. https://doi.org/10.3390/cryst15100837
Yasemin A, Karabulut AF. Calcium Hexaboride Synthesis from Anhydrous Colemanite by Mechanochemical Method. Crystals. 2025; 15(10):837. https://doi.org/10.3390/cryst15100837
Chicago/Turabian StyleYasemin, Aylin, and Ahmet F. Karabulut. 2025. "Calcium Hexaboride Synthesis from Anhydrous Colemanite by Mechanochemical Method" Crystals 15, no. 10: 837. https://doi.org/10.3390/cryst15100837
APA StyleYasemin, A., & Karabulut, A. F. (2025). Calcium Hexaboride Synthesis from Anhydrous Colemanite by Mechanochemical Method. Crystals, 15(10), 837. https://doi.org/10.3390/cryst15100837






